Abstract
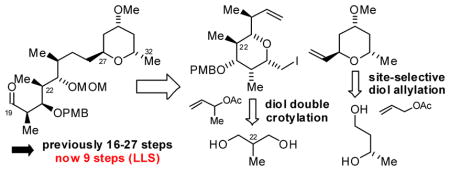
Using stereo- and site-selective C-H allylation and crotylation of unprotected diols, an intermediate in the synthesis of premisakinolide A (bistheonellic acid B) that was previously made in 16–27 (LLS) steps is now prepared in only 9 steps. This fragment also represents a synthesis of C(19)-C(32) of the actin-binding macrodiolide swinholide A.
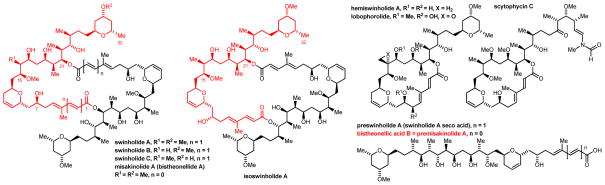
The efficacy of anti-cancer agents that perturb microtubule dynamics (e.g. paclitaxel, docetaxel, ixabepilone, eribulin mesylate) 1 suggests actin-binding agents hold promise for cancer therapy.2 Swinholide A, an actin-binding marine polyketide isolated in 1985 from the Okinawen marine sponge Theonella swinhoei, 3a was first assumed to be secreted by symbiotic cyanobacteria3c but was later shown using Palauan specimens of the sponge to be linked to the presence heterotrophic of unicellular bacteria. 4 Swinholide A was initially mistaken for the monomeric macrolide hemiswinholide A2a until subsequent work3b–e revealed it to be a 44-membered macrodiolide (Figure 1). Other members of this class have been isolated (swinholides B-K, ankaraholides A and B), 5 including isoswinholide5a and their presumed biogenic precursor preswinholide (swinholide A seco acid).6 Many marine natural products, the miskinolides (bistheonellides)7 and scytophycin C,8 have structural features in common with swinholide A (Figure 1).
Figure 1.
Swinholide A and selected naturally occurring congeners.a
aFor the structures of swinholides D–K, see reference 5.
Swinholide A is the most potent member of its class, with cytotoxicity against diverse tumor cell lines in the ng/mL range9 due to its ability to dimerize actin (Kd ~ 50 nM)10a and cleave actin filaments.10 High levels of potency are critically dependent on the symmetric 44-membered diolide ring of swinholide A, as congeners such as isoswinholide A are significantly less potent.9 A high resolution (2Ǻ) crystal structure of swinholide A bound to two actin molecules has been obtained,10c enabling the rational design of simplified actin-binding compounds.11
Total syntheses of swinholide A were reported by Paterson 12 and Nicolaou. 13 The total synthesis of preswinholide A was reported by Nakata,14 and numerous syntheses of swinholide A substructures have been disclosed.15 Broadly speaking, these syntheses all exploit classical carbonyl additions involving stoichiometric organometallic reagents. We have developed catalytic enantioselective methods that enable direct stereo- and site-selective conversion of lower alcohols to higher alcohols.16 These methods streamline or eliminate protecting group manipulations and discrete alcohol-to-carbonyl redox reactions, providing the most concise routes ever reported to diverse polyketide natural products.16b Here, we apply these methods to the construction of the C(19)-C(32) fragment of swinholide A and the formal synthesis of premisakinolide A (bistheonellic acid B),15h the monomer of the macrodiolide misakinolide A (bistheonellide A).7
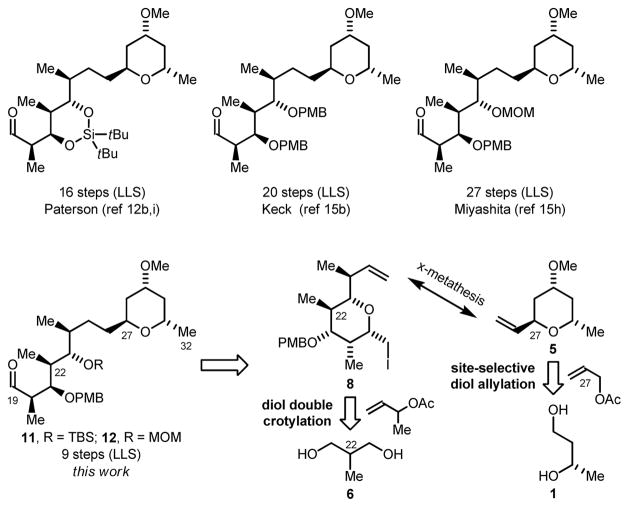
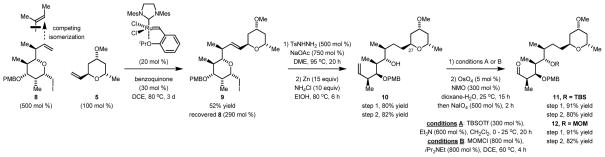
Our retrosynthetic analysis of premisakinolide A (bistheonellic acid B) and the C(19)-C(32) substructure of swinholide A is as follows (Scheme 1). Aldehydes 11 and 12 were envisioned to arise through cross-metathesis of vinyl pyran 5 with iodoether 8. The synthesis of vinyl pyran 5 takes advantage of site-selective C-allylation of (S)-1,3-butane diol 1.17 The iodoether 8 is readily prepared from 2-methyl-1,3-propane diol 6 via bidirectional enantioselective double anti-crotylation. 18 The strategic advantage of these methods is borne out by the brevity our route, which delivers Miyashita’s premisakinolide A (bistheonellic acid B) intermediate 1215h in 9 steps – a substructure previously prepared in 16–27 steps.12b,i,15b,15h
Scheme 1.
Retrosynthetic analysis of premisakinolide A (bistheonellic acid B) and C(19)-C(32) of swinholide A and prior fragment syntheses.a
aFor graphical summaries of prior total syntheses, see Supporting Information. Longest Linear Sequence (LLS). The carbon numbering scheme of swinholide A is used.
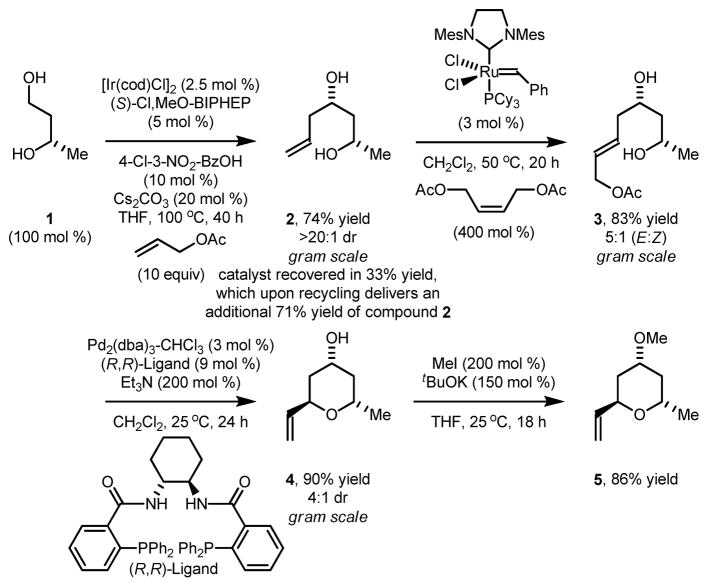
The synthesis of vinyl pyran 5 begins with the established stereo- and site-selective allylation of commercially available (S)-1,3-butane diol 1.17 Exposure of the resulting homoallylic alcohol 2 to the second generation Grubbs catalyst in the presence of cis-1,4-diacetoxy-2-butene delivered the product of cross metathesis 3 as a 5:1 mixture alkene geometrical isomers.19 Tsuji-Trost cyclization of 3 using the chiral palladium catalyst modified by the indicated chiral ligand delivered the 2,6-trans-disubstituted pyran 4 in 99% yield as a 4:1 mixture of diastereomers. As previously established, the presence of alkene geometrical isomers at the stage of compound 3 does not influence diastereoselectivity in the cyclization to form pyran 4. While pyran 4 was previously prepared in our laboratory,17 the present route is an improved gram-scale synthesis with recycling of the iridium catalyst. Finally, methylation of the 4-hydroxyl moiety delivers vinyl pyran 5 (Scheme 2).
Scheme 2.
Synthesis of vinyl pyran 5 via site-selective allylation of diol 1.a
aCited yields are of diastereomeric mixtures isolated by silica gel chromatography. Diastereomeric ratios were determined by 1H NMR analysis of crude reaction mixtures. See Supporting Information for further experimental details, including recovery and recycling of the iridium catalyst.
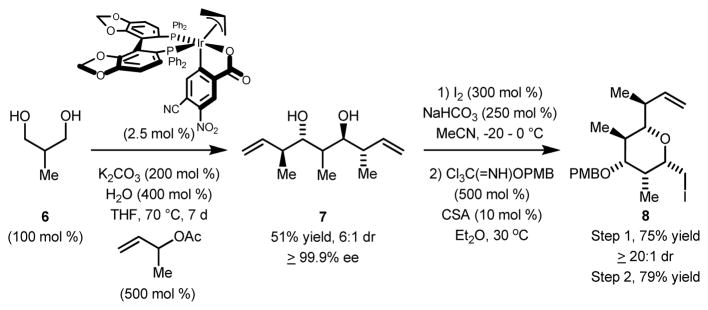
The PMB-protected iodoether 8 was prepared was prepared by a procedure previously established in our laboratory (Scheme 3).18 Specifically, diol 6 was subjected to double anti-crotylation to provide 7, which incorporates a triketide stereopolyad. A single enantiomer of 7 is formed due to Horeau’s principle. 20 That is, the minor diastereomer of the mono-adduct is converted predominantly to the meso-stereoisomer. 21 In the conversion of diol 6 to adduct 7, it was found that use of α-methyl allyl acetate prepared from the corresponding alcohol using acetic anhydride and triethylamine (rather than pyridine) gave optimal results. Iodoetherification differentiates the diol and terminal olefin moieties and defines the chirotopic nonstereogenic center at C(22). Conversion of the remaining free hydroxyl to the PMB-ether delivers compound 8.
Scheme 3.
Synthesis of iodoether 8.a
aCited yields are of diastereomeric mixtures isolated by silica gel chromatography. Diastereomeric ratios were determined by 1H NMR analysis of crude reaction mixtures. See Supporting Information for further experimental details.
With vinyl pyran 5 and iodoether 8 in hand, conversion to aldehydes 11 and 12 (Miyashita’s intermediate) was attempted. The cross-metathesis of vinyl pyran 5 and iodoether 8 was especially challenging due to competing isomerization of the terminal olefin of iodoether 8 to the internal olefin. Although related cross-metatheses of vinyl pyrans are known, 22 they involve equatorially disposed vinyl moieties, unlike the vinyl moiety of pyran 5. After considerable optimization, it was found that use of the second generation Grubbs-Hoveyda catalyst in combination with 1,4-benzoquinone23 delivered the desired product of metathesis 9 in 52% yield. Hydrogenation of the C(25)-C(26) double bond of compound 9 using palladium on carbon resulted in partial hydrogenation of the carbon-iodine bond. Use of Crabtree’s catalyst24 prevented this side reaction but led to complete epimerization of C(27). In contrast, diimide-mediated hydrogenation of the C(25)-C(26) double bond occurred smoothly. 25 Subsequent Bernet-Vasella cleavage26 of the iodoether delivered the terminal olefin 10, which upon TBS-protection followed by Johnson-Lemieux oxidative cleavage provides the aldehyde 11. Similarly, Miyashita’s premisakinolide A (bistheonellic acid B) intermediate 12 is prepared through MOM-protection followed by alkene oxidative cleavage in a total of 9 steps (LLS) from (S)-1,3-butane diol 1.
In summary, using technology for the direct stereo- and site-selective conversion of lower alcohols to higher alcohols via C-C bond forming transfer hydrogenation, we report a formal synthesis of premisakinolide A (bistheonellic acid B). The intercepted substructure, which was previously made in 16–27 steps (LLS),12b,i,15b,15h is now made in only 9 steps (LLS). This fragment also represents a synthesis of C(19)-C(32) of the actin-binding macrodiolide swinholide A. This study, along with prior work from our laboratory,16b highlights the strategic advantages of site-selectivity and redox-economy in chemical synthesis.
Supplementary Material
Scheme 4.
Formal synthesis of premisakinolide A (bistheonellic acid B) and synthesis of the C(19)-C(32) substructure of swinholide A.a
aCited yields are of diastereomeric mixtures isolated by silica gel chromatography. Diastereomeric ratios were determined by 1H NMR analysis of crude reaction mixtures. See Supporting Information for further experimental details.
Acknowledgments
Acknowledgment is made to the Robert A. Welch Foundation (F-0038), the NIH-NIGMS (RO1-GM093905) and the Center for Green Chemistry and Catalysis for partial support of this research.
Footnotes
Supporting Information Available. Spectral data for all new compounds (1H NMR, 13C NMR, IR, HRMS). This material is available free of charge via the internet at http://pubs.acs.org.
References
- 1.For reviews, see: Jordan MA, Wilson L. Nat Rev Cancer. 2004;4:253. doi: 10.1038/nrc1317.Dumontet C, Jordan MA. Nat Rev Drug Discovery. 2010;9:790. doi: 10.1038/nrd3253.Rohena CC, Mooberry SL. Nat Prod Rep. 2014;31:335. doi: 10.1039/c3np70092e.
- 2.For a review, see: Spector I, Braet F, Shochet NR, Bubb MR. Microsc Res Tech. 1999;47:18. doi: 10.1002/(SICI)1097-0029(19991001)47:1<18::AID-JEMT3>3.0.CO;2-E.Yeung KS, Paterson I. Angew Chem Int Ed. 2002;41:4632. doi: 10.1002/anie.200290057.Giganti A, Friederich E. Prog Cell Cycle Res. 2003;5:511.Kita M, Kigoshi H. Nat Prod Rep. 2015;32:534. doi: 10.1039/c4np00129j.
- 3.For isolation and structure determination of swinholide A, see: Carmely S, Kashman Y. Tetrahedron Lett. 1985;26:511.Kobayashi M, Tanaka J, Katori T, Matsuura M, Kitagawa I. Tetrahedron Lett. 1989;30:2963.Kitagawa I, Kobayashi M, Katori T, Yamashita M, Tanaka J, Doi M, Ishida T. J Am Chem Soc. 1990;112:3710.Kobayashi M, Tanaka J, Katori T, Matsuura M, Yamashita M, Kitagawa I. Chem Pharm Bull. 1990;38:2409. doi: 10.1248/cpb.38.2960.Doi M, Ishida T, Kobayashi M, Kitagawa I. J Org Chem. 1991;56:3629.
- 4.Bewley CA, Holland ND, Faulkner DJ. Experientia. 1996;52:716. doi: 10.1007/BF01925581. [DOI] [PubMed] [Google Scholar]
- 5.Swinholides B-K: Kobayashi M, Tanaka J-i, Katori T, Kitagawa I. Chem Pharm Bull. 1990;38:2960. doi: 10.1248/cpb.38.2960.Tsukamoto S, Ishibashi M, Sasaki T, Kobayashi J-i. J Chem Soc Perkin Trans. 1991 Jan;:3185.Dumdei EJ, Blunt JW, Munro MHG, Pannell LK. J Org Chem. 1997;62:2636. doi: 10.1021/jo961745j.Youssef DTA, Mooberry SL. J Nat Prod. 2006;69:154. doi: 10.1021/np050404a.De Marino S, Festa C, D’Auria MV, Cresteil T, Debitus C, Zampella A. Mar Drugs. 2011;9:1133. doi: 10.3390/md9061133.Sinisi A, Calcinai B, Cerrano C, Dien HA, Zampella A, D’Amore C, Renga B, Fiorucci S, Taglialatela-Scafati O. Bioorg Med Chem. 2013;21:5332. doi: 10.1016/j.bmc.2013.06.015.Andrianasolo EH, Gross H, Goeger D, Musafija-Girt M, McPhail K, Leal RM, Mooberry SL, Gerwick WH. Org Lett. 2005;7:1375. doi: 10.1021/ol050188x.
- 6.Todd JS, Alvi KA, Crews P. Tetrahedron Lett. 1992;33:441. [Google Scholar]
- 7.(a) Sakai R, Higa T, Kashman Y. Chem Lett. 1986:1499. [Google Scholar]; (b) Kato Y, Fusetani N, Matsunaga S, Hashimoto K, Sakai R, Higa T, Kashman Y. Tetrahedron Lett. 1987;28:6225. [Google Scholar]; (c) Kobayashi J, Tsukamoto S, Tanabe A, Sasaki T, Ishibashi M. J Chem Soc Perkin Trans. 1991 Jan;:2379. [Google Scholar]
- 8.(a) Moore RE, Patterson GML, Mynderse JS, Barchi J, Jr, Norton TR, Furusawa E, Furusawa S. Pure Appl Chem. 1986;58:263. [Google Scholar]; (b) Ishibashi M, Moore RE, Patterson GML, Xu C, Clardy J. J Org Chem. 1986;51:5300. [Google Scholar]
- 9.Kobayashi M, Kawazoe K, Okamoto T, Sasaki T, Kitagawa I. Chem Pharm Bull. 1994;42:19. doi: 10.1248/cpb.42.19. [DOI] [PubMed] [Google Scholar]
- 10.(a) Bubb MR, Spector I, Bershadsky AD, Korn ED. J Biol Chem. 1995;270:3463. doi: 10.1074/jbc.270.8.3463. [DOI] [PubMed] [Google Scholar]; (b) Saito S-y, Karaki H. Clin Exp Pharmacol Physiol. 1996;23:743. doi: 10.1111/j.1440-1681.1996.tb01770.x. [DOI] [PubMed] [Google Scholar]; (c) Klenchin VA, King R, Tanaka J, Marriott G, Rayment I. Chem Biol. 2005;12:287. doi: 10.1016/j.chembiol.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 11.(a) Perrins RD, Cecere G, Paterson I, Marriott G. Chem Biol. 2008;15:287. doi: 10.1016/j.chembiol.2008.01.010. [DOI] [PubMed] [Google Scholar]; (b) Herkommer D, Dreisigacker S, Sergeev G, Sasse F, Gohlke H, Menche D. ChemMedChem. 2015;10:470. doi: 10.1002/cmdc.201402508. and references cited therein. [DOI] [PubMed] [Google Scholar]
- 12.(a) Paterson I. Pure Appl Chem. 1992;64:1821. [Google Scholar]; (b) Paterson I, Cumming JG. Tetrahedron Lett. 1992;33:2847. [Google Scholar]; (c) Paterson I, Smith JD. J Org Chem. 1992;57:3261. [Google Scholar]; (d) Paterson I, Smith JD. Tetrahedron Lett. 1993;34:5351. [Google Scholar]; (e) Paterson I, Cumming JG, Smith JD, Ward RA. Tetrahedron Lett. 1994;35:441. [Google Scholar]; (f) Paterson I, Cumming JG, Smith JD, Ward RA, Yeung KS. Tetrahedron Lett. 1994;35:3405. [Google Scholar]; (g) Paterson I, Smith JD, Ward RA, Cumming JG. J Am Chem Soc. 1994;116:2615. [Google Scholar]; (h) Paterson I, Yeung KS, Ward RA, Cumming JG, Smith JD. J Am Chem Soc. 1994;116:9391. [Google Scholar]; (i) Paterson I, Cumming JG, Ward RA, Lamboley S. Tetrahedron. 1995;51:9393. [Google Scholar]; (j) Paterson I, Smith JD, Ward RA. Tetrahedron. 1995;51:9413. [Google Scholar]; (k) Paterson I, Ward RA, Smith JD, Cumming JG, Yeung KS. Tetrahedron. 1995;51:9437. [Google Scholar]; (l) Paterson I, Yeung KS, Ward RA, Smith JD, Cumming JG, Lamboley S. Tetrahedron. 1995;51:9467. [Google Scholar]
- 13.(a) Patron AP, Richter PK, Tomaszewski MJ, Miller RA, Nicolaou KC. J Chem Soc Chem Commun. 1994:1147. [Google Scholar]; (b) Richter PK, Tomaszewski MJ, Miller RA, Patron AP, Nicolaou KC. J Chem Soc Chem Commun. 1994:1151. [Google Scholar]; (c) Nicolaou KC, Patron AP, Ajito K, Richter PK, Khatuya H, Bertinato P, Miller RA, Tomaszewski MJ. Chem Eur J. 1996;2:847. [Google Scholar]; (d) Nicolaou KC, Ajito K, Patron AP, Khatuya H, Richter PK, Bertinato P. J Am Chem Soc. 1996;118:3059. [Google Scholar]
- 14.(a) Nakata T, Komatsu T, Nagasawa K. Chem Pharm Bull. 1994;42:2403. [Google Scholar]; (b) Nakata T, Komatsu T, Nagasawa K, Yamada H, Takahashi T. Tetrahedron Lett. 1994;35:8225. [Google Scholar]; (c) Nagasawa K, Shimizu I, Nakata T. Tetrahedron Lett. 1996;37:6881. [Google Scholar]; (d) Nagasawa K, Shimizu I, Nakata T. Tetrahedron Lett. 1996;37:6885. [Google Scholar]
- 15.(a) Mulzer J, Meyer F, Buschmann J, Luger P. Tetrahedron Lett. 1995;36:3503. [Google Scholar]; (b) Keck GE, Lundquist GD. J Org Chem. 1999;64:4482. [Google Scholar]; (c) Kartika R, Frein JD, Taylor RE. J Org Chem. 2008;73:5592. doi: 10.1021/jo800704d. [DOI] [PubMed] [Google Scholar]; (d) Hayakawa H, Miyashita M. J Chem Soc, Perkin Trans. 1999 Jan;:3399. [Google Scholar]; (e) Hayakawa H, Iida K, Miyazawa M, Miyashita M. Chem Lett. 1999:601. [Google Scholar]; (f) Hayakawa H, Miyashita M. Tetrahedron Lett. 2000;41:707. [Google Scholar]; (g) Nakamura R, Tanino K, Miyashita M. Org Lett. 2003;5:3579. doi: 10.1021/ol035227o. [DOI] [PubMed] [Google Scholar]; (h) Nakamura R, Tanino K, Miyashita M. Org Lett. 2005;7:2929. doi: 10.1021/ol050864v. [DOI] [PubMed] [Google Scholar]
- 16.Selected reviews: Ketcham JM, Shin I, Montgomery TP, Krische MJ. Angew Chem Int Ed. 2014;53:9142. doi: 10.1002/anie.201403873.Dechert-Schmitt AMR, Schmitt DC, Gao X, Itoh T, Krische MJ. Nat Prod Rep. 2014;31:504. doi: 10.1039/c3np70076c.
- 17.Shin I, Wang G, Krische MJ. Chem Eur J. 2014;20:13382. doi: 10.1002/chem.201404065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.(a) Gao X, Han H, Krische MJ. J Am Chem Soc. 2011;133:12795. doi: 10.1021/ja204570w. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gao X, Woo SK, Krische MJ. J Am Chem Soc. 2013;135:4223. doi: 10.1021/ja4008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.For related cross-metatheses, see: Lucas BS, Burke SD. Org Lett. 2003;5:3915. doi: 10.1021/ol0354775.Lee K, Kim H, Hong J. Org Lett. 2009;11:5202. doi: 10.1021/ol902125d.
- 20.Vigneron JP, Dhaenens M, Horeau A. Tetrahedron. 1973;29:1055.For a historical review, see: Heller D, Drexler H-J, Fischer C, Buschmann H, Baumann W, Heller B. Angew Chem Int Ed. 2000;39:495. doi: 10.1002/(sici)1521-3773(20000204)39:3<495::aid-anie495>3.0.co;2-g.
- 21.(a) Kogure T, Eliel EL. J Org Chem. 1984;49:576. [Google Scholar]; (b) Midland MM, Gabriel J. J Org Chem. 1985;50:1143. [Google Scholar]; (c) Poss CS, Schreiber SL. Acc Chem Res. 1994;27:9. [Google Scholar]
- 22.Ghosh AK, Cheng X, Bal R, Hamel E. Eur J Org Chem. 2012;4130 doi: 10.1002/ejoc.201200286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hong SH, Sanders DP, Lee CW, Grubbs RH. J Am Chem Soc. 2005;127:17160. doi: 10.1021/ja052939w. [DOI] [PubMed] [Google Scholar]
- 24.Review: Brown JM. Angew Chem Int Ed. 1987;26:190.
- 25.(a) Dewey RS, van Tamelen EE. J Am Chem Soc. 1961;83:3729. [Google Scholar]; (b) Zhu K, Panek JS. Org Lett. 2011;13:4652. doi: 10.1021/ol201863b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Cheung LL, Marumoto S, Anderson CD, Rychnovsky SD. Org Lett. 2008;10:3101. doi: 10.1021/ol8011474. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Kang EJ, Cho EJ, Ji MK, Lee YE, Shin DM, Choi SY, Chung YK, Kim JS, Kim HJ, Lee SG, Lah MS, Lee E. J Org Chem. 2005;70:6321. doi: 10.1021/jo0507993. [DOI] [PubMed] [Google Scholar]
- 26.(a) Bernet B, Vasella A. Helv Chim Acta. 1979;62:1990. [Google Scholar]; (b) Bernet B, Vasella A. Helv Chim Acta. 1979;62:2400. [Google Scholar]; (c) Bernet B, Vasella A. Helv Chim Acta. 1984;67:1328. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.