Abstract
This current concepts review outlines the role of different imaging modalities in the diagnosis, preoperative planning, and follow-up of osteochondral ankle defects. An osteochondral ankle defect involves the articular cartilage and subchondral bone (usually of the talus) and is mostly caused by an ankle supination trauma. Conventional radiographs are useful as an initial imaging tool in the diagnostic process, but have only moderate sensitivity for the detection of osteochondral defects. Computed tomography (CT) and magnetic resonance imaging (MRI) are more accurate imaging modalities. Recently, ultrasonography and single photon emission CT have been described for the evaluation of osteochondral talar defects. CT is the most valuable modality for assessing the exact location and size of bony lesions. Cartilage and subchondral bone damage can be visualized using MRI, but the defect size tends to be overestimated due to bone edema. CT with the ankle in full plantar flexion has been shown a reliable tool for preoperative planning of the surgical approach. Postoperative imaging is useful for objective assessment of repair tissue or degenerative changes of the ankle joint. Plain radiography, CT and MRI have been used in outcome studies, and different scoring systems are available.
Keywords: Cartilage, Subchondral bone, Imaging, Ankle, Talus, Radiography, Computed tomography, Magnetic resonance imaging, Outcome assessment
Core tip: This current concepts review aims to summarize the literature on imaging modalities in the diagnosis, preoperative planning, and follow-up of osteochondral ankle defects. There have been recent developments in this field, including the use of sophisticated methods for diagnosis [such as single photon emission computed tomography (CT)] and the use of imaging for outcome assessment (such as CT and certain magnetic resonance imaging techniques). These are all discussed in the article, which may help the reader to optimize his/her preoperative and postoperative strategy.
INTRODUCTION
Osteochondral defects (OCDs) of the ankle mostly affect the talus and involve the articular hyaline cartilage and the subchondral bone. These defects often cause deep ankle pain on weight bearing, impairing sports and daily activities of the young and active population. The diagnosis is often delayed because of low index of suspicion and the possible absence of radiographic signs on standard radiographs[1]. Deep ankle pain that persists months after supination trauma should alert the physician of a possible OCD of the talus.
Various imaging studies are available to diagnose ankle OCDs. Plain radiographs are a common initial diagnostic tool but may not depict the OCD[2]. Computed tomography (CT) and magnetic resonance imaging (MRI) are more accurate[1]. In recent years, alternative imaging methods have become available, including ultrasonography and single photon emission computed tomography (SPECT)[3,4].
The choice of treatment of OCDs depends on the duration of symptoms, the size, location, and stability of the defect, and whether it concerns a primary or secondary OCD[5]. In general, OCDs smaller than 15 mm (diameter) are amenable for arthroscopic debridement and bone marrow stimulation (BMS) techniques, while bigger or secondary OCDs (those after failed primary surgical treatment) require a more invasive strategy, such as osteochondral autograft transfer[5-7]. For preoperative planning of the surgical procedure and the surgical approach it is important to determine precisely the extent and localization of the OCD[8-10].
After treatment, not only is the subjective functional outcome of importance, but also has objective assessment of repair tissue become of interest. Plain radiography, CT and MRI have been used in outcome studies, and different scoring systems are available.
This current concepts review outlines the role of various imaging modalities in the diagnosis, preoperative planning and follow-up of osteochondral ankle defects.
DIAGNOSIS
Radiography
Conventional radiographs usually consist of anterior-posterior (AP) mortise view and lateral weight-bearing views of the ankle. The AP mortise view is not a true AP projection but the patient’s leg is projected in 15 degrees of internal rotation to optimize visualization of the ankle joint (Figure 1). The radiographs may show an area of detached bone surrounded by radiolucency but initially the damage may be too small to be visualized[11].
Figure 1.
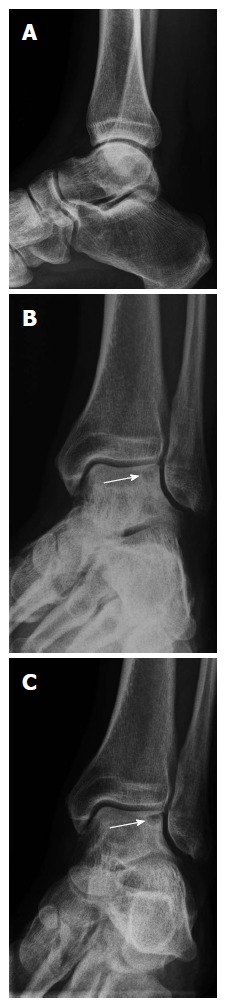
Weight-bearing radiographs [lateral (A), anterior-posterior mortise (B), and 4-cm heel-rise (C) views] showing an osteochondral defect (arrows).
Routine radiological examination fails to detect 30%-50% of OCDs[1,12-14]. Thompson and Loomer[15] hypothesized that most of the missed OCDs were located posteriorly. They developed a heel-rise view to visualize the posterior part of the talar dome. This view is projected as an AP mortise view with a heel rise of 4 cm (Figure 1). Verhagen et al[1] prospectively compared the efficacy of diagnostic methods in the evaluation of OCDs. They demonstrated that a heel-rise view doubled the diagnostic odds ratio in comparison with standard radiographs (Table 1).
Table 1.
Accuracy of different imaging techniques in diagnosing talar osteochondral defects[1]
| Sensitivity | Specificity | Positive predictive value | Negative predictive value | |
| Standard radiography | 0.59 | 0.91 | 0.70 | 0.86 |
| Heel-rise radiography | 0.70 | 0.94 | 0.79 | 0.90 |
| Computed tomography | 0.81 | 0.99 | 0.96 | 0.94 |
| Magnetic resonance imaging | 0.96 | 0.96 | 0.89 | 0.99 |
The differences between computed tomography and magnetic resonance imaging were not statistically significant (P = 0.33).
The frequent absence of radiological changes has led to the use of more sensitive methods[16].
CT
CT is a technology that uses computer-processed X-rays. A helical or spiral CT is mostly used. This permits continuous rotation of the X-ray source and detector while the patient is moved slowly through the X-ray ring. The scanning protocol for ankle CTs involves “ultra high resolution” axial slices with an increment of 0.3 mm and a thickness of 0.6 mm. Multiplanar coronal and sagittal reconstructions should be 1 mm[5].
Verhagen et al[1] showed a sensitivity and specificity of 0.81 and 0.99, respectively, for detecting OCDs on a helical CT (Table 1). The size and location of the bone defect and the detachment of a fragment can be visualized (Figure 2). CT is the most effective method for evaluating the osseous anatomy but it lacks the ability to visualize cartilage directly. However, focus on the condition of the subchondral bone plate seems more important in diagnosing and treating OCDs, because the pain of an OCD originates in the subchondral bone, and the integrity of the subchondral bone plate is crucial for the vitality of articular cartilage[17,18]. Nakasa et al[19] showed that the evaluation of subchondral bone using CT correlates with chondral damage in OCDs. There was no significant difference between CT findings and International Cartilage Repair Society grade or arthroscopic findings in their evaluation of 31 ankles[19].
Figure 2.
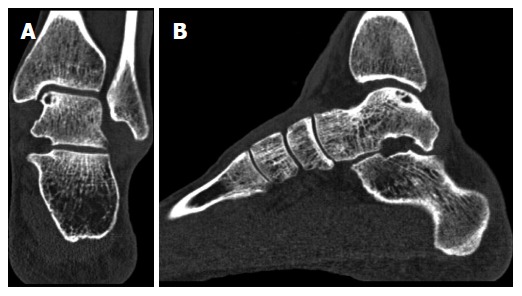
Coronal (A) and sagittal (B) computed tomography scans of a left ankle, showing an osteochondral defect of the posteromedial talar dome. Note the clear visualization of the cyst with an intact subchondral bone plate.
MRI
MRI uses a radiowave frequency that causes the hydrogen nuclei to resonate. Multiple transmitted radiofrequency pulses can be used to differentiate between different tissues because they have different relaxation times. It is possible to suppress tissue (e.g., fat) on MRI. A cartilage-sensitive pulse sequence visualizes the articular cartilage. MRI has the capability of detecting cartilage damage and subchondral bone, which may not be visible on conventional radiographs. Also, MRI has the advantage of not utilizing ionizing radiation and gives a superior detail of the surrounding soft tissue[20].
A high accuracy of diagnosing OCD with MRI has been reported in the literature. Verhagen et al[1] reported a sensitivity and specificity of 96% (Table 1). Mintz et al[21] analyzed patients who had an MRI and in whom arthroscopy was performed. They reported a 100% specificity and a 95% sensitivity of the MRI to identify OCDs[21]. Additionally, De Smet et al[22] showed that MRI is a reliable diagnostic and an accurate predictor of stability of the fragment. However, due to bony edema, the true extent of the lesion can be overestimated, which may affect the treatment decision (Figure 3)[23,24].
Figure 3.
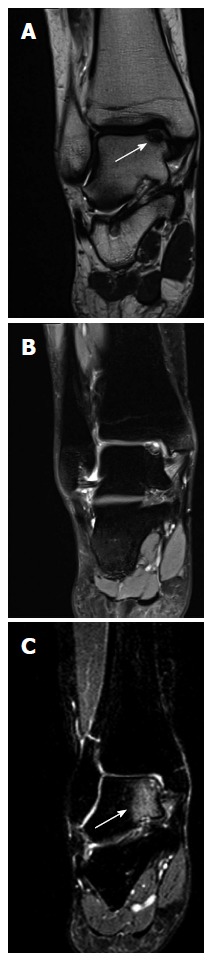
Magnetic resonance imaging scans of coronal T1 (A) and T2 (B) and bone edema on a T2 image (C). Coronal T1 (A) and T2 (B) magnetic resonance imaging scans of a right ankle with an osteochondral lesion of the medial talar dome. Note that the extent of the bony defect is difficult to assess precisely due to the bone edema on a T2 image (C).
Alternative imaging modalities
Arthrography: CT and MRI scans can be supplemented with intra-articular contrast[25]. The contrast may improve assessment of OCD stability by situating between the osteochondral fragment and underlying bone. In a comparative study, Schmid et al[25] concluded that CT arthrography is more reliable than MR arthrography in the detection of cartilage lesions of the ankle. In comparison with conventional MRI, MR arthrography is more accurate in the evaluation of the stability of osteochondral lesions and the detection of intra-articular bodies[26,27]. However, in our opinion, there is no indication for arthrography as it is an invasive technique with risk of complications, and does not influence the treatment decision.
SPECT: SPECT is a nuclear tomographic imaging technique using gamma rays and is able to provide a 3-dimensional visualization due to the radiation distribution of bone-specific radioactive tracers in combination with the CT. Meftah et al[3] assessed the role of SPECT-CT in the management of OCDs. Twenty-two patients with OCDs of the talus had a SPECT-CT and an MRI. With the SPECT-CT they were able to differentiate between an active area and a non-active area of the OCD, which is useful when a patient has chronic OCD and pain after recent trauma. In two patients the MRI showed minimal subchondral edema, while the SPECT-CT showed a significant activity over the subchondral surface, which can implicate an early onset of the OCD[3]. Leumann et al[24] evaluated SPECT-CT in comparison with MRI with respect to decision-making in the treatment of talar OCDs. In this study, the area of activity in SPECT-CT was 56% smaller in the coronal plane and 52% smaller in the sagittal plane than the bone edema in MRI[24]. Thus, SPECT-CT can be of additional value for surgical decision-making when there is a complex case with co-existing pathology.
Ultrasonography: Ultrasound can detect typical OCD morphology, such as cortex irregularities and loose fragments, and is noninvasive and cost-effective, which makes it an interesting alternative (Figure 4)[4,27]. In a human cadaveric study there was a high sensitivity and specificity for detection of 3 to 15-mm artificially created cartilage defects[28]. However, with ultrasound, only anterior and central lesions can be detected even with maximum plantar flexion of the ankle[9]. However, although ultrasound is not yet generally applicable for detecting OCDs in the ankle, it already has the potential to act as a good noninvasive monitoring tool for the healing of an OCD after treatment.
Figure 4.
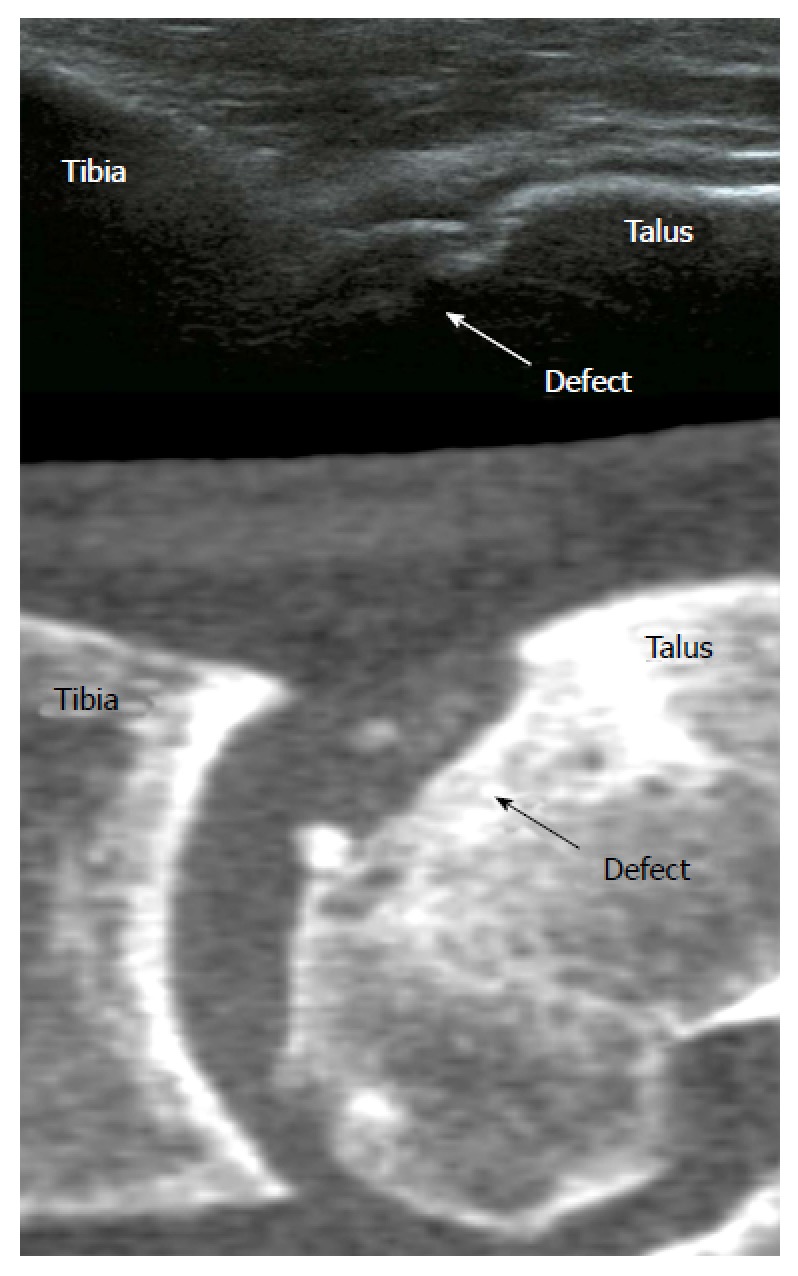
Ultrasound image (top) showing an osteochondral talar defect. A computed tomography scan (bottom) is shown for comparison.
PREOPERATIVE PLANNING
For planning the optimal treatment and surgical approach, different (combinations of) diagnostic techniques as described above can be used. We typically use plain radiography and CT. Alternatively to CT, MRI can be obtained, especially in suspected concomitant soft tissue pathology. However, CT is preferred to measure the precise OCD size and localization. To plan the surgical approach, the ankle is scanned in maximum plantar flexion.
CT plantar flexion
A diagnostic CT scan is usually made with the ankle in a plantigrade position. van Bergen et al[9,10] described the use of a CT scan made of the ankle in full plantar flexion for preoperative planning (Figure 5). A CT of the ankle in full plantar flexion mimics the situation of the ankle during anterior arthroscopic treatment. The scanning technique was shown to determine preoperatively the in-situ arthroscopic location and accessibility of a talar OCD[9]. With use of the CT, the anterior arthroscopic reach was shown to be 48.2% and 47.8% of the medial and lateral talar dome, respectively[10]. The anterior arthroscopic reach was dependent on the degree of plantar flexion movement of the ankle. We recommend the use of this technique for planning surgical treatment of OCDs localized in the posterior half of the talus and of OCDs in ankles with limited range of motion.
Figure 5.
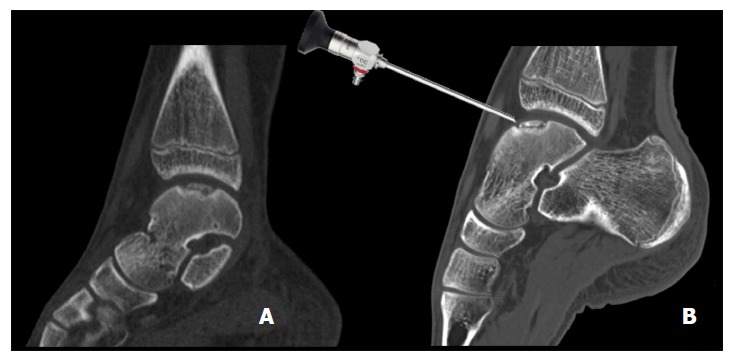
Sagittal computed tomography images of a 14-year-old patient with an osteochondral defect of the medial talar dome. Normal helical CT (A) and a CT made in full plantar flexion (B) showing arthroscopic accessibility. CT: Computed tomography.
POSTOPERATIVE IMAGING
In order to obtain objective assessment of repair after OCD treatment, postoperative imaging is important to evaluate the outcome following surgical procedures. However, in the literature, follow-up imaging is used inconsistently[29,30]. A systematic review showed that 58% of studies reported follow-up radiographs and 25% reported MRI after microfracture treatment[29]. After osteochondral autograft transfer, postoperative radiographs, CT and MRI were described in 65%, 80% and 20%, respectively[30]. More awareness of postoperative imaging possibilities thus seems crucial.
Radiography
Radiographs are frequently obtained in the postoperative assessment of talar OCDs (Figure 6), especially to evaluate degenerative changes in the joint. Several radiographic grading systems have been developed for the osteoarthritic (OA) ankle joint[31-33]. The purpose of the scales is to allow objective assessment of OA changes. The scales focus on the presence of osteophytes and joint space narrowing. The Kellgren-Lawrence system was not designed specifically for the ankle[31]. The five OA grades were originally described as None (0), Doubtful (1), Minimal (2), Moderate (3), and Severe (4), without further detail[31]. Kijowski et al[34] published a more detailed description of the Kellgren-Lawrence scale (Table 2). The Takakura system focuses mainly on the medial joint space[32]. Tanaka et al[35] further classified Takakura stage 3 (Obliteration of the joint space with subchondral bone contact medially) into stage 3a and 3b (Table 2). The van Dijk OA classification is used to evaluate the complete talocrural joint, and has been used for the evaluation of talar OCD in outcome studies[2,36].
Figure 6.
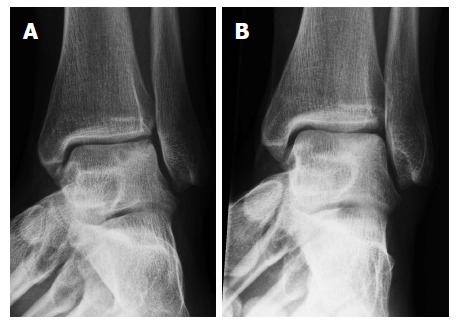
Radiograph of a left ankle with an ostechondral defect in the lateral talar dome, preoperative (A) and postoperative (B).
Table 2.
Overview of several radiographic and magnetic resonance imaging scoring systems for the ankle joint
| Van Dijk Scale[33] | MOCART[41] |
| (0) Normal joint or subchondral sclerosis | Degree of defect repair and filling of the defect |
| (1) Osteophytes without joint space narrowing | Complete (on a level with adjacent cartilage) |
| (2) Joint space narrowing with or without osteophytes | Hypertrophy (over the level of the adjacent cartilage) |
| (3) (Sub)total disappearance or deformation of the joint space | Incomplete (under the level of the adjacent cartilage; underfilling) |
| > 50% of the adjacent cartilage | |
| < 50% of the adjacent cartilage | |
| Modified Takakura Scale[35] | Subchondral bone exposed (complete delamination or dislocation and/or loose body) |
| (1) No joint space narrowing but early sclerosis and osteophyte formation | Integration to border zone |
| (2) Narrowing of the joint space medially | Complete (complete integration with adjacent cartilage) |
| Incomplete (incomplete integration with adjacent cartilage) | |
| (3a) Obliteration of the joint space limited to the facet of medial malleolus with subchondral bone contact | Demarcating border visible (split-like) Defect visible |
| (3b) Obliteration of the joint space advanced to the roof of the talar dome with subchondral bone contact | < 50% of the length of the repair tissue > 50% of the length of the repair tissue |
| (4) Obliteration of the whole joint space with complete bone contact | |
| Modified Kellgren-Lawrence Scale[34] | Surface of the repair tissue |
| (0) No radiographic findings of osteoarthritis | Surface intact (lamina splendens intact) |
| (1) Minute osteophytes of doubtful clinical significance | Surface damaged (fibrillations, fissures and ulcerations) |
| (2) Definite osteophytes with unimpaired joint space | < 50% of repair tissue depth |
| (3) Definite osteophytes with moderate joint space narrowing | > 50% of repair tissue depth or total degeneration |
| (4) Definite osteophytes with severe joint space narrowing and subchondral sclerosis | |
| Structure of the repair tissue | |
| Homogenous | |
| Inhomogenous or cleft formation | |
| Signal intensity of the repair tissue | |
| Dual T2-FSE | |
| Isointense | |
| Moderately hyperintense | |
| Markedly hyperintense | |
| 3D-GE-FS | |
| Isointense | |
| Moderately hypointense | |
| Markedly hypointense | |
| Subchondral lamina | |
| Intact | |
| Not intact | |
| Subchondral bone | |
| Intact | |
| Non-intact (edema, granulation tissue, cysts, sclerosis) | |
| Adhesions | |
| No | |
| Yes | |
| Effusion | |
| No | |
| Yes |
MOCART: Magnetic resonance observation of cartilage repair tissue; FSE: Fast spin-echo; 3D: Three-dimensional; FS: Fat-suppressed.
Moon et al[37] compared the van Dijk scale[33], the modified Kellgren-Lawrence scale[34], and modified Takakura scale[35], and concluded that all these scales were reliable and valid[35]. Interobserver and intraobserver comparisons (weighted Kappa) of each scale were found to be satisfactory (Kellgren-Lawrence, 0.51 to 0.81; Takakura, 0.65 to 0.88; van Dijk, 0.64 to 0.89). However, the predictability of the scales for cartilage damage, as observed by arthroscopy, was only moderate (intraclass correlation coefficients, 0.42 to 0.51)[37]. Therefore, postoperative radiography appears to be useful for the assessment of degenerative changes but less useful for the assessment of OCD repair tissue.
CT
To objectively assess the bone repair, multislice helical CT scans can be obtained during the postoperative follow-up (Figure 7)[38]. CT has been shown to be accurate in the follow-up of talar OCDs[39]. The scanning protocol is the same as in the preoperative situation[5]. One can measure the completeness, thickness, and level of the subchondral plate (i.e., flush, depressed, or proud), as well as bone volume filling of the defect and postoperative loose bony particles[38,40]. However, to our knowledge, a postoperative grading system based on CT is unavailable.
Figure 7.
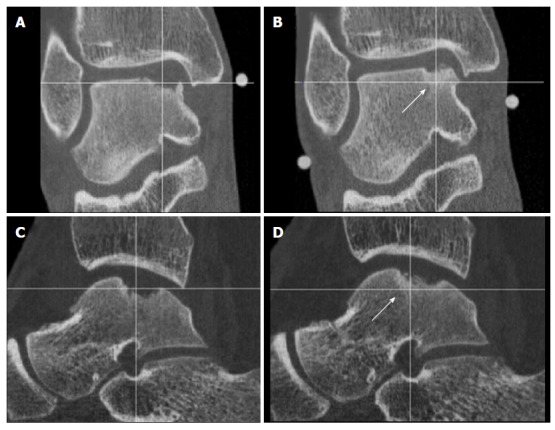
Coronal (A) and Sagittal (C) computed tomography-scans obtained 2 wk postoperatively, showing a medial osteochondral defect of the talus treated with arthroscopic debridement and microfracturing, these can be compared with 1-year postoperative computed tomography scans (B, D). Note the partial bony ingrowth of the defect.
MRI
MRI evaluation of OCD repair tissue has gained popularity in recent years, as the development of high-field MR systems and the use of dedicated coils have improved the visualization of the articular cartilage morphology. The scanning protocol can incorporate several sequences. Proton density-weighted fast spin-echo (FSE-PD) and three-dimensional (3D) fat-suppressed (FS) T1-weighted gradient-echo (3D-FS T1W GRE) sequences are the ones most commonly used[41,42]. New innovative quantitative MRI techniques, like T2 mapping and delayed Gadolinium-Enhanced MR Imaging of Cartilage (dGEMRIC), are frequently used to depict early cartilage degeneration before morphologic cartilage loss occurs. These techniques have been applied increasingly in recent years to evaluate the cartilage status after cartilage repair surgery[43].
T2 mapping: T2 mapping evaluation is a biochemical imaging technique that is complement to morphological imaging in monitoring the biomechanical properties of cartilage repair tissue[44-46]. This technique does not require the injection of contrast medium and is sensitive to the collagen fiber network, including the concentration, orientation and integrity of collagen, as well as to the water content in repair tissue. Good organization of collagen is considered to indicate the maturation of repair tissue and is associated with a good clinical outcome[47], while poor cartilage organization predicts poor clinical outcomes[46,48]. A recent study demonstrated also that T2 mapping is an effective noninvasive tool for evaluating cartilage repair after microfracture treatment for full-thickness cartilage defect models in rabbit knee joints[49].
dGEMRIC: dGEMRIC is also a qualitative MR imaging technique that is recognized as a reliable tool for the assessment of cartilage status[50]. This technique was first described in 2001 and is able to provide a direct measurement of the concentration glycosaminoglycan (GAG) in articular cartilage by using the T1 relaxation time[51]. dGEMRIC requires the injection of a negatively charged intravenous gadolinium-based contrast medium and depends on the distribution of this negatively charged contrast agent through the extracellular matrix of hyaline cartilage[50]. The diffusion of intravenous gadolinium in inverse proportion to the GAG content of cartilage tissue results in a proportionate change in the T1 relaxation times measured by MRI. Several studies have shown that dGEMRIC has the potential to assess the cartilage quality in repair tissue after cartilage repair techniques[43].
Magnetic resonance observation of cartilage repair tissue: Some investigators have quantified MRI results by self-developed criteria[52,53], but a more objective, well-known, and frequently used method is the magnetic resonance observation of cartilage repair tissue (MOCART) (Table 2)[41,54]. Nine variables describe the morphology and signal intensity of the repair tissue compared with the adjacent native cartilage, the degree of filling of the defect, the integration to the border zone, the description of the surface and structure, the signal intensity, the status of the subchondral lamina and subchondral bone, the appearance of adhesions and the presence of synovitis[41]. This system has good interobserver reliability, with intraclass correlation coefficients of > 0.81 in eight of nine variables[54]. However, the association of the MOCART with the clinical situation is not exactly clear. In a study by Aurich et al[55] there was no relation between the MOCART and clinical outcome after matrix-associated chondrocyte implantation of the talus. In another study, three out of five variables of the modified MOCART showed good correlation with second-look arthroscopy after autologous chondrocyte implantation in the ankle, while two out of five variables showed poor correlation[56].
CONCLUSION
In the initial evaluation of patients with acute or chronic ankle pain, conventional radiographs can be useful. However, the sensitivity of detecting OCDs is limited. Although a heel-rise view improves the sensitivity, the use of more sensitive diagnostic methods is essential. Radiographs are frequently obtained in the postoperative assessment of OCDs but are primarily used for the assessment of degenerative changes to the joint.
CT is the most effective for the assessment of osseous structures. Size, location, detachment of a fragment, as well as the integrity of the subchondral bone can be assessed. The appearance of subchondral bone in CT is correlated with cartilage damage at arthroscopic evaluation. SPECT-CT allows a 3D localization of osteoblastic activity, providing additional information about the involvement of the subchondral bone, and can be of additional value in complex cases with co-existing pathology. A plantar flexion CT can be a viable tool to assess preoperatively the arthroscopic accessibility of an OCD. The use of intra-articular contrast can be helpful in the assessment of OCD stability but arthrography is an invasive method.
MRI has the advantage of not utilizing ionizing radiation and gives a superior detail of the surrounding soft tissue. However, the true extent of the OCD may be obscured by concomitant bone-marrow edema. New innovative quantitative MRI techniques, like T2 mapping and dGEMRIC, are frequently used to depict early cartilage degeneration before morphologic cartilage loss occurs.
Ultrasound can detect cortex irregularities and loose fragments, and is noninvasive and cost-effective, which makes it an interesting alternative. However, only anterior and central lesions can be detected.
FUTURE DIRECTIONS
Because of advancements in imaging sensitivity, OCDs of the talus are increasingly detected. Conservative treatment options are available, but a review by Tol et al[57] showed that the average success rate of nonoperative treatment was only 45%. Current surgical treatment options consist of either reparative or restorative techniques. Arthroscopic debridement and BMS is a widely used first-line reparative treatment for OCDs. While Zengerink et al[6] reported that the short- and medium-term results of BMS are successful in 85% of cases, authors have questioned the long-term results. In particular the long-term viability of fibrocartilage, with fibrillation and fissuring being recognized by cartilage sensitive imaging modalities[58]. Biological adjuncts, like concentrated bone marrow aspirate and platelet-rich plasma, may promote hyaline-like tissue development and improve the biological environment in which cartilage can heal. Also, new surgical techniques to restore the natural congruency of the subchondral bone and to preserve hyaline cartilage are developed. Kerkhoffs et al[59] showed promising results with an arthroscopic fixation technique. For this “lift drill fill fix” technique, more long-term follow-up research is needed to confirm the excellent short-term results and to show more pitfalls.
We believe that restoration of the subchondral bone and the preservation or restoring of hyaline cartilage will be the main focus of the treatment of OCDs in the future. The subchondral bone should be addressed to support the overlying cartilage[17]. Imaging modalities like CT, MRI T2 mapping and dGEMRIC, will play an important role in the postoperative assessment of the subchondral bone plate and cartilage and may lead to new insights in the treatment of OCDs of the talus.
Footnotes
Conflict-of-interest statement: The authors declare that they do not have a conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 1, 2015
First decision: August 4, 2015
Article in press: October 13, 2015
P- Reviewer: Franklyn MJ, Kennedy JG S- Editor: Ji FF L- Editor: A E- Editor: Jiao XK
References
- 1.Verhagen RA, Maas M, Dijkgraaf MG, Tol JL, Krips R, van Dijk CN. Prospective study on diagnostic strategies in osteochondral lesions of the talus. Is MRI superior to helical CT? J Bone Joint Surg Br. 2005;87:41–46. [PubMed] [Google Scholar]
- 2.Schuman L, Struijs PA, van Dijk CN. Arthroscopic treatment for osteochondral defects of the talus. Results at follow-up at 2 to 11 years. J Bone Joint Surg Br. 2002;84:364–368. doi: 10.1302/0301-620x.84b3.11723. [DOI] [PubMed] [Google Scholar]
- 3.Meftah M, Katchis SD, Scharf SC, Mintz DN, Klein DA, Weiner LS. SPECT/CT in the management of osteochondral lesions of the talus. Foot Ankle Int. 2011;32:233–238. doi: 10.3113/FAI.2011.0233. [DOI] [PubMed] [Google Scholar]
- 4.Kok AC, Terra MP, Muller S, Askeland C, van Dijk CN, Kerkhoffs GM, Tuijthof GJ. Feasibility of ultrasound imaging of osteochondral defects in the ankle: a clinical pilot study. Ultrasound Med Biol. 2014;40:2530–2536. doi: 10.1016/j.ultrasmedbio.2014.03.022. [DOI] [PubMed] [Google Scholar]
- 5.van Bergen CJ, de Leeuw PA, van Dijk CN. Treatment of osteochondral defects of the talus. Rev Chir Orthop Reparatrice Appar Mot. 2008;94:398–408. doi: 10.1016/j.rco.2008.09.003. [DOI] [PubMed] [Google Scholar]
- 6.Zengerink M, Struijs PA, Tol JL, van Dijk CN. Treatment of osteochondral lesions of the talus: a systematic review. Knee Surg Sports Traumatol Arthrosc. 2010;18:238–246. doi: 10.1007/s00167-009-0942-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hannon CP, Smyth NA, Murawski CD, Savage-Elliott I, Deyer TW, Calder JD, Kennedy JG. Osteochondral lesions of the talus: aspects of current management. Bone Joint J. 2014;96-B:164–171. doi: 10.1302/0301-620X.96B2.31637. [DOI] [PubMed] [Google Scholar]
- 8.van Dijk CN. Ankle arthroscopy: Techniques developed by the Amsterdam Foot and Ankle School 2014. Springer Heidelberg, Germany, 2014 [Google Scholar]
- 9.van Bergen CJ, Tuijthof GJ, Blankevoort L, Maas M, Kerkhoffs GM, van Dijk CN. Computed tomography of the ankle in full plantar flexion: a reliable method for preoperative planning of arthroscopic access to osteochondral defects of the talus. Arthroscopy. 2012;28:985–992. doi: 10.1016/j.arthro.2011.11.030. [DOI] [PubMed] [Google Scholar]
- 10.van Bergen CJ, Tuijthof GJ, Maas M, Sierevelt IN, van Dijk CN. Arthroscopic accessibility of the talus quantified by computed tomography simulation. Am J Sports Med. 2012;40:2318–2324. doi: 10.1177/0363546512455403. [DOI] [PubMed] [Google Scholar]
- 11.Nelson DW, DiPaola J, Colville M, Schmidgall J. Osteochondritis dissecans of the talus and knee: prospective comparison of MR and arthroscopic classifications. J Comput Assist Tomogr. 1990;14:804–808. doi: 10.1097/00004728-199009000-00026. [DOI] [PubMed] [Google Scholar]
- 12.Hepple S, Winson IG, Glew D. Osteochondral lesions of the talus: a revised classification. Foot Ankle Int. 1999;20:789–793. doi: 10.1177/107110079902001206. [DOI] [PubMed] [Google Scholar]
- 13.Flick AB, Gould N. Osteochondritis dissecans of the talus (transchondral fractures of the talus): review of the literature and new surgical approach for medial dome lesions. Foot Ankle. 1985;5:165–185. doi: 10.1177/107110078500500403. [DOI] [PubMed] [Google Scholar]
- 14.Loomer R, Fisher C, Lloyd-Smith R, Sisler J, Cooney T. Osteochondral lesions of the talus. Am J Sports Med. 1993;21:13–19. doi: 10.1177/036354659302100103. [DOI] [PubMed] [Google Scholar]
- 15.Thompson JP, Loomer RL. Osteochondral lesions of the talus in a sports medicine clinic. A new radiographic technique and surgical approach. Am J Sports Med. 1984;12:460–463. doi: 10.1177/036354658401200611. [DOI] [PubMed] [Google Scholar]
- 16.McCullough RW, Gandsman EJ, Litchman HE, Schatz SL. Dynamic bone scintigraphy in osteochondritis dissecans. Int Orthop. 1988;12:317–322. doi: 10.1007/BF00317831. [DOI] [PubMed] [Google Scholar]
- 17.van Dijk CN, Reilingh ML, Zengerink M, van Bergen CJ. Osteochondral defects in the ankle: why painful? Knee Surg Sports Traumatol Arthrosc. 2010;18:570–580. doi: 10.1007/s00167-010-1064-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madry H, van Dijk CN, Mueller-Gerbl M. The basic science of the subchondral bone. Knee Surg Sports Traumatol Arthrosc. 2010;18:419–433. doi: 10.1007/s00167-010-1054-z. [DOI] [PubMed] [Google Scholar]
- 19.Nakasa T, Adachi N, Kato T, Ochi M. Appearance of Subchondral Bone in Computed Tomography Is Related to Cartilage Damage in Osteochondral Lesions of the Talar Dome. Foot Ankle Int. 2014;35:600–606. doi: 10.1177/1071100714528493. [DOI] [PubMed] [Google Scholar]
- 20.O’Loughlin PF, Heyworth BE, Kennedy JG. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am J Sports Med. 2010;38:392–404. doi: 10.1177/0363546509336336. [DOI] [PubMed] [Google Scholar]
- 21.Mintz DN, Tashjian GS, Connell DA, Deland JT, O’Malley M, Potter HG. Osteochondral lesions of the talus: a new magnetic resonance grading system with arthroscopic correlation. Arthroscopy. 2003;19:353–359. doi: 10.1053/jars.2003.50041. [DOI] [PubMed] [Google Scholar]
- 22.De Smet AA, Ilahi OA, Graf BK. Reassessment of the MR criteria for stability of osteochondritis dissecans in the knee and ankle. Skeletal Radiol. 1996;25:159–163. doi: 10.1007/s002560050054. [DOI] [PubMed] [Google Scholar]
- 23.Tan TC, Wilcox DM, Frank L, Shih C, Trudell DJ, Sartoris DJ, Resnick D. MR imaging of articular cartilage in the ankle: comparison of available imaging sequences and methods of measurement in cadavers. Skeletal Radiol. 1996;25:749–755. doi: 10.1007/s002560050173. [DOI] [PubMed] [Google Scholar]
- 24.Leumann A, Valderrabano V, Plaass C, Rasch H, Studler U, Hintermann B, Pagenstert GI. A novel imaging method for osteochondral lesions of the talus--comparison of SPECT-CT with MRI. Am J Sports Med. 2011;39:1095–1101. doi: 10.1177/0363546510392709. [DOI] [PubMed] [Google Scholar]
- 25.Schmid MR, Pfirrmann CW, Hodler J, Vienne P, Zanetti M. Cartilage lesions in the ankle joint: comparison of MR arthrography and CT arthrography. Skeletal Radiol. 2003;32:259–265. doi: 10.1007/s00256-003-0628-y. [DOI] [PubMed] [Google Scholar]
- 26.Cerezal L, Llopis E, Canga A, Rolón A. MR arthrography of the ankle: indications and technique. Radiol Clin North Am. 2008;46:973–974, v. doi: 10.1016/j.rcl.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 27.Sarkalkan N, Loeve AJ, van Dongen KW, Tuijthof GJ, Zadpoor AA. A novel ultrasound technique for detection of osteochondral defects in the ankle joint: a parametric and feasibility study. Sensors (Basel) 2015;15:148–165. doi: 10.3390/s150100148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tuijthof GJ, Kok AC, Terra MP, Aaftink JF, Streekstra GJ, van Dijk CN, Kerkhoffs GM. Sensitivity and specificity of ultrasound in detecting (osteo)chondral defects: a cadaveric study. Ultrasound Med Biol. 2013;39:1368–1375. doi: 10.1016/j.ultrasmedbio.2013.02.011. [DOI] [PubMed] [Google Scholar]
- 29.Hannon CP, Murawski CD, Fansa AM, Smyth NA, Do H, Kennedy JG. Microfracture for osteochondral lesions of the talus: a systematic review of reporting of outcome data. Am J Sports Med. 2013;41:689–695. doi: 10.1177/0363546512458218. [DOI] [PubMed] [Google Scholar]
- 30.Hannon CP, Baksh N, Newman H, Murawski CD, Smyth NA, Kennedy JG. A systematic review on the reporting of outcome data in studies on autologous osteochondral transplantation for the treatment of osteochondral lesions of the talus. Foot Ankle Spec. 2013;6:226–231. doi: 10.1177/1938640013484796. [DOI] [PubMed] [Google Scholar]
- 31.Kellgren JH, Lawrence JS. Radiological assessment of osteo-arthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Takakura Y, Tanaka Y, Kumai T, Tamai S. Low tibial osteotomy for osteoarthritis of the ankle. Results of a new operation in 18 patients. J Bone Joint Surg Br. 1995;77:50–54. [PubMed] [Google Scholar]
- 33.van Dijk CN, Tol JL, Verheyen CC. A prospective study of prognostic factors concerning the outcome of arthroscopic surgery for anterior ankle impingement. Am J Sports Med. 1997;25:737–745. doi: 10.1177/036354659702500603. [DOI] [PubMed] [Google Scholar]
- 34.Kijowski R, Blankenbaker D, Stanton P, Fine J, De Smet A. Arthroscopic validation of radiographic grading scales of osteoarthritis of the tibiofemoral joint. AJR Am J Roentgenol. 2006;187:794–799. doi: 10.2214/AJR.05.1123. [DOI] [PubMed] [Google Scholar]
- 35.Tanaka Y, Takakura Y, Hayashi K, Taniguchi A, Kumai T, Sugimoto K. Low tibial osteotomy for varus-type osteoarthritis of the ankle. J Bone Joint Surg Br. 2006;88:909–913. doi: 10.1302/0301-620X.88B7.17325. [DOI] [PubMed] [Google Scholar]
- 36.van Bergen CJ, Kox LS, Maas M, Sierevelt IN, Kerkhoffs GM, van Dijk CN. Arthroscopic treatment of osteochondral defects of the talus: outcomes at eight to twenty years of follow-up. J Bone Joint Surg Am. 2013;95:519–525. doi: 10.2106/JBJS.L.00675. [DOI] [PubMed] [Google Scholar]
- 37.Moon JS, Shim JC, Suh JS, Lee WC. Radiographic predictability of cartilage damage in medial ankle osteoarthritis. Clin Orthop Relat Res. 2010;468:2188–2197. doi: 10.1007/s11999-010-1352-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.van Bergen CJ, Blankevoort L, de Haan RJ, Sierevelt IN, Meuffels DE, d’Hooghe PR, Krips R, van Damme G, van Dijk CN. Pulsed electromagnetic fields after arthroscopic treatment for osteochondral defects of the talus: double-blind randomized controlled multicenter trial. BMC Musculoskelet Disord. 2009;10:83. doi: 10.1186/1471-2474-10-83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zinman C, Wolfson N, Reis ND. Osteochondritis dissecans of the dome of the talus. Computed tomography scanning in diagnosis and follow-up. J Bone Joint Surg Am. 1988;70:1017–1019. [PubMed] [Google Scholar]
- 40.van Bergen CJ, de Leeuw PA, van Dijk CN. Potential pitfall in the microfracturing technique during the arthroscopic treatment of an osteochondral lesion. Knee Surg Sports Traumatol Arthrosc. 2009;17:184–187. doi: 10.1007/s00167-008-0594-y. [DOI] [PubMed] [Google Scholar]
- 41.Marlovits S, Striessnig G, Resinger CT, Aldrian SM, Vecsei V, Imhof H, Trattnig S. Definition of pertinent parameters for the evaluation of articular cartilage repair tissue with high-resolution magnetic resonance imaging. Eur J Radiol. 2004;52:310–319. doi: 10.1016/j.ejrad.2004.03.014. [DOI] [PubMed] [Google Scholar]
- 42.Ronga M, Angeretti G, Ferraro S, DE Falco G, Genovese EA, Cherubino P. Imaging of articular cartilage: current concepts. Joints. 2014;2:137–140. doi: 10.11138/jts/2014.2.3.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jungmann PM, Baum T, Bauer JS, Karampinos DC, Erdle B, Link TM, Li X, Trattnig S, Rummeny EJ, Woertler K, et al. Cartilage repair surgery: outcome evaluation by using noninvasive cartilage biomarkers based on quantitative MRI techniques? Biomed Res Int. 2014;2014:840170. doi: 10.1155/2014/840170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Domayer SE, Apprich S, Stelzeneder D, Hirschfeld C, Sokolowski M, Kronnerwetter C, Chiari C, Windhager R, Trattnig S. Cartilage repair of the ankle: first results of T2 mapping at 7.0 T after microfracture and matrix associated autologous cartilage transplantation. Osteoarthritis Cartilage. 2012;20:829–836. doi: 10.1016/j.joca.2012.04.015. [DOI] [PubMed] [Google Scholar]
- 45.Glaser C. New techniques for cartilage imaging: T2 relaxation time and diffusion-weighted MR imaging. Radiol Clin North Am. 2005;43:641–653, vii. doi: 10.1016/j.rcl.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 46.Trattnig S, Mamisch TC, Welsch GH, Glaser C, Szomolanyi P, Gebetsroither S, Stastny O, Horger W, Millington S, Marlovits S. Quantitative T2 mapping of matrix-associated autologous chondrocyte transplantation at 3 Tesla: an in vivo cross-sectional study. Invest Radiol. 2007;42:442–448. doi: 10.1097/01.rli.0000262088.67368.49. [DOI] [PubMed] [Google Scholar]
- 47.Theologis AA, Schairer WW, Carballido-Gamio J, Majumdar S, Li X, Ma CB. Longitudinal analysis of T1ρ and T2 quantitative MRI of knee cartilage laminar organization following microfracture surgery. Knee. 2012;19:652–657. doi: 10.1016/j.knee.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.White LM, Sussman MS, Hurtig M, Probyn L, Tomlinson G, Kandel R. Cartilage T2 assessment: differentiation of normal hyaline cartilage and reparative tissue after arthroscopic cartilage repair in equine subjects. Radiology. 2006;241:407–414. doi: 10.1148/radiol.2412051750. [DOI] [PubMed] [Google Scholar]
- 49.Tao H, Li H, Hua Y, Chen Z, Feng X, Chen S. Quantitative magnetic resonance imaging (MRI) evaluation of cartilage repair after microfracture treatment for full-thickness cartilage defect models in rabbit knee joints: correlations with histological findings. Skeletal Radiol. 2015;44:393–402. doi: 10.1007/s00256-014-2062-8. [DOI] [PubMed] [Google Scholar]
- 50.Rutgers M, Bartels LW, Tsuchida AI, Castelein RM, Dhert WJ, Vincken KL, van Heerwaarden RJ, Saris DB. dGEMRIC as a tool for measuring changes in cartilage quality following high tibial osteotomy: a feasibility study. Osteoarthritis Cartilage. 2012;20:1134–1141. doi: 10.1016/j.joca.2012.07.001. [DOI] [PubMed] [Google Scholar]
- 51.Burstein D, Velyvis J, Scott KT, Stock KW, Kim YJ, Jaramillo D, Boutin RD, Gray ML. Protocol issues for delayed Gd(DTPA)(2-) -enhanced MRI (dGEMRIC) for clinical evaluation of articular cartilage. Magn Reson Med. 2001;45:36–41. doi: 10.1002/1522-2594(200101)45:1<36::aid-mrm1006>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 52.Becher C, Burger UL, Allenberg JR, Kaufmann GW, Thermann H. Delayed diagnosis of a pseudoaneurysm with recurrent hemarthrosis of the knee joint. Knee Surg Sports Traumatol Arthrosc. 2008;16:561–564. doi: 10.1007/s00167-008-0505-2. [DOI] [PubMed] [Google Scholar]
- 53.Imhoff AB, Paul J, Ottinger B, Wörtler K, Lämmle L, Spang J, Hinterwimmer S. Osteochondral transplantation of the talus: long-term clinical and magnetic resonance imaging evaluation. Am J Sports Med. 2011;39:1487–1493. doi: 10.1177/0363546510397726. [DOI] [PubMed] [Google Scholar]
- 54.Marlovits S, Singer P, Zeller P, Mandl I, Haller J, Trattnig S. Magnetic resonance observation of cartilage repair tissue (MOCART) for the evaluation of autologous chondrocyte transplantation: determination of interobserver variability and correlation to clinical outcome after 2 years. Eur J Radiol. 2006;57:16–23. doi: 10.1016/j.ejrad.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 55.Aurich M, Bedi HS, Smith PJ, Rolauffs B, Mückley T, Clayton J, Blackney M. Arthroscopic treatment of osteochondral lesions of the ankle with matrix-associated chondrocyte implantation: early clinical and magnetic resonance imaging results. Am J Sports Med. 2011;39:311–319. doi: 10.1177/0363546510381575. [DOI] [PubMed] [Google Scholar]
- 56.Lee KT, Choi YS, Lee YK, Cha SD, Koo HM. Comparison of MRI and arthroscopy in modified MOCART scoring system after autologous chondrocyte implantation for osteochondral lesion of the talus. Orthopedics. 2011;34:e356–e362. doi: 10.3928/01477447-20110627-10. [DOI] [PubMed] [Google Scholar]
- 57.Tol JL, Struijs PA, Bossuyt PM, Verhagen RA, van Dijk CN. Treatment strategies in osteochondral defects of the talar dome: a systematic review. Foot Ankle Int. 2000;21:119–126. doi: 10.1177/107110070002100205. [DOI] [PubMed] [Google Scholar]
- 58.Savage-Elliott I, Ross KA, Smyth NA, Murawski CD, Kennedy JG. Osteochondral lesions of the talus: a current concepts review and evidence-based treatment paradigm. Foot Ankle Spec. 2014;7:414–422. doi: 10.1177/1938640014543362. [DOI] [PubMed] [Google Scholar]
- 59.Kerkhoffs GM, Reilingh ML, Gerards RM, de Leeuw PA. Lift, drill, fill and fix (LDFF): a new arthroscopic treatment for talar osteochondral defects. Knee Surg Sports Traumatol Arthrosc. 2014:Epub ahead of print. doi: 10.1007/s00167-014-3057-7. [DOI] [PubMed] [Google Scholar]


