Abstract
Brain oscillations are supposedly crucial for normal cognitive functioning and alterations are associated with cognitive dysfunctions. To demonstrate their causal role on behavior, entrainment approaches in particular aim at driving endogenous oscillations via rhythmic stimulation. Within this context, transcranial electrical stimulation, especially transcranial alternating current stimulation (tACS), has received renewed attention. This is likely due to the possibility of defining oscillatory stimulation properties precisely. Also, measurements comparing pre-tACS with post-tACS electroencephalography (EEG) have shown impressive modulations. However, the period during tACS has remained a blackbox until now, due to the enormous stimulation artifact. By means of application of beamforming to magnetoencephalography (MEG) data, we successfully recovered modulations of the amplitude of brain oscillations during weak and strong tACS. Additionally, we demonstrate that also evoked responses to visual and auditory stimuli can be recovered during tACS. The main contribution of the present study is to provide critical evidence that during ongoing tACS, subtle modulations of oscillatory brain activity can be reconstructed even at the stimulation frequency. Future tACS experiments will be able to deliver direct physiological insights in order to further the understanding of the contribution of brain oscillations to cognition and behavior.
Keywords: Transcranial alternating current stimulation, tACS, Magnetoencephalography, MEG, Artifact rejection, Entrainment
Introduction
Normal cognitive functioning requires a temporally precise coordination of neuronal ensembles at relatively local scales as well as over long distances. It has been proposed that brain oscillations play an essential role in these synchronization processes, and failures of the mechanisms enabling precise synchronization have been implicated in disordered cognition and psychiatric disorders (Herrmann and Demiralp, 2005, Schnitzler and Gross, 2005, Uhlhaas et al., 2008). However, the majority of literature relating oscillatory brain processes and behavior, in humans in particular, has been correlative. Recently, an increasing number of studies have employed brain stimulation techniques to “entrain” brain rhythms at natural frequencies to probe the effects on behavior. Contrary to conventional neuroscientific experiments, the entrainment approach claims to use brain activity as the independent variable and behavior as the dependent variable allowing for more causal inferences. Most common techniques are transcranial magnetic stimulation (TMS) (Thut and Miniussi, 2009) and transcranial alternating current stimulation (tACS; recently reviewed in Antal and Paulus, 2013, Herrmann et al., 2013, Marshall and Binder, 2013). Knowledge about the mechanism of action of tACS mainly stems from animal research: Although the effect of tACS on single neurons is small, the rhythmic structure of tACS is able to modulate neuronal networks (Fröhlich and McCormick, 2010, Reato et al., 2010). Comparing pre- and post-tACS interventions, recent electroencephalography (EEG) studies have shown successful modulations of amplitude, phase, and coherence of oscillatory brain activity (e.g., Marshall et al., 2006, Neuling et al., 2012, Neuling et al., 2013, Polania et al., 2012, Zaehle et al., 2010). These offline effects have clinical relevance, because of the opportunity to induce long-term changes of dysbalanced brain activity (Kuo et al., 2014). To extend this offline approach and to make a fully convincing case of the impact of tACS on brain activity, scientists need to be capable of uncovering the electrophysiological brain dynamics during stimulation (Herrmann et al., 2013). This would also allow disentangling online entrainment effects and after-effects (Vossen et al., 2014). Measuring brain activity during tACS has, however, proven to be a challenge, due to the enormous artifact, several orders of magnitude higher in amplitude than the brain signal, introduced during electrical stimulation. In a worst-case scenario, this would cause a clipping of the signal, prohibiting separation of the artifact and the brain signal from the outset.
Regarding the latter issue of separating stimulation artifact from brain activity, recent EEG studies have made important advances. Helfrich et al. (2014) utilized average subtraction and independent and principal component analysis (ICA/PCA) to remove the stimulation artifact and demonstrated online effects of tACS, among others the recovery of evoked potentials and an increase of spectral power at the stimulation frequency. Voss et al. (2014) subtracted the signal of a reference electrode and notch filtered the remaining signal centered on the stimulation frequency to show online effects. Critically, neither study demonstrated modulations of oscillatory brain activity during tACS (in particular at the actual stimulation frequency which a priori is a frequency of interest), only that oscillatory brain activity was different during tACS as compared to before or after stimulation. This distinction is crucial, because the former is a foundation for future studies utilizing tACS, especially for adaptive tACS protocols.
Although the first steps towards monitoring electrophysiological effects online during tACS have been taken, EEG poses important disadvantages: for example, the stimulation electrodes are attached to the highly conductive scalp, just like the recording electrodes that capture the artifact. The electrical coupling between the electrodes severely limits the intensity at which tACS can be applied (Helfrich et al., 2014, Voss et al., 2014), posing experimental design limitations and diminishing tACS efficacy (Fröhlich and McCormick, 2010). In our experience, various factors (e.g., distance of electrodes, skin conductivity, electrode impedance) can cause clipping to happen at intensities as low as .1 mA (even though under favorable conditions 1 mA can be reached; see Helrich et al., 2014). Another disadvantage is that the stimulation electrodes can cover a large area of the scalp (one electrode is usually 7 by 5 cm), which cannot be covered by EEG electrodes. This leads to reduced spatial sampling of the signal. Importantly, it has to be pointed out that while the current approaches (Helfrich et al., 2014, Voss et al., 2014) based on EEG data of separating brain signals from artifact have shown a general increase of power at the stimulation frequency during tACS as compared to a pre-tACS period, it cannot be excluded with certainty whether these are still related to residual artifacts. The demonstration of an experimental modulation of power at the stimulation frequency during tACS is required to build a case that electrophysiological signals can be monitored during tACS. The evidence has not been provided so far.
Apart from “hardware” aspects, the reconstruction of oscillatory brain activity during tACS adds a further challenge: if the phases of the brain oscillations and the tACS signal align, previously used simple subtraction (e.g., Helfrich et al., 2014, Voss et al., 2014) will cancel the artifact along with the to be analyzed brain activity. Here, we demonstrate that magnetoencephalography (MEG), combined with advanced source imaging approaches, is capable of overcoming the aforementioned limitations. Our work is based on a recent demonstration of combined transcranial direct current stimulation (tDCS) and MEG (Soekadar et al., 2013). The authors utilized spatial filtering (synthetic aperture magnetometry) to suppress artifactual activity outside the brain in order to reconstruct and localize oscillatory activity inside the brain. It is an open issue whether, due to the high synchrony of the brain signal and the stimulation artifact, it would be possible to separate these two and uncover subtle modulations of brain activity during tACS. Using interventions that lead to well-established and robust modulations of alpha power (eyes open vs. closed and stimulus induced alpha power decrease), we demonstrate for the first time that MEG in combination with a similar spatial filtering technique as used by Soekadar et al. (2013) can be utilized to disentangle oscillatory brain activity from the highly correlated tACS signal. The possibility of studying brain activity, even at the stimulation frequency during the ongoing tACS-stimulation, opens up new avenues for understanding tACS related effects on brain functioning with far reaching consequences for cognitive and clinical neuroscience.
Methods
Subjects
Seventeen healthy volunteers (9 males, 28 ± 4 years old; all right-handed) without psychiatric or neurological disorders took part in this study. The experimental protocol was approved by the ethics committee of the University of Trento, and all participants gave written informed consent before the beginning of the experiment.
Stimuli and procedure
After applying the MEG coils and tACS electrodes to the head, the stimulation intensity was determined (see next section). Participants were seated in an upright position in the MEG room. Before the first block, a three-minute resting condition with the eyes open was performed. The data from this measurement were used to estimate the the individual alpha frequency (IAF) of the subject (see below). Three blocks with identical basic setup followed during which either sham-, weak-, or strong tACS was applied (see next section): In the first part of a block, subjects were asked to keep their eyes open for 2 min until a tone and a visual instruction presented on a screen asked subjects to close their eyes for another 2 min. This resting-state measurement was followed by a second part consisting of passive viewing and listening. 100 auditory and 100 visual stimuli were presented in random order, divided into two subblocks separated by a self-paced break. The visual stimuli were moving Garbor patches, subtending 0.46° of visual angle, with 3 cycles (resulting in 6.5 cycles/°), with a Gaussian envelope of .07°, and oriented at − 45%, moving upward at 4.43°/s with randomized initial phase. The Garbor patches were projected centrally on a screen (62 × 35 cm, 1920 × 1080 pixel resolution, 120 Hz refresh rate) at 50% contrast and the background was a mean-luminance uniform gray. The auditory stimuli were pure tones of 1 kHz, sinusoidal, sampled at 44.1 kHz, and presented binaurally via air-conducting tubes with ear inserts. The duration of all stimuli was 2 s and the interstimulus interval was set to 2.5 ± 0.5 s (uniformly distributed) which amounts to 16 min per block. Since the participants experienced the sensation of suprathreshold stimulation during the threshold assessment (see below), we could ask them after each block to indicate whether they perceived the stimulation. In the end, three minutes of resting state (eyes open) without any stimulation was recorded. After the experiment, subjects were asked to fill out a translated questionnaire that captures the possible adverse effect of transcranial electrical stimulation (Brunoni et al., 2011).
tACS parameters
A battery-operated stimulator system (DC-Stimulator Plus, NeuroConn GmbH, Ilmenau, Germany) was placed outside the magnetically shielded room. It was connected to the stimulation electrodes via the MRI module (NeuroConn GmbH, Ilmenau, Germany). The stimulator delivered an alternating, sinusoidal current at the IAF via two conductive-rubber electrodes (NeuroConn GmbH) centered at electrode positions Cz and Oz of the international 10–20 system (Fig. 1a). These positions were chosen for maximal stimulation intensity in the parieto-occipital cortex (Neuling et al., 2012). The electrodes had a size of 7 by 5 cm and were applied with a conductive paste (Ten20, D.O. Weaver, Aurora, CO, USA) resulting in impedance values of 6.13 ± 0.8 kΩ (mean ± se). The electrode cables were located on the right side of the participant's head. The stimulation intensity was kept below each subject's sensation and phosphene threshold in order to keep them naive regarding the stimulation condition (for individual parameters, cf. Supplementary Table 1). To obtain the threshold, the subject was first familiarized with the skin sensation. The subject was then stimulated with an intensity of 400 μA (peak-to-peak) at 10 Hz for 5 s. The intensity was increased by steps of 100 μA until the subject indicated skin sensation or phosphene perception or an intensity of 1500 μA was reached. In the two cases in which the subject already reported an adverse effect at 400 μA, the intensity was reduced to a start level of 100 μA and increased by steps of 100 μA in line with the start level of 400 μA. The staircase procedure resulted in average stimulation intensities of 653 ± 447 μA (mean ± standard deviation). As mentioned above, the experiment comprised three different stimulation blocks (sham, weak, and strong tACS). While the order of the first two blocks (sham and weak tACS) was pseudorandomized, the strong tACS block was always the last block in order to avoid after-effects during the sham- and weak stimulation blocks. During the sham block, the experimental setup was the same as in the other blocks, but no electrical stimulation was applied. A stimulation intensity of 50 μA was delivered at IAF during the weak stimulation block to induce an artifact, but likely without an effect on brain activity (Reato et al., 2013). The individual estimated threshold level − 100 μA was used as stimulation intensity in the strong tACS block.
Fig. 1.
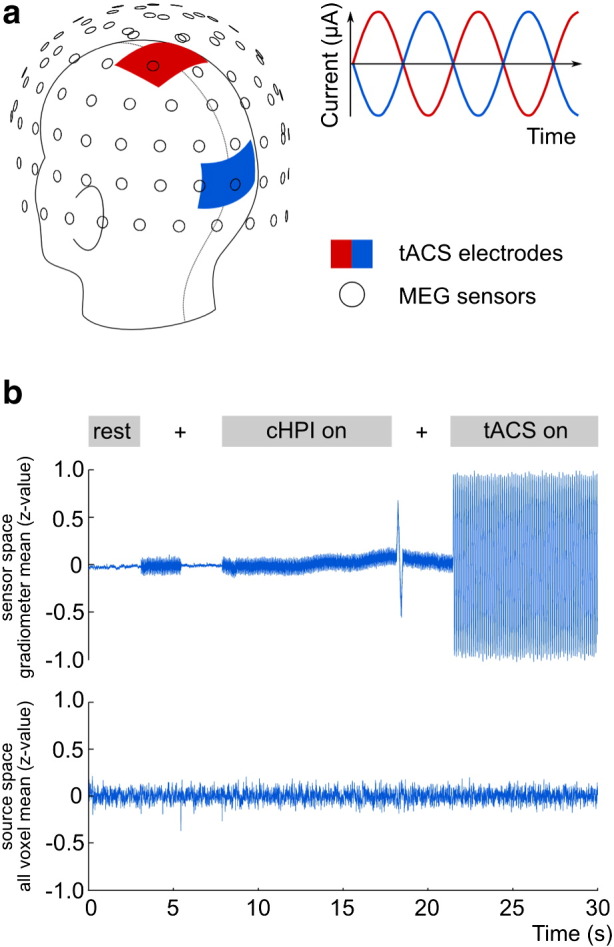
Experimental setup and stimulation artifact.
a: Schematic illustration of the electrode and MEG sensor positions: Stimulation electrodes were centered at Oz and Cz according to the international 10–20 system. b: Time series depicting the tACS artifact in sensor space (upper) and after transformation into source space (lower), both z-normalized. b: Upper: During tACS (tACS on), the artifact is several magnitudes higher than the resting state brain activity (rest) and the signal emitted by the head positioning coils (cHPI on). Note the initial head position estimation signal (around 3–6 s), and the sharp transition at around 18 s corresponding to the impedance check of the tACS device. b: Lower: The same time series in source space. Note the suppressed tACS artifact. For illustrative purposes, the time series in source space was band-pass filtered between 1 and 100 Hz.
MEG data recording
Magnetic brain activity was recorded at 1000 Hz (hardware filters: 0.1–330 Hz) using whole head MEG (Elekta Neuromag Vectorview, Elekta Oy, Finland), spatially sampling the signals at 102 positions. Each position consists of a channel triplet of one magnetometer and two orthogonal planar gradiometers yielding 306 sensors overall. The MEG system is housed in a magnetically shielded room (AK3b, Vacuumschmelze, Germany). Fiducials (nasion and left and right pre-auricular points), the location of five head position indicator (HPI) coils and > 200 headshape samples were digitized prior to the experiment. These points served for later head modeling as well as for determining the head position within the helmet prior to each run. The latter controls for large head movements over the course of the experiment.
IAF determination
For each subject we acquired 3 min of resting state activity with the eyes open as first measurement. In order to determine each subject's IAF , these data were analyzed offline immediately after the measurement. First, continuous data were cut into segments of 2 s each, and frequency analysis (1 to 25 Hz, .25 Hz resolution, Hanning window, 4 s padding) was subsequently performed on each trial to estimate the power spectrum. Then we manually chose a few gradiometers showing a prominent alpha peak on the averaged trials, and assessed the frequency of the peak. Two subjects did not exhibit a clear alpha peak in the resting state data with the eyes open, and therefore we had to determine their alpha frequency on a subsequent resting state block with the eyes open and eyes closed conditions.
Offline MEG data analysis
Preprocessing
Continuous data were offline band-pass filtered between 1 and 200 Hz and downsampled to 512 Hz. Then the data were segmented into non-overlapping epochs of 2 s for the eyes open vs. eyes closed data or − 2 to 3 s relative to stimulus onset for the auditory/visual stimulation condition, respectively. Noisy and dead sensors were identified in the sham block and excluded for the other two blocks (weak and strong tACS). Epochs containing artifacts (caused by, e.g., blinks/muscle activity) were removed from the stimulation free block. Since the electrical stimulation leads to signals of several orders of magnitude higher than actual physiological data (see Fig. 1b), we refrained from removing further artifacts from the tACS blocks.
Source projection of raw data
Sensor level data were projected into source space using linearly constrained minimum variance (LCMV) beamformer filters (van Veen et al., 1997), which is a standard procedure in source analysis of electrophysiological data. We followed a procedure described here for single virtual sensors (http://www.fieldtriptoolbox.org/tutorial/shared/virtual_sensors) and extended it to 889 points covering the whole brain (see below). For this, epoched raw data were filtered from 1 to 40 Hz and the covariance matrix of each single trial was calculated and averaged across trials. Together with single-shell head models (Nolte, 2003) derived from the individual head shape and the lead field matrix, the covariance matrix was used to obtain the beamformer filters. These were subsequently multiplied with the sensor level time series to obtain time series for each source location. Using this process, we were able to perform identical analyses on the sensor as well as on the source level. A favorable feature, for our purposes, of beamformers is that they are geared to optimally estimate activity at a source point while suppressing activity originating from elsewhere. Importantly, beamformers effectively suppress noise sources that are correlated over sensors, which is evident in the case of tACS. We used a grid with a size of 889 points equally spaced by a 1.5 cm distance in Montreal Neurological Institute (MNI) space and warped these positions into individual head space. An MNI template brain was used for subsequent visualization purposes.
Resting-state spectral power
For the eyes open/eyes closed resting-state data, spectral power estimations (for both sensor and source space) were performed after Hanning-tapering the epochs (see Preprocessing), for frequencies ranging from 2 to 30 Hz in 1 Hz steps. Subsequently, averages of the power spectra were calculated for all stimulation conditions (sham, weak tACS, and strong tACS), and eyes open/eyes closed data per subject. For visualization purposes, the source space spectral power results for the eyes open vs. eyes closed conditions were then statistically compared in the alpha range (8–12 Hz) as described below (see Statistical analysis).
Stimulus-evoked responses
In sensor and source space (see Preprocessing above) epochs were low-pass filtered at 25 Hz and reduced to − 0.2 to 1 s relative to stimulus onset. Then, all remaining epochs per stimulus (visual/auditory) and condition (sham, weak tACS, strong tACS) were averaged for each individual subject yielding evoked responses (ER) in sensor and source space. For sensor space data all epochs were baseline normalized by subtracting the average baseline (− 0.2 to 0 s) amplitude from the whole epoch. In source space, baseline normalization was accomplished by computing the absolute value of the signal (thus discarding potential polarity differences of a source across participants) and computing a relative change, i.e., by subtracting the average baseline amplitude from the epoch and dividing this difference by the average baseline amplitude. Using relative change as baseline normalization effectively abolishes the well-known depth bias of the beamformer (van Veen et al., 1997). For visualization purposes, the source space results for the auditory M90 (peak at 90 ms) and the visual M150 (peak at 150 ms) were then statistically compared as described below (see Statistical analysis).
Stimulus-induced power modulations
Spectral power estimation in sensor and source space in single epochs was performed on Hanning-tapered time windows from − 0.5 to 2.5 s (in steps of 0.05 s) relative to the stimulus (visual/auditory) onset. The sliding window had a fixed length of 0.4 s. Frequencies of interest ranged from 2 to 30 Hz in steps of 1 Hz. Then, power estimates of trials belonging to the same stimulus and condition were averaged for each individual subject. In source space, stimulus related alpha band (8–12 Hz) suppressions in the time window of 0.3–1.5 s post-stimulus were compared against baseline. To do that we computed the normalized difference as described in the following paragraph.
Statistical analysis
Source space statistical results were accomplished in a similar manner for the resting state and the stimulus evoked/induced activity. We calculated the normalized difference (cf. Spaak et al., 2014) for each time/voxel point for the ER and each time/frequency/voxel point for the spectral estimates. The normalized difference is computed as (A − B) / (A + B). A and B corresponded to the eyes open and eyes closed, visual M150 and auditory M90, and post-stimulus alpha and baseline, respectively. Following a permutation test approach the normalized difference was computed both for the observed data and for all possible (131,072) permutations of the above described conditions. Based on the per-voxel permutation distribution, we obtained individual probabilities for the observed data. All p-values were corrected for multiple comparisons across voxels using the false discovery rate procedure (FDR, Benjamini and Hochberg, 1995, Genovese et al., 2002).
Signal processing and statistical analysis were performed using the Fieldtrip toolbox (Oostenveld et al., 2011).
Results
To demonstrate that our approach is capable of uncovering subtle modulations of oscillatory brain activity during tACS, we combined non-invasive brain stimulation with concurrent MEG. We faced a challenge to disentangle oscillatory brain activity from the artifact caused by tACS, which is several magnitudes higher (Fig. 1b) and in the same frequency range (8–12 Hz) as the frequencies we aimed to analyze. One promising technique to overcome the challenge is spatial filtering by means of LCMV beamforming (van Veen et al., 1997). Beamformers are especially suited to remove the tACS artifact because they suppress noise sources that are correlated over sensors. We used well-established interventions to modulate alpha power without tACS and during tACS, which was adjusted to the IAF and sensation threshold. We subsequently analyzed alpha modulations and compared the results in sensor space and source space.
Eyes open/closed related alpha modulations
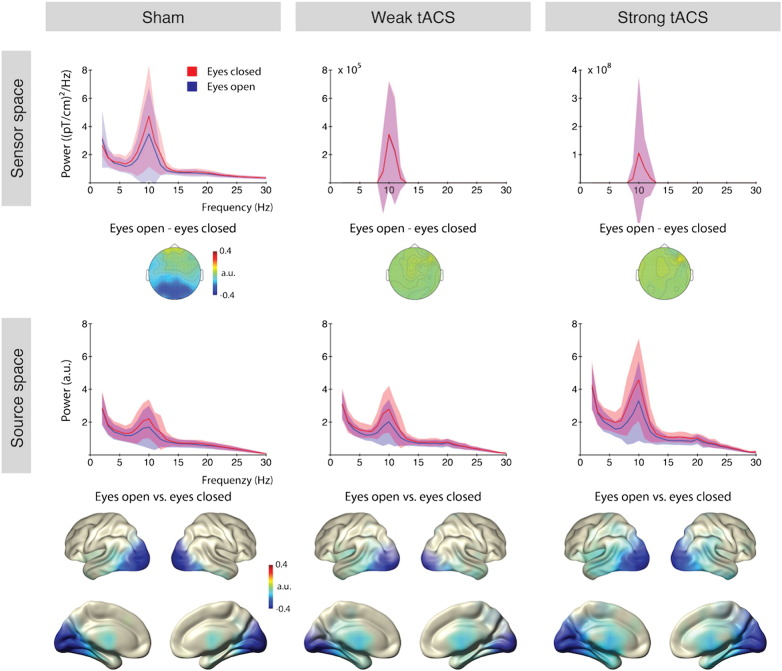
Modulations of cortical alpha power caused by closing the eyes were investigated in sensor and source space. In the sham condition clear occipital modulations were observed in the alpha range (8–12 Hz) in sensor space. During weak and strong tACS, the sensor space data were completely dominated by the stimulation artifact, thus rendering any endogenous alpha modulation invisible (Fig. 2, top middle and right). Note that the scales in the spectra change drastically from sham to weak and strong stimulation (Fig. 2, top). Thus on a sensor level, the stimulation artifact completely covers neurophysiological effects (see also Fig. 1b). In source space, however, both weak and strong tACS showed spectra with similar morphology as the sham condition (Fig. 2, bottom). Statistical contrast revealed similar sources mainly localized to visual cortices along the calcarine fissure (pFDR < .05). Interestingly, but not within the scope of this proof of principle manuscript, strong tACS seems to affect alpha power in both conditions, eyes open and eyes closed. This could be an indicator of entrainment on the neuronal level; however, we did not further investigate the effect at this point. For a demonstration that possible effects are not residual artifacts, see Supplemental Figure 2 for a comparison of two subjects that received weak and strong tACS intensities.
Fig. 2.
Resting state alpha modulations.
Top — Sensor level average spectra across all gradiometers (combined) in the three stimulation conditions. The typical increase of alpha band power when the eyes are closed is only observable without stimulation. Topographies show the difference of alpha power (8–12 Hz) in the eyes open minus the eyes closed condition. Only without stimulation (sham) a parieto-occipital decrease is observable; under weak- and strong tACS the topographies reflect the stimulation artifact. Note the different scales in the spectra depending on the stimulation. Bottom — Source space spectra across all cortical sources. The typical increase of alpha band power when the eyes are closed is clearly observable in all stimulation conditions. The statistical maps show a contrast (normalized difference) of the eyes open vs. eyes closed condition; FDR corrected significant effects were observed in all stimulation conditions. For illustrative purposes the maps are thresholded at p < 0.001. Shaded areas represent the standard deviation. a.u. — arbitrary unit.
Visual and auditory evoked responses
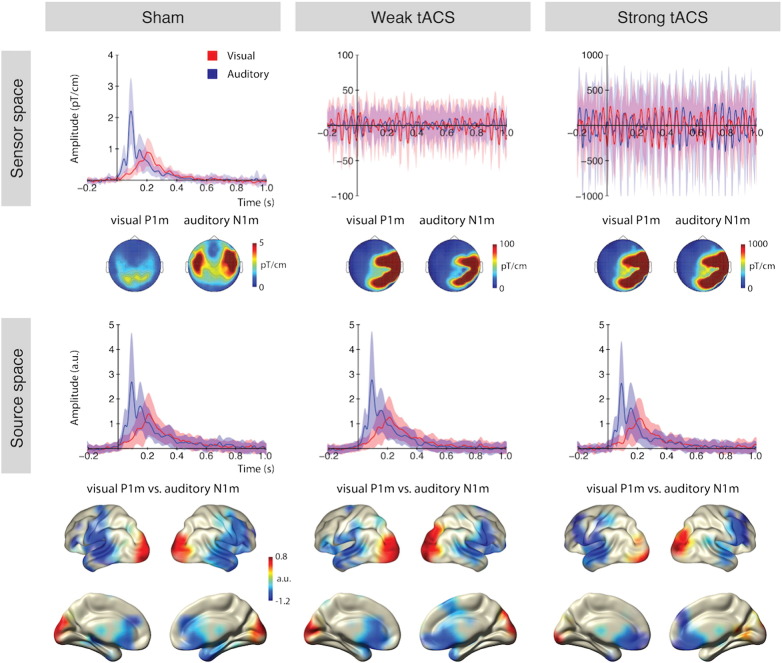
Although not the focus of our interest, evoked activity time-locked to visual (Gabor gratings) and auditory stimuli (sine tones) was investigated in sensor and source space. In the sham condition (Fig. 3, left), clear auditory and visual ER could be observed on sensor and source levels. Statistical contrasts of the visual M150 (peak at around 150 ms) and the auditory M90 (peak at 90 ms) demonstrated that the M150 was mainly generated in primary and secondary visual areas in the occipital cortex (pFDR < .05), and the M90 was generated by sources in the superior temporal gyrus (including primary auditory cortex) and additional sources in the frontal cortex (pFDR < .05). During weak and strong tACS, the sensor space data were completely dominated by the stimulus artifact without any clear ER being discernible (Fig. 3, top middle and right). Analogous to Fig. 2, please notice in Fig. 3 (top panel) the drastic change in scales: even following averaging of ~ 100 trials, the ER in the strong tACS condition is still a factor ~ 250 times larger than the “real” ER. In dramatic contrast to the sensor level, source space analysis of weak and strong tACS conditions revealed clear modality specific ERs with strikingly similar time courses and spatial distributions as the sham condition (Fig. 3, bottom).
Fig. 3.
Event-related fields to visual and auditory stimuli.
Top — Sensor level average across all gradiometers (combined, which is the squared root-mean across gradiometer pairs) in the three stimulation conditions. Topographies show the visual M150 (30 ms window around the peak at 150 ms) and the auditory M90 (30 ms window around the peak at 90 ms). Modality specific ERs and topographies are observable only without stimulation (sham); under weak and strong tACS stimulation the topographies reflect the stimulation artifact, especially on the right side where the electrode cables were located. Note the different scales in the three conditions. Bottom — Source space averages across all cortical sources. Visual and auditory ERs are clearly observable in all stimulation conditions. The statistical maps show a contrast (normalized difference) of the visual M150 (red colors) vs. the auditory M90 (blue colors), for contrasts against baseline, see Supplemental Figure 3; FDR corrected significant effects were observed for both modalities. Auditory source activity was stronger than visual source activity, thus, for illustrative purposes the auditory activity was thresholded at p < 0.001 and visual activity was thresholded at p < 0.05. Shaded areas represent the standard deviation. a.u. — arbitrary unit.
Stimulus induced alpha decreases
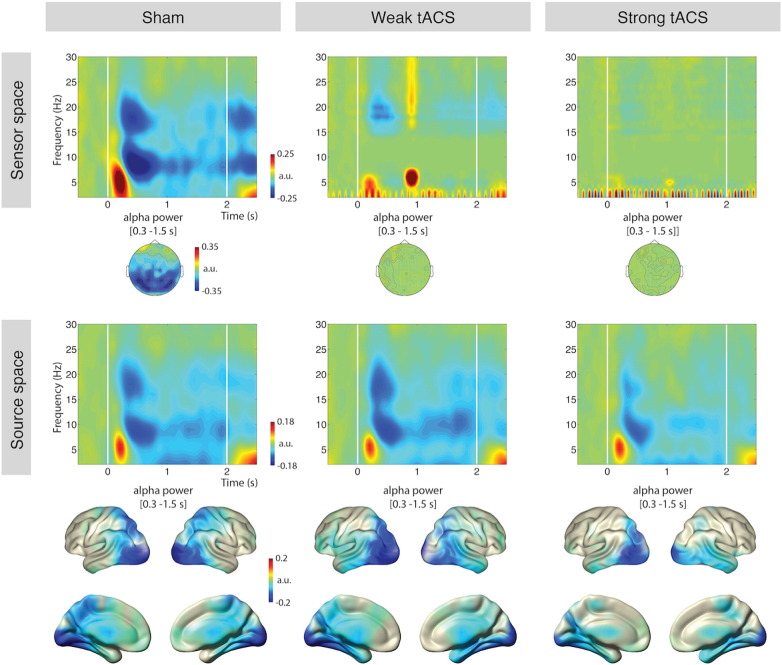
Next, we investigated alpha power decreases induced by visual stimulation (a similar analysis for alpha decrease induced by auditory stimuli can be found in Fig. S1). In sensor space, the alpha decrease after stimulus onset was observable only in the sham condition (Fig. 4, top left). As expected, the alpha suppression, as evaluated with statistical contrasts of post-stimulus alpha power (8–12 Hz) vs. baseline, was dominant in bilateral visual cortical regions (pFDR < .05, Fig. 4, bottom left). With stimulation, the tACS artifact dominated the time–frequency representations making it impossible to observe any modulations in the alpha range. Even worse, due to the tACS artifact being constant over the course of the trial, the act of baseline normalization effectively removes any activity in the alpha range (Fig. 4, top middle and right). Time–frequency analysis on our source space data, on the other hand, yielded clear alpha decreases. Similar to the sham condition, statistical contrasts of post-stimulus alpha power (8–12 Hz) vs. baseline in the tACS conditions yielded dominant generators in posterior regions (pFDR < .05, Fig. 4, bottom middle and right). On a descriptive level, the alpha suppression in source space appears less pronounced for the strong tACS condition compared to the two others, which could be an actual effect of the neurostimulation leading to increased inhibitory states (Klimesch et al., 2007). As addressing this issue was not the goal of the study, we did not follow-up this descriptive pattern.
Fig. 4.
Alpha decrease induced by visual stimuli.
Top — Sensor level average across all gradiometers (combined) in the three stimulation conditions. Topographies show alpha band (8–12 Hz) activity during stimulus presentation (0.3–1.5 s). Parieto-occipital alpha desynchronization is observable only in the sham condition. The time–frequency representations are baseline corrected (relative change). Note, the strong signal at around 1 s in the middle is a broadband artifact present only in one subject. Bottom — Source space averages across all cortical sources. Stimulus induced alpha decrease (normalized difference of post-stimulus alpha vs. baseline) is observable in all stimulation conditions in parieto-occipital areas. The statistical maps are thresholded at p < .001 for descriptive purposes, FDR corrected significant effects were observed in all conditions. a.u. — arbitrary unit.
Discussion
In this study, we demonstrated, for the first time, the feasibility of using MEG to uncover brain dynamics during tACS. It was not our intention to reveal actual immediate effects of tACS on oscillatory brain activity, but provide a critical proof of concept for future studies. The essential component of our approach is the successful removal of the stimulation artifact to uncover subtle modulations of oscillatory brain activity during oscillatory brain stimulation in the same frequency range. In order to provide a validation of our approach, we resorted to well-established paradigms known to elicit very robust alpha modulations. As expected, in the sham condition robust ERs and alpha modulations (both for eyes open vs. eyes closed and stimulus-induced power decrease) were obtained on the sensor as well as at source level. However, already at weak stimulation, sensor level data were utterly unusable, with the artifact dominating all results. Importantly for the resting state data, even the statistical contrast between eyes open and eyes closed did not remove the “common” influence of the artifact, yielding no meaningful difference between the conditions during real tACS. Also, since tACS was constant throughout one block, baseline normalization in the time–frequency analysis effectively removed any traces of alpha modulations following auditory and visual stimulation. In contrast to the sensor level, source level analysis provided strikingly similar patterns to those obtained for the sham condition. Not only did we obtain the well-established ERs, we also showed the known alpha modulation patterns, i.e., power increases during eyes closed and power reductions during sensory stimulation. Importantly, the results presented in Fig. 2, Fig. 3, Fig. 4 show contrasts. That means that even if there is contamination by the tACS it should be similarly affecting both conditions (be it eyes open vs. closed or sensory stimulation). Thus, the crucial comparison is not the curves but the statistical parametric source maps below the curves. Overall, our study goes significantly beyond a previous EEG approach, which for alpha tACS showed alpha increases only relative to a condition without stimulation (Helfrich et al., 2014; see Voss et al., 2014, for a study in the gamma frequency range). An additional advantage of our beamforming approach is that it can be applied objectively, whereas the approach introduced by Helfrich et al. (2014) relies on subjectively removing ICA components for each subject. As suggested by Schmidt et al. (2014), it is of utmost importance for future studies to measure brain activity and brain modulations during tACS stimulation. This is particularly important for the stimulation frequency itself, which is at the center of interest in most tACS studies. Showing that alpha modulations can be faithfully obtained while undergoing alpha tACS (at intensities common in the literature) is the most important contribution of this study.
The critical step in our analysis pipeline is the projection of the sensor data into source space using the LCMV beamformer, which allows for a separation of brain activity from the stimulation artifact even during high levels of tACS. This well established and frequently- used source projection exploits a feature of beamformers, which suppress perfectly correlating data, since these are physiologically improbable (van Veen et al., 1997). As tACS introduces signals that perfectly correlate over basically all sensors, it is in principle well suited to remove the stimulation artifact. Our approach from a signal processing perspective is not innovative and very similar to the one reported by Soekadar et al. (2013), who showed it was feasible to remove artifacts introduced by tDCS. However, as long as sensors do not saturate, high-pass filtering may already be sufficient in the case of DC stimulation, whereas in the case of tACS filtering the sensor level data would lead to an unacceptable loss of information. While opening up new avenues to investigate experimental effects during neurostimulation, the potential feasibility of our approach may have been foreshadowed by other applications of beamformers in the case of time-varying artifacts. For example, Wong and Gordon (2009) demonstrated the successful use of beamformers for suppressing artifacts introduced by cochlear implants on EEG data. Furthermore, in a combined EEG/magnetic resonance imaging (MRI) measurement, beamformers were successfully used to cancel out the gradient and cardioballistic artifacts (Brookes et al., 2008). This shows that the presented beamforming approach is not limited to MEG and could also be realized with EEG, however, likely with greater problems (see Introduction) and less spatial accuracy.
Until now, human tACS studies mostly have relied on offline measurements of brain activity which required stimulation protocols of long duration that produce after-effects (Zaehle et al., 2010, Neuling et al., 2012, Neuling et al., 2013), or indirect measures that correlate with changes of brain activity were used, e.g., behavior or TMS triggered motor evoked potentials (Feurra et al., 2011, Neuling et al., 2012, Polania et al., 2012, Strüber et al., 2014). With our approach, it will be possible to demonstrate how tACS directly modulates oscillatory brain activity and subsequent behavior: Future research will be capable of transporting cognitive neuroscientific experiments more straightforwardly in a combined MEG-neurostimulation setting. The same line of thought applies to the investigation of how entrainment may act on diverse disorders for which dysfunctional oscillations have been assumed to be critical, such as tinnitus (Weisz et al., 2005), schizophrenia (Uhlhaas& Singer, 2012), and Parkinson's disease (Hammond et al., 2007).
Finally, our approach allows the investigation of the impact of tACS directly on the human brain at an unprecedented level. This issue is currently poorly understood and relies on the extension of animal findings onto humans (Reato et al., 2013), which may be more or less valid. As an example, we will describe two unresolved and pressing problems with regard to tACS efficacy: dosage (i.e., duration and intensity) and interindividual differences (Krause and Cohen Kadosh, 2014, López-Alonso et al., 2014). Thus far, considerations to adjust the dosage of tACS have been rather elusive: stimulation was either applied with fixed parameters for all participants (e.g., Antal et al., 2008) to keep the dosage seemingly constant, or specific parameters were adjusted individually considering, for instance, endogenous frequency or sensation threshold in order to maximize tACS efficacy and enable sham control (Neuling et al., 2012, Neuling et al., 2013, Zaehle et al., 2010). There is only sparse evidence on how external stimulation parameters and internal parameters of the participant (e.g., brain state, individual oscillatory power and frequency) contribute to tACS efficacy (Fröhlich and McCormick, 2010, Neuling et al., 2013, Schmidt et al., 2014). In future studies, brain activity recordings during tACS, as presented here, will reveal the interplay of dosage, internal parameters, and efficacy. Furthermore, dosage control will have groundbreaking consequences for future therapeutic interventions that utilize tACS in order to enhance beneficial brain oscillations or suppress pathological brain activity (Fröhlich, 2014, Kuo et al., 2014), because it allows for patient-tailored stimulation protocols.
To conclude: Until now, the progress of tACS in neuroscientific research has been hindered by the seemingly insurmountable challenge to analyze brain activity during stimulation, in particular at the stimulation frequency. In the present study, using well-established alpha effects; we have presented a proof of concept to overcome this limitation. This will allow unprecedented insights into understanding the online impact of tACS on brain function.
Competing financial interests
The authors declare no competing financial interests.
Authors' contributions
TN, PR, MF, GD & NW designed the experiments; TN & MF performed the experiments and collected the data; TN, PR, MF, GD & NW analyzed data; TN, PR, NW & CSH wrote the manuscript. TN/PR & NW/CSH contributed equally to this work.
Acknowledgments
This research was funded by the European Research Council (ERC StG 283404) and the German Research Foundation (Deutsche Forschungsgemeinschaft, DFG Cluster of Excellence 1077 “Hearing4all”). T.N. was further supported by a stipend of the Lienert Stiftung.
Footnotes
Supplementary data to this article can be found online at http://dx.doi.org/10.1016/j.neuroimage.2015.06.026.
Appendix A. Supplementary data
Supplementary material.
References
- Antal A., Paulus W. Transcranial alternating current stimulation (tACS) Front. Hum. Neurosci. 2013;31(7) doi: 10.3389/fnhum.2013.00317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antal A., Boros K., Poreisz C., Chaieb L., Terney D., Paulus W. Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul. 2008;1:97–105. doi: 10.1016/j.brs.2007.10.001. [DOI] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: a practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B. 1995;57(1):289–300. [Google Scholar]
- Brookes M.J., Mullinger K.J., Stevenson C.M., Morris P.G., Bowtell R. Simultaneous EEG source localisation and artifact rejection during concurrent fMRI by means of spatial filtering. NeuroImage. 2008;40(3):1090–1104. doi: 10.1016/j.neuroimage.2007.12.030. [DOI] [PubMed] [Google Scholar]
- Brunoni A.R., Amadera J., Berbel B., Volz M., Rizzerio B., Fregni F. A systematic review on reporting and assessment of adverse effects associated with transcranial direct current stimulation. Int. J. Neuropsychopharmacol. 2011;14:1133–1145. doi: 10.1017/S1461145710001690. [DOI] [PubMed] [Google Scholar]
- Feurra M., Paulus W., Walsh V., Kanai R. Frequency specific modulation of human somatosensory cortex. Front. Psychol. 2011;2(13) doi: 10.3389/fpsyg.2011.00013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich F., McCormick D.A. Endogenous electric fields may guide neocortical network activity. Neuron. 2010;67(1):129–143. doi: 10.1016/j.neuron.2010.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Genovese C.R., Lazar N.A., Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. NeuroImage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- Hammond C., Bergman H., Brown P. Pathological synchronization in Parkinson's disease: networks, models and treatments. Trends Neurosci. 2007;30:357–364. doi: 10.1016/j.tins.2007.05.004. [DOI] [PubMed] [Google Scholar]
- Helfrich R.F., Schneider T.R., Rach S., Trautmann-Lengsfeld S.A., Engel A.K., Herrmann C.S. Entrainment of brain oscillations by transcranial alternating current stimulation. Curr. Biol. 2014;24(3):333–339. doi: 10.1016/j.cub.2013.12.041. [DOI] [PubMed] [Google Scholar]
- Herrmann C.S., Demiralp T. Human EEG gamma oscillations in neuropsychiatric disorders. Clin. Neurophysiol. 2005;116:2719–2733. doi: 10.1016/j.clinph.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Herrmann C.S., Rach S., Neuling T., Strüber D. Transcranial alternating current stimulation: a review of the underlying mechanisms and modulation of cognitive processes. Front. Hum. Neurosci. 2013;7(279) doi: 10.3389/fnhum.2013.00279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fröhlich F. Endogenous and exogenous electric fields as modifiers of brain activity: rational design of noninvasive brain stimulation with transcranial alternating current stimulation. Dialogues Clin. Neurosci. 2014;16(1):93–102. doi: 10.31887/DCNS.2014.16.1/ffroehlich. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klimesch W., Sauseng P., Hanslmayr S. EEG alpha oscillations: the inhibition-timing hypothesis. Brain Res. Rev. 2007;53(1):63–88. doi: 10.1016/j.brainresrev.2006.06.003. [DOI] [PubMed] [Google Scholar]
- Krause B., Cohen Kadosh R. Not all brains are created equal: the relevance of individual differences in responsiveness to transcranial electrical stimulation. Front. Syst. Neurosci. 2014;8(25) doi: 10.3389/fnsys.2014.00025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo M.F., Paulus W., Nitsche M.A. Therapeutic effects of non-invasive brain stimulation with direct currents (tDCS) in neuropsychiatric diseases. NeuroImage. 2014;85(3):948–960. doi: 10.1016/j.neuroimage.2013.05.117. [DOI] [PubMed] [Google Scholar]
- López-Alonso V., Cheeran B., Río-Rodríguez D., Fernández-del-Olmo M. Inter-individual variability in response to non-invasive brain stimulation paradigms. Brain Stimul. 2014;7(3):372–380. doi: 10.1016/j.brs.2014.02.004. [DOI] [PubMed] [Google Scholar]
- Marshall L., Helgadottir H., Molle M., Born J. Boosting slow oscillations during sleep potentiates memory. Nature. 2006;444:610–613. doi: 10.1038/nature05278. [DOI] [PubMed] [Google Scholar]
- Marshall L., Binder S. Contribution of transcranial oscillatory stimulation to research on neural networks: an emphasis on hippocampo-neocortical rhythms. Front. Hum. Neurosci. 2013;7(614) doi: 10.3389/fnhum.2013.00614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neuling T., Rach S., Herrmann C.S. Good vibrations: oscillatory phase shapes perception. NeuroImage. 2012;63:771–778. doi: 10.1016/j.neuroimage.2012.07.024. [DOI] [PubMed] [Google Scholar]
- Neuling T., Rach S., Herrmann C.S. Orchestrating neuronal networks: sustained after-effects of transcranial alternating current stimulation depend upon brain states. Front. Hum. Neurosci. 2013;7(161) doi: 10.3389/fnhum.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolte G. The magnetic lead field theorem in the quasi-static approximation and its use for magnetoencephalography forward calculation in realistic volume conductors. Phys. Med. Biol. 2003;48:3637–3652. doi: 10.1088/0031-9155/48/22/002. [DOI] [PubMed] [Google Scholar]
- Oostenveld R., Fries P., Maris E., Schoffelen J.-M. FieldTrip: open source software for advanced analysis of MEG, EEG, and invasive electrophysiological data. Comput. Intell. Neurosci. 2011 doi: 10.1155/2011/156869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polania R., Nitsche M.A., Korman C., Batsikadze G., Paulus W. The importance of timing in segregated theta phase-coupling for cognitive performance. Curr. Biol. 2012;22:1314–1318. doi: 10.1016/j.cub.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Reato D., Rahman A., Bikson M., Parra L.C. Low-intensity electrical stimulation affects network dynamics by modulating population rate and spike timing. J. Neurosci. 2010;30(45):15067–15079. doi: 10.1523/JNEUROSCI.2059-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reato D., Rahman A., Bikson M., Parra L.C. Effects of weak transcranial alternating current stimulation on brain activity—a review of known mechanisms from animal studies. Front. Hum. Neurosci. 2013;7(687) doi: 10.3389/fnhum.2013.00687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt S.L., Iyengar A.K., Foulser A.A., Boyle M.R., Fröhlich F. Endogenous cortical oscillations constrain neuromodulation by weak electric fields. Brain Stimul. 2014 doi: 10.1016/j.brs.2014.07.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnitzler A., Gross J. Normal and pathological oscillatory communication in the brain. Nat. Rev. Neurosci. 2005;6:285–296. doi: 10.1038/nrn1650. [DOI] [PubMed] [Google Scholar]
- Soekadar S.R., Witkowski M., Cossio E.G., Birbaumer N., Robinson S.E., Cohen L.G. In vivo assessment of human brain oscillations during application of transcranial electric currents. Nat. Commun. 2013;4(2032) doi: 10.1038/ncomms3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spaak E., de Lange F.P., Jensen O. Local entrainment of α oscillations by visual stimuli causes cyclic modulation of perception. J. Neurosci. 2014;34(10):3536–3544. doi: 10.1523/JNEUROSCI.4385-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strüber D., Rach S., Trautmann-Lengsfeld S.A., Engel A.K., Herrmann C.S. Antiphasic 40 Hz oscillatory current stimulation affects bistable motion perception. Brain Topogr. 2014;27(1):158–171. doi: 10.1007/s10548-013-0294-x. [DOI] [PubMed] [Google Scholar]
- Thut G., Miniussi C. New insights into rhythmic brain activity from TMS-EEG studies. Trends Cogn. Sci. 2009;13(4):182–189. doi: 10.1016/j.tics.2009.01.004. [DOI] [PubMed] [Google Scholar]
- Uhlhaas P.J., Haenschel C., Nikoli'c D., Singer W. The role of oscillations and synchrony in cortical networks and their putative relevance for the pathophysiology of schizophrenia. Schizophr. Bull. 2008;34:927–943. doi: 10.1093/schbul/sbn062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uhlhaas P.J., Singer W. Neuronal dynamics and neuropsychiatric disorders: toward a translational paradigm for dysfunctional large-scale networks. Neuron. 2012;75(6):963–980. doi: 10.1016/j.neuron.2012.09.004. [DOI] [PubMed] [Google Scholar]
- Van Veen B.D., van Drongelen W., Yuchtman M., Suzuki A. Localization of brain electrical activity via linearly constrained minimum variance spatial filtering. IEEE Trans. Biomed. Eng. 1997;44(9):867–880. doi: 10.1109/10.623056. [DOI] [PubMed] [Google Scholar]
- Voss U., Holzmann R., Hobson A., Paulus W., Koppehele-Gossel J., Klimke A., Nitsche M.A. Induction of self awareness in dreams through frontal low current stimulation of gamma activity. Nat. Neurosci. 2014;17(6):810–812. doi: 10.1038/nn.3719. [DOI] [PubMed] [Google Scholar]
- Vossen A., Gross J., Thut G. Alpha power increase after transcranial alternating current stimulation at alpha frequency (α-tACS) reflects plastic changes rather than entrainment. Brain Stimul. 2014 doi: 10.1016/j.brs.2014.12.004. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisz N., Moratti S., Meinzer M., Dohrmann K., Elbert T. Tinnitus perception and distress is related to abnormal spontaneous brain activity as measured by magnetoencephalography. PLoS Med. 2005;2(6):e153. doi: 10.1371/journal.pmed.0020153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong D.D., Gordon K.A. Beamformer suppression of cochlear implant artifacts in an electroencephalography dataset. IEEE Trans. Biomed. Eng. 2009;56(12):2851–2857. doi: 10.1109/TBME.2009.2029239. [DOI] [PubMed] [Google Scholar]
- Zaehle T., Rach S., Herrmann C. Transcranial alternating current stimulation enhances individual alpha activity in human EEG. PLoS One. 2010;5:e13766. doi: 10.1371/journal.pone.0013766. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.