Abstract
Objective(s):
Auraptene (7-geranyloxycoumarin) (AUR), from Citrus species has shown anti-inflammatory, neuroprotective, and acetylcholinesterase (AChE) and beta-secretase inhibitory effects. Scopolamine is a nonselective muscarinic receptor antagonist which causes short-term memory impairments and is used for inducing animal model of Alzheimer’s disease (AD). This research aimed to investigate the effect of AUR on scopolamine-induced avoidance memory retention deficits in step-through task in mice.
Materials and Methods:
The effect of four-day pre-training injections of AUR (50, 75, and 100 mg/kg, subcutaneous (SC)) and scopolamine (1 mg/kg, IP), and their co-administration on avoidance memory retention in step-through passive avoidance task, was investigated by measuring the latency to enter to the dark chamber.
Results:
Pre-training administration of AUR caused significant increase in step-through latency in comparison with control group, 48, 96, and 168 hr after training trial. The findings of this study showed that scopolamine (1 mg/kg, IP, for four consecutive days) impaired passive avoidance memory retention compared to saline-treated animals. Step-through passive avoidance task results showed that AUR markedly reversed scopolamine-induced avoidance memory retention impairments, 24 and 168 hr after training trial in step-through task.
Conclusion:
Results from co-administration of AUR and scopolamine showed that AUR reversed scopolamine-induced passive avoidance memory retention impairments.
Keywords: Auraptene, Avoidance memory retention, Mice, Scopolamine, Shuttle box
Introduction
Alzheimer’s disease (AD) is the most common neurodegenerative disease especially among elderly people. Aging is the most important etiological factor in AD which is rapidly becoming very common all around the world especially in industrialized countries. Two major hallmarks of AD are β-amyloid plaques deposition and cholinergic system down-regulation. Beta-secretase is an enzyme involved in the cleavage of amyloid beta protein precursor (APP) into β-amyloid and the production of β-amyloid plaques. Multiple neurotransmitter systems seem to be affected in AD. One of these systems is brain cholinergic system that has been implicated in the pathophysiology and treatment of memory deficits (1-4).
Auraptene (7-geranyloxycoumarin) (AUR) is a coumarin derivative which could be found in a variety of citrus fruits. Several studies have mentioned valuable properties of AUR such as anti-inflammatory and anticarcinogenesis effects in peripheral tissues. Furthermore, AUR exhibits multiple protective activities in the brain including neurotrophic, acetylcholinesterase (AChE) and beta-secretase inhibitory effects, and strong antioxidant activity. Moreover, AUR can suppress microglia activation in the hippocampus and inhibit N-methyl-D-aspartate (NMDA)-induced neurotoxicity (5-12). In addition, AUR has shown neurotrophic activity in PC12 cell line via activation of ERK1/2 and promotion of neurite outgrowth (12). It has also protective effects against brain global ischemia in mice through inhibition of inflammation, the main cause of cognitive defects. Naturally occurring coumarins inhibit beta-secretase enzyme which is regarded as a valuable therapeutic target for the treatment of AD (7, 8).
Scopolamine is a nonselective muscarinic receptor antagonist. It decreases acetylcholine level in the hippocampus and causes learning acquisition deficits, which make it a useful pharmacological tool to induce a partial model of the AD (13).
This study assessed the effects of AUR on the cognitive performance of scopolamine-treated animals. As AUR was found to cause dose-dependent neuroprotective effects, different doses of AUR (50, 75, and 100 mg/kg, subcutaneous (SC)) were chosen to investigate its effects on scopolamine-induced avoidance memory retention deficits in step-through passive avoidance task in mice.
Materials and Methods
Animals
Adult male mice (30-35 g, n=7) were obtained from Zabol University of Medical Sciences, Zabol, Iran, and housed at normal ambient temperature (22-23 °C) with a 12:12 hr light:dark cycle. Animals were trained and tested during the light period. All methods used in this study were in accordance with Helsinki declaration.
Drugs
AUR was provided by Dr. Iranshahi, Mashhad University of Medical Sciences, Mashhad, Iran. Briefly, it was synthesized through the reaction of 7-hydroxy-coumarin (1 M) with trans-geranyl bromide (1.5 M) in acetone, at room temperature. The reaction was done in the presence of 1,8-Diazabicyclo[5.4.0]undec-7-ene (DBU) (2M). The resulting mixture was concentrated under reduced pressure and purified by column chromatography using petroleum ether/ethyl acetate (9:1, v/v) to produce white crystals (9). To provide injection preparation, AUR was dissolved in dimethyl sulfoxide (DMSO)/polyethylene glycol (PEG) 300 (1:1) solutions (10). Scopolamine was purchased from Sigma (St Luis, MO, USA) and dissolved in sodium chloride 0.9%.
Apparatus; passive avoidance training and testing
One-trial passive avoidance learning task was selected as the tool for behavioral assessment to measure associative memory retention in mice. For this purpose, an apparatus consisted of two identical illuminated and non-illuminated boxes was used. At first, all animals were allowed to habituate in the experimental chamber without shock and mice with no tendency to enter the dark room were excluded. For the acquisition trial, animals were placed individually in the illuminated compartment of automated passive avoidance system (UgoBasile, Comerio, Italy). The apparatus was activated by pressing the pedal. The guillotine door was raised after 10 sec allowing the animal to enter the dark compartment. After that, the guillotine door was closed and animals were given an inescapable foot-shock (0.2 mA), for 2 sec (on training day). The retention tests were conducted 24, 48, 96, and 168 hr after the training session (without shock). The latency time (cut off 300 sec) to enter the dark compartment was recorded.
Experiment 1
In this part, effects of AUR were assessed by passive avoidance task. Animals were treated with AUR (50, 75, and 100 mg/kg, SC) for four consecutive days. On the 4th day, 30 min after the last injection, animals were trained in step-through passive avoidance task and 24, 48, 96, and 168 hr after the training, passive avoidance memory retention was assessed. Control animals received DMSO/PEG 300 (1:1) (AUR was dissolved in DMSO/PEG) and tested in the same manner.

Experiment 2
Here, scopolamine (1 mg/kg, IP) was administered for four consecutive days. At the end of the 4th day and 30 min after the last injection, animals were trained in step-through passive avoidance task and 24, 48, 96, and 168 hr after the training, passive avoidance memory retention was evaluated. Control animals received saline (scopolamine was dissolved in saline) and tested in the same manner.

Finally, effects of AUR on scopolamine-induced memory impairment were assessed. At first, AUR (50, 75, and 100 mg/kg, SC) was injected and 15 min later, scopolamine (1 mg/kg, IP) was administered. AUR and scopolamine were administered for four consecutive days. However, 30 min after the last injection, animals were trained in step-through passive avoidance task and 24, 48, 96, and 168 hr after the training, passive avoidance memory retention was assessed.

Data analysis
Data are expressed as means±SEM and were analyzed using one-way analysis of variance (ANOVA). For multiple comparisons, Newman–Keuls post–hoc test was used. Statistical significance was set at P<0.05.
Results
Pre-training administration effects of AUR on passive avoidance memory retention in step-through task in mice
Four-days pre-training administration of AUR (50, 75, and 100 mg/kg, SC) caused significant increase in step-through latency in comparison with control group after 48 (Figure 1B), 96 (Figure 1C), and 168 hr (Figure 1D).
Figure 1.

(A-D). Pre-training effects of auraptene (AUR) administration (4 days, SC) on avoidance memory retention after 24 (A), 48 (B), 96 (C), and 168 hr (D) in step-through passive avoidance task. Each value represents the mean±SEM for 7 mice. *P<0.0.5, **P<0.01 and ***P<0.001 show significant differences from control [DMSO/PEG 300 (1:1)] group
The effects of AUR on scopolamine-induced avoidance memory retention impairments in step-through task in mice
The results showed that pre-training administra-tion of scopolamine (1 mg/kg, IP, for four consecutive days) impaired passive avoidance memory retention in step-through task. There were significant differences between scopolamine and control (saline-treated) animals, 24 (Figure 2A, P<0.001), 48 (Figure 2B, P<0.05), 96 (Figure 2C, P<0.01), and 168 hr (Figure 2D, P<0.001) after training trial in step-through task.
Figure 2.
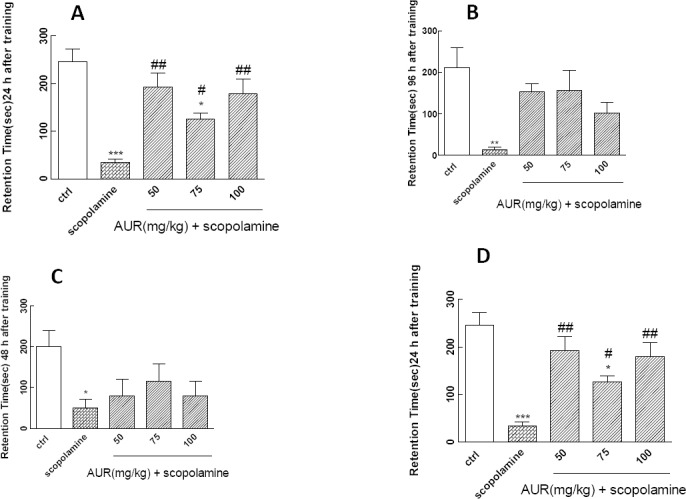
(A–D). The effects of auraptene (AUR) on scopolamine–induced memory retention impairments in step-through task after 24 (A), 48 (B), 96 (C), and 168 hr (D). * P<0.05 and **P<0.01 show significant differences from control (saline-treated) animals. #P<0.05, ##P<0.01, and ###P<0.001 show significant differences from scopolamine–treated group. Each value represents the mean±SEM for 7 mice
AUR (50, 75, and 100 mg/kg, SC) was administered 15 min before scopolamine (1 mg/kg, IP) for four consecutive days. Step-through passive avoidance task results showed that AUR markedly reversed scopolamine-induced avoidance memory retention impairments, 24 (Figure 2A) and 168 hr (Figure 2D) after training trial in step-through task.
Discussion
In the present study, we investigated the effects of AUR on scopolamine-induced avoidance memory retention impairment in the passive avoidance task in mice. Our results showed that administration of scopolamine (1 mg/kg, IP) for four consecutive days, induced impairment of passive avoidance retention which was able to impair retention memory processes. We found that AUR can consolidate memory and reverse avoidance memory impairment induced by scopolamine. Central and peripheral modification of neurotransmission is involved in learning ability. It has been suggested that cholinergic system plays an important role in learning and memory processes. Scopolamine is a nonselective muscarinic receptor antagonist that induces senile and Alzheimer-type dementia (14, 15).
It has been reported that AUR has strong antioxidant effects (10). Many studies confirmed that AUR also has antioxidant effects on central nervous system as well as peripheral tissues. It has been already found that AUR can suppress microglial activation and ameliorate expression of cyclooxygenase-2 (COX-2) in astrocytes in the hippocampus following brain ischemia (10).
Memory consolidating effects are not solely mediated via antioxidant and free radical scavenging effects of AUR, but it has been reported that inhibition of COX-2 enzyme protects delayed neuronal cell death in neurodegenerative diseases including Parkinson’s and Alzheimer’s disease (16-18). In addition, AUR has shown neuroprotective effects against NMDA-induced neuro-degeneration (5).
Also, AUR potentiates central muscarinic cholinergic system. It was cleared that muscarinic system is one of the most important systems involved in memory especially in acquisition and cognition. It has been shown that AUR inhibits AChE enzyme moderately. Cholinergic hypothesis is the most widely accepted theory of AD development (19-21).
The beta-secretase enzyme has been recognized as a valuable target for the treatment of AD. This enzyme is known to be incorporated in the production of amyloid beta plaques via cleavage of amyloid beta protein precursor. Beta-secretase inhibitors are strongly related to drugs that have been used for the treatment of AD. A study done by Marumoto and colleagues showed that naturally occurring coumarins like AUR at different concentrations inhibit beta-secretase (8, 7).
cAMP has an important role in the long-term potentiation (LTP) via activation of cAMP response element binding (CREB) through protein synthesis (22). Activated CREB recruits the CREB binding protein (CBP) to the promoter regions of cAMP-responsive genes associated with growth of dendrite, changing morphology, improving synaptic plasticity, and long-term memory (12, 23). It is known that AUR strongly induces ERK1/2, consequently phosphorylates CREB in PC12 cell line and cortical neuronal cell line, resulting in memory consolidation (12).
AUR has anti-inflammatory, anti-hypertensive, antioxidant, anti-bacterial, and immunomodulatory effects (5, 24-30). Inflammation is implicated in the progress of neurodegenerative diseases including AD and Parkinson’s disease (18). The other possible mechanisms for memory consolidating effects of AUR are modulation of estrogen receptor (ER) and inhibition of acyl-CoA:cholesterol acyltransferase (ACAT). Based on the ACAT enzyme inhibitory and ER modulatory effects of AUR, Medina et al suggested that AUR could be considered as a useful agent in the treatment of AD (31).
Conclusion
Our study showed that AUR reverses scopolamine-induced avoidance memory retention deficits in step-through task in mice. Findings of this study suggest that AUR may restore memory processes partly via modulation of cholinergic pathways.
Acknowledgment
The results described in this paper were part of a Pharm D thesis which was supported by Zabol University of Medical Sciences, Zabol, Iran.
References
- 1.Gutierres JM, Carvalho FB, Schetinger MR, Agostinho P, Marisco PC, Vieira JM, et al. Neuroprotective effect of anthocyanins on acetylcholinesterase activity and attenuation of scopolamine-induced amnesia in rats. Int J Dev Neurosci. 2014;33:88–97. doi: 10.1016/j.ijdevneu.2013.12.006. [DOI] [PubMed] [Google Scholar]
- 2.Warburton EC, Koder T, Cho K, Massey PV, Duguid G, Barker GR, et al. Cholinergic neurotransmission is essential for perirhinal cortical plasticity and recognition memory. Neuron. 2003;38:987–996. doi: 10.1016/s0896-6273(03)00358-1. [DOI] [PubMed] [Google Scholar]
- 3.Murphy KJ, Foley AG, O’connell AW, Regan CM. Chronic exposure of rats to cognition enhancing drugs produces a neuroplastic response identical to that obtained by complex environment rearing. Neuropsychopharmacology. 2006;31:90–100. doi: 10.1038/sj.npp.1300810. [DOI] [PubMed] [Google Scholar]
- 4.Jafari MR, Zarrindast MR, Djahanguiri B. Influence of cholinergic system modulators on morphine state-dependent memory of passive avoidance in mice. Physiol Behav. 2006;88:146–151. doi: 10.1016/j.physbeh.2006.03.026. [DOI] [PubMed] [Google Scholar]
- 5.Epifano F, Molinaro G, Genovese S, Ngomba RT, Nicoletti F, Curini M. Neuroprotective effect of prenyloxycoumarins from edible vegetables. Neurosci Lett. 2008;443:57–60. doi: 10.1016/j.neulet.2008.07.062. [DOI] [PubMed] [Google Scholar]
- 6.Furukawa Y, Okuyama S, Amakura Y, Watanabe S, Fukata T, Nakajima M, et al. Isolation and characterization of activators of ERK/MAPK from citrus plants. Int J Mol Sci. 2012;13:1832–1845. doi: 10.3390/ijms13021832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marumoto S, Miyazawa M. beta-secretase inhibitory effects of furanocoumarins from the root of Angelica dahurica. Phytother Res. 2010;24:510–513. doi: 10.1002/ptr.2967. [DOI] [PubMed] [Google Scholar]
- 8.Marumoto S, Miyazawa M. Structure-activity relationships for naturally occurring coumarins as β-secretase inhibitor. Bioorg Med Chem. 2012;20:784–788. doi: 10.1016/j.bmc.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Askari M, Sahebkar A, Iranshahi M. Synthesis and purification of 7-prenyloxycoumarins and herniarin as bioactive natural coumarins. Iran J Basic Med Sci. 2009;12:63–69. [Google Scholar]
- 10.Okuyama S, Minami S, Shimada N, Makihata N, Nakajima M, Furukawa Y. Anti-inflammatory and neuroprotective effects of auraptene, a citrus coumarin, following cerebral global ischemia in mice. Eur J Pharmacol. 2013;699:118–123. doi: 10.1016/j.ejphar.2012.11.043. [DOI] [PubMed] [Google Scholar]
- 11.Onozuka H, Nakajima A, Matsuzaki K, Shin RW, Ogino K, Saigusa D, et al. Nobiletin, a citrus flavonoid, improves memory impairment and Abeta pathology in a transgenic mouse model of Alzheimer’s disease. J Pharmacol Exp Ther. 2008;326:739–744. doi: 10.1124/jpet.108.140293. [DOI] [PubMed] [Google Scholar]
- 12.Furukawa Y, Watanabe S, Okuyama S, Nakajima M. Neurotrophic effect of citrus auraptene: neuritogenic activity in PC12 cells. Int J Mol Sci. 2012;13:5338–5347. doi: 10.3390/ijms13055338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seifhosseini S, Jahanshahi M, Moghimi A, Aazami NS. The effect of scopolamine on avoidance memory and hippocampal neurons in male wistar rats. Basic Clin Neurosci. 2011;3:9–15. [Google Scholar]
- 14.Azami NS, Piri M, Oryan S, Jahanshahi M, Babapour V, Zarrindast MR. Involvement of dorsal hippocampal alpha-adrenergic receptors in the effect of scopolamine on memory retrieval in inhibitory avoidance task. Neurobiol Learn Mem. 2010;93:455–462. doi: 10.1016/j.nlm.2010.01.003. [DOI] [PubMed] [Google Scholar]
- 15.Klinkenberg I, Blokland A. A comparison of scopolamine and biperiden as a rodent model for cholinergic cognitive impairment. Psychopharmaco-logy (Berl) 2011;215:549–566. doi: 10.1007/s00213-011-2171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nakayama M, Uchimura K, Zhu RL, Nagayama T, Rose ME, Stetler RA, et al. Cyclooxygenase-2 inhibition prevents delayed death of CA1 hippocampal neurons following global ischemia. Proc Natl Acad Sci USA. 1998;95:10954–10959. doi: 10.1073/pnas.95.18.10954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McGeer EG, Klegeris A, McGeer PL. Inflammation, the complement system and the diseases of aging. Neurobiol Aging. 2005;26:94–97. doi: 10.1016/j.neurobiolaging.2005.08.008. [DOI] [PubMed] [Google Scholar]
- 18.Qin L, Wu X, Block ML, Liu Y, Breese GR, Hong JS, et al. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Loizzo MR, Tundis R, Menichini F, Menichini F. Natural products and their derivatives as cholinesterase inhibitors in the treatment of neurodegenerative disorders: an update. Curr Med Chem. 2008;15:1209–1228. doi: 10.2174/092986708784310422. [DOI] [PubMed] [Google Scholar]
- 20.Miyazawa M, Tougo H, Ishihara M. Inhibition of acetylcholinesterase activity by essential oil from Citrus paradisi. Nat Prod Lett. 2001;15:205–210. doi: 10.1080/10575630108041281. [DOI] [PubMed] [Google Scholar]
- 21.Karimi Gh, Iranshahi M, Hosseinalizadeh F, Riahi B, Sahebkar A. Screening of acetylcholinesterase inhibitory activity of terpenoid and coumarin derivatives from the genus Ferula. Pharmacologyonline. 2010;1:566–574. [Google Scholar]
- 22.Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- 23.Sharifzadeh M, Tavasoli M, Naghdi N, Ghanbari A, Amini M, Roghani A. Post-training intrahippocampal infusion of nicotine prevents spatial memory retention deficits induced by the cyclo-oxygenase-2-specific inhibitor celecoxib in rats. J Neurochem. 2005;95:1078–1090. doi: 10.1111/j.1471-4159.2005.03454.x. [DOI] [PubMed] [Google Scholar]
- 24.Iranshahi M, Kalategi F, Rezaee R, Shahverdi AR, Ito C, Furukawa H, et al. Cancer chemopreventive activity of terpenoid coumarins from Ferula species. Planta Med. 2008;74:147–150. doi: 10.1055/s-2008-1034293. [DOI] [PubMed] [Google Scholar]
- 25.Soltani F, Mosaffa F, Iranshahi M, Karimi G, Malekaneh M, Haghighi F, et al. Auraptene from Ferula szowitsiana protects human peripheral lymphocytes against oxidative stress. Phythoter Res. 2010;24:85–89. doi: 10.1002/ptr.2874. [DOI] [PubMed] [Google Scholar]
- 26.Nazari ZE, Iranshahi M. Biologically active sesquiterpene coumarins from Ferula species. Phytother Res. 2011;25:315–323. doi: 10.1002/ptr.3311. [DOI] [PubMed] [Google Scholar]
- 27.Iranshahi M, Jabbari A, Orafaie A, Mehri R, Zeraatkar S, Ahmadi T, et al. Synthesis and SAR studies of mono O-prenylated coumarins as potent 15-lipoxygenase inhibitors. Eur J Med Chem. 2012;57:134–142. doi: 10.1016/j.ejmech.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 28.Imenshahidi M, Eghbal M, Sahebkar A, Iranshahi M. Hypotensive activity of auraptene, a monoterpene coumarin from Citrus spp. Pharm Biol. 2013;51:545–549. doi: 10.3109/13880209.2012.747546. [DOI] [PubMed] [Google Scholar]
- 29.Valiahdi SM, Iranshahi M, Sahebkar A. Cytotoxic activities of phytochemicals from Ferula species. Daru J Pharm Sci. 2013;21:39–46. doi: 10.1186/2008-2231-21-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Razavi BM, Arasteh E, Imenshahidi M, Iranshahi M. Antihypertensive effect of auraptene, a monoterpene coumarin from the genus Citrus, upon chronic administration. Iran J Basic Med Sci. 2015;18:153–158. [PMC free article] [PubMed] [Google Scholar]
- 31.de Medina P, Genovese S, Paillasse MR, Mazaheri M, Caze-Subra S, Bystricky K, et al. Auraptene is an inhibitor of cholesterol esterification and a modulator of estrogen receptors. Mol Pharmacol. 2010;78:827–836. doi: 10.1124/mol.110.065250. [DOI] [PubMed] [Google Scholar]


