Abstract
Objective(s):
In this study the effect of crocin, a carotenoid isolated from saffron, on malathion (an organophosphate insecticide) induced depressive- like behavior in subacute exposure was investigated. Moreover the molecular mechanism of malathion induced depressive- like behavior and its decreasing effect on the level of brain derived neurotrophic factor (BDNF) in rat hippocampus and cerebral cortex were evaluated.
Materials and Methods:
Male Wistar rats were exposed to malathion (50 mg/kg/day, IP) alone or in combination with crocin (10, 20 and 40 mg/kg/day, IP), imipramine (20 mg/kg/day, IP) and vitamin E (200 mg/kg, three times a week, IP) respectively for 14 days. The forced swimming test (FST) was performed on days 1st, 7th and 14st. The level of malondealdehyde (MDA) and reduced glutathione (GSH) were measured in cerebral cortex and hippocampus of rats. The protein level of BDNF was evaluated using Western blot analysis.
Results:
Malathion (50 mg/kg, IP) increased immobility time in the FST, without affecting total locomotor activity in open-field test. Malathion increased the malondealdehyde (MDA) and decreased the glutathione (GSH), whereas these effects were reversed by crocin and vitamin E. Malathion decreased plasma acetylcholinesterase activity, however this effect was not reversed by crocin or vitamin E. Malathion reduced the protein level of BDNF in rat hippocampus. Imipramine and crocin prevented the decreasing effect of malathion on BDNF.
Conclusion:
These results showed that crocin attenuates some neurochemical and behavioral effects induced by malathion. This neuroprotective effect of crocin may be in part due to its effect on BDNF.
Keywords: BDNF, Crocin, Depression, Forced swimming test, Malathion, Saffron
Introduction
Malathion, an organophosphorus (OPs) compound, is widely used for agricultural crops to control pests and public health programs (1). Besides neurotoxicity caused by inhibition of acetylcholinesterase (AChE) activity in acute exposure, neuropsychiatric symptoms including affective disorders such as anxiety and depression may be produced by long-term exposure to low levels of OPs (2).
Depression is a common disorder and an important cause of disability. According to the World Health Organization (WHO), depression will be the most common cause of illness induced disability by the year 2030 (3). The mechanism of depression is quite complex. In addition to the monoamine theory, the changes in gene expression following an increase in monoamine levels are important for the therapeutic effects of anti-depressants (4). Moreover intracellular pathways regulating neuroplasticity and neuro-degeneration are implicated in the etiology of mood disorders. Based on this hypothesis, depression occurs as a result of stress, neuronal atrophy and decreased neurogenesis (5). Also it was reported that antidepressants can increase the expression of neurotrophic factors, mainly brain-derived neur- otrophic factor (BDNF) by stimulating intracellular pathways (5).
Crocus sativus L., commonly known as saffron, is a perennial stemless herb of the Iridaceae family. Crocin, a carotenoid isolate of this herb, is responsible for red color of saffron (6).
Several studies have demonstrated that crocin exhibits antitumor (7), antioxidant, radical scavenging (8, 9), hypolipidemic (10), antinociceptive (11), anticonvulsant (12) and memory-improving effects (13, 14). Crocin also showed protective effects on diazinon and acrylamide induced oxidative stress both in in vitro and in vivo experiments (15-17). Antidepressant effects of saffron and its components have been established both in traditional and modern pharmacological studies. It has been reported that antidepressant effect of saffron was significantly more effective than placebo in the treatment of major depression. It was also found to be comparable to imipramine in the treatment of mild to moderate depression (18). In a recent study, crocin significantly reduced immobility time in FST in subacute exposure (19).
As mechanism of depression is quite complex, many currently available synthetic antidepressants have low response rates and severe adverse effects. Accordingly, natural plants and their active components may be considered as new antidepressant drugs with trivial side effects than that of synthetic antidepressants (20).
In this study, a possible antidepressant effect of crocin against malathion induced depression was evaluated. Moreover, the neuroprotective effect of crocin against malathion was assessed by evaluating of its effect on antioxidant defense system and lipid peroxidation in the hippocampus and cerebral cortex of rats. We also investigated the molecular mechanisms of malathion induced depression and its decreasing effect on the level of BDNF in rat hippocampus and cerebral cortex.
Materials and Methods
Chemicals
Malathion (purity 96%), vitamin E (OSVE Pharmaceutical Co. Tehran, Iran) and imipramine hydrochloride (Marham Daru, Iran) were obtained. TBA (2-thiobarbituric acid), n-butanol, phosphoric acid, potassium chloride and MDA were obtained from Merck. Reduced GSH were obtained from Sigma-Aldrich. Stigmas of C. sativus L. from Novin Saffron (collected from Ghaen, Khorasan province, Northeast of Iran) was obtained and analyzed in accordance to the ISO/TS 3632-2. Crocin was extracted and purified as previously reported (21). Rabbit polyclonal BDNF (Abcam, # ab46176) and β-actin (Cell Signaling, # 4967) IgG, were provided. Antirabbit IgG labeled with horseradish peroxidase were purchased from Cell Signaling. BradFord Protein assay kit (#500-0002) and polyvinylidene fluoride (PVDF) membrane were purchased from BioRad (#162-0177). ECL Western blotting substrate was obtained from Pierce (#32106). All chemicals used in this study were analytical grade and purchased from Sigma-Aldrich.
Animals and treatment
Adult male Wistar rats (weight 200–250 g) were provided by animal center (School of Pharmacy, Mashhad University of Medical Sciences). They were kept on a 12 hr light/dark cycle and at a temperature of 23±1 °C with free access to food and water. These conditions were maintained constant throughout the experiments. All animal experiments were carried out in accordance to Mashhad University of Medical Sciences, Ethical Committee Acts.
Rats were randomly divided into seven groups: 1) control (normal saline); 2) malathion (50 mg/kg); 3) malathion + crocin 10 mg/kg; 4) malathion + crocin 20 mg/kg; 5) malathion + crocin 40 mg/kg; 6) malathion+ imipramine 20 mg/kg and 7) malathion+ vitamin E 200 IU/kg. All groups consisted of six rats. Treatments were continued for two weeks. Malathion, crocin, imipramine (once a day) and vitamin E (three days a week) were administrated via intraperitoneal (IP) injections. Control rats received normal saline in the same way.
Forced swimming test (FST)
The FST was performed according to the method described by Porsolt et al (22). Each rat was introduced for 15 min into separate transparent cylindrical containers (diameter 30 cm and height 45 cm) that were filled with water to 30 cm so that rats could only touch the bottom with the tip of the tail. Fresh water was introduced prior to each test and water temperature was maintained at 25±2 °C. They were removed 15 min. later, dried and placed in their home cage. Twenty-four hrs later, a subsequent 6-min exposure was carried out and time of immobility was measured. All the experiments were performed during the morning in a noiseless room and recorded with a video camera. Videotapes were subsequently scored. The rats were considered immobile when they remained motionless, and make movements only to keep their heads out of the water. The FST was performed on days 1st, 7th and 14th of experiment.
Open field test
The open field apparatus, made of white wood, had a floor of 100×100 cm divided by red lines into 25 squares of 20×20 cm. The walls (50 cm high) were also painted in white. The test room was illuminated at the same intensity at the colony room. Each rat was placed in the center of the open field, and its behavior was observed for 5 min. The parameters evaluated were the total number of squares crossed, the number of outer squares (those adjacent to the walls) crossed and the number of inner squares crossed; the three measures were referred to as total, peripheral, and central locomotion, respectively. The open-field test was carried out at the first day and 24 hr after the last treatments. At the end of each test, the whole area was cleaned with a wet sponge and a dry paper towel (23).
Measurement of MDA
At the end of treatments, animals were sacrificed by decapitation. Hippocampus and cerebral cortex were dissected on ice immediately following decapitation. Then, brain specimens were rapidly frozen in liquid nitrogen and stored at -80°C. To measure MDA in rat hippocampus and cerebral cortex, the tissues from different groups were homogenized for 2 min at 4 °C (POLYTRON® PT 10-35, Kinematica, Switzerland) in 1.15% KCl in order to provide a 10% homogenate. These homogenates were centrifuged (Hettich, Germany) at 6000×g for 10 min to obtain supernatants. Total protein contents and MDA were measured in supernatants. The protein contents of homogenates were determined using Bio-Rad Protein Assay Kit according to the manufacturer protocol. MDA levels were determined according to the method of Fernandez et al (24). This method is based on the spectrophotometric measurement of the color developed by reaction of MDA to thiobarbituric acid (TBA). Briefly, 3 ml of phosphoric acid (1%) and 1ml TBA (0.6%) were added to 0.5 ml of supernatant in a falcon tube and the mixture was incubated for 45 min in a boiling water bath. After cooling, 4ml of n-butanol was added to the mixture and vortex-mixed for 1 min followed by centrifugation at 3000×g for 20 min. The organic layer was transferred to a fresh tube and its absorbance was measured at 532 nm.
Measurement of reduced GSH
GSH content in rat hippocampus and cerebral cortex was measured according to the method of Moron et al (25). The tissues were homogenized in ice cold phosphate buffered saline (PBS), pH 7.4, to obtain 10% homogenate (w/v). The homogenate was centrifuged at 3000×g for 10min, and then the contents of protein and GSH were determined in the supernatants. Reduced GSH content was measured using 5,5′-dithiobis(2-nitrobenzoic acid) (DTNB) which produced a yellow-colored 5-thio-2-nitrobenzoic acid (TNB).
Briefly, equal amounts of sample and 10% trichloroacetic acid (TCA) were mixed and centrifuged at 3000×g for 5 min. 0.5 ml of 0.04% DTNB reagent was added to the 0.5 ml of supernatant plus 2 ml PBS (0.1M, pH 8.0). Then, the absorbance of yellow colored TNB was measured at 412 nm. Tissue GSH content was expressed as nmol/mg protein.
Determination of AChE activity
After two weeks of treatment, rats were killed and sera were collected. The plasma cholinesterase activity was analyzed using Ellman method (26). This method is based on the hydrolysis of acetylthiocholine iodide. The reaction of the thoil compound with 5,5’-Dithio-bis(2-nitrobenzoic acid) (DTNB) produces a color-forming compound with absorbance at 405 nm. The absorbance of different samples was recorded at 0.5 min intervals for 2 min. The cholinesterase activity was calculated as: Cholinesterase (mU/ml, at 25 °C)= change in absorbance in 30 sec × 23400.
Western blot analysis
Western blot analysis was carried out on protein extracts from the hippocampus and cerebral cortex for BDNF. Tissues were homogenized in the homogenization buffer containing Tris 50 mM pH 7.4, 2 mM EDTA, 10 mM NaF, 1 m M Na3VO4, 10 m M β- glycerol-phosphate, 0.2% W/V sodium deoxycholate, 1 mM phenylmethylsulfonyl fluoride (PMSF), and complete protease inhibitor cocktail (Sigma, P8340) using polytron homogenizer (POLYTRON® PT 10-35, Kinematica, Switzerland) in ice and then were centrifuged at 4 °C for 15 min at 10000× g. The total protein content in supernatants was determined using Bradford protein assay kit (Bio-Rad). Supernatants were stored at -80 °C until further use.
Level of BDNF protein was measured by immuno blotting analysis. Briefly, equal amount of protein extract (50 µg) was loaded to SDS-PAGE gel. After electrophoresis, protein was transferred to PVDF membrane. Membranes were blocked with 5% non-fat dry milk in Tris-buffered saline tween for 3 hr. Primary antibodies were rabbit polyclonal anti-serum against BDNF and rabbit monoclonal anti-serum against β-actin. All antibodies were diluted 1:1000. Anti rabbit horseradish peroxidase labeled IgG was used as secondary antibody. Protein bands were visualized using an enhanced chemiluminescence and Alliance Gel-doc (Alliance 4.7 Gel doc, UK). UV tec software (UK) was used to semi quantify protein bands. All protein bands were normalized against corresponding β-actin protein bands.
Statistical analysis
Data are expressed as mean±SEM. Statistical analysis was performed using one-way ANOVA followed by Tukey–Kramer post test for multiple comparisons. The P-values less than 0.05 were considered to be statistically significant.
Results
Malathion induced depressive-like behavior, which was prevented by crocin
In compassion to control group, malathion (50 mg/kg) increased the immobility time on 7th and 14th days (P<0.001). Crocin (20 and 40 mg/kg) and imipramine decreased the immobility time in the FST as compared with the malathion group (P<0.001 and P< 0.01, respectively) (Figure 1).
Figure 1.
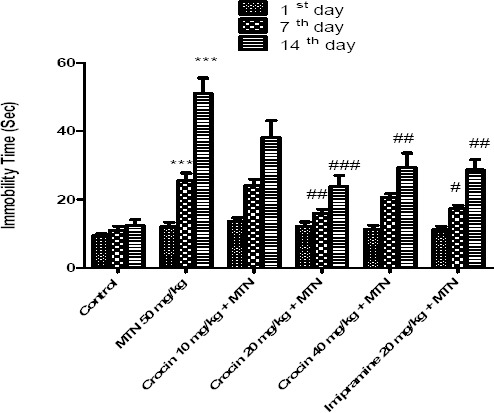
Effects of malathion (50 mg/kg) and crocin on the immobility time in the FST. Data are presented as mean ± SEM. *** P<0.001 as compared with control (normal saline) group, # P<0.05, ##P<0.01 and ###P<0.001 as compared with MTN (malathion) group
Effect of malathion and crocin on locomotor activity using Open-Field Test
Malathion alone or in combination with crocin did not show a significant effect on total locomotion, however malathion (50 mg/kg, IP) reduced the central locomotion compared to control group (P<0.001). Crocin plus malathion significantly increased the central locomotion (P<0.01and P<0.001, respectively) (Figure 2).
Figure 2.
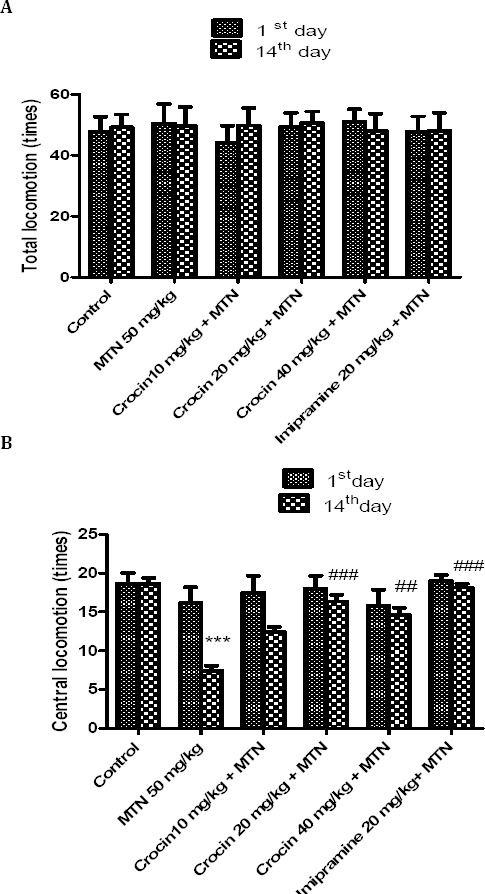
Effects of malathion (50 mg/kg) and crocin on total locomotion times (TL) (A) and central locomotion times (CL) (B) in the open field test. Data are presented as mean ± SEM. *** P<0.001 as compared with control (normal saline) group after 14 days, ##P<0.01 and ###P<0.001 as compared with MTN (malathion) group after 14 days
Effect of malathion and crocin on AChE Activity
As shown in Figure 3, malathion reduced significantly AChE activity in comparison with control (P<0.001). Neither crocin nor vitamin E shows significant effects on the activity of AChE in the sera of rats.
Figure 3.
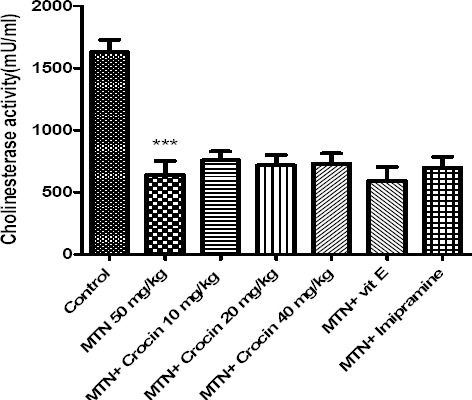
Effects of malathion (50 mg/kg) and crocin on AChE activity. Data are presented as mean ± SEM. *** P<0.001 as compared with control (normal saline) group
Effect of malathion and crocin on oxidative stress markers Measurement of MDA
Malathion increased significantly the level of MDA in rat hippocampus and cerebral cortex in comparison with control (P<0.001). Both crocin (20 and 40 mg/kg) and vitamin E in combination with malathion could decrease the level of MDA in rat hippocampus and cerebral cortex (Figure 4).
Figure 4.
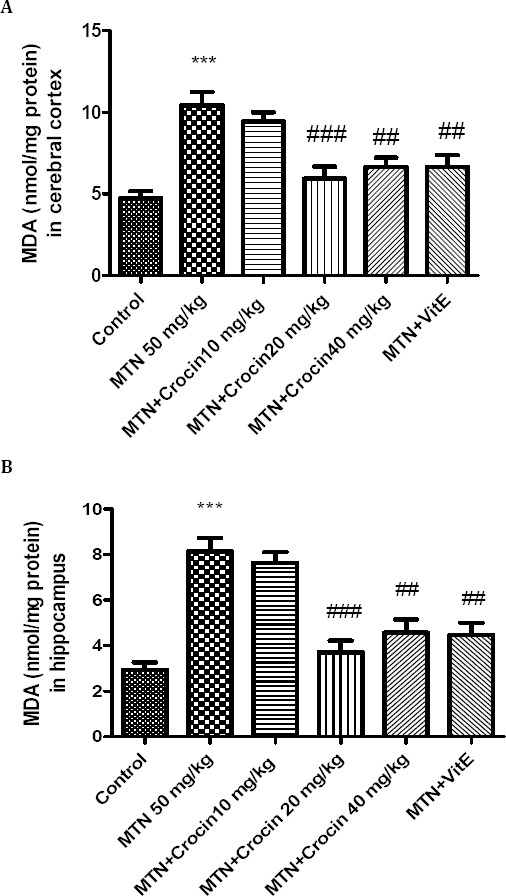
Effects of malathion (50 mg/kg) and crocin on the level of MDA in rat cerebral cortex (A) and hippocampus (B). Data are presented as mean ± SEM. *** P<0.001 as compared with control (normal saline) group after 14 days, ##P<0.01 and ###P<0.001 as compared with MTN (malathion) group after 14 days
Measurement of reduced GSH
Compared to control, in malathion treated rats, hippocampus and cerebral cortex contents of GSH were significantly decreased (P<0.001). Both crocin (20 and 40 mg/kg) and vitamin E plus malathion significantly increased the content of GSH (Figure 5).
Figure 5.
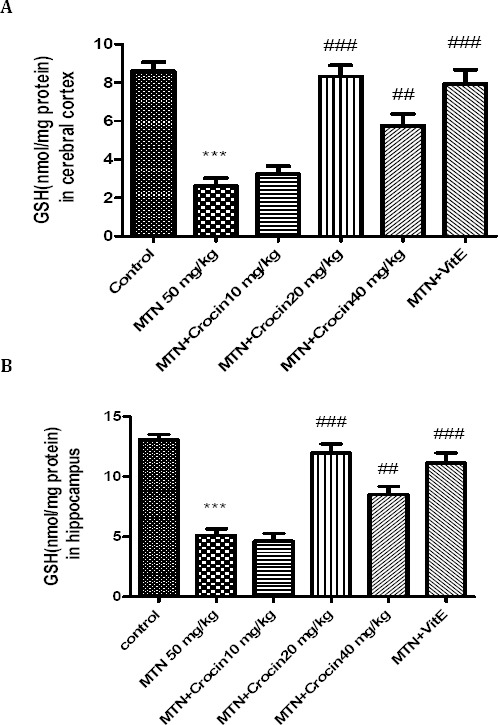
Effects of malathion (50 mg/kg) and crocin on the GSH content in rat cerebral cortex (A) and hippocampus (B). Data are presented as mean ± SEM. *** P<0.001 as compared with control (normal saline) group after 14 days, ##P<0.01 and ###P<0.001 as compared with MTN (malathion) group after 14 days
Western blot analysis
Malathion significantly decreased protein level of BDNF in rat hippocampus (P<0.001). Both imipramine and crocin (20 mg/kg) could increase the protein level of BDNF in comparison with malathion in rat hippocampus (P<0.05 and P<0.01, respectively) (Figure 7). The decreasing effect of malathion and increasing effect of crocin on BDNF protein levels were not significant in rat cerebral cortex (Figure 6).
Figure 7.
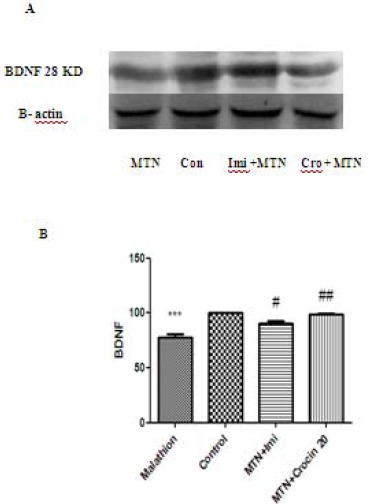
Effects of malathion (50 mg/kg) and crocin on the BDNF level in the rat hippocampus by western blotting (Con=control, Cro=crocin, IMI=imipramine, MTN=malathion). (A) Representative western blots showing specific bands for BDNF and β-actin as an internal control. These bands are representative of four separate experiments. (B) Densitometric data of protein analysis. Data are expressed as the mean ± SEM. *** P<0.001 as compared with control (normal saline) group, #P<0.05 and ##P<0.01 as compared with MTN (malathion) group
Figure 6.
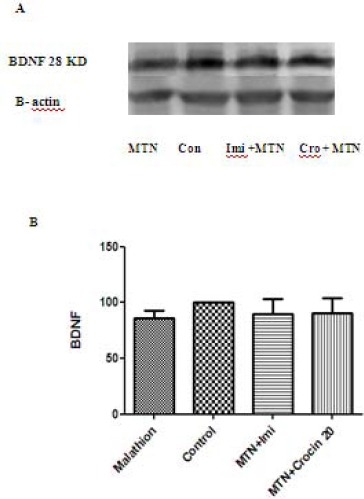
Effects of malathion (50 mg/kg) and crocin on the BDNF level in the rat cerebral cortex by western blotting (Con=control, Cro=crocin, IMI=imipramine, MTN=malathion). (A) Representative western blots showing specific bands for BDNF and β-actin as an internal control. These bands are representative of four separate experiments. (B) Densitometric data of protein analysis. Data are expressed as the mean ± SEM
Discussion
Pesticide exposure is neurotoxic and can induce neuropsychiatric symptoms including affective disorders such as anxiety and depression (2, 27). Incidence of depression is increased dramatically in volunteers frequently exposed to pesticides and is thought to be a possible cause of suicides (27, 28).
Possible neurobehavioral impairments induced by malathion, were evaluated using FST. FST, is an animal test commonly used to predict antidepressant effect (22). Agents who can decrease the immobility time in the FST, exhibit antidepressant effects (22). In this study, malathion (50 mg/kg, IP) increased immobility time in the FST, in comparison to the control group after 14 days in rats. Previous studies also revealed that malathion caused depressant like behavior in acute and chronic exposures. Brocardo et al (2007) showed that female rats exposed to malathion (50-100 mg/kg, IP) for 3 days demonstrated a remarkable increase in FST (28) or another study by Ramos et al (2006), reported that malathion (25-150 mg/kg, IP) exhibited a depressant-like behavior in the FST after 28 days (29).
The results of the open field test showed that malathion did not change total locomotion, since, the increase of immobility time induced by malathion in the FST cannot be related to the locomotor activity induced by malathion. Our results also showed that malathion reduced the central locomotion in the open field test. It has been justified that more anxious and emotional animals tend to avoid the central part in the open field apparatus and the central zone is usually considered to be a stressful location (30). So the decrease in the central locomotion induced by malathion may be attributed to the anxiogenic-like effects. These results are in consistent with Brocardo et al (2007) that showed the malathion reduced the central locomotion at dose of 100 mg /kg in the open field test (28).
Moreover anxiogenic-like effects induced by malathion have been shown in the elevated plus-maze after acute (100 mg/kg, IP) and repeated (25 mg/kg, IP) exposure to malathion (31).
Crocin, a carotenoid isolated from Crocus sativus L. (saffron), exhibits numerous beneficial effects both in animals and human studies (32). Antidepressant effect of saffron and its constituents have been previously shown. It was reported that ethanolic (200 and 800 mg/kg) and aqueous (160 and 320 mg/kg) extracts of saffron decrease the immobility time compared to the control (33). In a recent study, the antidepressant effect of crocin (12.5-50 mg/kg/day, IP) has been shown in FST after 21 days (19). In our study crocin attenuated neurobehavioral impairments induced by malathion in FST and open field studies. In this study imipramine, a tricyclic antidepressant was used as a positive control (34). Crocin decreased immobility time similar to imipramine in rats exposed to malathion.
The results of AChE activity measurement showed that malathion (50 mg/kg, IP) decreased the activity of this enzyme after 14 days treatment. Although crocin alleviated malathion induced neurobehavioral impairments, but it could not increase the AChE activity. Based on these results, other mechanisms rather than cholinergic stimulation may be involved in neurobehavioral impairments induced by malathion.
Research has shown that acute and chronic exposure to malathion induce oxidative stress leading to the free radicals generation and change in antioxidants or reactive oxygen species (ROS) scavenging enzymes in different tissues including erythrocytes, liver, and brain (1, 35).
Oxidative stress has been suggested as a possible alternative mechanism of malathion toxicity (1, 36-38). Malathion increased the levels of MDA, the end product of lipid peroxidation, and decreased the level of reduced glutathione, as free radical scavengers, in rat hippocampus and cortex. Our results are in agreement with the findings of another study that showed malathion leads to an obvious imbalance in the brain antioxidant status (39). It has been reported that malathion caused a mild reduction in the activities of glutathione peroxidase and glutathione reductase in rat cerebral cortex and hippocampus (28).
This study showed that crocin and vitamin E improved all toxic effects of malathion in rat hippocampus and cortex. Vitamin E or α-tocopherol is one of the most biologically active antioxidants in the biological system. Antioxidants play an important role in preventing free radical mediated damages by directly scavenging them (40). Many studies show that vitamin E could protect tissues against toxicity induced by malathion (41). Furthermore the protective effects of zinc, as an antioxidant, against malathion induced oxidative damages in rat brain was reported in a previous study (28). The antioxidant effects of crocin has also been shown previously (9). In our study, crocin like vitamin E, as an antioxidant, protected brain against malathion induced oxidative damages by alleviating lipid peroxidation and increase in reduced glutathione.
The protective effects of crocin against oxidative damages induced by some toxic agents such as diazinon and acrylamide have been shown in our previous studies (16, 17, 42). Crocin in combination with diazinon (15 mg/kg/day, 28 days, gavage) protected cardiovascular system and liver tissues via antioxidant effects (17, 42, 43). Similar to the previous findings, the protective effect of crocin was more obvious at higher doses especially at 20 mg/kg.
In this study, in addition to the role of oxidative stress in depressant like behavior of malathion, the protein level of BDNF has been also evaluated. It is believed that depression occurs as a result of stress, neuronal atrophy and decreased neurogenesis and antidepressants increase neurogenesis in the hippocampus. Furtheremore intracellular pathways regulating neuroplasticity and neurodegeneration are implicated in the etiology of depression (5).
BDNF, a member of neurotrophin family, is a small dimmer neuroprotective protein. In depressed patients, the reduced levels of neurotrophic factors, particularly BDNF, has been shown and antidepressant treatment increases BDNF levels (44). It is documented that long term exposures to the pesticide caused neural atrophy and cell loss (27). Therefore the role of neurotrophic factors, as one probable mechanisms of malathion induced depressant- like behavior have been evaluated in rat hippocampus and cerebral cortex. Our results showed that malathion increased BDNF protein level in rat hippocampus and crocin as well as imipramine increased the level of BDNF.
The role of BDNF in antidepressant effects of crocin in subactue exposure (21 days) was reported in rats in our previous study (19). The data presented in this research also revealed that crocin in combination with malathion could ameliorate the depressant -like effect of malathion through increase in BDNF level similar to the imipramine in rat hippocampus but not significantly in cerebral cortex.
It is identified that the behavioral impairments in depression are related to the changes in the hippocampus function (45). Broncko et al (2007) showed that malathion-induced morphological damages in the dentate gyrus of hippocampus and zinc improved it (28). Although both hippocampus and cerebral cortex are affected by oxidative damages induced by malathion (50 mg/kg, IP) administration for 14 days, but the decrease level of BDNF was only observed significantly in rat hippocampus. It may be concluded that hippocampus has a critical role in malathion induced neurobehavioral changes such as depression. Moreover other neuroregulatory pathways may be involved in rat cerebral cortex treated by malathion for 14 days.
Conclusion
These results showed that crocin is able to attenuate some neurochemical and behavioral effects induced by malathion in subacute exposure, particularly the malathion-induced depressant-like effect in FST. Crocin ameliorated brain oxidative damages by malathion via antioxidant effects. Furthermore the neuroprotective effect of crocin may be in part due to its effect on BDNF. In this regard, crocin could be considered as a promising therapeutic agent in population exposed to pesticides to prevent neurobehavioral impairments.
Acknowledgment
The authors are thankful to the Vice Chancellor of Research, Mashhad University of Medical Sciences Mashhad, Iran for financial support.
References
- 1.Delgado EHB, Streck EL, Quevedo JL, Dal-Pizzol F. Mitochondrial respiratory dysfunction and oxidative stress after chronic malathion exposure. Neurochem Res. 2006;31:1021–1025. doi: 10.1007/s11064-006-9111-1. [DOI] [PubMed] [Google Scholar]
- 2.Salvi RM, Lara DR, Ghisolfi ES, Portela LV, Dias RD, Souza DO. Neuropsychiatric evaluation in subjects chronically exposed to organophosphate pesticides. Toxicol Sci. 2003;72:267–271. doi: 10.1093/toxsci/kfg034. [DOI] [PubMed] [Google Scholar]
- 3.Haenisch B, Bönisch H. Depression and antidepressants. Insights from knockout of dopamine, serotonin or noradrenaline re-uptake transporters. Pharmacol Ther. 2011;129:352–368. doi: 10.1016/j.pharmthera.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 4.Hisaoka K, Maeda N, Tsuchioka M, Takebayashi M. Antidepressants induce acute CREB phosphorylation and CRE-mediated gene expression in glial cells: a possible contribution to BDNF production. Brain Res. 2008;1196:53–58. doi: 10.1016/j.brainres.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 5.Aydemir O, Devecia A, Tanelib F. The effect of chronic antidepressant treatment on serum brain-derived neurotrophic factor levels in depressed patients: a preliminary study. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:261–265. doi: 10.1016/j.pnpbp.2004.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Soeda S, Ochiai T, Shimeno H, Saito H, Abe K, Tanaka H, Shoyama Y. Pharmacollogical activities of crocin in saffron. J Nat Med. 2007;61:102–111. [Google Scholar]
- 7.Fernández J. Anticancer properties of saffron, Crocus sativus Linn. Adv Phytomed. 2006:313–30. [Google Scholar]
- 8.Assimopoulou A, Sinakos Z, Papageorgiou V. Radical scavenging activity of Crocus sativus L. extract and its bioactive constituents. Phytother Res. 2005;19:997–1000. doi: 10.1002/ptr.1749. [DOI] [PubMed] [Google Scholar]
- 9.Hosseinzadeh H, Shamsaie F, Mehri S. Antioxidant activity of aqueous and ethanolic extracts of Crocus sativus L. stigma and its bioactive constituents crocin and safranal. Pharmacogn Mag. 2010;5:419–424. [Google Scholar]
- 10.Sheng L, Qian Z, Zheng S, Xi L. Mechanism of hypolipidemic effect of crocin in rats: crocin inhibits pancreatic lipase. Eur J Pharmacol. 2006;543:116–122. doi: 10.1016/j.ejphar.2006.05.038. [DOI] [PubMed] [Google Scholar]
- 11.Hosseinzadeh H, Younesi MH. Antinociceptive and anti-inflammatory effects of Crocus sativus L. stigma and petal extracts in mice. BMC Pharmacol. 2002;2:7. doi: 10.1186/1471-2210-2-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hosseinzadeh H, Khosravan V. Anticonvulsant effects of aqueous and ethanolic extracts of Crocus sativus L. stigmas in mice. Arch Iran Med. 2002:44–47. [Google Scholar]
- 13.Abe K, Saito H. Effects of saffron extract and its constituent crocin on learning behaviour and long-term potentiation. Phytother Res. 2000;14:149–52. doi: 10.1002/(sici)1099-1573(200005)14:3<149::aid-ptr665>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 14.Hosseinzadeh H, Ziaei T. Effects of Crocus sativus stigma extract and its constituents, crocin and safranal, on intact memory and scopolamine-induced learning deficits in rats performing the Morris water maze task. J Med Plants. 2006;5:40–50. [Google Scholar]
- 15.Hariri A, Moallem S, Mahmoudi M, Memar B, Hosseinzadeh H. Sub-acute effects of diazinon on biochemical indices and specific biomarkers in rats: Protective effects of crocin and safranal. Food Chem Toxicol. 2010;48:2803–8. doi: 10.1016/j.fct.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 16.Mehri S, Abnous K, Mousavi S, Motamed Shariaty V, Hosseinzadeh H. Neuroprotective effect of crocin on acrylamide-induced cytotoxicity in PC12 cells. Cell Mol Neurobiol. 2012;32:227–35. doi: 10.1007/s10571-011-9752-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Razavi B, Hosseinzadeh H, Movassaghi A, Imenshahidi M, Abnous K. Protective effect of crocin on diazinon induced cardiotoxicity in subcronic exposure. Chem Boil Inter. 2013;25:547–55. doi: 10.1016/j.cbi.2013.03.010. [DOI] [PubMed] [Google Scholar]
- 18.Akhondzadeh S, Fallah-Pour H, Afkham K, Jamshidi A, Khalighi-Cigaroudi F. Comparison of Crocus sativus L. and imipramine in the treatment of mild to moderate depression: A pilot double-blind randomized trial. BMC Complem Altern Med. 2004;4:12–17. doi: 10.1186/1472-6882-4-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vahdati Hassani F, Naseri V, Razavi BM, Mehri S, Abnous K, Hosseinzadeh H. Antidepressant effects of crocin and its effects on transcript and protein levels of CREB, BDNF, and VGF in rat hippocampus. Daru. 2014;8:22. doi: 10.1186/2008-2231-22-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sanchez-Mateo C, Bonkanka C, Prado B, Rabanal R. Antidepressant activity of some Hypericum reflexum L. fil. extracts in the forced swimming test in mice. J Ethnopharmacol. 2007;112:115–121. doi: 10.1016/j.jep.2007.02.019. [DOI] [PubMed] [Google Scholar]
- 21.Hadizadeh F, Mohajeri S, Saifi M. Extraction and purification of crocin from saffron stigmas employing a simple and efficient crystallization method. Pak J Biol Sci. 2010;13:691–698. doi: 10.3923/pjbs.2010.691.698. [DOI] [PubMed] [Google Scholar]
- 22.Porsolt D, Le P, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 23.Pardon M, Perez Diaz F, Joubert C, Cohen Salmon C. Age dependent effects of a chronic ultramild stress procedure on open field behavior in B6D2F1 female mice. Physiol Behavio. 2000;70:7–13. doi: 10.1016/s0031-9384(00)00216-x. [DOI] [PubMed] [Google Scholar]
- 24.Fernandez J, Perez-Alvarez J, Fernandez-lopez J. Thiobarbituric acid test for monitoring lipid oxidation in meat. Food Chem Toxicol. 1997;99:345–53. [Google Scholar]
- 25.Moron M, Depierre J, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 26.Rezg R, Mornagui B, El-Fazaa S, Gharbi N. Biochemical evaluation of hepatic damage in subchronic exposure to malathion in rats: effect on superoxide dismutase and catalase activities using native PAGE. C R Biol. 2008;331:655–62. doi: 10.1016/j.crvi.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 27.Chen N, Luo D, Yao X, Yu C, Wang Y, Wang Q. Pesticides Induce Spatial Memory Deficits with Synaptic Impairments and an Imbalanced Tau Phosphorylation in Rats. J Alzheimers Dis. 2012;30:585–594. doi: 10.3233/JAD-2012-111946. [DOI] [PubMed] [Google Scholar]
- 28.Brocardo P, Assini F, Franco J, Pandolfo P, Muller M, Takahashi RN, et al. Zinc attenuates Malathion-Induced Depressant-like Behavior and Confers Neuroprotection in the Rat Brain. Toxicology. 2007;97:140–8. doi: 10.1093/toxsci/kfm024. [DOI] [PubMed] [Google Scholar]
- 29.Ramos ZR, Fortunato JJ, Agostinho FR, Martins MR, Correa M, Schetinger MR, et al. Influence of malathion on acetylcholinesterase activity in rats submitted to a forced swimming test. Neurotox Res. 2006;9:285–290. doi: 10.1007/BF03033318. [DOI] [PubMed] [Google Scholar]
- 30.Walsh RN, Cummins RA. The open-field test: A critical review. Psychol Bull. 1976;83:482–504. [PubMed] [Google Scholar]
- 31.Assini FL, Zanette KD, Brocardo PS, Pandolfo P, Rodrigues ALS, Takahashi RN. Behavioral effects and ChE measures after acute and repeated administration of malathion. Environ Toxicol Pharmacol. 2005;20:443–449. doi: 10.1016/j.etap.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 32.Alavizadeh S, Hosseinzadeh H. Bioactivity assessment and toxicity of crocin: a Comprehensive Review. Food Chem Toxicol. 2014;64:65–80. doi: 10.1016/j.fct.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 33.Hosseinzadeh H, Karimi G, Niapoor M. Anti-depressant effects of Crocus sativus stigma extracts and its constituents, crocin and safranal, in mice. J Med Plants. 2004;3:48–58. [Google Scholar]
- 34.Nakamura K, Tanaka Y. Antidepressant-like effects of aniracetam in aged ratsand its mode of action. Psychopharmacol J. 2001;158:205–212. doi: 10.1007/s002130100849. [DOI] [PubMed] [Google Scholar]
- 35.Akhgari M, Abdollahi M, Kebryaeezadeh A, Hosseini R, Sazevari O. Biochemical evidence for free radical-induced lipid peroxidation as a mechanism for subchronic toxicity of malathion in blood and liver of rats. Hum Exp Toxicol. 2003;22:205–211. doi: 10.1191/0960327103ht346oa. [DOI] [PubMed] [Google Scholar]
- 36.Possamai FP, Fortunato JJ, Feier G, Agostinho FR, Quevedo J, Filho DW, et al. Oxidative stress after acute and sub-chronic malathion intoxication in Wistar rats. Env Toxicol Pharmacol. 2007;23:198–204. doi: 10.1016/j.etap.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Fortunato J J, Feier G, Vitali A, Petronilho F, Dal-Pizzol F, Quevedo J. Malathion-induced Oxidative Stress in Rat Brain Regions. Neurochem Res. 2006;31:671–678. doi: 10.1007/s11064-006-9065-3. [DOI] [PubMed] [Google Scholar]
- 38.Ghazi-Khansari M, Baeeri M, Abdollahi M. Protection by pentoxifylline of malathion-induced toxic stress and mitochondrial damage in rat brain. Hum Exp Toxicol. 2010;29:851–864. doi: 10.1177/0960327110363836. [DOI] [PubMed] [Google Scholar]
- 39.Abdollahi M, Mostafalou S, Pournourmohammadi SH, Shadnia SH. Oxidative stress and cholinesterase inhibition in saliva and plasma of rats following subchronic exposure to malathion. Comp Biochem Physiol Part C: Toxicol Pharmacol. 2004;137:29–34. doi: 10.1016/j.cca.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 40.Ogutcu A, Uzunhisarcikli M, Kalender S, Durak D, Bayrakdar F, Kalender Y. The effects of organophosphate insecticide diazinon on malondialdehyde levels and myocardial cells in rat heart tissue and protective role of vitamin E. Pest Biochemi Physiol. 2006;86:93–98. [Google Scholar]
- 41.John S, Kale M, Rathore N, Bhatnagar D. Protective effect of Vitamin E in dimethoate and malathion induced oxidative stress in rat erythrocytes. J Nutr Biochem. 2001;12:500–504. doi: 10.1016/s0955-2863(01)00160-7. [DOI] [PubMed] [Google Scholar]
- 42.Razavi BM, Hosseinzadeh H, Abnous K, Imenshahidi M. Protective effect of crocin on diazinon induced vascular toxicity in subchronic exposure in rat aorta ex-vivo. Drug Chem Toxicol Early Online. 2014:1–6. doi: 10.3109/01480545.2013.866139. [DOI] [PubMed] [Google Scholar]
- 43.Lari P, Abnous K, Imenshahidi M, Rashedinia M, Razavi M, Hosseinzadeh H. Evaluation of diazinon-induced hepatotoxicity and protective effects of crocin. Toxicol Ind Health. 2013 doi: 10.1177/0748233713475519. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 44.Wang J, Dranovsky A, Hen R. The When and Where of BDNF and the Antidepressant Response. Biol Psychiatry. 2008;63:640–641. doi: 10.1016/j.biopsych.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 45.Thakker-Varia S, Alder J. Neuropeptides in depression: role of VGF. Behav Brain Res. 2009;197:262–278. doi: 10.1016/j.bbr.2008.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]


