Abstract
Objective(s):
Nitric oxide (NO), a product of inducible nitric oxide synthase (iNOS), contributes in germ cell apoptosis. This study was aimed to evaluate the effects of Aloe vera gel (AVG) on male Wistar rat reproductive organ, serum NO level, and expression of iNOS gene in leydig cells.
Materials and Methods:
Adult male Wistar rats (n=36) were used for experiments in three groups. The experimental groups were orally administered with the AVG extract solution once-daily as follow: 150 mg.kg-1; group A, 300 mg.kg-1; group B, and only normal saline; group C (control group). They were mated with untreated females and the reproductive and chemical parameters were assessed for each group, including semen quality, serum testosterone, sperm fertility, gonad and body weight, serum NO concentration (by the Griess method), and iNOS gene expression (using RT-PCR).
Results:
The testes weight, serum testosterone, as well as sperm count and fertility of the AVG treated groups were significantly reduced when compared to the control (P<0.001). Concentration of serum NO was significantly increased (37.1±4.63 µM) in the administrated group with higher AVG concentration, compared to the control group (P<0.001; 10.19±0.87 µM); however, iNOS mRNA expression was increased in the treated animals (P<0.001).
Conclusion:
iNOS may play a functional role in spermatogenesis via apoptosis, reducing sperm count, but further studies are needed to illustrate the mechanisms by which AVG exerts its negative effects on spermatogenesis and sperm quality.
Keywords: Aloe vera gel, Inducible nitric oxide synthase, Wistar rat, Testis
Introduction
Nowadays, many plants are increasingly being used as medical plants (1-4). Aloe vera shows significant recuperative activities, such as repairing radiation skin damages and wounds, cancer therapeutic effects, as well as improving decubitus ulcers (2, 5-9). A. vera gel (AVG) contains high levels of carbohydrates (composed of long-chain polydispersed mucopoly-saccharides and a mannose monomer/acetyl), calcium malate, and protein as its predominant compositions (10-12). AVG enhances the release of several cytokines, including interleukins (IL-1, 2, and 6), interferon (IFN), granulocyte/monocyte-colony stimulating factor (GM-CSF) and tumor necrosis factor (TNF), as well as nitric oxide (NO) (11). Acemannan (ACM) is an important polydispersed mucopolysaccharide in AVG, known to have many pharmacological properties, including immune-stimulant, antiviral, antineoplastic, and gastrointestinal activities. Through stimulating the release of cytokines (e.g., IL-1, IL-6, TNF-α), AVG is able to activate macrophages and monocytes and produce NO (11, 13, 14). Recently, NO has shown several intra and inter cellular functions as a messenger and a basic role in regulation of male reproductive system, especially in human and rats; it has displayed autocrine and paracrine control over steroidogenesis of leydig cells (14-19).
In testis, macrophages are the most important sources of NO and control testicular NO signaling; indeed, during different phases of the reproductive cycle, a direct relationship exists between testicular macrophages and leydig cells (20, 21). Increasing of testicular macrophages at the beginning of testis recrudescence is related to the enhancement of leydig cells proliferation, reaching maximum number during breeding phase. However, throughout the reproductive cycle, the ratio of these reproductive cells to macrophages remains stable (1:3) (22). Low and high concentrations of NO stimulate leydig cells steroidogenesis, through the reproductive cycle soluble guanylatecyclase (GUCY1), and suppress their function, respectively (23).
To the author’s knowledge, in spite of these early observations, the molecular mechanism of A. vera in male reproductive system has remained unclear. Since A. vera has an important role on NO signaling pathway in leydig cells, this paper evaluates the quantitative expression of nitric oxide synthase (NOS) in these cells. Moreover, it focuses on the possible side effects of AVG on rat male reproductive organ, sperm count and fertility, concentration of serum testosterone, gene expression of iNOS mRNA in testis, and serum NO concentration. The relation between variations in gene expression of inducible nitric oxide synthase (iNOS) mRNA in the testis with the relative weight of reproductive organ, sperm count and fertility, and concentration of serum testosterone has been also evaluated in this study.
Materials and Methods
Preparation of AVG extract
AVG was prepared from the fresh leaves (70-90 cm) as follow: the leaves were washed with clean water and cut transversely into slices and then the thick epidermis was selectively removed. The achieved gel was homogenized and lyophilized and thereafter was extracted using 95% ethanol. Its ethanol was evaporated in a rotary, under low pressure and dry condition, and the achieved extract was stored at 4 °C (24).
DPPH radical scavenging assay
The antioxidant activity of the prepared AVG was evaluated based on the radical scavenging ability of 2, 2-diphenyl-1-picrylhydrazyl (DPPH). The AVG stock solution was diluted with methanol at different concentrations: 10, 20, 30, 40, 50, 60, 70, 80, 90, and 100 µg/ml. The prepared DPPH methanol solution was added to these AVG solutions and allowed to react at room temperature. Finally, after 15 min, the absorbance values were measured at 517 nm and the activity was calculated by the following equation (25):
Ic50 (%) = 100 × (Acontrol-Asample)/Acontrol
Ic50 represents the sample concentration at which 50% of the DPPH radical was scavenged.
Total phenolic compounds of AVG
Total phenolic compounds were determined using a modified version of the Folin–Ciocalteu method. A low volume (0.1 ml) of the extract was added to 0.5 ml of Folin–Ciocalteu phenol reagent. The mixture was then allowed to stand for 5 min and 0.4 ml sodium carbonate was added to the mixture. The final mixture became blue and its absorbance was measured at 680 nm. The standard calibration curve was plotted with 12.5, 25, 50, 62.5, 100, and 125 mg.l-1 of gallic acid in methanol and water (60:40, v/v). The amount of phenols was expressed based on mg.g-1 in gallic acid equivalent (26).
Total flavonoid of AVG
Total flavonoid content of the methanolic extract was determined according to a colorimetric method. Briefly, a solution (1.5, 1, and 6 ml of 60% methanol, 2% aluminum, and 5% potassium acetate, respectively) was added to 1 ml of AVG extract, and kept at room temperature for 40 min. The absorbance of the final mixture was measured at 415 nm and the total flavonoids were expressed as the mg equivalent of Rutin per g of extracts (27).
Animals and treatment
Male Wistar rats (n=36; 8-week old) were purchased from Razi Institute, (Tehran, Iran) and randomly divided into 3 groups. The experimental groups were orally administered with the AVG extract solution as follow: 150 mg.kg-1; group A, 300 mg.kg-1; group B, and only normal saline; group C (control group) (28, 29). The rats were housed in a standard air-conditioned animal room; constant room temperature: 25±3 °C, humidity level: 10%, and a 12 hr light: 12 hr dark cycle, as well as food available ad libitum.
Tissue preparation
At the end of the experiment, the rats were weighted and deeply anesthetized with ketamine (110 mg.kg-1) and xylazine (5 mg.kg-1). Their testes were dissected out, weighted, and immediately frozen (-70 °C) until extracting their RNA content.
Sperm counting
Sperm counting was performed after dissecting out the epididymides. Briefly, the ducts were incised and their sperm content and epididymal fluid were mixed with saline solution (1 ml; pH=7.2) (30). The suspension was filtered through a nylon mesh and the number of sperms was evaluated using a counting chamber Neubauer (Deep 0.1 mm, LABART, Germany) (30). Five small squares per sample were counted in triplicate; the total sperm count was determined and expressed as million per milliliter.
Fertility and pregnancy test
At the end of experiment, each of the treated male was mated with three fertile females. Detection of sperm in the vaginal smear, as an excellent predictor of pregnancy and the presence of a vaginal plug, were the indicators of a successful mating. During pregnancy, changes in the body weight of female possessed vaginal plug were checked.
Radioimmunoassay of serum testosterone
The serum was separated from the blood specimens, which had been collected from the abdominal aorta of the treated rats, and stored at -20 °C. Testosterone kit (Demeditec Diagnostics GmbH. Lise-Meitner-Straße 2. D-24145Kiel, Germany), with an accuracy of 0.066 ng.dl, was used to measure the serum testosterone.
RNA extraction
According to the protocol of the manufacturer, total RNA was isolated from the rat testes using the Biozol total RNA extraction reagent (Bioflux, Japan).
Quantitative analysis of iNOS mRNA in the rat testis using real-time PCR (RT-PCR)
An aliquot containing 0.2 μg of total RNA was used for the reverse transcription reaction, which was conducted using the SuperScript first-strand cDNA synthesis system (Fermentas, Finland). The sequences of oligonucleotide primer and probe are shown in Table 1. The quantification of iNOS mRNA levels was analyzed using a Rotor-Gene 3000 (Corbett). RT-PCR reactions were performed in a total reaction volume of 10 μl, containing 1 μl of synthesized cDNA, 5 μl of Rotor-Gene Probe PCR Master Mix (Qiagen, Germany), 0.4 μl of each primer, and 0.2 µl of the TaqMan probe. Amplification program included a pre-warming step (5 min at 94 °C), denaturation step (94 °C for 15 sec), and an annealing/extension step (60 °C for 1 min). The mRNA expression levels of the other genes were normalized via quantification of GAPDH (glyceraldehyde 3-phosphate dehydrogenase), as a reference gene. The relative quantification of gene expression in each sample was analyzed using the 2ΔΔCt protocol and expressed as the ratio of related gene to GAPDH mRNA.
Table 1.
Primers and probes used for RT-PCR of specific gene expression
| Name | Primer and probe sequence |
|---|---|
| GAPDH | F: 5’-CCGAGGGCCCACTAAAGG-3’ |
| R:5’-GCTGTTGAAGTCACAGGAGACAA-3’ | |
| 5’-(FAM) CATCCTGGGCTACACTGAGGACCA-3’ (TAMRA) | |
| iNOS | F: 5’-TGG TCC AAC CTG CAG GTC TT-3’ |
| R : 5’-CAG TAA TGG CCG ACC TGA TGT-3’ | |
| 5’-(FAM) TGCCCGGAGCTGTAGCACTGCAT-3’ (TAMRA) |
NO assays
Serum NO metabolites were assessed according to the Griess reaction supplemented by the enzymatic reaction of nitrite and nitrate with copper-plated cadmium (31). Briefly, Griess reagent was prepared from two solutions, including sulfanilamide and N-(1-naphthyl) ethylenediamine dihydrochloride, and stored at 4 °C. Nitrite and Griess reagent were mixed to form a purple azo dye in a reaction coil; its absorbance was measured at 540 nm by a flow-through spectrophotometer after 10 min. Finally, NO concentra-tions were determined relative to a standard graph derived from different concentrations of NaNO3.
Statistical analysis
For the statistical analysis of the data, one-way analysis of variance (ANOVA) was used to compare the differences between the experimental groups and the control group (P<0.05 and 0.001), and the results were expressed as the mean±standard error (SEM).
Results
Standardization of A. vera extract
Total amount of phenolic compounds in A. vera extract was 17.2 mg galic acid equivalent per one g dried extract. Total amount of flavonoid compounds were 12.26 mg per one g of dry matter.
Antioxidant activity of A. vera extract against DPPH
Different concentrations of A. vera extract showed low levels of radical scavenging activity against DPPH; calculation of IC50 was impossible in the higher concentrations (Table 2).
Table 2.
Antioxidant activity of A. vera extract and Butylated Hydroxy Toluene (BHT), as a positive control)
| Sample | Concentration (µg/ml) | Radical scavenging activity DPPH (µg/ml) (IC50%) |
|---|---|---|
| Aloe vera extract | 300 | 15.32 |
| 400 | 12.98 | |
| 500 | 11.68 | |
| 600 | 12.20 | |
| 700 | 10.12 | |
| 800 | 9.87 | |
| 900 | 6.49 | |
| 1000 | 1.29 | |
| Butylated Hydroxy Toluene | 10 | 22 |
| 20 | 40.09 | |
| 25.41 | 50 (IC50) | |
| 30 | 55.5 | |
| 40 | 78.3 | |
| 50 | 90.8 |
Relative body and reproductive organ weights in male adult rats
At the end of experiment, weight of testes in the AVG treated rats was significantly reduced compared to the control group (P<0.001), although body weight and seminal vesicle weight showed no significance difference between all of the groups (Table 3).
Table 3.
Reproductive organ weight of male rats in different concentrations of Aloe vera gel (AVG) extract
| Groups | Body weight (g) |
|---|---|
| Control | 167.37±5.42 |
| AVG 150 mg.kg-1 | 165.45±3.44 |
| AVG 300 mg.kg-1 | 165.78±2.24 |
* Values are mean±SEM. Significant differences are indicated by *** (P<0.001)
Spermatogenesis and sperm fertility
Spermatozoa concentration in the caudal epidi-dymis of the treated rats was significantly decreased compared to the control group (P<0.05; Figure 1). In addition, according to the percentages of the pregnant rats with vaginal plug, sperm fertility in the treated groups was significantly decreased (even more than 50 percent in 300 mg.kg-1 AVG concentration) when compared to the control group (P<0.05; Table 4).
Figure 1.
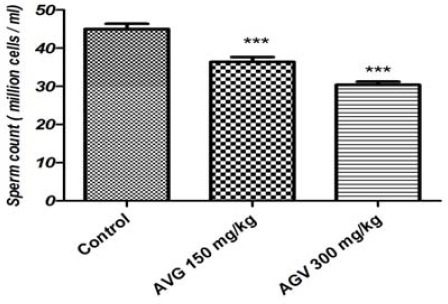
Sperm count in epididymis of the Aloe vera gel (AVG) treated rats and control group
*Values are mean±SEM. Significant differences are indicated by *** (P<0.001)
Table 4.
Effects of oral administration of Aloe vera gel (AVG) on fertility in adult rats
| Treatment groups | Pregnant female rats (%a) |
|---|---|
| Control | 100% |
| AVG 150 mg/kg | 66.6% |
| AVG 300 mg/kg | 45.5% |
Serum testosterone and NO levels
After 8 weeks of AVG administration, concentration of serum testosterone in the treated groups was statistically reduced in a dose-dependent manner, compared to the control group (Figure 2).
Figure 2.
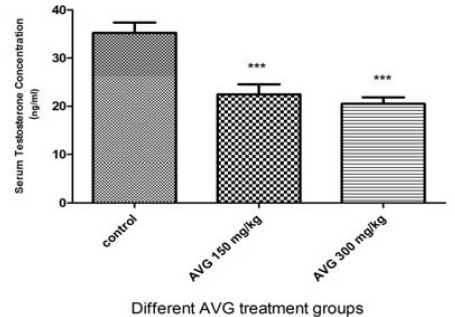
Effects of Aloe vera gel (AVG) on serum testosterone concentrations in the experimental groups
* Values are mean±SEM. Significant differences are indicated by *** (P<0.001)
Figure 3.
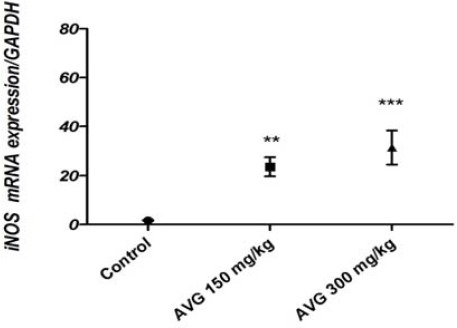
Quantitative expression of iNOS in leydig cells of the experimental groups
Figure 4 shows that higher concentration of AVG (300 mg.kg-1) resulted in a dramatic increase in serum NO level, about four times higher than the control group (P<0.001).
Figure 4.
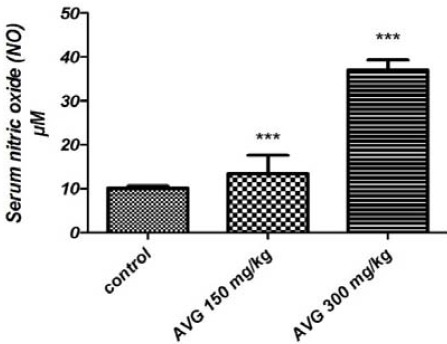
Serum NO concentrations in the experimental groups after 8-week administration of AVG
* Values are mean±SEM. Significant differences are indicated by *** (P<0.001)
Quantitative RT-PCR analysis of the iNOS mRNA expression
Expression of iNOS mRNA in ledyig cells of the AVG treated rats was significantly increased compared to the control group (P<0.001). Expression of GAPDH served as a control to normalize the expressed mRNA of ledyig cells in all experimental groups.
Discussion
Nitric oxide plays several important functions in organisms and is synthesized from l-argenine through an enzymatic reaction of NOS. NOS possess three isoforms, including inducible (iNOS), neural (nNOS), and endothelial (eNOS) (32). The iNOS isoform, involved in immune response, releases high levels of NO, and its progressive generation leads to cytotoxic and neurotoxic effects. NO directly leads to DNA damage and thereby induces apoptosis in various cells (e.g., thymocytes, macrophages, B cells, neurocytes, and germ cells) (33-36). DNA damage is associated with increasing expression of the p53 tumor suppressor protein, known as the first indicator of NO induced apoptosis (37, 38).
Literature suggests that some compounds in A. vera, such as polysaccharides, stimulate the activity of testicular macrophages to produce iNOS. However, leydig cells steroidogenesis appears to be highly sensitive to the paracrine NO (39-41); NOS suppresses the conversion of cholesterol into pregnenolone through inhibition of the heme containing stero-idogenic enzyme (CYP17A1), thereby inhibits testosterone production. Furthermore, in vitro studies show that NO suppresses leydig cell functions through inhibiting the expression of CYP11A1, known as a side-chain cleavage enzyme of cytochrome p450 (42, 43). Therefore, the suppression of testosterone production in the AVG treated rats could be as a result of high iNOS level in these cells.
Mitochondria are the most important regulators in cell apoptosis. The key regulatory proteins of apoptosis are Bcl2 and Bclx that promote cell survival by inhibiting the mitochondrial release of proapoptotic factors including Bax and Bak (44). NO is involved in mitochondrial dependent intrinsic signaling pathway, a key apoptotic pathway for male germ cell apoptosis (45, 46); iNOS induces release of cytochrome C and consequently the activation of the inhibitor caspase 9, the executioner caspases (3, 6, 7), as well as poly (ADP-ribose) polymerase (PARP) cleavage (33, 47-49). Therefore, our results showed a significant reduction in the relative weight of reproductive organ of the AVG treated groups. Moreover, epididymal sperm count decreased and iNOS expression increased in these groups. Therefore, it could be suggested that the probable induced apoptosis by AVG was the main cause of the reduction in sperm count, which in turn reduced the reproductive organ weight. These results are inconsistent with previous study showed that due to deficiency of iNOS in mice, a remarkable increase in testis weight and sperm output occurred after a testicular warming treatment (43 °C for 15 min) (36, 50).
Conclusion
The data confirm sever deleterious effects of AVG on fertility of male rat through overexpression of iNOs gene signaling pathway. Moreover, iNOS may play a functional role in rat spermatogenesis via apoptosis, and reduce the sperm count, but further studies are needed to illustrate the mechanisms and signaling pathway by which AVG exerts its negative effects of spermatogenesis and sperm quality.
Acknowledgment
This research was financially supported by Medical Sciences Department, Shahrekord University, Shahrekord, Iran.
References
- 1.Weissman BA, Sottas CM, Zhou P, Iadecola C, Hardy MP. Testosterone production in mice lacking inducible nitric oxide synthase expression is sensitive to restraint stress. Am J Physiol Endocrinol. 2007;292:615–620. doi: 10.1152/ajpendo.00412.2006. [DOI] [PubMed] [Google Scholar]
- 2.Shirzad H, Nasri H. Toxicity and safety of medicinal plants. J Herb Med Pharmacol. 2013;2:21–22. [Google Scholar]
- 3.Rafieian-kopaei M, Shahinfard N, Rouhi-Boroujeni H, Gharipour M, Darvishzadeh-Boroujeni P. Effects of Ferulago angulata extract on serum lipids and lipid peroxidation. J Evid Based Complementary Altern Med. 2014;2014:4. doi: 10.1155/2014/680856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bahmani M, Zargaran A, Rafieian-Kopaei M, Saki M. Ethnobotanical study of medicinal plants used in the management of diabetes mellitus in the Urmia, Northwest Iran. Asian Pac J Trop Med. 2014;7:348–354. doi: 10.1016/S1995-7645(14)60257-1. [DOI] [PubMed] [Google Scholar]
- 5.Haniadka R, Kamble PS, Azmidha A, Mane PP, Geevarughese NM, Palatty PL, et al. Review on the use of Aloe vera (Aloe) in dermatology. Nutr Health. 2013:125–133. [Google Scholar]
- 6.Yagi A. Possible prophylactic efficacy of Aloe vera for γ-ray radiation-induced damages. J Gastroen Hepatol. 2013:2. [Google Scholar]
- 7.Suganya S, Venugopal J, Mary SA, Ramakrishna S, Lakshmi B, Dev VG. Aloe vera incorporated biomimetic nanofibrous scaffold: a regenerative approach for skin tissue engineering. Iran Polym J. 2014;23:237–248. [Google Scholar]
- 8.Baradaran A, Nasri H, Nematbakhsh M, Rafieian-Kopaei M. Antioxidant activity and preventive effect of aqueous leaf extract of Aloe vera on gentamicin-induced nephrotoxicity in male Wistar rats. Clin Ter. 2014;165:7–11. doi: 10.7471/CT.2014.1653. [DOI] [PubMed] [Google Scholar]
- 9.Delfan B, Bahmani M, Eftekhari Z, Jelodari M, Saki K, Mohammadi T. Effective herbs on the wound and skin disorders: a ethnobotanical study in Lorestan province, west of Iran. Asian Pac J Trop Dis. 2014;4:938–942. [Google Scholar]
- 10.Grace OM, Dzajic A, Jäger AK, Nyberg NT, Önder A, Rønsted N. Monosaccharide analysis of succulent leaf tissue in Aloe. Phytochemistry. 2013;93:79–87. doi: 10.1016/j.phytochem.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 11.Ray A, Aswatha SM. An analysis of the influence of growth periods on physical appearance, and acemannan and elemental distribution of Aloe vera gel. Ind Crops Prod. 2013;48:36–42. [Google Scholar]
- 12.Djeraba A, Quere P. In vivo macrophage activation in chickens with Acemannan, a complex carbohydrate extracted from Aloe vera. Int J Immunopharmacol. 2000;22:365–372. doi: 10.1016/s0192-0561(99)00091-0. [DOI] [PubMed] [Google Scholar]
- 13.Budai MM, Varga A, Milesz S, Tőzsér J, Benkő S. Aloe vera downregulates LPS-induced inflammatory cytokine production and expression of NLRP3 inflammasome in human macrophages. Mol Immunol. 2013;56:471–479. doi: 10.1016/j.molimm.2013.05.005. [DOI] [PubMed] [Google Scholar]
- 14.Klein-Wieringa IR, Andersen NS, Kwekkeboom JC, Giera M, de Lange-Brokaar BJ, van Osch GJ, et al. Adipocytes modulate the phenotype of human macrophages through secreted lipids. J Immunol. 2013;191:1356–1363. doi: 10.4049/jimmunol.1203074. [DOI] [PubMed] [Google Scholar]
- 15.Yan L, Li Z. Mannose receptor ligands regulate the gene expression of Toll-like receptors in chicken monocytes. J Poult Sci. 2013;50:388–395. [Google Scholar]
- 16.Pitsikas N. The role of nitric oxide in the object recognition memory. Behav Brain Res. 2015;285:200–207. doi: 10.1016/j.bbr.2014.06.008. [DOI] [PubMed] [Google Scholar]
- 17.Jarazo-Dietrich S, Jacobo P, Pérez C, Guazzone V, Lustig L, Theas M. Up regulation of nitric oxide synthase–nitric oxide system in the testis of rats undergoing autoimmune orchitis. Immunobiology. 2012;217:778–787. doi: 10.1016/j.imbio.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 18.Andric SA, Janjic MM, Stojkov NJ, Kostic TS. Testosterone-induced modulation of nitric oxide-cGMP signaling pathway and androgenesis in the rat Leydig cells. Biol Reprod. 2010;83:434–442. doi: 10.1095/biolreprod.110.083626. [DOI] [PubMed] [Google Scholar]
- 19.Ikegami H, Kajikawa S, Ito K, Nii A, Okamiya H, Nakayama H, et al. Immunohistochemical study on inducible type of nitric oxide (iNOS), basic fibroblast growth factor (bFGF) and tumor growth factor-β1 (TGF-β1) in arteritis induced in rats by fenoldopam and theophylline, vasodilators. Exp Toxicol Pathol. 2002;54:1–7. doi: 10.1078/0940-2993-00238. [DOI] [PubMed] [Google Scholar]
- 20.Lal B, Dubey N. Existence of a nitric oxide synthase/nitric oxide system in fish testis and its role in modulation of androgenesis. Fish Physiol Biochem. 2013;39:65–69. doi: 10.1007/s10695-012-9648-7. [DOI] [PubMed] [Google Scholar]
- 21.Auharek S, Lara N, Avelar G, Sharpe R, França L. Effects of inducible nitric oxide synthase (iNOS) deficiency in mice on Sertoli cell proliferation and perinatal testis development. Int J Androl. 2012;35:741–751. doi: 10.1111/j.1365-2605.2012.01264.x. [DOI] [PubMed] [Google Scholar]
- 22.Yu W, Zheng H, Lin W, Tajima A, Zhang Y, Zhang X, et al. Estrogen promotes Leydig cell engulfment by macrophages in male infertility. J Clin Invest. 2014;124:2709–27210. doi: 10.1172/JCI59901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Valenti S, Cuttica CM, Fazzuoli L, Giordano G, Giusti M. Biphasic effect of nitric oxide on testosterone and cyclic GMP production by purified rat Leydig cells cultured in vitro. Int J Androl. 1999;22:336–41. doi: 10.1046/j.1365-2605.1999.00189.x. [DOI] [PubMed] [Google Scholar]
- 24.Rajasekaran S, Sivagnanam K, Subramanian S. Antioxidant effect of Aloe vera gel extract in streptozotocin-induced diabetes in rats. Pharmacol Report. 2005;57:90–96. [PubMed] [Google Scholar]
- 25.Rabiei Z, Rafieian-kopaei M, Heidarian E, Saghaei E, Mokhtari S. Effects of zizyphus jujube extract on memory and learning impairment induced by bilateral electric lesions of the nucleus basalis of meynert in rat. Neurochem Res. 2014;39:353–360. doi: 10.1007/s11064-013-1232-8. [DOI] [PubMed] [Google Scholar]
- 26.Rabiei Z, Rafieian-Kopaei M. Neuroprotective effect of pretreatment with Lavandula officinalis ethanolic extract on blood-brain barrier permeability in a rat stroke model. Asian Pac J Trop Med. 2014;7:421–426. doi: 10.1016/S1995-7645(14)60269-8. [DOI] [PubMed] [Google Scholar]
- 27.Rabiei Z, Rafieian-kopaei M, Mokhtari S, Shahrani M. Effect of dietary ethanolic extract of lavandula officinalis on serum lipids profile in pats. Iran J Pharm Res. 2014;13:1295–1301. [PMC free article] [PubMed] [Google Scholar]
- 28.Rajasekaran S, Sivagnanam K, Ravi K, Subramanian S. Hypoglycemic effect of Aloe vera gel on streptozotocin-induced diabetes in experimental rats. J Med Food. 2004;7:61–66. doi: 10.1089/109662004322984725. [DOI] [PubMed] [Google Scholar]
- 29.Rajasekaran S, Sivagnanam K, Ravi K, Subramanian S. Beneficial effects of Aloe vera leaf gel extract on lipid profile status in rats with streptozotocin diabetes. Clin Exp Pharmacol Physiol. 2006;33:232–237. doi: 10.1111/j.1440-1681.2006.04351.x. [DOI] [PubMed] [Google Scholar]
- 30.Lucio RA, Tlachi-López JL, Eguibar JR, Ågmo A. Sperm count and sperm motility decrease in old rats. (73-79).Physiol Behav. 2013:110–111. doi: 10.1016/j.physbeh.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 31.Navarro-Gonzálvez JA, García-Benayas C, Arenas J. Semiautomated measurement of nitrate in biological fluids. Clin Chem. 1998;44:679–681. [PubMed] [Google Scholar]
- 32.Dimmeler S, Zeiher A. Nitric Oxide and Apoptosis: Another paradigm for the double-edged role of nitric Oxide. Nitric Oxide. 1997;2:75–281. doi: 10.1006/niox.1997.0133. [DOI] [PubMed] [Google Scholar]
- 33.Cohen O, Ish-Shalom E, Kfir-Erenfeld S, Herr I, Yefenof E. Nitric oxide and glucocorticoids synergize in inducing apoptosis of CD4+8+thymocytes: implications for ‘Death by Neglect’ and T-cell function. Int Immunol. 2012;24:783–791. doi: 10.1093/intimm/dxs083. [DOI] [PubMed] [Google Scholar]
- 34.F Ni B, Liu M, Feng Z, Xiong Q, Xiao S, Shao G. Mycoplasma hyopneumoniae-derived lipid-associated membrane proteins induce apoptosis in porcine alveolar macrophage via increasing nitric oxide production, oxidative stress, and caspase-3 activation. Vet Immunopathol. 2013:55–161. doi: 10.1016/j.vetimm.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 35.Saini A, Shenoy G, Rath S, Bal V, George A. Inducible nitric oxide synthase is a major intermediate in signaling pathways for the survival of plasma cells. Nat Immunol. 2014;15:275–228. doi: 10.1038/ni.2806. [DOI] [PubMed] [Google Scholar]
- 36.Lue Y, Sinha Hikim AP, Wang C, Leung A, Swerdloff RS. Functional role of inducible nitric oxide synthase in the induction of male germ cell apoptosis, regulation of sperm number, and determination of testes size: evidence from null mutant mice. J Endocrinol. 2003;144:3092–3100. doi: 10.1210/en.2002-0142. [DOI] [PubMed] [Google Scholar]
- 37.Chen D, Zheng W, Lin A, Uyhazi K, Zhao H, Lin H. Pumilio 1 suppresses multiple activators of p53 to safeguard spermatogenesis. Curr Biol. 2012;22:420–425. doi: 10.1016/j.cub.2012.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Broniowska KA, Diers AR, Corbett JA, Hogg N. Effect of nitric oxide on naphthoquinone toxicity in endothelial cells: role of bioenergetic dysfunction and poly(ADP-ribose) polymerase activation. Biochemistry. 2013;52:4364–4372. doi: 10.1021/bi400342t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nee Pathak ND, Lal B. Paracrine role of macrophage produced-nitric oxide (NO) in Leydig cell steroidogenesis in a teleost, Clarias batrachus: Impact of gonadotropin, growth hormone and insulin on NO production by testicular macrophages. Gen Comp Endocrinol. 2009;160:12–18. doi: 10.1016/j.ygcen.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Karimi Jashni HH, Najmadini N, Hooshm F. Effect of alcoholic extract of Aloe vera plant on serum testosterone and gonadotropin levels in rats. J Jahrom Univ Med Sci. 2012;10:1–8. [Google Scholar]
- 41.Skarzynski DJ, Kobayashi S, Okuda K. Influence of nitric oxide and noradrenaline on prostaglandin F2α-induced oxytocin secretion and intracellular calcium mobilization in cultured bovine luteal cells. Biol Reprod. 2000;63:1000–1005. doi: 10.1095/biolreprod63.4.1000. [DOI] [PubMed] [Google Scholar]
- 42.Mondillo C, Pagotto RM, Piotrkowski B, Reche CG, Patrignani ZJ, Cymeryng CB, et al. Involvement of nitric oxide synthase in the mechanism of histamine-induced inhibition of Leydig cell steroidogenesis via histamine receptor subtypes in Sprague-Dawley rats. Biol Reprod. 2009;80:144–152. doi: 10.1095/biolreprod.108.069484. [DOI] [PubMed] [Google Scholar]
- 43.Nee Pathak ND, Lal B. Nitric oxide: An autocrine regulator of Leydig cell steroidogenesis in the Asian catfish, Clarias batrachus. Gen Comp Endocrinol. 2008;158:161–167. doi: 10.1016/j.ygcen.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 44.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brookes PS, Levonen AL, Shiva S, Sarti S, Darley-Usmar VM. Mitochondria: regulators of signal transduction by reactive oxygen and nitrogen species. Free Radic Biol Med. 2002;33:755–764. doi: 10.1016/s0891-5849(02)00901-2. [DOI] [PubMed] [Google Scholar]
- 46.Sinha-Hikim I, Braga M, Shen R, Hikim APS. Involvement of c-Jun NH2-terminal kinase and nitric oxide-mediated mitochondria-dependent intrinsic pathway signaling in cardiotoxin-induced muscle cell death: role of testosterone. Apoptosis. 2007;12:1965–1978. doi: 10.1007/s10495-007-0120-6. [DOI] [PubMed] [Google Scholar]
- 47.Ghatan S, Larner S, Kinoshita Y, Hetman M, Patel L, Xia Z, et al. P38 MAP kinase mediates BAX translocation in nitric oxide–induced apoptosis in neurons. J Cell Biol. 2000;150:335–348. doi: 10.1083/jcb.150.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hikim APS, Lue Y, Diaz-Romero M, Yen PH, Wang C, Swerdloff RS. Deciphering the pathways of germ cell apoptosis in the testis. J Steroid Biochem Mol Biol. 2003;85:175–182. doi: 10.1016/s0960-0760(03)00193-6. [DOI] [PubMed] [Google Scholar]
- 49.Tripathi R, Mishra DP, Shaha C. Male germ cell development: turning on the apoptotic pathways. J Reprod Immunol. 2009;83:31–35. doi: 10.1016/j.jri.2009.05.009. [DOI] [PubMed] [Google Scholar]
- 50.Yamamoto CM, Sinha Hikim AP, Huynh PN, Shapiro B, Lue YH, Salameh WA, et al. Redistribution of Bax is an early step in an apoptotic pathway leading to germ cell death in rats, triggered by mild testicular hyperthermia. Biol Reprod. 2000;63:1683–1690. doi: 10.1095/biolreprod63.6.1683. [DOI] [PubMed] [Google Scholar]


