Abstract
Background:
Based on biochemical properties, Enterobacter cloacae represents a large complex of at least 13 variant species, subspecies, and genotypes that progressively identified as the most species causing hospital-acquired infections. The aim of this study was to determine the relevance between phylogenetically related strains within the E. cloacae complex and the frequency of urinary tract infection caused by them.
Methods:
A 268-bp fragment was obtained from hsp60 gene for 50 clinical E. cloacae isolates from urine cultures of inpatients that admitted to six hospitals in Tehran, Iran during December 2012 to November 2013. The 107 nucleotide sequences were analyzed and the evolutionary distances of sequences were computed and neighbor-joining tree was calculated.
Results:
It showed that all of the genetic clusters have not an equal involvement in pathogenesis of urinary tract infections. Three superior clusters were found, together representing more than two third (80%) of the isolates (cluster VI with 25 members; clusters III and VIII with 9 and 6 members, respectively) and some genetic clusters were absent (IV, X, XII, and xiii), some of which are supposed to be associated with plants and no human infection has been reported.
Conclusions:
This study, for the first time, reports the unequal contribution of E. cloacae complex subspecies and clusters in urinary tract infections in Iran and together with studies from other countries suggest that the subspecies of E.hormaechei subsp. Oharae is the most prevalent E. cloacae complex subspecies regardless of country under study.
Key Words: Enterobacter cloacae complex, Urinary tract infection, hsp60
INTRODUCTION
Enterobacter spp. are Gram-negative and facultative anaerobic bacteria that are saprophytic in the environment, as they are found in soil and sewage. These bacteria are also parts of the commensal flora of the human gastrointestinal tract and can be considered as pathogens of plants, insects, and humans[1].
Taxonomy of the genus Enterobacter has been repetitively updated[2]. Within a genetic complex, referred to as the “Enterobacter cloacae complex”, six genetically related and phenotypically similar species have been merged, i.e. E. cloacae, E. asburiae, E. dissolvens, E. hormaechei, E. kobei, and E. nimipressuralis. Most of them share a DNA relatedness with E. cloacae ranging from 61 to 67%[1]. In addition to these species, at least six genetic clusters are phylogenetically defined within the complex[3]. These clusters are routinely identified as ‘E. cloacae’ using commercial biochemical kits such as API20E. However, the exact identification of the isolates within this taxon is difficult. Analysis of the 16SrRNA gene is widely used for bacterial recognition, but it is poorly discriminative for closely related members of the Enterobacteriaceae family and more specifically, for members of the Enterobacter genus[4]. Other targets for molecular recognition of the isolates within the Enterobacter genus are oriC[5], gyrB[6], rpoB[3,7], and hsp60 locus[3]. The hsp60 gene sequencing-based identification seemed to be both discriminatory and easily carry out; however, for the identification of Enterobacter, other sequence-based molecular methods were not as accurate. Sequence analysis of rpoB and DNA gyrB genes were also supposed to be discriminatory for E. cloacae complex, but the lack of unanimity and consistency for the analysis of results has led us and other investigators to consider hsp60 as a powerful tool for assigning the sub-species and clusters[6].
Sequence analysis of a segment of the hsp60 gene demonstrated that the E. cloacae complex could be divided into 12 genetic clusters (I to XII) and one sequence crowd (xiii)[4,8]. Their specific names of some of these clusters are as follows: E. asburiae (cluster I), E. kobei (cluster II), E. ludwigii (cluster V), E. nimipressuralis (cluster X), E. cloacae subsp. cloacae (cluster XI), and E. cloacae subsp. dissolvens (cluster XII)[3,8,9]. However, the name E. hormaechei was sometimes applied as a generic name for strains belonging to various hsp60 gene sequencing-based clusters, including VI, VII, and VIII, which are related to three subspecies: oharae, hormaechei, and steigerwallti, respectively[10]. Species names were not ascribed to clusters III, IV, and IX and to sequence crowd xiii.
Based on biochemical properties, what we identify in the laboratory as E. cloacae represents a large complex of at least 13 variant species, subspecies, and genotypes. E. cloacae has been progressively identified as the 10 most frequent species causing hospital-acquired wound, pneumonia, urinary tract infections, and sepsis in intensive care units. In spite of this relevancy, little is known about the correlation between phylogenetically related strains within the E. cloacae complex and the frequency of diseases caused by these[11]. The members of the E. cloacae complex differ in pathogenicity to humans, and some members have been reported to cause an epidemic outbreak[12].
To our knowledge, there are no published data on molecular epidemiology of E. cloacae genotypes in Iran. Therefore, the current study was conducted to identify the specific distribution within the E. cloacae complex of strains isolated from the patients with urinary tract infection in Tehran, Iran.
MATERIALS AND METHODS
Bacterial strains and epidemiological data
In total, 50 E. cloacae isolates were collected from urine cultures of inpatients suffering from urinary tract infection admitted to six large academic and governmental hospitals in Tehran, Iran during December 2012 to November 2013. The samples were systematically and prospectively collected and stored. The identification of the infecting organism as E. cloacae was confirmed using a routine phenotypic identification system (the API20E, BioMerieux, France). E. cloacae PTCC (Persian Type Culture Collection) 1003 and E. cloacae PTCC 1798 were used as standard strains.
Molecular identification methods
Bacterial DNA was prepared for PCR analysis with using boiling method in which fresh bacteria colonies were suspended in 500 μl sterile distilled water (molecular grade) and boiled for 10 minutes. The suspension was centrifuged (at 10,000 ×g at room temperature for 10 min), and the 200-μl supernatant was transferred to a microtube and used directly for PCR assay. Partial sequencing of the hsp60 gene was performed by a previously described protocol[3]. Briefly, oligonucleotide primers Hsp60-F (5_-GTAGAAGAA GGCGTGGTTGC-3_) and Hsp60-R (5_ATGCATT CGGTGGTGATCATCAG-3_) were used for genomic amplification of a 341-bp fragment of the hsp60 gene. Amplification was also performed in a reaction mixture with total volume of 25 μl, containing 15.6 μl sterile water (molecular grade), 2.5 μl 10× Taq polymerase buffer, 0.3 μl dNTPs (10 mmol l-1), 0.5 U Taq DNA polymerase, 25 pmol each primer, 0.6 µL MgCl2 (50 mM), and 5 μl template DNA. Amplification was performed as follows: initial denaturation step at 94°C for 5 min, followed by 30 cycles consisting of denaturation (94°C for 30 s), annealing (57°C for 30 s) (separately adjusted for each set of primer pairs), and extension (72°C for 60 s) with a final extension step at 72°C for 5 min. PCR products were visualized on 1% agarose gels after electro-phoresis and staining. PCR was performed. Forward strand of the amplified DNA fragment was used for direct sequencing using the ABI 3730X capillary sequencer (Genfanavaran, Macrogen, Seoul, Korea).
Nucleotide sequence accession numbers
The sequences of the following reference and type strains were retrieved from the GenBank database. The number of genotype and reference strains of E. cloacae complex in Table 1 refer to Hoffmann and Roggenkamp study[3]. A 268-bp sequence of the hsp60 gene was obtained from 50 clinical and 2 PTCC strains. The sequences were compared with 44 reference sequences from the strains previously described in taxonomic studies by Hoffmann and Roggenkamp[3], four reference sequences for every cluster except cluster X that has 1 sequence, and 11 sequence type strains[3,13]. All of the 50 clinical and 2 PTCC sequences were deposited in the GenBank under the accession numbers KM202107, KM222360 through KM222408, KM278223 and KM278224.
Table 1.
Reference and type strains retrieved from the GenBank database and used in this study
|
Reference/
type strains |
Species | Nucleotide sequence accession numbers |
|---|---|---|
| Cluster I | E. asburiae | (AJ567893.1, AJ567846.1, AJ417140.1, AJ417141.1) |
| Cluster II | E. kobei | (AJ567888.1, AJ567886.1, AJ567862.1,AJ567849.1) |
| Cluster III | E. cloacae III | (AJ567880.1, AJ567877.1, AJ567872.1, AJ567871.1) |
| Cluster IV | E. cloacae IV | (AJ543893.1, AJ543807.1, AJ543889.1, AJ543877.1) |
| Cluster V | E. ludwigii | (AJ862859.1, AJ862861.1, AJ862862.1, AJ862863.1) |
| Cluster VI | E. hormaechei subsp. oharae | (AJ567891.1, AJ567885.1, AJ567878.1, AJ567876.1) |
| Cluster VII | E. hormaechei subsp. hormaechei | (AJ866491.1, AJ862866.1, AJ862867.1,AJ417108.1) |
| Cluster VIII | E. hormaechei subsp. steigerwaltii | (AJ567892.1 , AJ567890.1, AJ567889.1,AJ567884.1) |
| Cluster IX | E. cloacae IX | (AJ543878.1, AJ543819.1, AJ543881.1, AJ543820.1) |
| Cluster X | E. nimipressuralis | (AJ567900.1) |
| Cluster XI | E. cloacae subsp. cloacae | (AJ543855.1, AJ417139.1, AJ417142.1, AJ543768.1) |
| Cluster XII | E. cloacae subsp. dissolvens | (AJ417143.1, AJ862872.1, AJ543817.1, AJ543847.1) |
| Cluster xiii | E. cloacae sequencecrowd | (AJ543872.1, AJ543870.1, AJ417128.1, AJ543837.1) |
| Outgroup | E. cancerogenus | (ATCC 33241, AJ567895) |
| Outgroup | E. amnigenus | (ATCC 3072, AJ567894) |
| Outgroup | E. cowanii | (ATCC 107300T, AJ567896) |
| Outgroup | E. gergoviae | (ATCC 33028, AJ567897) |
| Outgroup | E. pyrinus | (ATCC 49851, AJ567901) |
| Outgroup | C. sakazaki | (ATCC 29544, AJ567902) |
Type strains are shown in bold.
Statistical analysis
The evolutionary history was inferred using the Neighbor-Joining method[1]. The analysis involved 105 nucleotide sequences. The evolutionary distances computed using the Maximum Composite Likelihood method[3] are in units of the number of base substitutions per site. There were a total of 268 positions with 74 variables in the final dataset. Evolutionary analyses were conducted in MEGA (version 6)[14].
RESULTS
Prevalence of species and genotypes
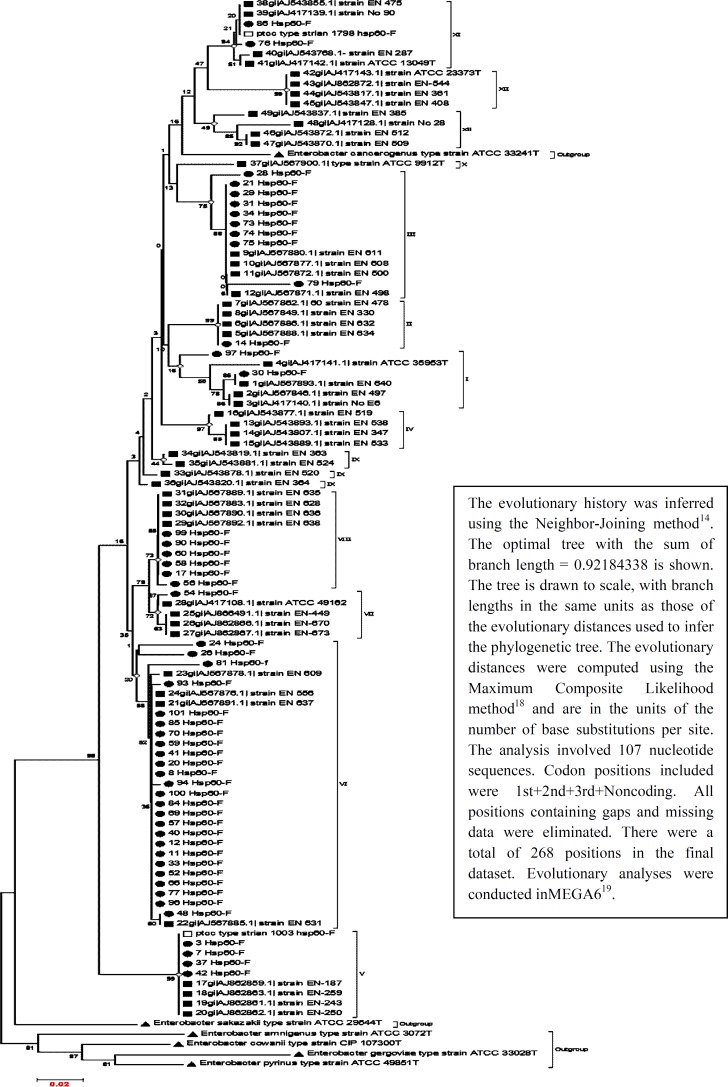
Each isolate was allotted to its individual species, subspecies, or genotypes by sequence analysis of 268-bp hsp60. A neighbor-joining tree was calculated, including all clinical, types, and reference strains of the E. cloacae complex as well as type strains of the Enterobacter genus (105 nucleotide sequences). The optimal tree with the sum of branch length = 0.92199563 is shown in Figure 1. The percentage of replicate trees in which the associated taxa clustered together in the bootstrap test (100 replicates) is shown next to the branches. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree. Of 13 genotypes and species delineated so far, 8 were found in the present study.
Fig. 1.
The evolutionary history of analysis 268 nucleotides (74 variable) of the hsp60 gene sequences from 50 clinical strains (urine) and 44 reference and 11 type strains of the genus Enterobacter (105 nucleotide sequences ) was inferred using the Neighbor-Joining method[15]. The optimal tree with the sum of branch length = 0.92184338 is shown. The tree is drawn to scale, with branch lengths in the same units as those of the evolutionary distances used to infer the phylogenetic tree
Thirty two (64%) isolates belonged to three E. hormaechei subspecies, explaining E. hormaechei by far the most eminent species of our study collection. Furthermore, only one isolate (2%) clustered with the E. hormaechei type strain (E. hormaechei subsp. hormaechei), 25 isolates (50%) were identified as E. hormaechei subsp. Oharae, and 6 isolates (12%) as E. hormaechei subsp. Steigerwaltii, suggesting that E. hormaechei subsp. oharae was the subspecies with the highest clinical relevance in urinary tract infection.
Nine isolates (18%) clustered within genotype III, making it the second most frequent genotype of the E. cloacae complex. In addition, 4 isolates (8%) grouped with cluster V (E. ludwigii). E. cloacae subsp. cloacae, E. asburiae, and E. kobei (clusters XI , I , II, and VII, respectively) were found in 2 (4%), 2 (4%), 1 (2%), and 1 (2%) respectively, while clusters IV, IX, X (E. nimipressuralis), XII (E. cloacae subsp. dissolvens), and xiii (E. cloacae sequence crowd) were absent among the isolates in this study (Table 2).
Table 2.
Distribution of clinical strains within the genetic clusters of the E. cloacae complex
| Strains | Cluster |
No. of
urinary strains |
Frequency (%) |
|---|---|---|---|
| E. asburiae | I | 2 | 4 |
| E. kobei | II | 1 | 2 |
| E. cloacae III | III | 9 | 18 |
| E. lodwigii | V | 4 | 8 |
| E. hormaechei subsp. oharae | VI | 25 | 50 |
| E. hormaechei subsp. hormaechei | VII | 1 | 2 |
| E. hormaechei subsp. steigerwaltii | VIII | 6 | 12 |
| E. cloacae subsp. cloacae | XI | 2 | 4 |
| Total | 8 | 50 | 100 |
DISCUSSION
The current study demonstrates distribution of the strains involved in urinary tract infection within the genetic clusters of the E. cloacae complex. All of the 50 isolates, which were phenotypically identified as E. cloacae using API 20E, harbored the hsp60 gene. It can be suggested that API 20E is a reliable tool for primary identification of E. cloacae complex; however, it should not be ignored that only sequence-based methods could discriminate the genotypes and clusters.
On the basis of hsp60 analysis, we showed that all of the genetic clusters have not equal involvement in the pathogenesis of urinary tract infections. Therefore, this fact underlines the necessity for more accurate, routine methods for bacterial identification and for better understanding of the etiology of urinary tract infection and pathogenesis of the E. cloacae complex[11,13]. Three superior clusters were found, together with representing more than two third (80%) of the isolates (cluster VI with 25 members and clusters III and VIII with 9 and 6 members, respectively). Other investigators from Euroupe have shown that the E. hormaechei subsp. oharae (cluster VI), E. cloacae cluster III, and E. hormaechei subsp. steigerwaltii (cluster VIII) are the species most frequently recovered from clinical specimens[3,11,13]. This finding suggests the equivalent distribution of genotypes in different geographical locations.
Morand and colleagues[13] indicated that the most common genetic clusters were clusters III, VI, and VIII, which was detected among clinical strains routinely identified as E. cloacae in the clinical laboratory. However, clusters VI and VIII (E. hormaechei) but not cluster III had a dominant association with the infections of orthopedic implants and specifically, with the infected material in the hip (P=0.019)[13].
Analysis based on microarray comparative genomic hybridization (CGH) showed two genetically distinct clades. Most strains related to the clinical diseases belonged to the youngest CGH-based clade (clade 2), which comprises clusters III, VI, and VII based on hsp60 gene sequencing. The second older clade (clade 1) includes heterogeneous strains, some of which are commensal[12]. Therefore, it can be inferred that Iranian urinary tract infection, which is prevalent in isolated strains, are derived from the younger CGH clade.
Paauw et al.[12] reported Enterobacter hormaechei outbreak strain as the cause of a nationwide outbreak in the Netherlands, which carried a wide range of virulence and antimicrobial-associated genetic elements[12]. In total, 32 cases (64%) of E. hormaechei subspecies (clusters VI, VII, and VIII) in our study were dominant, indicating an outbreak of hospital-acquired urinary tract infection in Tehran that Pulsed-field Gel Electrophoresis genotyping findings rejected the hypothesis (unpublished data). Our data together with the finding of Paauw et al.[12] emphasizes the need for routine monitoring of E. cloacae genotypes and clusters within the clinical isolates due to fear of clonal dissemination or outbreak occurrence.
Enterobacter asburiae is a normal flora in the gastrointestinal tract that also is separated in water and soil. Also, it is most usually found in immune-compromised patients and associated with antibiotic use, enervated states, and chronic respiratory conditions[15]. Two strains of this organism isolated in our study were separated from two female newborns in two different hospitals, which further supports that E. asburiae is associated with immunocompromised patients newborns (in this study).
In the presrnt study, some genetic clusters IV, X, XII, and xiii were absent. These strains, which are mainly less prevalent, are found in nature. Cluster XII (E. cloacae subsp. dissolvens) is associated with plants and no human infections[8,16]. Cluster X (E. nimipressuralis) is found in potable water reservoirs but, to our knowledge, it has never been associated with human disease[17].
In conclusion, this study, for the first time, reports the unequal contribution of E. cloacae complex subspecies and clusters in urinary tract infections. These results are consistent with other similar studies and suggest that the subspecies of E. hormaechei subsp. Oharae is the most prevalent E. cloacae complex genotype regardless of country under study. Other genotyping methods could be beneficial to assess the clonal correlation of strains within one E. cloacae cluster, which is mandatory for outbreak monitoring.
ACKNOWLEDGMENTS
We thank the Research Council of Tarbiat Modares University (Tehran, Iran) for financial support of the project. This work is a part of thesis project of Majid Akbari, Ph.D. student of Faculty of Medical Sciences, Tarbiat Modares University, Tehran, Iran.
CONFLICT OF INTEREST. None declared.
References
- 1.Mezzatesta ML, Gona F, Stefani S. Enterobacter cloacae complex: clinical impact and emerging antibiotic resistance. Future microbiology. 2012;7(7):887–902. doi: 10.2217/fmb.12.61. [DOI] [PubMed] [Google Scholar]
- 2.O'Hara CM, Steigerwalt AG, Hill BC, Farmer JJ 3rd, Fanning GR, Brenner, DJ. Enterobacter hormaechei, a new species of the family Enterobacteriaceae formerly known as enteric group 75. Journal of clinical microbiology. 1989;27(9):2046–20469. doi: 10.1128/jcm.27.9.2046-2049.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoffmann H, Roggenkamp A. Population genetics of the nomenspecies Enterobacter cloacae. Applied and environmental microbiology. 2003;69(9):5306–5318. doi: 10.1128/AEM.69.9.5306-5318.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tang YW, Ellis NM, Hopkins MK, Smith DH, Dodge DE, Persing DH. Comparison of phenotypic and genotypic techniques for identification of unusual aerobic pathogenic gram-negative bacilli. Journal of clinical microbiology. 1998;36(12):3674–3679. doi: 10.1128/jcm.36.12.3674-3679.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Roggenkam A. Phylogenetic analysis of enteric species of the family Enterobacteriaceae using the oriC-locus. Systematic and Applied microbiology. 2007;30(3):180–188. doi: 10.1016/j.syapm.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 6.Delmas J, Breysse F, Devulder G, Flandrois JP, Chomarat M. Rapid identification of enterobacteriaceae by sequencing DNA gyrase subunit B encoding gene. Diagostic microbiology and infecious disease. 2006;55(4):263–268. doi: 10.1016/j.diagmicrobio.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 7.Paauw A, Caspers MP, Schuren FH, Leverstein Hall MA, Deletoile A, Montijn RC, Verhoef J, Fluit AC. Genomic diversity within the Enterobacter cloacae complex. PLoS one. 2008;3(8):e3018. doi: 10.1371/journal.pone.0003018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hoffmann H, Stindl S, Ludwig W, Stumpf A, Mehlen A, Heesemann J, Monget D, Schleifer KH, Roggenkamp A. Reassignment of enterobacter dissolvens to Enterobacter cloacae as E. cloacae subspecies dissolvens comb. Nov. and emended description of Enterobacter asburiae and Enterobacter kobei. Systematic and applied microbiology. 2005;28(3):196–205. doi: 10.1016/j.syapm.2004.12.010. [DOI] [PubMed] [Google Scholar]
- 9.Hoffmann H, Stindl S, Stumpf A, Mehlen A, Monget D, Heesemann J, Schleifer KH, Roggenkamp A. Description of Enterobacter ludwigii sp. nov., a novel Enterobacter species of clinical relevance. Systematic and applied microbiology. 2005;28(3):206–212. doi: 10.1016/j.syapm.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 10.Hoffmann, H, Stindl, S, Ludwig, W, Stumpf, A, Mehlen, A, Monget, D, Pierard, D, Ziesing, S, Heesemann, J, Roggenkamp, A, Schleifer, KH. Enterobacter hormaechei subsp. oharae subsp. nov., E. hormaechei subsp. hormaechei comb. nov., and E. hormaechei subsp. steigerwaltii subsp. nov., three new subspecies of clinical importance. Journal of clinical microbiology. 2005;43:3297–303. doi: 10.1128/JCM.43.7.3297-3303.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kremer A, Hoffmann H. Prevalences of the Enterobacter cloacae complex and its phylogenetic derivatives in the nosocomial environment. European journal of clinical microbiology diseases. 2012;31(11):2951–2955. doi: 10.1007/s10096-012-1646-2. [DOI] [PubMed] [Google Scholar]
- 12.Paauw A, Caspers P, Leverstein-van Hall MA, Schuren FH, Montijn RC, Verhoef J, Fluit AC. Identification of resistance and virulence factors in an epidemic enterobacter hormaechei outbreak strain. Microbiology. 2009;155(Pt 5):1478–1488. doi: 10.1099/mic.0.024828-0. [DOI] [PubMed] [Google Scholar]
- 13.Morand PC, Billoet A, Rottman M, Sivadon-Tardy V, Eyrolle L, Jeanne L, Tazi A, Anract P, Courpied JP, Poyart C, Dumaine V. Specific distribution within the Enterobacter cloacae complex of strains isolated from infected orthopedic implants. Journal of clinical microbiology. 2009;47(8):2489–2495. doi: 10.1128/JCM.00290-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tamura K, Stecher G, Peterson D, Filipski A, Kumar S. MEGA6: molecular evolutionary genetics analysis version 6.0. Molecular biology and evolution. 2013;30(12):2725–2729. doi: 10.1093/molbev/mst197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koth K, Boniface J, Chance EA, Hanes MC. Enterobacter asburiae and aeromonas hydrophila: soft tissue infection requiring debridement. Orthopedics. 2012;35(6):e996–e999. doi: 10.3928/01477447-20120525-52. [DOI] [PubMed] [Google Scholar]
- 16.Frank HA, Lum NA, Cruz AS. Bacteria responsible for mucilage-layer decomposition in Kona coffee cherries. Applied microbiology. 1965;13:201–207. doi: 10.1128/am.13.2.201-207.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kämpfer P, Nienhüser A, Packroff G, Wernicke F, Mehling A, Nixdorf K, Fiedler S, Kolauch C, Esser M. Molecular identification of coliform bacteria isolated from drinking water reservoirs with traditional methods and the Colilert-18 system. International journal of Hygiene and environmental health. 2008;211(3-4):374–384. doi: 10.1016/j.ijheh.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 18.Saitou N, Nei M. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Molecular Biolology and Evolution. 1987;4(4):406–425. doi: 10.1093/oxfordjournals.molbev.a040454. [DOI] [PubMed] [Google Scholar]
- 19.Tamura, K, Nei, M, Kumar, S. Prospects for inferring very large phylogenies by using the neighbor-joining method. Proceeding of the national academy of the United States of America. 2004;101(30):11030–11035. doi: 10.1073/pnas.0404206101. [DOI] [PMC free article] [PubMed] [Google Scholar]