Abstract
DICER1 is an enzyme that generates mature microRNAs (miRNAs), which regulate gene expression post-transcriptionally in brain and other tissues and is involved in synaptic maturation and plasticity. Here, through genome-wide differential gene expression survey of post-traumatic stress disorder (PTSD) with comorbid depression (PTSD&Dep), we find that blood DICER1 expression is significantly reduced in cases versus controls, and replicate this in two independent cohorts. Our follow-up studies find that lower blood DICER1 expression is significantly associated with increased amygdala activation to fearful stimuli, a neural correlate for PTSD. Additionally, a genetic variant in the 3′ un-translated region of DICER1, rs10144436, is significantly associated with DICER1 expression and with PTSD&Dep, and the latter is replicated in an independent cohort. Furthermore, genome-wide differential expression survey of miRNAs in blood in PTSD&Dep reveals miRNAs to be significantly downregulated in cases versus controls. Together, our novel data suggest DICER1 plays a role in molecular mechanisms of PTSD&Dep through the DICER1 and the miRNA regulation pathway.
 DICER1 is required for the maturation of miRNAs which regulate expression of thousands of genes. Here the authors show significantly reduced levels of DICER1 in individuals having post-traumatic stress disorder and comorbid depression suggestive of a role in the molecular mechanism of the condition.
DICER1 is required for the maturation of miRNAs which regulate expression of thousands of genes. Here the authors show significantly reduced levels of DICER1 in individuals having post-traumatic stress disorder and comorbid depression suggestive of a role in the molecular mechanism of the condition.
Exposure to traumatic life events and its sequelae, such as post-traumatic stress disorder (PTSD) and depression, are a significant source of suffering and impairment around the world and major contributors to the global burden of disease1,2. However, our understanding of biological mechanisms underlying PTSD and depression is still incomplete. Gene expression profiles reflect dynamic interplay between genes and the environment and thus can provide a window into molecular mechanisms underlying these stress-related disorders. Though gene expression is tissue specific, expression levels of many genes have moderate correlations between blood and other tissues, including post-mortem brain3,4. Moreover, stress-related transcriptomic changes in peripheral blood mononuclear cells were paralleled by stress-related gene expression changes in the relevant brain regions in mice5. Since it is difficult to obtain brain tissues in live human subjects, gene expression profiles in blood may provide a useful surrogate to offer information on biological mechanisms of neuropsychological disorders as well as their effects on physical health.
PTSD with comorbid depression (referred to as PTSD&Dep from here on) is highly prevalent among susceptible individuals following traumatic life experiences. For example, in the Grady Trauma Project (GTP) sample of 6863 at-risk inner city participants with high rates of trauma exposure, the prevalence of PTSD&Dep was 28.4%. In another instance, among the 29,486 post-9–11World Trade Center Health Registry enrolees, 4.8% had depression only, 5.1% had PTSD only and 10.1% had PTSD&Dep at 10 years after the September 11 disaster6. Nosologic distinctions among different psychiatric disorders so far have largely been based on descriptive studies and not on neural circuits or biological mechanisms. Hence, in this study, we aimed to increase our understanding of molecular mechanisms underlying PTSD&Dep, a relatively common sequel of traumatic life events.
To that end, we survey genome-wide differential gene expression profiles in blood in cases of PTSD&Dep and traumatized controls with no PTSD and no depression. Our hypothesis is that genes differentially expressed in PTSD&Dep inform biological pathways underlying this psychiatric phenotype. We find that expression of DICER1 is significantly reduced in the cases relative to the controls, and this finding is replicated in two independent cohorts. This is quite intriguing given prior possible association of DICER1 to depression-related phenotypes in humans and mice7,8. To follow-up on the DICER1 finding, we perform expression quantitative trait locus (eQTL) analysis for DICER1 and find an eQTL, rs10144436, located in DICER1's three prime un-translated region (3′UTR), significantly associated with PTSD&Dep and replicate this finding in another cohort. Additionally, we find that lower blood DICER1 expression is significantly associated with increased activation of the amygdala to threat stimuli, a neural correlate of PTSD, in a functional magnetic resonance imaging (fMRI) study. DICER1 is notable as an enzyme that is central to the post-transcriptional regulation of gene expression as it cleaves precursor microRNAs (miRNAs) and double-stranded RNAs into mature miRNAs and short-interfering RNAs, both of which regulate expression of thousands of genes post-transcriptionally9. Given DICER1 functions, we survey genome-wide differential miRNA expression profiles in blood in a smaller discovery sample of PTSD&Dep and find two miRNAs significantly reduced in abundance in the cases. Of these, miR-3130-5p is also significantly downregulated in PTSD&Dep cases versus controls in a replication sample, and one of its mRNA targets is upregulated in the PTSD&Dep cases. Together, our data show that DICER1 and miRNAs are involved in molecular mechanisms of PTSD&Dep via the DICER1 and the miRNA regulation pathway. This stress-related DICER1 and miRNA regulation in blood is paralleled by recent findings of stress-related DICER1 and miRNA regulation in brain in a mouse model8.
Results
Characteristics of the discovery cohort
There were 184 participants in the GTP discovery cohort (Table 1). Of these, 112 were cases of current PTSD&Dep and 72 were traumatized controls with no current PTSD and no current depression. Specifically, mean PTSD symptom scores among the cases and controls were 28.7 and 2.1, respectively, and mean depression scores among the cases and controls were 28.2 and 2.7, respectively (Table 1). The cases and controls had comparable age and proportions of men and women (Table 1). As expected, the cases were exposed to more traumatic life events than the controls (7.3 versus 3.8; Table 1). In a subset of these participants (n=145–150), we had information on current alcohol and drug use disorders, bipolar disorder and current psychotic disorder assessed by the Mini International Neuropsychiatric Interview (MINI)10 and Structure Clinical Interview for DSM-IV (SCID)11. More cases had substance use disorder, psychotic disorder and bipolar disorder than controls, but these differences did not reach statistical significance (Table 1). Lastly, the cases had slightly more percentage of African Americans than the controls (Table 1). We controlled for these psychiatric variables and ancestry in our differential expression analysis as detailed below.
Table 1. Characteristics of the GTP discovery sample of cases of PTSD&Dep and controls with no PTSD and no depression (n=184).
| Characteristics | Cases (n=112) | Controls (n=72) | P-value* |
|---|---|---|---|
| Age | 41.9±10.7 | 43.3±14.9 | 0.28 |
| Sex (% female) | 77.7% | 70.8% | 0.29 |
| Race (% African American) | 91.1% | 98.6% | 0.04 |
| PSS† score | 28.7±9.2 | 2.1±2.3 | <0.0001 |
| BDI‡ score | 28.2±10.1 | 2.7±2.1 | <0.0001 |
| Current substance use disorder§ (yes) | 18.0% | 9.8% | 0.17 |
| Current psychotic disorder|| (yes) | 16.3% | 6.8% | 0.12 |
| Bipolar disorder¶ (yes) | 2.3% | 0.0% | 0.51 |
| Number of traumatic experiences | 7.3±3.8 | 3.4±2.8 | <0.0001 |
BDI; Beck Depression Inventory; GTP, Grady Trauma Project; MINI, Mini International Neuropsychiatric Interview; PSS, PTSD symptom scale; PTSD, post-traumatic stress disorder; PTSD&Dep, PTSD with comorbid depression; SCID, Structure Clinical Interview for DSM-IV.
*P values were from Wilcoxon Rank Sums or Fisher exact test.
†PSS: PTSD symptom severity score from the PSS.
‡BDI: depression severity score from the BDI.
§Available in a subset of 150 subjects; assessed by the SCID and MINI.
||Available in a subset of 145 subjects; assessed by the SCID and MINI.
¶Available in a subset of 148 subjects; assessed by the SCID and MINI.
Genome-wide differential expression profiles in PTSD&Dep
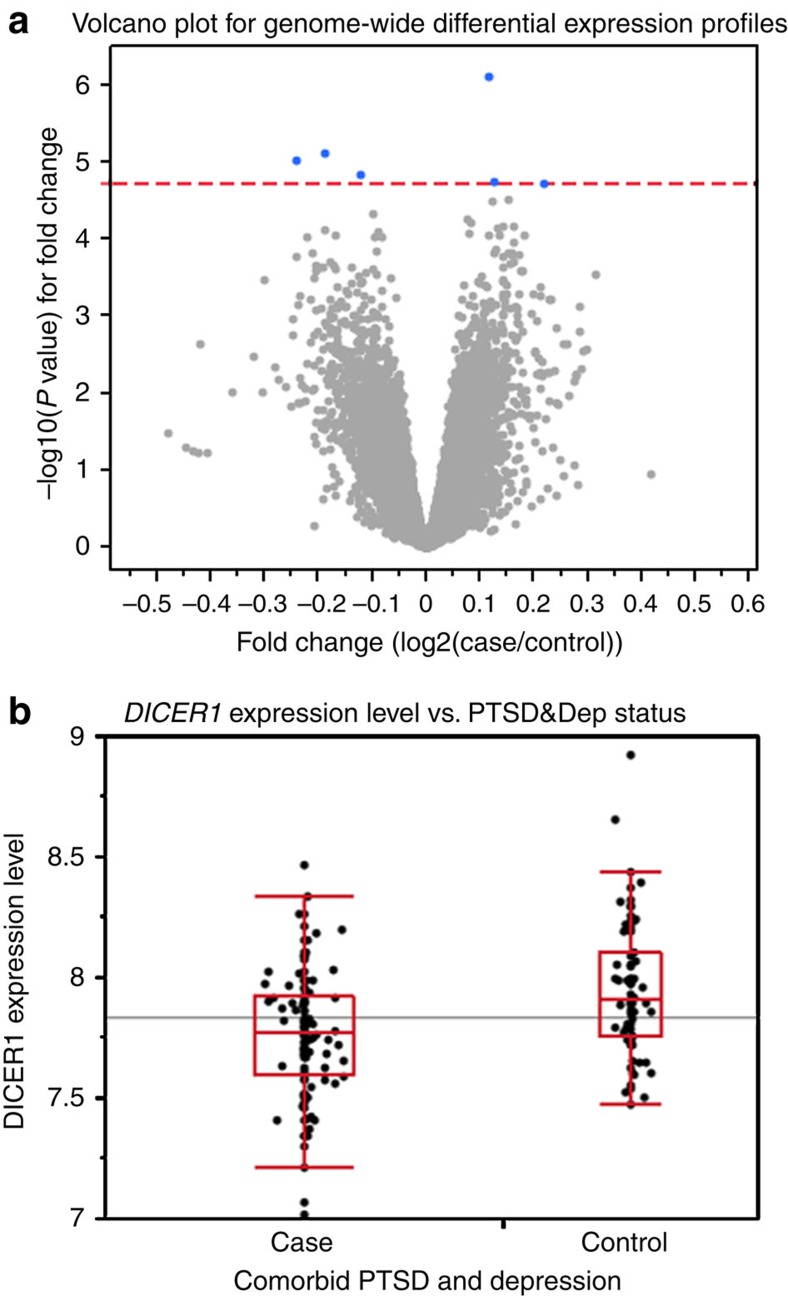
In our primary analysis, at a genome-wide false discovery rate (FDR) <0.05, we found six genes significantly differentially expressed between cases and controls after controlling simultaneously for sex, age and population substructure via genotypic principal components (Fig. 1). In order of the lowest to highest adjusted P value, these genes were MRPS23 (mitochondrial ribosomal protein S23), DICER1 (DICER1, ribonuclease type III), CHST15 (carbohydrate N-acetylgalactosamine 4-sulfate 6-o sulfotransferase 15), ZXDC (ZXD family zinc finger C), PDCD5 (programmed cell death 5), and LOC649548 (a pseudogene) (Fig. 1a). DICER1 had lower expression in cases relative to controls (F-test, P=7.93E−06, genome-wide adjusted P=0.040, Fig. 1b).
Figure 1. Genome-wide differential gene expression profiles in PTSD&Dep cases vs controls.
(a) Volcano plot for genome-wide differential gene expression profiles between cases of PTSD&Dep and controls, adjusting for sex, age and population substructure. The red line represents P value at FDR of 0.05 (−log10p=4.729). The blue dots represent genes significantly differentially expressed between cases and controls. These genes are MRPS23, DICER1, CHST15, ZXDC, PDCD5 and LOC649548. Cases had reduced DICER1 expression compared with controls. (b) Box plot for DICER1 expression level in cases of PTSD&Dep and controls. The horizontal line represents the mean DICER1 level in the overall sample. These were derived from expression microarrays.
Our a priori plan was to follow-up on the genes with the most promising evidence of being involved in PTSD and depression using a curated literature search approach. We decided to examine DICER1 more closely for several reasons. First, DICER1 is a conserved ribonuclease enzyme that processes precursor RNAs into mature short-interfering RNAs and miRNAs9,12, which regulates the expression of thousands of genes post-transcriptionally and involve in synaptic development, maturation and plasticity13,14. Second, DICER1 expression was twofold reduced in the temporal cortex of patients with major depressive disorder relative to controls7. Third, regulation of expression of DICER1 and miRNAs in the nucleus accumbens was found to be important in mediating vulnerability or resilience to stress in a chronic social defeat mouse model8. Lastly, studies have shown that decreasing DICER1 expression in human peripheral cell lines stimulated the innate immune system via the upregulation of the major histocompatibility complex class I-related molecules15, and causal signatures for PTSD development was enriched for functions of the innate immune response in a prospective transcriptomic study16.
Next, since gene expression profiles can be affected by alcohol use, drug use, other psychiatric disorders or proportion of blood cell counts, we performed a secondary analysis in a subset of participants who had data on all of these variables. To that end, in a subset of 121 participants of the GTP discovery cohort, we performed genome-wide differential gene expression analysis between cases of PTSD&Dep and controls, adjusting simultaneously for sex, age, genotypic principal components, current alcohol and drug use disorders, bipolar disorder, current psychotic disorder and estimated cell counts. Consistent with our DICER1 result from the primary analysis, we found that at genome-wide FDR<0.05, DICER1 was significantly downregulated in cases compared with controls (F-test, P=0.0001, adjusted P=0.048), suggesting that the reduced expression of DICER1 in the cases was not likely due to effects of drug or alcohol use, other psychiatric disorders or variation in immune cell proportions.
Subsequently we performed a validation of the downregulation of DICER1 mRNA level in cases of PTSD&Dep compared with controls with quantitative PCR (qPCR) and found consistent results (1.3-fold downregulation in cases at t-test P=0.001). Since DICER1 expression was significantly diminished in PTSD&Dep after sex, age, population substructure, alcohol and drug use, other psychiatric disorders and estimated blood cell count were adjusted for, we focused on DICER1 expression, as reflected by the normalized intensity of the ILMN_1772692 probe, for follow-up studies. Hence, the genetic and imaging studies described in subsequent sections are targeted, hypothesis driven analyses to follow-up on our DICER1 expression finding.
DICER1 SNPs versus DICER1 expression in blood
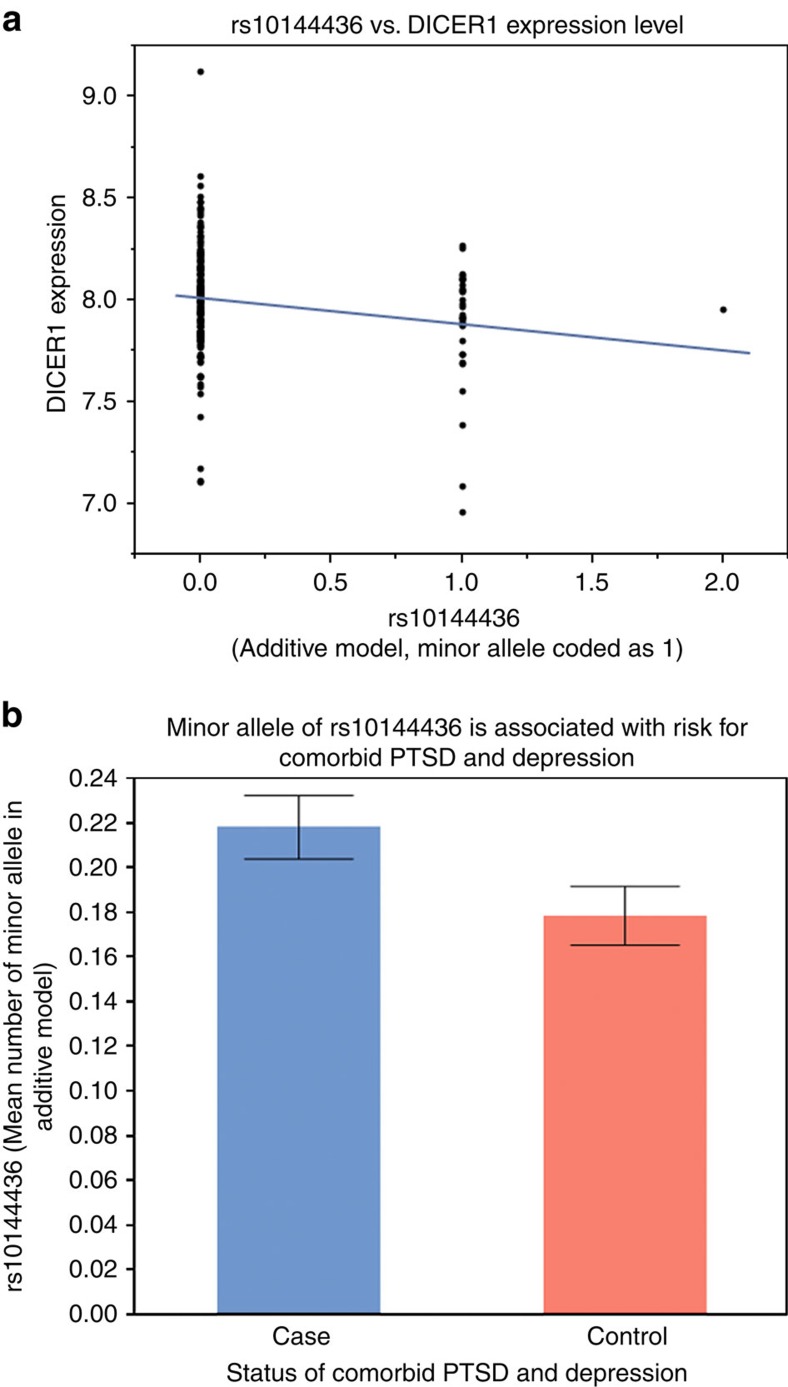
Given our finding of reduced DICER1 expression in cases of PTSD&Dep versus controls, we next asked which single-nucleotide polymorphisms (SNPs) of the DICER1 gene might influence its blood mRNA level. In our discovery GTP sample, the Illumina HumanOmni1-Quad BeadChip generated 14 DICER1 tagging SNPs after standard genotype quality control as described in Methods. Out of these 14 SNPs, two were located in the coding regions, four in the 3′UTR and eight in the intronic regions. We found three SNPs significantly associated with DICER1 expression level after controlling for sex and the principal components: rs10144436 (Wald test P=0.0034; n=191), rs1209904 (Wald test P=P=0.0018; n=191) and rs11160231 (Wald test P=0.0082; n=191). After we performed permutation to adjust for multiple testing17, only two SNPs remained significantly associated with DICER1 expression: rs10144436 (P=0.0034; adjusted P=0.0452) and rs1209904 (P=0.0018; adjusted P=0.0277). These two eQTLs were in linkage disequilibrium with each other at D'=0.98 and r2=0.05. Specifically, rs1209904 is an intronic SNP and its minor allele (T) was associated with decreased DICER1 expression. The other SNP, rs10144436, is located in the 3′UTR of DICER1 and its minor allele (A) was also associated with lower DICER1 mRNA level (Fig. 2a).
Figure 2. The SNP rs10144436, located in the 3′UTR of DICER1, is associated with DICER1 mRNA expression level and with PTSD&Dep.
(a) The minor allele of rs10144436 was associated with lower DICER1 expression level. (b) The minor allele of rs10144436 was associated with PTSD&Dep. Of note the minor allele was coded as 1 in the additive model. Bar graph shows mean±s.e.m.
DICER1 eQTLs versus PTSD&Dep phenotype
Having identified two DICER1 eQTL SNPs, rs10144436 (minor allele frequency (MAF)=0.098, Hardy–Weinberg equilibrium (HWE) P value=0.93, minor allele=A) and rs1209904 (MAF=0.337; HWE P value=0.03, minor allele=T), we next examined whether they were associated with PTSD&Dep in our 1876 all African American participants. Out of these two SNPs, only one, rs10144436, was significantly associated with PTSD&Dep after sex and population substructure via principal components were adjusted for (odds ratio=1.32, Wald test P=0.012, Bonferroni-adjusted P=0.024; n=1,874). Its minor allele (A) was associated with higher risk for PTSD&Dep. This is consistent with the expression finding, in which the minor allele of rs10144436 was associated with lower DICER1 expression level, which was also associated with PTSD&Dep (Fig. 2b).
Gene set enrichment analysis (GSEA) in PTSD&Dep
We next performed gene set enrichment analysis (GSEA)18 to gain insights into biological processes involving the differentially expressed genes in PTSD&Dep. Genes differentially expressed at P values<0.01 (P values ranged from 0.0098 to 7.92E−07) were included in the GSEA. We found many gene sets significantly enriched at FDR of 0.05 (Supplementary Table 1). Given the critical role of DICER1 in the formation of mature miRNAs, it is notable that 13 sets of genes that were targets of various miRNAs and one set involving brain miRNA biogenesis in a mouse model of schizophrenia were significantly enriched, suggesting that genes that are targets of different miRNAs were differentially expressed in PTSD&Dep (Supplementary Table 1). Additionally, three gene sets related to the immune system were enriched: (a) genes involved in cytokine signalling, (b) genes upregulated in polymorphonuclear leukocytes after Francisella tularensis vaccination in human and (c) genes involved in the innate immune system (Supplementary Table 1).
microRNA regulation in PTSD&Dep
Given the above GSEA results and our DICER1 expression and genotype findings, which were replicated in three independent cohorts as described in the subsequent sections, we aimed to obtain more evidence towards our hypothesis that the DICER1 and the miRNA pathway is involved in molecular mechanisms of PTSD&Dep by measuring blood miRNA levels in PTSD&Dep cases and controls. Using an RNA extraction protocol that preserves total RNA, we had 78 participants with available small RNAs and PTSD&Dep case or control status. We randomly selected 24 participants out of these 78 for a discovery sample, and the remaining 54 participants served as the replication sample. There was no difference in percentage of case status or percent of female between the 24 and 78 participants (Fisher Exact Test P=0.805 for percent of case status; P=0.800 for percent of female).
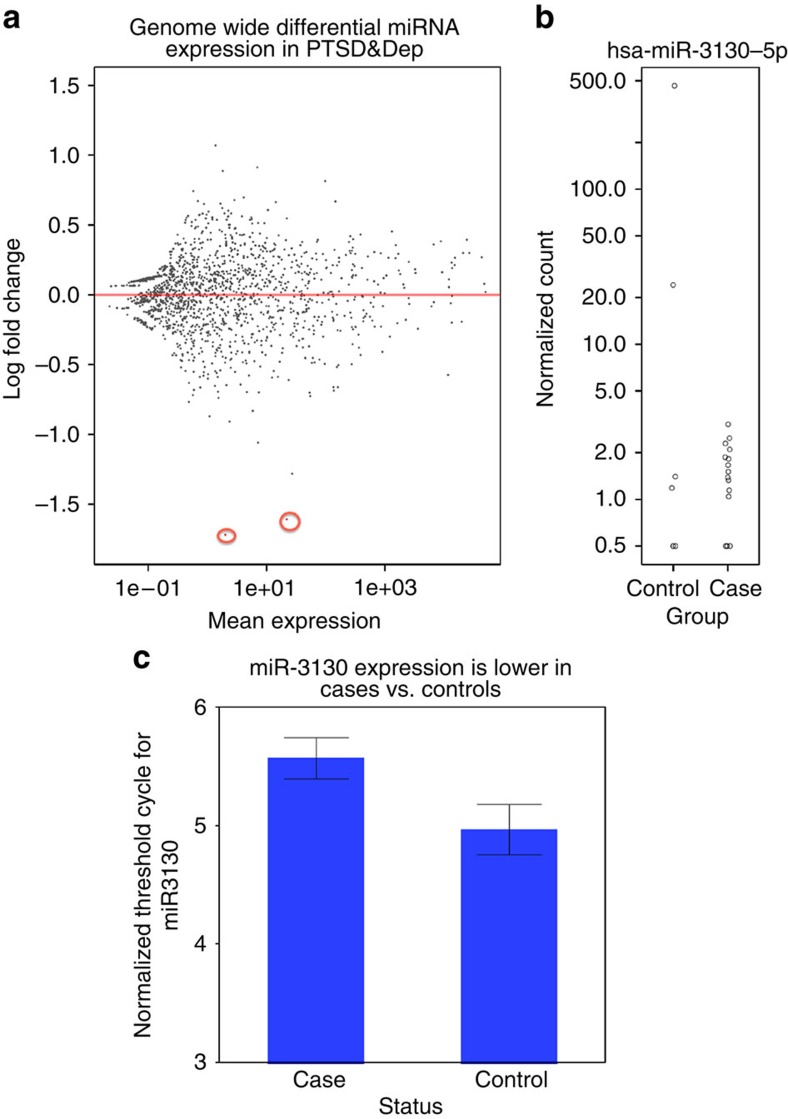
We next generated small RNA libraries for the discovery sample to perform small RNA sequencing. After standard quality control as described in Methods, we performed genome-wide differential miRNA expression analysis using DESeq2 (ref. 19), adjusting for sex, age and genotypic principal components. All 24 participants were African American, and there were 7 controls and 17 cases of PTSD&Dep. At genome-wide FDR<0.05, two miRNAs were significantly differentially expressed, and both were downregulated in the cases compared with the controls after sex, age and genotypic principal components were adjusted for: miR-212-3p (log2 fold change=−1.72; Wald test P=4.48E−05, adjusted P=0.048) and miR-3130-5p (log2 fold change=−1.61; Wald test P=4.97E−05, adjusted P=0.048; Fig. 3a,b).
Figure 3. miRNA levels in PTSD&Dep cases vs. controls in the discovery (n=24) and replication sample (n=54).
(a) MA plot for genome-wide differential expression of miRNAs in the discovery sample. The two points coloured in red are the two miRNAs with genome-wide adjusted P value<0.05 (miR-212-3p and miR-3130-5p). (b) miR-3130-5p expression was significantly lower in cases vs. controls in the discovery sample after sex, age and principal components were adjusted for. (c) miR-3130-5p expression was significantly downregulated in cases vs. controls in the replication sample after sex, age and genotypic principal components were adjusted for (P=0.030). Note, higher threshold cycle (CT) indicates lower abundance level. Error bar represents one s.e.m.
The replication sample had 54 participants, all of whom were African American and 20 were controls and 34 were cases. Using qPCR, we examined the two miRNAs having adjusted P<0.05 above in the replication sample. We found that miR-3130-5p was significantly downregulated, with 1.57-fold downregulation, in the PTSD&Dep cases relative to controls after sex, age and genotypic principal components were adjusted for (β=0.284; χ2-test P=0.030; Fig. 3c). There was no significant association between miR-212-3p and PTSD&Dep in the replication sample. Of great interest, these two miRNAs have been studied in other neuropsychiatric disorders as described further in Discussion.
DICER1 and miRNA regulation in PTSD&Dep
Using miRDB (miRDB.org), we identified 138 mRNAs that are potential targets of the miR-3130-5p at target score ⩾60 (list A, a.k.a Supplementary Table 2). From our original genome-wide differential gene expression analysis, 59 mRNAs were upregulated in the PTSD&Dep cases versus controls at FDR<0.1 (list B, a.k.a Supplementary Table 3). Since DICER1 expression was reduced in the cases, we infer that levels of mature miRNAs in the cases would be reduced, and as the result, levels of their corresponding mRNA targets would be increased in the cases. The intersection of the list A (potential mRNA targets of miR-3130-5p) and list B (59 upregulated mRNAs in the cases) yielded one overlap: MRPL35 (mitochondrial ribosomal protein L35). In this proof-of-concept study we found that in the cases of PTSD&Dep DICER1 expression level was reduced, and consistently at least one mature miRNA had decreased abundance level, and one of its corresponding mRNA target had increased expression level relative to controls. Since miRNA-mediated gene silencing of targeted mRNA occurs through a combination of translational repression, mRNA deadenylation, decapping and decay12, it is not surprising that only a limited number of mRNA targets changed their abundance at the mRNA transcript level. In sum, this novel data support the notion that DICER1 and the miRNA pathway is implicated in molecular mechanisms of PTSD&Dep.
Neural correlates of DICER1 gene expression
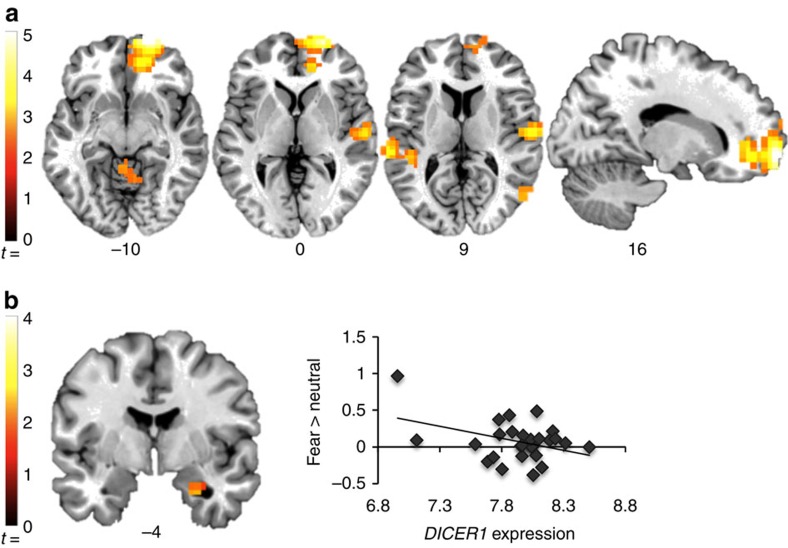
As our primary interest was to understand mechanisms underlying effects of DICER1 expression on symptoms of PTSD and depression, we investigated how DICER1 blood mRNA level might be related to neural responses to threat stimuli using fMRI. Specifically, we conducted a whole-brain analysis of linear correlation between DICER1 expression levels and the contrast of fearful>neutral face stimuli in a subset of 28 traumatized women who provided both blood mRNA and fMRI data (Fig. 4, Table 2). Whole-brain analysis showed an inverse correlation between DICER1 expression and activation in the right medial prefrontal cortex, bilateral temporal cortex, right posterior parietal cortex and cerebellum. No region correlated positively with DICER1 expression levels. Since functional neuroimaging evidence has shown involvement of the amygdala, particularly its increased activation, in PTSD20,21, we selected this region for further examination. Amygdala region of interest analyses indicated a cluster in the right amygdala that correlated negatively with DICER1 expression level (x, y, z=28, −4, −28; Z=2.19; k=11). No part of the amygdala showed a positive correlation with DICER1 expression. In sum, we found that lower DICER1 expression level was significantly associated with more amygdala activation to fearful stimuli, an intermediate phenotype for PTSD, which is consistent with our above finding of lower DICER1 expression being associated with PTSD&Dep.
Figure 4. Lower DICER1 expression in blood is significantly associated with more activation of the right amygdala in response to fearful faces, an intermediate phenotype for PTSD.
Regions in which the response to threat (fearful>neutral face stimuli) correlated negatively with expression, P<0.05, corrected. (a) Clusters showing a significant correlation with expression in whole-brain analysis. Correction for multiple comparisons used a combined height-extent threshold calculated using Alphasim Monte-Carlo simulation, with 1,000 iterations and a cluster-forming threshold of P<0.01. (b) A significant negative correlation with expression in the amygdala region of interest (ROI). Amygdala ROI analyses used a small-volume correction implemented in Alphasim, with 1,000 iterations and a cluster-forming threshold of P<0.05. Scatter plot shows the average contrast estimate for the fear>neutral contrast, from voxels in the activated cluster. Images are shown in neurological orientation, overlaid on a single-subject template in Montreal Neurological Institute (MNI) space.
Table 2. Whole-brain analysis of linear correlation between DICER1 expression level and the contrast of fearful>neutral face stimuli in 28 female participants.
|
MNI coordinates |
||||||
|---|---|---|---|---|---|---|
| Region | HEM | x | y | z | Z | k |
| Positive correlation with DICER1 expression | ||||||
| No significant clusters | ||||||
| Negative correlation with DICER1 expression | ||||||
| Sup. frontal G. | R | 20 | 68 | −8 | 4.27 | 211 |
| Orbitofrontal G. | R | 16 | 44 | −8 | 3.23 | (LM) |
| Sup. frontal G. | R | 12 | 52 | −24 | 2.56 | (LM) |
| Sup. temporal G. | R | 60 | −16 | 4 | 3.27 | 85 |
| Sup. temporal G. | R | 68 | −28 | 20 | 3.01 | (LM) |
| Supramarginal G. | R | 64 | −20 | 44 | 2.9 | (LM) |
| Mid. temporal G. | L | −64 | −32 | 8 | 3.17 | 53 |
| Sup. temporal G. | L | −44 | −36 | 8 | 2.77 | (LM) |
| Sup. temporal G. | L | −48 | −40 | 20 | 2.45 | (LM) |
| Mid. temporal G. | R | 56 | −68 | 12 | 2.91 | 22 |
| Angular G. | R | 48 | −68 | 44 | 3 | 27 |
| Angular G. | R | 48 | −72 | 36 | 2.97 | (LM) |
| Cerebellum | L | −4 | −44 | −8 | 2.59 | 24 |
| Cerebellum | L | 8 | −52 | −8 | 2.48 | (LM) |
MNI, Montreal Neurological Institute; HEM, Hemisphere; LM, Local Maximum.
Replication of reduced DICER1 expression in PTSD&Dep
We examined DICER1 expression level in the Detroit Neighborhood Health Study (DNHS) cohort of 111 participants, out of whom 15 were cases of current PTSD (and 60% of these cases had comorbid current depression) and 96 were controls with no current PTSD and no current depression. Mean age among cases and controls was 48.5±12 and 54.7±15.4, proportion of female was 60% and 52%, percentage of African Americans was 66.7% and 86.5%, of European Americans was 33.3% and 12.5%, and of other ethnic group was 0 and 1.0%, respectively. Blood DICER1 expression level, as reflected by normalized intensity levels of the ILMN_1772692 probe, was significantly reduced in cases relative to controls (β=−0.138, s.e.m.=0.062, F-test P=0.029) after sex, age and self-reported race were controlled for in a linear regression model (Fig. 5a). In a subset of 94 participants in whom we had principal components derived from genome-wide genotypes, DICER1 expression continued to be significantly reduced in cases compared with controls after sex, age and principal components were adjusted for (β=−0.180, s.e.m.=0.072, F-test P=0.014). This DICER1 expression finding is consistent with, and thus replicated, our finding of reduced DICER1 blood expression level in cases of PTSD&Dep.
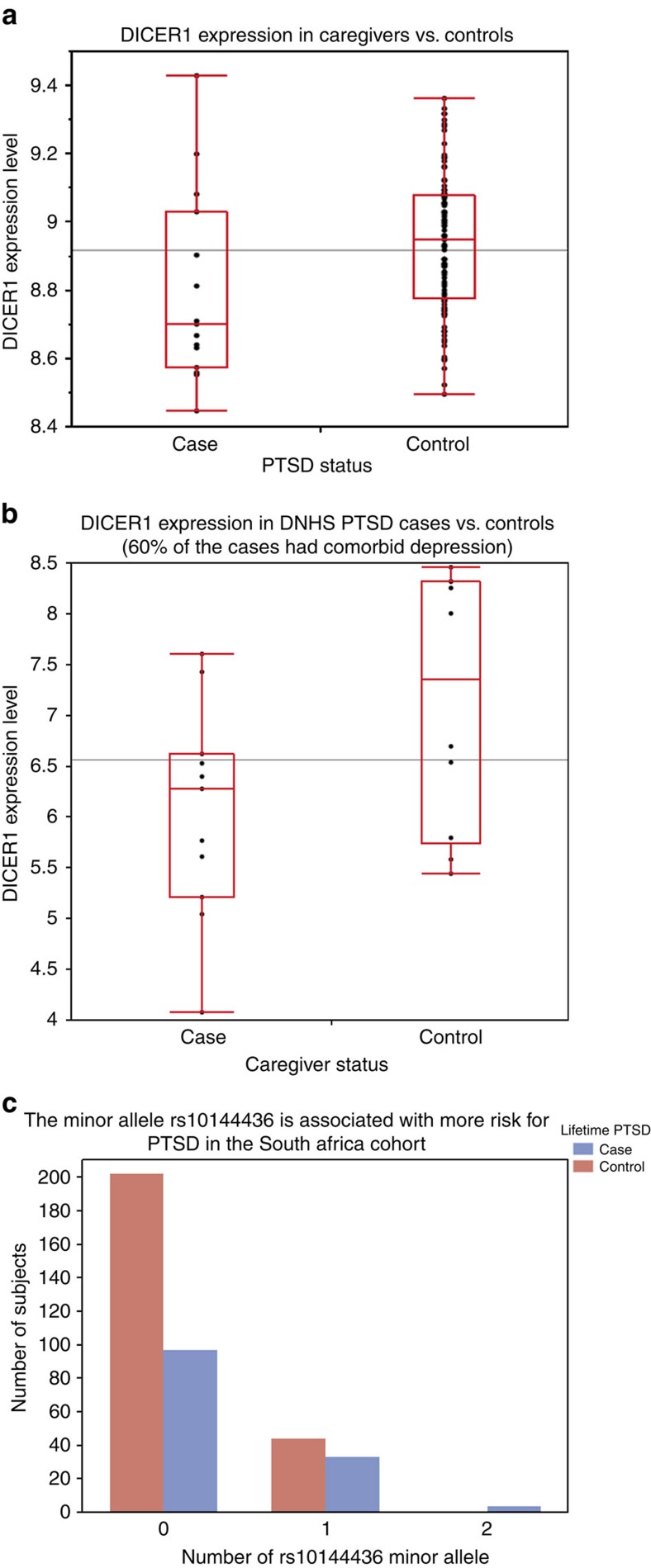
Figure 5. Replication of reduced DICER1 expression level in.
(a) The Detroit Neighborhood Health Study (DNHS) and (b) The Caregivers of family member with aggressive brain cancer (data from the publicly available data set GSE7893). The black horizontal lines represent the means of the overall samples. (c) Replication of the association between the DICER1 eQTL rs10144436 and PTSD&Dep in the Drakenstein, South Africa cohort (minor allele was coded as 1 in the additive model).
Replication of reduced DICER1 expression in another sample
We searched the publicly available datasets deposited in the Gene Expression Omnibus (GEO, in January of 2015) and found a relevant study on blood gene expression in a stressed cohort by Miller et al.22 (GSE7893). The authors investigated effects of severe psychological stress on transcriptional control pathways by surveying genome-wide differential gene expression profiles of caregivers of family members with aggressive brain tumours and matched controls22. The caregivers had significantly higher psychological distress, as reflected by higher perceived stress score (t-test P=0.003), decreased satisfaction with life (t-test P=0.04), and fewer positive emotions (t-test P<0.004) compared with controls22. Controls were matched for age, gender, ethnicity, marital status, cigarette and alcohol use, exercise, sleep habits, body mass index and history of cardiovascular diseases, as well as being free of major stressors such as divorce, bereavement, unemployment and family illness during the prior year22. Study subjects were recruited from the British Columbia in Vancouver. We downloaded the data and performed a secondary analysis on DICER1 expression in this sample and found that DICER1 expression was reduced in stressed cases versus controls (β=−0.54, t-test P=0.04, Fig. 5b). Further reading of the study publication confirmed our finding, as DICER1 was listed in Supplementary Table S1 in the supplemental data as underexpressed in stressed cases versus controls at an expression ratio of 0.47 and P<0.05. Assessment of PTSD was not done in this study; however, we think the severe, chronic and traumatizing stress caregivers experienced is arguably consistent with stress-related PTSD and depression. This finding is consistent with our finding of decreased DICER1 expression in cases of PTSD&Dep.
Replication of association between DICER1 eQTL and PTSD&Dep
Here, we examined the association between the DICER1 eQTL rs10144436 and PTSD&Dep in the Drakenstein, South African cohort. The Drakenstein cohort consists of pregnant women recruited from the peri-urban community of the Drakenstein subdistrict in South Africa. There is a high prevalence of trauma exposure in these at-risk women who were assessed longitudinally for trauma-related psychiatric disorders23. Post-traumatic stress disorder was assessed with the modified PTSD symptom scale (PSS)24 and the MINI10. Among the 380 participants with available genome-wide genotype markers from the PsychArray chip, there were 134 cases with lifetime PTSD and 246 controls with no lifetime PTSD. Among the 134 PTSD cases, ∼64% also had significant depression in their lifetime, as assessed by the MINI10 or having a score of ⩾14 on the Beck Depression Inventory (BDI)25,26. There were 60.4% Black and 39.6% mixed race among the cases and 56.1% Black and 43.9% mixed race among the controls. In this cohort, the DICER1 rs10144436 had an MAF of 0.101, HWE P value of 0.82, and A as the minor allele. We found that rs10144436 was significantly associated with lifetime PTSD after population substructure was controlled for via principal components from genome-wide genotype markers (odds ratio=1.877, Wald test P=0.008, n=380). Consistent with our finding, the minor allele of rs10144436 was associated with increased risk for PTSD (Fig. 5c). Gene expression data are not available for this cohort.
Discussion
Post-traumatic stress disorder with comorbid depression is highly prevalent among susceptible individuals following traumatic life events6. Here, we surveyed genome-wide differential gene expression profiles in blood in cases of PTSD&Dep and controls with no PTSD and no depression. We found that DICER1 mRNA level was significantly reduced in the cases relative to the controls after we adjusted simultaneously for sex, age, population substructure, current alcohol and drug use disorders, current psychotic disorder, bipolar disorder and estimated blood cell counts. Importantly, downregulation of DICER1 expression in PTSD&Dep was replicated in two independent cohorts, the DNHS27 and the Vancouver caregivers for family members with aggressive malignant brain cancer22. Consistently, our follow-up fMRI study found that lower DICER1 blood mRNA level was significantly associated with increased amygdala activation to fearful stimuli, a neural correlate for PTSD. Furthermore, we found that a DICER1 eQTL, rs10144436, located in its 3′UTR, was significantly associated with PTSD&Dep, and this association was replicated in a South African cohort of lifetime PTSD cases and controls23. Taken together, we infer that DICER1 plays a role in the mechanism or manifestation of PTSD&Dep.
DICER1 is a highly conserved RNAse III enzyme that is crucial for the biogenesis of noncoding, regulatory small RNAs, including miRNAs and short-interfering RNAs, in different tissues including the brain8,9,12. Specifically, DICER1 converts precursor miRNAs to mature miRNAs, which not only fine-tune expression of thousands of genes via post-transcriptional repression in various tissues, including the nervous system but also involve in synaptic development, maturation and plasticity13,14. A growing number of studies have suggested the involvement of miRNAs in major depressive disorder, stress-related disorders and other psychiatric disorders28,29,30. Consistently, in our proof-of-concept study, we found that at least one miRNA, miR-3130-5p, had significantly reduced abundance level in the PTSD&Dep cases in both the discovery and replication samples, and one of its mRNA targets was upregulated in the PTSD&Dep cases versus controls after we controlled for sex, age and population substructure. Taken together, our findings support the notion that the DICER1 and the miRNA pathway plays a role in the pathogenesis or manifestation of PTSD&Dep, potentially by regulating expression of the corresponding downstream mRNA targets and influencing cellular responses.
Of note, miR-3130 has been reported in other neuropsychiatric disorders. Specifically, a genome-wide association study of memory and hippocampal structure of 14,781 older adults found a genome-wide significant SNP that is located in the intronic region of the FASTKD2 gene that is overlapped by miR-3130 (ref. 31). Although not replicated in our replication sample, miR-212 has been studied in Alzheimer's disease patients32. That study found that decreasing or increasing miR-212 expression in the temporal cortex and hippocampal neurons could induce apoptosis or neuroprotective effects respectively against oxidative stress32.
Stress-related DICER1 and miRNA regulation in blood and brain may occur in parallel in human subjects, as our stress-related DICER1 and miRNA findings in blood are consistent with stress-related DICER1 and miRNA findings in the nucleus accumbens, a brain region involved in motivation, pleasure, reward, reinforcement learning and addiction, in a chronic social defeat stress mouse model8. In this study, Dias et al.8 found that β-catenin mediated vulnerability or resilience to stress through DICER1 and miRNA regulation in the nucleus accumbens. Specifically, the authors found that DICER1 was a robust target of β-catenin, that mice with DICER1 knockdown (thus reduced or absent DICER1 mRNA level) was easily susceptible to stress, and that in resilient mice, β-catenin turned on DICER1 transcription8. Our finding is also consistent with another mouse study in which depletion of DICER1 in the central amygdala led to an increase in anxiety-like behaviour, and that after acute restraint stress, expression profiles of miRNAs in the amygdala were changed33. Our findings are also in line with two human studies, in which DICER1 expression was twofold reduced in the temporal cortex of patients with major depressive disorder relative to controls7, and global miRNA expression was reduced in the prefrontal cortex of depressed suicide completers relative to controls34.
Interestingly, DICER1 mRNA level was found to be upregulated in lymphoblastoid cell lines of schizophrenia patients35 and also upregulated in the dorsolateral prefrontal cortex of schizophrenia patients36, suggesting the possibility of a positive correlation of DICER1 expression between blood and brain; however, this remains to be investigated.
Given these findings, studies are needed to elucidate mechanisms linking the regulation of DICER1 and miRNA in blood to that in the brain as well as to complex neurobehavioral phenotypes of PTSD and depression in humans. We hypothesize that this occurs via multiple pathways, and one potential pathway is the brain-immune interactions given the connection between DICER1 expression and the innate immune system15,37,38. Specifically, decreased DICER1 expression in human peripheral tissues (that is, embryonic kidney, dermal foreskin fibroblast or lung-tissue fibroblast) induces a DNA damage response, which stimulates the innate immune response by upregulation of the major histocompatibility complex class I-related molecules A and B15,37,38. The activated innate immune system creates a pro-inflammatory state, recruiting and activating neutrophils, and releasing cytokines from activated macrophages, among other processes39. A chronic inflammatory state, including elevated levels of pro-inflammatory cytokines or activated monocytes, has been shown to contribute to anxiety and depressive disorders by affecting metabolism of neurotransmitters in the brain or via monocyte trafficking to the brain in both mice and human studies40,41,42. Another potential mechanism is the regulation of the tightness or leakiness of the blood–brain barrier (BBB) by central or peripheral miRNAs43,44. A recent study of the mechanism of migration of breast cancer cells through the BBB in brain metastasis suggests that breast cancer cells with high metastatic capacity release extracellular vesicles containing proteins and miRNAs that can trigger the breakdown of the BBB44. Particularly, the study authors found that miR-181c in these peripheral tissue promotes the destruction of the BBB by regulating the actin dynamics44.
Prospective studies observed an increased risk of early-age cardiovascular disease, such as early-onset heart disease, stroke and myocardial ischaemia, in PTSD patients45,46. The involvement of DICER1 and the miRNA pathway in PTSD is consistent with the overall psychosomatic picture of PTSD reported by these epidemiological studies, since both DICER1 and miRNAs have been implicated in cardiovascular diseases. For instance, dysregulation of DICER1-dependent miRNA expression has been suggested to be a potential mechanism of impaired angiogenesis47 and impaired vascular remodelling48. Likewise, miRNAs have been shown to control important processes that contribute to cardiac hypertrophy, myocardial infarction, neovascularization, cardiac fibrosis, arrhythmia, angiogenesis and cholesterol regulation49,50.
Several studies have linked PTSD to increased activation of the amygdala to negative or fearful stimuli20,21. Here we found that decreased DICER1 expression in blood was associated with greater amygdala reactivity to threat stimuli and to PTSD and depression symptom severity. Additionally, we found that diminished DICER1 expression was associated with increased activation of the posterior parietal cortex, which engages in a host of cognitive functions including selective attention51. This is consistent with findings from another study, where war and torture survivors suffering from PTSD had hyperactivation of the superior parietal cortex selectively in response to aversive stimuli52. We also found an inverse correlation between DICER1 expression and activation of the medial prefrontal cortex, which is not consistent with what is typically observed in PTSD20. However, this finding is consistent with hyperactivity of the medial prefrontal cortex during the self-referential processing of negative words in major depressed patients relative to controls53. Our fMRI sample included women having been exposed to traumatic experiences and developed high levels of PTSD and depression, and thus our neuroimaging DICER1 findings are specific to this sample of participants; influence of DICER1 expression on brain activation might be different in healthy participants. In future research, it would be interesting to investigate potential effects of DICER1 expression on threat-processing circuitry using structural connectivity measures such as diffusion tensor imaging.
Our study has some limitations that should be taken into consideration when interpreting the results. Specifically, we did not have information on medications and tobacco use to control for in the gene expression analyses as they may influence gene expression profiles. We also did not assess for generalized anxiety disorder, social anxiety disorder or panic disorder to adjust for in the differential analysis. Additionally, the replication samples for DICER1 expression level, the DNHS and caregiver samples, have relatively small sample sizes and thus higher potential for technical variation. Further, our genome-wide expression profiling of miRNAs was done in a small sample and thus likely lack the power to discover more miRNAs differentially regulated between PTSD&Dep cases and controls. A larger sample size is needed to discover more miRNAs differentially expressed in stress-related disorders such as PTSD or PTSD&Dep. Lastly, the observed differential expression of DICER1 in PTSD&Dep could be reflective of an underlying genetic or epigenetic mechanism or a combination of both. Here, we presented evidence for an underlying genetic mechanism by showing a significant association between a polymorphism on the 3′UTR of DICER1, rs10144436, and PTSD&Dep. Even though this association remained significant after multiple testing corrections and was replicated in an independent South African cohort, it is worth mentioning that our finding still remains at the level of candidate gene analysis and not genome-wide association. Stronger evidence at the genome-wide association level is needed to confirm that a genetic mechanism underlies the association between DICER1 expression and PTSD&Dep. It would be interesting for future studies to investigate the hypothesis that epigenetic mechanisms underlie the association between differential expression of DICER1 and PTSD&Dep.
In summary, this is the first human study to our knowledge to suggest that DICER1 and the miRNA regulation pathway are implicated in biological mechanisms of PTSD with comorbid depression. Studies are needed to elucidate the relationship between blood and brain DICER1 and miRNA regulation as well as mechanisms underlying the connection between blood DICER1 and miRNA regulation and stress-related psychiatric disorders to contribute to their prevention and treatment efforts.
Methods
Grady trauma project study participants
This study was part of a larger GTP investigating genetic and environmental factors contributing to PTSD in a population of inner city, low income and high stress and trauma exposed individuals. Inclusion criteria consisted of being 18 or older, understanding English and being able to give informed consent. Exclusion criteria included being acutely suicidal, psychotic or having acute medical problems. Participants gave informed consent, completed a battery of psychological measures and provided blood for genetic materials. This study has been approved by the Institutional Review Board of Emory University School of Medicine and Grady Memorial Hospital. A total of 184 men and women were included in this study based on availability of gene expression profiles and principal components derived from genome-wide genotype markers, and meeting the criteria for PTSD with comorbid depression (PTSD&Dep) as described in detail below. A subset of these participants was reported in a previous study focusing on childhood maltreatment and PTSD54.
PTSD with comorbid depression (PTSD&Dep) phenotype
PTSD was assessed with the modified PSS, a psychometrically validated, 18-item self-report scale with excellent internal consistency, high test-retest reliability and concurrent validity, as well as mirroring the DSM-IV criteria for PTSD24. A PSS cutoff score of 14 yields a sensitivity of 91% and specificity of 62% for identifying PTSD when compared with the diagnosis of PTSD made by the clinician-administered PTSD scale in 229 motor vehicle accident survivors55. Hence, we operationally defined PSS scores ⩾14 to indicate clinically significant current PTSD symptoms.
Depressive symptoms were measured with the psychometrically validated 21-item BDI25. The BDI has an excellent internal consistency (reliability coefficient of 0.93) and is commonly used in depression research studies25. Among African American primary care patients, a BDI cutoff score of 14 yielded a sensitivity of 88% and specificity of 84% when compared with a diagnosis of MDD made by PRIME-MD Mood Module, a focused interview for MDD using DSM-IV criteria26. Given these data and that 94% of our study participants are African Americans, we used a BDI score ⩾14 to indicate clinically significant current depression.
Cases of current PTSD&Dep were defined as having both a PSS⩾14 and BDI⩾14. Controls were defined as not having either significant PTSD or depression, as reflected by a PSS ≤7 and BDI ≤7. There were 112 cases of current PTSD&Dep and 72 controls in this study.
Other psychiatric disorders
Current alcohol or drug use disorder, bipolar disorder and current psychotic disorder were assessed using the SCID11 or MINI10, and was available in a subset of 145–150 participants.
Gene expression profiles in the GTP cohort
RNA was extracted from whole blood collected in Tempus tubes at about 0830, h for all participants. All samples had Bioanalyzer RNA Integrity Number (RIN) ⩾6. Complementary DNA was derived from RNA and then hybridized, and raw probe intensities were generated on Illumina HumanHT-12 v3 or v4 BeadChip arrays. Probes with detection P value>0.01 in at least 95% individuals were filtered out, leaving 15,877 probes from the v3 chip and 17,048 probes from the v4 chip. Combining probes from v3 and v4 chips yielded 13,048 probes in common to be included in the analysis. We then performed log2 transformation and normalization of probe intensities using the Supervised Normalization of Microarray algorithm, removing the effects of batch and RIN56. The gene expression data set has been deposited at the Gene Expression Omnibus with the accession number GSE67663.
Genotypes and principal components in the GTP cohort
DNA was extracted from saliva or blood, and genome-wide SNP genotyping was conducted using Illumina's HumanOmni1-Quad or OmniExpress BeadChips. Standard quality control of the genome-wide data was performed using PLINK57, removing individuals with greater than 2% missing data and removing one in each pair of related individuals with an identity by descent proportion>0.12 (indicating cousins or a closer relation). We removed SNPs with call rates ≤ 98%, MAF<0.01, and HWE P value<1 × 10−6 in PTSD controls. After restricting the SNPs to the autosomes, we used PLINK to prune the data in windows of 50 base pairs, removing one SNP from each pair of SNPs with r2>0.05 to obtain a set of roughly independent SNPs. This reduced set of SNPs was used in principal component analysis to infer axes of ancestry and remove outlier subjects58. The gene expression analysis utilized the principal components generated from combined genome-wide data from the two chips (HumanOmni1-Quad and OmniExpress) on the subset of subjects with expression data. The candidate gene analysis was conducted using DICER1 SNPs genotyped on the HumanOmni1-Quad Beadchip only, with similar quality control as described above (African American only, n=1,874 meeting definition of PTSD&Dep cases and controls).
Genome-wide differential gene expression analysis in GTP cohort
Genome-wide differential gene expression between cases of current PTSD&Dep and controls was surveyed using analysis of covariance, in which normalized gene expression level for each probe was the outcome, PTSD&Dep the dichotomous independent variable, sex, age and genotypic principal components as the covariates. Age was categorized into three groups, below 33rd percentile, from 33rd to 67th percentile and above 67th percentile. The first five principal components, described above, were used as covariates to control for population substructure. Multiple testing was accounted for with the Benjamini and Hochberg FDR of 0.05 (ref. 59). Analyses were performed using JMP Genomics version 7.
GSEA
To gain insight into biological processes involving the differentially expressed genes between cases and controls, we performed GSEA using the pre-ranked GSEA analytical software from the Broad Institute18. Genes that were differentially expressed between cases and controls at genome-wide FDR<0.20 (that is, genes with nominal P values ranging between 5.97E−07 and 0.0098, n=658 genes) were pre-ranked using their t-statistics derived from the analysis of covariance for differential expression between cases and controls as described above. Gene sets were obtained from the Molecular Signature Database (MSigD) v4.0 (ref. 18). We selected the gene sets from the curated, gene ontology, positional, motif, computational, oncogenic and immunologic signatures collections, particularly those with at least 15 genes in common with our pre-ranked gene list, and the weighted p option (P=1) for the running sum statistics for enrichment score. Pre-ranked GSEA assessed the significance for each gene set using 5000 permutations of case and the control status for each set, and multiple hypothesis testing was adjusted with Benjamini and Hochberg FDR18,59.
Cell count estimate
Cell type proportions were estimated from genome-wide DNA methylation data from blood samples drawn at the same visit as that used for the expression arrays. The proportions of granulocytes and lymphocytes in each sample were estimated using publically available data (GSE36069) and the method described by Houseman et al.60,61.
qPCR validation of DICER1 differential expression in PTSD&Dep
In a subset of 40 subjects (20 cases of PTSD&Dep and 20 controls), whose RNA was run on the Illumina HumanHT12 Beadchip arrays, total RNA from whole blood was reverse transcribed into cDNA using the RT2 First Strand Kit (Qiagen). DICER1 and GAPDH as housekeeping control were detected and quantified in duplicates using the TaqMan assays Hs00229023_m1 (DICER1) and Hs02758991_g1 (GAPDH) with TaqMan Gene Expression Master Mix on a ViiA7 real-time PCR system in 384-well format according to the instruction of the manufacturer (Applied Biosciences). Gene expression differences and fold changes were calculated using the 2−ΔΔCt method62.
Candidate gene and eQTL analyses in the GTP cohort
Associations between DICER1 SNPs and DICER1 expression levels were performed in PLINK using the linear regression model, in which normalized DICER1 expression level was the outcome, SNP genotype the independent variable and sex and the first five principal components as covariates. We used the additive model for SNP genotype, with the minor allele coded as 1. Multiple testing was addressed with permutation17. Analyses for association between DICER1 tagging SNPs and PTSD&Dep were performed in PLINK using logistic model, in which status of PTSD&Dep was the outcome, SNP genotype the independent variable and sex and the first five principal components as covariates. Likewise, we used the additive model for the SNP genotype, with the minor allele coded as 1, and addressing multiple testing with permutation17.
Small RNA sequencing and analysis in the GTP discovery sample
Total RNA was extracted from whole blood, which was collected in Tempus tubes at 0830, h for all participants, following the Norgen protocol (Norgen Biotek Inc.). Small RNA was size fractionated from total RNA using polyacrylamide gel and then used as the starting material to generate small RNA libraries following the Illumina TruSeq Small RNA protocol. The 24 small RNA libraries were then multiplexed and sequenced single-end reads on two lanes of the Illumina HiSeq1000.
Quality of the sequencing data was inspected with FastQC (bioinformatics.babraham.ac.uk/projects/fastqc/). Adapters were trimmed with cutadapt (https://cutadapt.readthedocs.org/en/stable/), and after trimming, sequences shorter than 14 nucleotides were discarded. Read length distribution after adapter trimming was visualized and observed to be centred at 22 nucleotides, indicating high-quality miRNA-seq library preparation63. Trimmed reads were aligned to the miRBase v.21 (ref. 64) with SHRiMP aligner ((http://compbio.cs.toronto.edu/shrimp/), and only reads with a minimum average PHRED score of 10 were considered for alignment. Percent reads mapped to mature miRNAs ranged from 85 to 95%, indicating high-quality libraries and sequencing data. The pipeline above was organized by the miRNA analysis application65. After removing an outlier sample with an extremely low raw count and four miRNAs with extremely high counts, genome-wide differential miRNA analysis was performed using DESeq2 (ref. 19), adjusting for sex, age and five genotypic principal components. Age was categorized into three groups, upper third, middle third and lower third. Multiple testing was addressed with Benjamini and Hochberg FDR19,59.
miRNA qPCR assays in the GTP replication sample (n=54)
Total RNA extracted from whole blood was reverse transcribed into cDNA using Qiagen miScript II RT kits and protocol. Hsa-miR-212-3p, hsa-miR-3130-5p and snRNA RNU6B as housekeeping control were detected and quantified in triplicates using Qiagen miScript Primer Assays Hs_miR-212_1, Hs_miR-3130-5p_1 and Hs_RNU6-2_11 with miScript SYBR Green PCR kit on a ViiA7 real-time PCR system in 384-well format according to the instruction of the manufacturer (Qiagen). Normalized threshold cycle (CT) values and fold change for miR-212-3p and miR-3130-5p were calculated using the 2−ΔΔCt method62. Differential expression analysis for each of the two miRNAs was performed using a generalized linear model in JMP Genomics, in which the normalized CT value was the outcome, PTSD&Dep status the independent variable, and sex, age, and five genotypic principal components as the covariates.
Functional MRI study in the GTP cohort
We examined neural correlates of DICER1 expression in a functional MRI study among a subset of traumatized women (n=28) for whom we had both mRNA and fMRI data, drawn from the larger GTP sample. Participants completed a task designed to engage threat-processing networks via passively viewing static fearful and neutral face stimuli. Blocks of either eight fearful or eight neutral face stimuli selected from the stimulus set of Ekman and Friesen66 were presented in a pseudo-random order, with each stimulus presented for 500 ms. These fearful and neutral stimuli were sorted in random order for each block. Participants were instructed to pay attention to the faces but not make any behavioural responses to minimize motion artefacts, neural activation unrelated to the processing of the visual stimulus, or dampening of emotional arousal67. Brain imaging data were acquired on a Siemens 3.0 Tesla Magnetom Trio TIM whole-body MR Scanner using a 12-channel head coil. Pre-processing and statistical analyses were performed using Statistical Parametric Mapping software (SPM5, Wellcome Trust Centre for Neuroimaging) and AFNI (http://afni.nimh.nih.gov/afni/). Statistical contrasts between conditions (for example, fearful versus neutral) were assessed with linear contrasts. First-level models included fearful and neutral task conditions and six motion parameters. Second-level models were conducted as a correlation between DICER1 expression levels (continuous variable) and the contrast of fearful>neutral. Correction for multiple comparisons was through a combined height-extent threshold calculated using Alphasim Monte-Carlo simulation with 1,000 iterations and a cluster-forming threshold of P<0.01 (ref. 68).
Detroit neighborhood health study (DNHS)
In the DNHS cohort structured telephone interviews were conducted to assess PTSD (with the PTSD Checklist Civilian Version (PCL-C)) and depression (with the Patient Health Questionnaire (PHQ-9))69. Post-traumatic stress disorder and depression diagnoses obtained from the telephone interview responses have been validated in a random subsample of participants via in-person clinical interview using the clinician-administered PTSD scale and Structured Clinical Interview for DSM-IV Disorders, and the comparison showed high internal consistency and concordance69.
Trained phlebotomists conducted venipuncture blood draws during scheduled home visits to consenting participants. Following the blood draw, all blood samples were sent to Wayne State University in Detroit, Michigan for processing. RNA was isolated from leukocytes using Leukolocks kits (Ambion, Austin, TX) following the manufacturer's alternative protocol to preserve total RNA. All included samples had RIN ⩾5, 28 s/18 s⩾1.0 and 260/280 ⩾1.7. Illumina HT-12 V4 Expression BeadChip was used to generate raw expression data, which was processed using the Bioconductor (http://bioconductor.org) Limma and ComBat packages to background correct, normalize and adjust for batch effects. The data was filtered to remove any low or unexpressed probes; after filtration, 28,352 probes remained.
In a subset of 94 participants (out of 111) in this cohort we had principal components derived from genome-wide genotypes. Participants' DNA was isolated from whole blood or saliva, and genome-wide genotypes were obtained using the Illumina HumanOmniExpress BeadChips. A total of 688,890 SNPs passed quality control filters, including call rate>95%, MAF>0.01, and HWE P>1 × 10−6. The Multidimensional scaling analysis of genome-wide identity-by-state data implemented in PLINK was used to determine ancestry in the whole sample. The first two components from the multidimensional scaling analysis distinguished African American from European American participants and others, and the second component distinguished Hispanic from non-Hispanic participants70. We therefore used the first two principal components to adjust for population substructure in the DICER1 expression replication analysis.
Drakenstein, South Africa cohort genotyping
Genome-wide genotype markers for this cohort were obtained using the Illumina Infinium PsychArray Beadchip. Quality control of the genotypes was performed to exclude individuals with>5% missing data, SNPs with call rates<95%, SNPs with MAF<0.05, and SNPs with HWE P value<1 × 10−6 in controls and<1 × 10−10 in PTSD cases, as well as removing duplicates and one in each pair of cryptically related individuals who had an identity by descent proportion>0.12 (indicating cousins or a closer relation). Principal components were created using 40,000 random and independent genome-wide SNPs. As only the first principal component had the Tracy–Widom test P value<0.05, it was used as a covariate in the association analysis to adjust for population substructure. DICER1 SNP rs10144436 was extracted from the above genotypes to perform association analysis with lifetime PTSD in PLINK using logistic model, co-varying for the first principal component. All participants were women (n=380).
Additional information
Accession codes: The gene expression data and small RNA sequencing data have been deposited at the Gene Expression Omnibus with the accession numbers GSE67663 and GSE74162 respectively.
How to cite this article: Wingo, A. P. et al. DICER1 and microRNA regulation in post-traumatic stress disorder with comorbid depression. Nat. Commun. 6:10106 doi: 10.1038/ncomms10106 (2015).
Supplementary Material
Supplementary Tables 1-3.
Acknowledgments
We appreciate the technical support of all of the staff and volunteers of the Grady Trauma Project, particularly Kimberly Kerley, BA, Jennifer Leveille, BA, Kristina Mercer, MS, Jordan Laird, BS, Allen W. Graham, BA, Angelo Brown and Claudia Klengel, MD. We thank Charles Gillespie, MD/PhD, Ann Schwartz, MD and Tamara Weiss, MD for medical support, Divya Mehta, PhD for contributing to generation of gene expression data, Abigail Lott, PhD for maintaining the database. Additionally, we thank Nirav Patel, MS, and Gregory Tharp, MS at the Emory Yerkes Genomics Core for technical support with the small RNA sequencing and data analysis. We thank the participants of the Grady Trauma Project for their time and effort. This study was supported in part by the Department of Veterans Affairs Career Development Award IK2CX000601 and the NARSAD Young Investigator Award (to A.P.W.). This work was primarily supported by the National Institutes of Mental Health (MH096764 and MH071537 to K.J.R.). Support was also received from Emory and Grady Memorial Hospital General Clinical Research Center, National Institutes of Health (NIH). The Drakenstein study was funded by the Bill and Melinda Gates Foundation (1017641), the NIH (R21 MH098662) and the Medical Research Council (MRC) of South Africa. The DNHS was funded by DA022720 and RC1MH088283 (to A.E.A.). T.K. is supported by a NARSAD YI Grant and an EMBO Long-Term Fellowship. The contents do not represent the views of the Department of Veterans Affairs or the United States Government.
Footnotes
Author contributions A.P.W. formulated the research question, performed the data analysis and wrote the initial version of the paper. K.J.R. obtained funding, oversees the GTP study, contacted collaborators and revised the paper. All authors edited and commented on the manuscript. G.G. provided consultant on the data analysis. B.B. contributed to conducting the GTP study. E.B.B. contributed to generating gene expression data. L.M.A. contributed to the genetic data analysis. A.L. performed the genetic replication analysis in the Drakenstein cohort. A.C.B. and M.U. performed the gene expression replication analysis. J.J.S. performed and wrote the fMRI data analysis. A.K.S. contributed to performing cell count estimate. T.K. performed the validation assays for DICER1 and miRNA expression and contributed to the design of the miRNA study. D.J.S. and N.K. obtained funding, conducted the study, and obtained genetic data for the South African cohort. A.E.A., K.C.K., D.E.W., M.U. and S.G. contributed to obtain funding and carrying out the DNHS. P.J. and Y.L. contributed to designing and conducting the miRNA study.
References
- Kessler R. C. Posttraumatic stress disorder: the burden to the individual and to society. J. Clin. Psychiatry 61, 4–12 (2000). [PubMed] [Google Scholar]
- Hidalgo R. B. & Davidson J. R. Posttraumatic stress disorder: epidemiology and health-related considerations. J. Clin. Psychiatry 61, 5–13 (2000). [PubMed] [Google Scholar]
- Sullivan P. F., Fan C. & Perou C. M. Evaluating the comparability of gene expression in blood and brain. Am. J. Med. Genet. B Neuropsychiatr. Genet. 141b, 261–268 (2006). [DOI] [PubMed] [Google Scholar]
- Rollins B., Martin M. V., Morgan L. & Vawter M. P. Analysis of whole genome biomarker expression in blood and brain. Am. J. Med. Genet. B. Neuropsychiatr. Genet. 153b, 919–936 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Heerden J. H. et al. Parallel changes in gene expression in peripheral blood mononuclear cells and the brain after maternal separation in the mouse. BMC Res. Notes 2, 195 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caramanica K., Brackbill R. M., Liao T. & Stellman S. D. Comorbidity of 9/11-related PTSD and depression in the World Trade Center Health Registry 10-11 years postdisaster. J. Trauma Stress 27, 680–688 (2014). [DOI] [PubMed] [Google Scholar]
- Aston C., Jiang L. & Sokolov B. P. Transcriptional profiling reveals evidence for signaling and oligodendroglial abnormalities in the temporal cortex from patients with major depressive disorder. Mol. Psychiatry 10, 309–322 (2005). [DOI] [PubMed] [Google Scholar]
- Dias C. et al. β-catenin mediates stress resilience through Dicer1/microRNA regulation. Nature 516, 51–55 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma E., Zhou K., Kidwell M. A. & Doudna J. A. Coordinated activities of human dicer domains in regulatory RNA processing. J. Mol. Biol. 422, 466–476 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheehan D. V. et al. The Mini-International Neuropsychiatric Interview (M.I.N.I.): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J. Clin. Psychiatry 59, 22–33 (1998). [PubMed] [Google Scholar]
- First M. B., Spitzer R. L., Gibbon M. & Williams J. B. W. Structured Clinical Interview for DSM-IV Axis I Disorders American Psychiatric Association (1997). [Google Scholar]
- Foulkes W. D., Priest J. R. & Duchaine T. F. DICER1: mutations, microRNAs and mechanisms. Nat. Rev. Cancer 14, 662–672 (2014). [DOI] [PubMed] [Google Scholar]
- Schratt G. microRNAs at the synapse. Nat. Rev. Neurosci. 10, 842–849 (2009). [DOI] [PubMed] [Google Scholar]
- Bredy T. W., Lin Q., Wei W., Baker-Andresen D. & Mattick J. S. MicroRNA regulation of neural plasticity and memory. Neurobiol. Learn. Mem. 96, 89–94 (2011). [DOI] [PubMed] [Google Scholar]
- Tang K. F. et al. Decreased Dicer expression elicits DNA damage and up-regulation of MICA and MICB. J. Cell. Biol. 182, 233–239 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breen M. S. et al. Gene networks specific for innate immunity define post-traumatic stress disorder. Mol. Psychiatry e-pub ahead of print . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conneely K. N. & Boehnke M. So many correlated tests, so little time! Rapid adjustment of P values for multiple correlated tests. Am. J. Hum. Genet. 81, 1158–1168 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A. et al. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl Acad. Sci. USA 102, 15545–15550 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love M. I., Huber W. & Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 15, 550 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin L. M. et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch. Gen. Psychiatry 62, 273–281 (2005). [DOI] [PubMed] [Google Scholar]
- Rauch S. L. et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol. Psychiatry 47, 769–776 (2000). [DOI] [PubMed] [Google Scholar]
- Miller G. E. et al. A functional genomic fingerprint of chronic stress in humans: blunted glucocorticoid and increased NF-kappaB signaling. Biol. Psychiatry 64, 266–272 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar H. J., Barnett W., Myer L., Stein D. J. & Nicol M. P. Investigating the early-life determinants of illness in Africa: the Drakenstein Child Health Study. Thorax 70, 592–594 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foa E. B., Riggs D. S., Dancu C. V. & Rothbaum B. O. Reliability and validity of a brief instrument for assessing post-traumatic stress disorder. J. Trauma Stress 6, 459–473 (1993). [Google Scholar]
- Beck A., Ward C., Mendelson M., Mock J. & Erbaugh J. An inventory for measuring depression. Arch. Gen. Psychiatry 4, 561–571 (1961). [DOI] [PubMed] [Google Scholar]
- Dutton G. R. et al. Use of the Beck Depression Inventory-II with African American primary care patients. Gen. Hosp. Psychiatry. 26, 437–442 (2004). [DOI] [PubMed] [Google Scholar]
- Goldmann E. et al. Pervasive exposure to violence and posttraumatic stress disorder in a predominantly African American Urban Community: the Detroit Neighborhood Health Study. J. Trauma Stress 24, 747–751 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini G. et al. The involvement of microRNAs in major depression, suicidal behavior, and related disorders: a focus on miR-185 and miR-491-3p. Cell. Mol. Neurobiol. 34, 17–30 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Issler O. & Chen A. Determining the role of microRNAs in psychiatric disorders. Nat. Rev. Neurosci. 16, 201–212 (2015). [DOI] [PubMed] [Google Scholar]
- Mulligan M. K. et al. Expression, covariation, and genetic regulation of miRNA Biogenesis genes in brain supports their role in addiction, psychiatric disorders, and disease. Front. Genet. 4, 126 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanan V. K. et al. FASTKD2 is associated with memory and hippocampal structure in older adults. Mol. Psychiatry 20, 1197–1204 (2015). [DOI] [PMC free article] [PubMed]
- Wong H. K. et al. De-repression of FOXO3a death axis by microRNA-132 and -212 causes neuronal apoptosis in Alzheimer's disease. Hum. Mol. Genet. 22, 3077–3092 (2013). [DOI] [PubMed] [Google Scholar]
- Haramati S. et al. MicroRNA as repressors of stress-induced anxiety: the case of amygdalar miR-34. J. Neurosci. 31, 14191–14203 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smalheiser N. R. et al. MicroRNA expression is down-regulated and reorganized in prefrontal cortex of depressed suicide subjects. PLoS ONE 7, e33201 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders A. R. et al. Transcriptome study of differential expression in schizophrenia. Hum. Mol. Genet. 22, 5001–5014 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santarelli D. M., Beveridge N. J., Tooney P. A. & Cairns M. J. Upregulation of dicer and microRNA expression in the dorsolateral prefrontal cortex Brodmann area 46 in schizophrenia. Biol. Psychiatry 69, 180–187 (2011). [DOI] [PubMed] [Google Scholar]
- Gasser S., Orsulic S., Brown E. J. & Raulet D. H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436, 1186–1190 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francia S. et al. Site-specific DICER and DROSHA RNA products control the DNA-damage response. Nature 488, 231–235 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin J. & Cohen B. An overview of the immune system. Lancet 357, 1777–1789 (2001). [DOI] [PubMed] [Google Scholar]
- Haroon E. et al. IFN-alpha-induced cortical and subcortical glutamate changes assessed by magnetic resonance spectroscopy. Neuropsychopharmacology 39, 1777–1785 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller A. H., Haroon E., Raison C. L. & Felger J. C. Cytokine targets in the brain: impact on neurotransmitters and neurocircuits. Depress. Anxiety. 30, 297–306 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohleb E. S., McKim D. B., Sheridan J. F. & Godbout J. P. Monocyte trafficking to the brain with stress and inflammation: a novel axis of immune-to-brain communication that influences mood and behavior. Front. Neurosci. 8, 447 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijerkerk A. et al. MicroRNAs regulate human brain endothelial cell-barrier function in inflammation: implications for multiple sclerosis. J. Neurosci. 33, 6857–6863 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tominaga N. et al. Brain metastatic cancer cells release microRNA-181c-containing extracellular vesicles capable of destructing blood-brain barrier. Nat. Commun. 6, 6716 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubzansky L. D., Koenen K. C., Spiro A. 3rd, Vokonas P. S. & Sparrow D. Prospective study of posttraumatic stress disorder symptoms and coronary heart disease in the Normative Aging Study. Arch. Gen. Psychiatry 64, 109–116 (2007). [DOI] [PubMed] [Google Scholar]
- Boscarino J. A. A prospective study of PTSD and early-age heart disease mortality among Vietnam veterans: implications for surveillance and prevention. Psychosom. Med. 70, 668–676 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ungvari Z. et al. Aging-induced dysregulation of dicer1-dependent microRNA expression impairs angiogenic capacity of rat cerebromicrovascular endothelial cells. J. Gerontol. A. Biol. Sci. Med. Sci. 68, 877–891 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y., Balazs L., Tigyi G. & Yue J. Conditional deletion of Dicer in vascular smooth muscle cells leads to the developmental delay and embryonic mortality. Biochem. Biophys. Res. Commun. 408, 369–374 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono K., Kuwabara Y. & Han J. MicroRNAs and cardiovascular diseases. FEBS J. 278, 1619–1633 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boon R. A. & Dimmeler S. MicroRNAs in myocardial infarction. Nat. Rev. Cardiol. 12, 135–4 (2014). [DOI] [PubMed] [Google Scholar]
- Behrmann M., Geng J. J. & Shomstein S. Parietal cortex and attention. Curr. Opin. Neurobiol. 14, 212–217 (2004). [DOI] [PubMed] [Google Scholar]
- Catani C., Adenauer H., Keil J., Aichinger H. & Neuner F. Pattern of cortical activation during processing of aversive stimuli in traumatized survivors of war and torture. Eur. Arch. Psychiatry Clin. Neurosci. 259, 340–351 (2009). [DOI] [PubMed] [Google Scholar]
- Yoshimura S. et al. Rostral anterior cingulate cortex activity mediates the relationship between the depressive symptoms and the medial prefrontal cortex activity. J. Affect. Disord. 122, 76–85 (2010). [DOI] [PubMed] [Google Scholar]
- Mehta D. et al. Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc. Natl Acad. Sci. USA 110, 8302–8307 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coffey S. F., Gudmundsdottir B., Beck J. G., Palyo S. A. & Miller L. Screening for PTSD in motor vehicle accident survivors using the PSS-SR and IES*. J. Trauma. Stress. 19, 119–128 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mecham B. H., Nelson P. S. & Storey J. D. Supervised normalization of microarrays. Bioinformatics 26, 1308–1315 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S. et al. PLINK: a toolset for whole-genome association and population-based linkage analysis. Am. J. Hum. Genet. 81, 559–575 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price A. L. et al. Principal components analysis corrects for stratification in genome-wide association studies. Nat. Genet. 38, 904–909 (2006). [DOI] [PubMed] [Google Scholar]
- Hochberg Y. & Benjamini Y. More powerful procedures for multiple significance testing. Stat. Med. 9, 811–818 (1990). [DOI] [PubMed] [Google Scholar]
- Houseman E. A. et al. DNA methylation arrays as surrogate measures of cell mixture distribution. BMC Bioinformatics 13, 86 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koestler D. C. et al. Blood-based profiles of DNA methylation predict the underlying distribution of cell types: a validation analysis. Epigenetics 8, 816–826 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K. J. & Schmittgen T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408 (2001). [DOI] [PubMed] [Google Scholar]
- Sun Z. et al. CAP-miRSeq: a comprehensive analysis pipeline for microRNA sequencing data. BMC Genomics 15, 423 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozomara A. & Griffiths-Jones S. miRBase: annotating high confidence microRNAs using deep sequencing data. Nucleic Acids Res. 42, D68–D73 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordero F., Beccuti M., Arigoni M., Donatelli S. & Calogero R. A. Optimizing a massive parallel sequencing workflow for quantitative miRNA expression analysis. PLoS ONE 7, e31630 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman P. & Friesen W. Pictures of Facial Affect Consulting Psychologists Press (1976). [Google Scholar]
- Lange K. et al. Task instructions modulate neural responses to fearful facial expressions. Biol. Psychiatry 53, 226–232 (2003). [DOI] [PubMed] [Google Scholar]
- Song X. W. et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS ONE 6, e25031 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uddin M. et al. Adcyap1r1 genotype, posttraumatic stress disorder, and depression among women exposed to childhood maltreatment. Depress. Anxiety 30, 251–258 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guffanti G. et al. Genome-wide association study implicates a novel RNA gene, the lincRNA AC068718.1, as a risk factor for post-traumatic stress disorder in women. Psychoneuroendocrinology 38, 3029–3038 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Tables 1-3.