Abstract
OBJECTIVE
To compare the effectiveness of diabetes prevention strategies addressing postpartum weight retention for women with gestational diabetes mellitus (GDM) delivered at the health system level: mailed recommendations (usual care) versus usual care plus a Diabetes Prevention Program (DPP)–derived lifestyle intervention.
RESEARCH DESIGN AND METHODS
This study was a cluster randomized controlled trial of 44 medical facilities (including 2,280 women with GDM) randomized to intervention or usual care. The intervention included mailed gestational weight gain recommendations plus 13 telephone sessions between 6 weeks and 6 months postpartum. Primary outcomes included the following: proportion meeting the postpartum goals of 1) reaching pregravid weight if pregravid BMI <25.0 kg/m2 or 2) losing 5% of pregravid weight if BMI ≥25.0 kg/m2; and pregravid to postpartum weight change.
RESULTS
On average, over the 12-month postpartum period, women in the intervention had significantly higher odds of meeting weight goals than women in usual care (odds ratio [OR] 1.28 [95% CI 1.10, 1.47]). The proportion meeting weight goals was significantly higher in the intervention than usual care at 6 weeks (25.5 vs. 22.4%; OR 1.17 [1.01, 1.36]) and 6 months (30.6 vs. 23.9%; OR 1.45 [1.14, 1.83]). Condition differences were reduced at 12 months (33.0 vs. 28.0%; OR 1.25 [0.96, 1.62]). At 6 months, women in the intervention retained significantly less weight than women in usual care (mean 0.39 kg [SD 5.5] vs. 0.95 kg [5.5]; mean condition difference −0.64 kg [95% CI −1.13, −0.14]) and had greater increases in vigorous-intensity physical activity (mean condition difference 15.4 min/week [4.9, 25.8]).
CONCLUSIONS
A DPP-derived lifestyle intervention modestly reduced postpartum weight retention and increased vigorous-intensity physical activity.
Introduction
Type 2 diabetes is a costly disease affecting ∼12.6 million women in the U.S. Randomized efficacy trials (1–4) demonstrated that weight loss programs can prevent diabetes. Diabetes prevention is critical for women with gestational diabetes mellitus (GDM), which affects 7–14% of pregnancies (5), as women with GDM are seven times more likely to develop type 2 diabetes than parous women without GDM (6). Postpartum weight retention increases diabetes risk (7), and postpartum weight management for women with GDM is recommended (8). Yet evidence to support health system adoption of postpartum diabetes prevention programs for women with GDM is lacking. In addition to traditional clinical trials enrolling carefully selected volunteers with a history of GDM under ideal conditions (4), pragmatic trials evaluating the effectiveness of diabetes prevention programs in real-world clinical settings among diverse populations are needed to increase generalizability and inform health system adoption (9).
The Gestational Diabetes’ Effects on Moms (GEM) cluster randomized controlled trial compared an existing postpartum diabetes prevention program of mailed recommendations delivered by the Kaiser Permanente Northern California (KPNC) Perinatal Center (10) (usual care) to usual care plus a Diabetes Prevention Program (DPP)–derived lifestyle intervention (1). The DPP-derived intervention was delivered on behalf of the KPNC Perinatal Center and offered as an optional routine care program (11). The goal of the intervention was to help women with GDM 1) reach pregravid weight if their pregravid BMI was <25.0 kg/m2 or 2) lose 5% of pregravid weight if their pregravid BMI was ≥25.0 kg/m2 (11). Primary outcomes were the proportion of women who reached postpartum weight goals and weight change from pregravid to postpartum across the 12-month postpartum follow-up period. Secondary outcomes included changes from pregnancy to postpartum in daily total energy intake, percent of calories from fat, physical activity, hypertension, and depression. An exploratory outcome was postpartum prediabetes or diabetes incidence.
Research Design and Methods
The rationale and methods of the GEM trial are described elsewhere (11). The Kaiser Foundation Research Institute Human Subjects Committee approved the trial and waived the requirement for informed consent for the intervention. Women provided verbal consent for the survey component of the trial after all medical facilities were randomized (11).
Setting, Randomization, and Masking
The setting was KPNC, an integrated health care delivery system with 44 medical facilities managing ∼33,000 births annually. KPNC membership closely approximates the surrounding population (12) and is dynamic with respect to enrollment. All 44 KPNC facilities were randomized to either usual care or intervention conditions. Randomization was blocked on facility size (i.e., the expected annual number of women with GDM, in three strata: <25, 25–74, and ≥75). A restricted randomization scheme was used to ensure acceptable between-condition balance (i.e., maximum between-condition relative difference) in expected racial/ethnic distributions and the number of women contacted by an ongoing unrelated observational study, both overall and within facility size stratum (11,13). All investigators, data collectors, and health care providers were blinded to condition assignment.
Participants
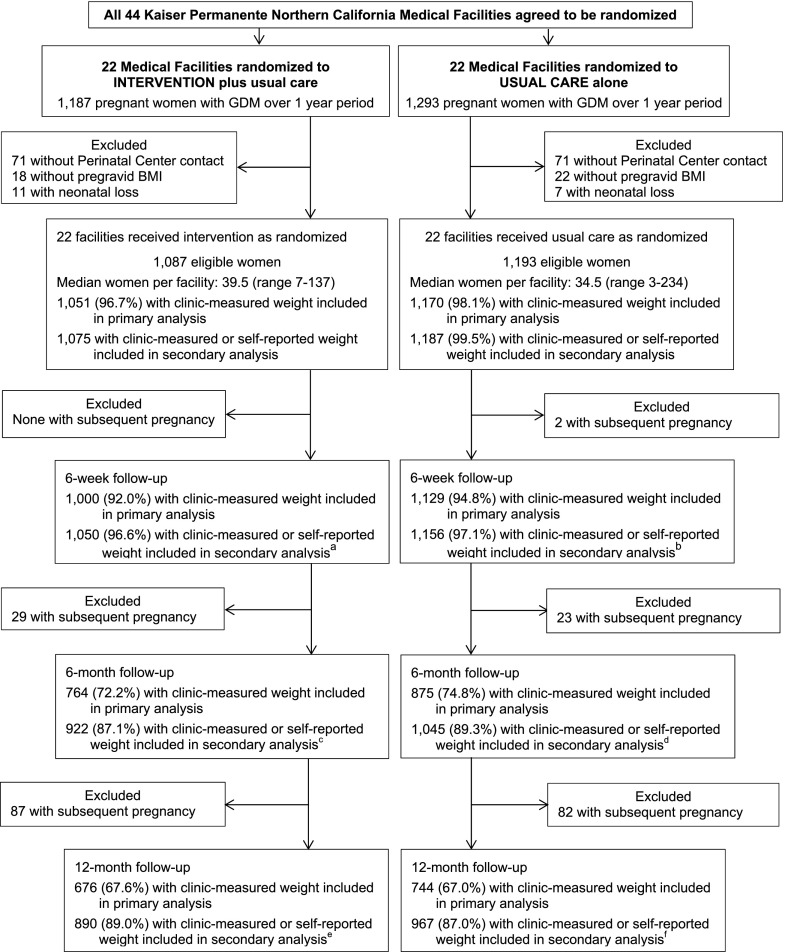
Potentially eligible women included pregnant women ≥18 years old diagnosed with GDM (n = 2,480) according to the Carpenter and Coustan criteria between March 2011 and March 2012, as recommended by the American College of Obstetricians and Gynecologists (14) during this period and implemented across all 44 KPNC medical facilities. Women were excluded if they did not have any telephone contact with the KPNC Perinatal Center during pregnancy (n = 142), were missing data on pregravid BMI (n = 40), or had a neonatal loss (n = 18), leaving 2,280 women: 1,087 in the intervention condition and 1,193 in usual care (Fig. 1). Follow-up ended on December 2013.
Figure 1.
Trial flow. a, 12 women were not health plan members and did not have clinic-measured weight, 5 of them provided self-reported weight; b, 9 women were not health plan members and did not have clinic-measured weight, 4 of them provided self-reported weight; c, 37 women were not health plan members and did not have clinic-measured weight, 26 of them provided self-reported weight; d, 36 women were not health plan members and did not have clinic-measured weight, 25 of them provided self-reported weight; e, 84 women were not health plan members and did not have clinic-measured weight, 61 of them provided self-reported weight; f, 101 women were not health plan members and did not have clinic-measured weight, 66 of them provided self-reported weight only.
Usual Care
All women with GDM at KPNC are offered supplemental care from the KPNC Perinatal Center consisting of telephone-based case management for glucose control during pregnancy (10). At 6 weeks postpartum, the KPNC Perinatal Center sends a letter encouraging women to be screened for diabetes and provides printed materials that emphasize a healthy BMI, participation in 30 min of physical activity daily, and healthy eating. If screening results indicate prediabetes, women are mailed materials about lifestyle prevention strategies and educational classes.
DPP-Derived Lifestyle Intervention
In addition to usual care, women attending medical facilities assigned to the intervention were mailed, within 2 weeks of the GDM diagnosis, a tailored letter detailing a personalized goal for gestational weight gain with healthy eating and physical activity tips (11). After delivery, women were offered a print/telephone-based lifestyle program modeled after the DPP intervention (11). The program was presented as optional routine care and delivered by GEM coaches on behalf of the KPNC Perinatal Center. During the core of the postpartum intervention (6 weeks to 6 months), the primary target was to help women achieve the postpartum weight goals: 1) reaching pregravid weight if their pregravid BMI was <25.0 kg/m2 or 2) losing 5% of pregravid weight if their pregravid BMI was ≥25.0 kg/m2 (11). Women were mailed a 13-session guidebook to review via telephone with a lifestyle coach, a registered dietitian employed for the trial and based at the KPNC Perinatal Center. Women were encouraged to set weekly goals for daily fat and caloric intake and to work up to or continue participating in 150 min of moderate- to vigorous-intensity physical activity per week (11). Behavior change techniques were individualized to women’s preferences, resources, and cultural context using motivational interviewing and theoretical constructs derived from social cognitive theory (15) and the Transtheoretical Model (16). All telephone sessions were audiotaped; a random 10% were coded using a brief checklist of intervention components to assess fidelity to the protocol. Fidelity was high (mean proportion of intervention components present = 95%, range 60–100%). During the maintenance phase (7–12 months postpartum), women were mailed three newsletters encouraging maintenance of healthy behaviors. Lifestyle coaches tracked the time spent scheduling and conducting telephone sessions. The direct cost of the intervention included coaches’ work hours, calculated using the May 2012 median annual wage for registered dietitians (17) plus fringe benefits (29% of annual wages), and the cost of printing and mailing materials.
Data Collection
All 2,280 eligible women in the 44 medical facilities were invited to complete study surveys (11); 1,783 (78.2%) completed the baseline survey during pregnancy after the GDM diagnosis (mean gestational age at completion 29.4 weeks [SD 6.1]). Survey responders did not differ from nonresponders except for being less likely to be of non-Hispanic white origin (11). Responders to the baseline survey were asked to complete surveys at 6 weeks, 6 months, and 12 months postpartum (11), with 91.6, 87.9, and 84.0% retention rates, respectively. Pregravid BMI was calculated from clinic-measured pregravid weight obtained through the electronic health record (EHR; 86.0%), weight ascertained at the first prenatal clinic visit before 10 weeks' gestation (10.5%, from the EHR), or self-reported pregravid weight on the GEM pregnancy survey (3.5%).
Clinic-measured postpartum weight was obtained through the EHR for each time point, regardless of participation in the intervention or the GEM surveys. Postpartum weight was also self-reported at each GEM survey.
The Block Food Frequency questionnaire (18) and the Pregnancy Physical Activity Questionnaire (19) were used at baseline and 6 months postpartum to assess usual daily total energy intake and weekly physical activity over the past 3 months. Hypertension was defined as present during pregnancy and at each postpartum time point by searching the EHR for any abnormal blood pressure measurements (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg) or for use of antihypertensive medications. Depression was defined as present during pregnancy and at each postpartum time point by searching the EHR for diagnoses of depression, use of antidepressant medications, or depression score ≥10 on Patient Health Questionnaire 9 (PHQ-9) (20) or score ≥10 on PHQ-8 (21) from surveys at each time point. Age, race/ethnicity, parity, and laboratory test results were also obtained from the EHR. Prediabetes and diabetes were defined according to American Diabetes Association glyc-emic thresholds (22) (see Supplementary Methods).
Power and Statistical Analysis
The sample size of 2,280, of which 97.4% had at least one clinic-measured weight during the 12-month postpartum follow-up period, allowed for robust estimation of the average effect of the intervention on postpartum weight goals assessed through clinic-measured weight across the 12-month postpartum follow-up period. We focused post hoc minimum detectable effect estimation at each point in time accounting for the cluster randomization. Of the sample, 93.4, 71.9, and 62.3% had clinic-measured weight at 6 weeks, 6 months, and 12 months postpartum, respectively, which led to a minimum detectable absolute difference in the proportion meeting postpartum weight goals ranging from 5.6 to 10.2%, 6.2 to 10.6%, and 6.6 to 11.0%, respectively, across the range in expected proportion meeting goals in the usual care condition (15–25%, based on our pilot trial [24%] and across the range in expected intraclass correlation [0.01–0.05]) (see Supplementary Methods). We note the additional power and precision gained in the analysis of treatment effects by incorporating the repeated measures of meeting postpartum weight goals.
Analyses were intent to treat. Analyses used marginal regression models to estimate population average intervention effects, using logistic regression with estimation via generalized estimating equations in analyses of meeting the weight goals and other dichotomous outcomes, and linear mixed regression in analyses of weight change and changes in other continuous outcomes after adjusting for baseline values. Regression models accounted for the within-medical facility correlation between patients and within-person correlation among repeated measurements for valid estimation of treatment effects and associated standard errors. Variations in treatment condition differences over time were examined via the introduction of appropriate cross-product terms. For all analyses, if women had a subsequent pregnancy, they were censored at the estimated time of conception.
Primary outcome analysis for the effect of the intervention included only clinic-measured postpartum weights. Sensitivity analyses to address potential bias from missing clinic-measured weight included clinic-measured or self-reported postpartum weight collected during postpartum surveys. Additional sensitivity analyses included clinic-measured or imputed postpartum weight (23). The fully conditional specification imputation method was used, with 10 imputations performed. Subgroup analyses were performed after stratifying by pregravid BMI. Two exploratory analyses assessed intervention effectiveness across number of sessions completed and using an instrumental variable to estimate the complier average causal effect, a measure of the effectiveness of the intervention among those who comply with assigned treatment, with the randomization indicator used as the instrumental variable (24). For this analysis, “all or none” compliance was defined as completing one or more telephone sessions (25). A key applicable assumption is that the outcomes among noncompliers are not affected by randomization condition assignment. Exploratory outcome analysis of prediabetes or diabetes incidence used Cox proportional hazards regression to estimate hazard ratios associated with the intervention, controlling for pregnancy fasting and 3-h glucose values and pregravid BMI. All analyses were conducted using SAS 9.3 (Cary, NC).
Results
The intervention and usual care conditions were comparable on prerandomization characteristics such as medical facility size (Fig. 1) and racial/ethnic and BMI distributions; however, they differed by fasting and 3-h glucose (Table 1). Among the 1,087 women in the intervention, 50.3% completed one or more telephone sessions; specifically, 18.8% completed 1–3 sessions, 16.2% completed 4–12 sessions, and 15.3% completed all 13 sessions.
Table 1.
Baseline characteristics by treatment condition: the GEM trial
| Intervention, N = 1,087 |
Usual care, N = 1,193 |
Entire sample, N = 2,280 |
|
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Age (years) | |||
| 18–24 | 59 (5.4) | 58 (4.9) | 117 (5.1) |
| 25–29 | 238 (21.9) | 289 (24.2) | 527 (23.1) |
| 30–34 | 425 (39.1) | 425 (35.6) | 850 (37.3) |
| 35–39 | 280 (25.8) | 330 (27.7) | 610 (26.8) |
| 40–50 | 85 (7.8) | 91 (7.6) | 176 (7.7) |
| Race-ethnicity | |||
| Asian | 458 (42.1) | 481 (40.3) | 939 (41.2) |
| Non-Hispanic white | 268 (24.7) | 305 (25.6) | 573 (25.1) |
| Hispanic | 236 (21.7) | 270 (22.6) | 506 (22.2) |
| African American | 47 (4.3) | 57 (4.8) | 104 (4.6) |
| Multiracial | 37 (3.4) | 37 (3.1) | 74 (3.3) |
| Other | 26 (2.4) | 16 (1.3) | 42 (1.8) |
| Pacific Islander | 13 (1.2) | 25 (2.1) | 38 (1.7) |
| Missing | 2 (0.2) | 2 (0.2) | 4 (0.2) |
| Pregravid BMI (kg/m2) | |||
| 15.9–19.9 | 61 (5.6) | 59 (5.0) | 120 (5.3) |
| 20.0–24.9 | 324 (29.8) | 343 (28.8) | 667 (29.3) |
| 25.0–29.9 | 319 (29.4) | 352 (29.5) | 671 (29.4) |
| 30.0–34.9 | 201 (18.5) | 216 (18.1) | 417 (18.3) |
| 35.0–59.7 | 182 (16.7) | 223 (18.7) | 405 (17.8) |
| Parity | |||
| 0 | 451 (41.5) | 501 (42.0) | 952 (41.8) |
| 1 | 363 (33.4) | 398 (33.4) | 761 (33.4) |
| 2 | 174 (16.0) | 157 (13.2) | 331 (14.5) |
| 3+ | 84 (7.7) | 117 (9.8) | 201 (8.8) |
| Missing | 15 (1.4) | 20 (1.7) | 35 (1.5) |
| Hypertension | |||
| Yes | 80 (7.4) | 82 (6.9) | 162 (7.1) |
| No | 1,005 (92.5) | 1,111 (93.1) | 2,116 (92.8) |
| Missing | 2 (0.2) | 0 (0) | 2 (0.1) |
| Depression | |||
| Yes | 206 (19.0) | 237 (19.9) | 443 (19.4) |
| No | 833 (76.6) | 887 (74.4) | 1,720 (75.4) |
| Missing |
48 (4.4) |
69 (5.8) |
117 (5.1) |
| Mean (SD) |
Mean (SD) |
||
| Pregravid body weight (kg) | 73.5 (19.9) | 74.7 (20.2) | 74.2 (20.0) |
| Pregravid BMI (kg/m2) | 28.5 (6.8) | 28.9 (6.9) | 28.7 (6.8) |
| BMI at GDM diagnosis (kg/m2) | 31.0 (6.5) | 31.4 (6.4) | 31.2 (6.5) |
| Gestational age at GDM diagnosis (weeks) | 25.2 (6.4) | 25.3 (6.6) | 25.3 (6.5) |
| 100-g, 3-h OGTT | |||
| Fasting glucose (mmol/L)* | 5.0 (0.7) | 5.1 (0.7) | 5.1 (0.7) |
| 1-h glucose (mmol/L) | 11.0 (1.4) | 11.1 (1.5) | 11.0 (1.4) |
| 2-h glucose (mmol/L) | 9.8 (1.5) | 9.8 (1.5) | 9.8 (1.5) |
| 3-h glucose (mmol/L)* | 7.3 (1.8) | 7.1 (1.8) | 7.2 (1.8) |
| Systolic blood pressure (mmHg) | 116.7 (13.0) | 116.7 (13.6) | 116.7 (13.3) |
| Diastolic blood pressure (mmHg) | 72.9 (10.0) | 72.6 (10.2) | 72.7 (10.1) |
| Daily total energy intake (kcal/day)† | 1,743.5 (662.5) | 1,736.7 (660.7) | 1,740.0 (661.4) |
| Daily percent of kcal from fat† | 42.2 (7.0) | 42.2 (7.0) | 42.2 (7.0) |
| Walking (min/week)‡ | 446.3 (355.2) | 427.9 (364.1) | 436.8 (359.8) |
| Moderate physical activity (min/week)‡ | 38.2 (62.9) | 32.1 (55.8) | 35.1 (59.4) |
| Vigorous physical activity (min/week)‡ | 23.5 (53.3) | 19.0 (46.2) | 21.2 (49.8) |
| Total volume of physical activity (MET min/week)‡ | 1,667.9 (1,312.9) | 1,554.4 (1,212.6) | 1,609.2 (1,262.7) |
Hypertension was defined as systolic blood pressure ≥140 mmHg, diastolic blood pressure ≥90 mmHg, or use of antihypertensive medications. Depression was defined as score ≥10 on PHQ-8 or PHQ-9, physician diagnosis, or use of antidepressant medications. OGTT, oral glucose tolerance test.
*P < 0.05 for the comparison with the intervention condition by two-tailed Student t test. All other comparisons had a P ≥ 0.05.
†639 women in the intervention and 668 women in usual care with valid diet data at baseline and 6 months postpartum (e.g., those with daily total energy intake ≥600 and ≤4,000 kcal at both time points).
‡522 women in the intervention and 560 women in usual care with valid physical activity data at baseline and 6 months postpartum (e.g., those with ≤20 h/week of total physical activity and ≤9 h/week of moderate physical activity at both time points).
Primary Outcomes
Clinic-measured postpartum weight was obtained at least once during the 12-month postpartum follow-up for 97.4% of women (n = 2,221). In the primary analysis that included only clinic-measured postpartum weight, on average, over the 12-month postpartum period, women in the medical facilities assigned to the intervention had a statistically significant 28% higher odds (odds ratio [OR] 1.28 [95% CI 1.10, 1.47]) of meeting postpartum weight goals than women in the medical facilities assigned to usual care (Table 2). The proportion meeting weight goals was significantly higher in the intervention facilities than usual care facilities at 6 weeks (25.5 vs. 22.4%; 1.17 [1.01, 1.36]) and 6 months postpartum (30.6 vs. 23.9%; 1.45 [1.14, 1.83]). At 12 months (6 months after the intervention ended), the condition difference was reduced (33.0 vs. 28.0%; 1.25 [0.96, 1.62]) and no longer statistically significant. The absolute difference between conditions was 3.1% at 6 weeks, 6.7% at 6 months, and 5.0% at 12 months postpartum. In analyses examining weight change from pregravid to postpartum (Table 2), women in the intervention facilities retained less weight than women in usual care facilities at 6 months postpartum (mean 0.39 kg [SD 5.5] vs. 0.95 kg [5.5]; adjusted mean condition difference −0.64 kg [95% CI −1.13, −0.14]); the condition difference was attenuated at 12 months postpartum and no longer statistically significant (−0.43 kg [−0.98, 0.11]) (Table 2). Sensitivity analyses that included self-reported or imputed weight if the clinic-measured weight was missing yielded similar results (Tables 2 and 3). The intervention was equally effective across racial/ethnic groups (P value for condition × race/ethnicity interactions >0.25). The intervention was also equally effective across BMI strata (Supplementary Tables 1 and 2).
Table 2.
Proportion of women meeting the postpartum weight goals with ORs estimating differences between conditions and mean changes in weight from pregravid to postpartum with mean differences between conditions: the GEM trial
| Primary analysis |
Sensitivity analysis |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measured postpartum weights |
Measured or self-reported postpartum weights |
Measured or imputed postpartum weights | ||||||||||||
| Intervention | Usual care | Intervention | Usual care | |||||||||||
|
n |
% meeting weight goals |
n |
% meeting weight goals |
OR (95% CI) |
P value* |
n |
% meeting weight goals |
n |
% meeting weight goals |
OR (95% CI) |
P value* |
OR (95% CI) |
P value* |
|
| Average effect of intervention | 1,051 | 1,170 | 1.28 (1.10, 1.47) | <0.01 | 1,075 | 1,187 | 1.23 (1.08, 1.39) | <0.01 | 1.25 (1.11, 1.42) | <0.01 | ||||
| 6 weeks postpartum | 1,000 | 25.5 | 1,129 | 22.4 | 1.17 (1.01, 1.36) | 0.04 | 1,050 | 25.0 | 1,156 | 22.7 | 1.16 (1.00, 1.34) | 0.05 | 1.18 (0.98, 1.44) | 0.09 |
| 6 months postpartum | 764 | 30.6 | 875 | 23.9 | 1.45 (1.14, 1.83) | <0.01 | 922 | 31.6 | 1,045 | 25.4 | 1.37 (1.13, 1.67) | <0.01 | 1.33 (1.09, 1.62) | 0.01 |
| 12 months postpartum |
676 |
33.0 |
744 |
28.0 |
1.25 (0.96, 1.62) |
0.09 |
890 |
33.5 |
967 |
30.2 |
1.16 (0.95, 1.41) |
0.14 |
1.24 (0.97, 1.59) |
0.08 |
|
n |
Mean weight (SD) |
n |
Mean weight (SD) |
Adjusted† mean weight condition difference (95% CI) |
P value* |
n |
Mean weight (SD) |
n |
Mean weight (SD) |
Adjusted† mean weight condition difference (95% CI) |
P value* |
Adjusted† mean weight condition difference (95% CI) |
P value* |
|
| Average effect of intervention | 1,051 | 1,170 | −0.29 (−0.69, 0.11) | 0.16 | 1,075 | 1,187 | −0.26 (−0.65, 0.13) | 0.19 | −0.24 (−0.67, 0.19) | 0.28 | ||||
| 6 weeks postpartum | 1,000 | 0.64 (5.57) | 1,129 | 0.75 (5.46) | −0.21 (−0.64, 0.23) | 0.35 | 1,050 | 0.64 (5.53) | 1,156 | 0.71 (5.44) | −0.20 (−0.63, 0.22) | 0.35 | −0.19 (−0.63, 0.26) | 0.41 |
| 6 months postpartum | 764 | 0.39 (5.55) | 875 | 0.95 (5.47) | −0.64 (−1.13, −0.14) | 0.01 | 922 | 0.17 (5.46) | 1,045 | 0.64 (5.54) | −0.55 (−1.02, −0.09) | 0.02 | −0.51 (−0.99, −0.02) | 0.04 |
| 12 months postpartum | 676 | 0.20 (5.84) | 744 | 0.50 (5.43) | −0.43 (−0.98, 0.11) | 0.12 | 890 | 0.01 (5.81) | 967 | 0.15 (5.73) | −0.32 (−0.82, 0.19) | 0.22 | −0.35 (−0.98, 0.29) | 0.28 |
*P value for condition differences; P values for treatment × follow-up time point interaction >0.05.
†Adjusted for pregravid weight.
Table 3.
Mean changes in physical activity and diet from pregnancy to 6 months postpartum with mean differences between conditions: the GEM trial
| Primary analysis |
Sensitivity analysis |
||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Self-reported data |
Self-reported or imputed data |
||||||||||
| Intervention |
Usual care |
Intervention |
Usual care |
||||||||
| n | Mean (SD) | n | Mean (SD) | Adjusted* mean condition difference (95% CI) | n | Mean (SD) | n | Mean (SD) | Adjusted* mean condition difference (95% CI) | ||
| Moderate activity (min/week) | |||||||||||
| During pregnancy | 522 | 38.2 (62.9) | 560 | 32.1 (55.8) | 1,083 | 39.1 (2.6) | 1,187 | 34.1 (2.5) | |||
| 6 months postpartum | 522 | 42.8 (67.2) | 560 | 33.7 (61.8) | 1,083 | 47.0 (2.5) | 1,187 | 35.4 (3.1) | |||
| Mean change (95% CI) | 4.5 (−1.8, 10.8) | 1.6 (−4.5, 7.7) | 7.0 (−0.8, 14.7) | 8.0 (1.8, 14.1) | 1.9 (−6.5, 10.3) | 9.5 (0.7, 18.3) | |||||
| Vigorous activity (min/week) | |||||||||||
| During pregnancy | 522 | 23.5 (53.3) | 560 | 19.0 (46.2) | 1,083 | 22.9 (1.8) | 1,187 | 19.6 (1.6) | |||
| 6 months postpartum | 522 | 47.2 (78.9) | 560 | 30.6 (68.4) | 1,083 | 47.6 (2.9) | 1,187 | 32.5 (2.9) | |||
| Mean change (95% CI) | 23.7 (16.9, 30.5) | 11.7 (5.1, 18.2) | 15.4 (4.9, 25.8) | 24.9 (18.0, 31.7) | 13.3 (6.3, 20.3) | 14.0 (5.0, 22.9) | |||||
| Total volume of physical activity (MET min/week) | |||||||||||
| During pregnancy | 522 | 1,667.9 (1,312.9) | 560 | 1,554.4 (1,212.6) | 1,816.6 (58.6) | 1,781.7 (53.3) | |||||
| 6 months postpartum | 522 | 1,753.9 (1,300.5) | 560 | 1,610.0 (1,354.7) | 1,843.0 (64.4) | 1,727.6 (54.0) | |||||
| Mean change (95% CI) | 88.0 (−48.5, 224.6) | 58.4 (−79.1, 195.9) | 113.8 (−78.0, 305.6) | 1,083 | 33.5 (−135.8, 202.8) | 1,187 | −47.4 (−203.0, 108.3) | 100.5 (−83.7, 284.7) | |||
| Daily total energy intake (kcal) | |||||||||||
| During pregnancy | 639 | 1,763.1 (655.7) | 668 | 1,740.2 (644.1) | 1,754.6 (28.4) | 1,785.7 (26.4) | |||||
| 6 months postpartum | 639 | 1,591.4 (614.7) | 668 | 1,583.4 (608.0) | 1,580.4 (27.5) | 1,622.7 (25.9) | |||||
| Mean change (95% CI) | −171.8 (−221.2, −122.4) | −153.9 (−203.7, −104.1) | −5.3 (−64.6, 53.9) | 1,083 | −175.5 (−232.9, −118.1) | 1,187 | −162.7 (−214.8, −110.6) | −26.6 (−88.3, 35.1) | |||
| Daily percent of calories from fat | |||||||||||
| During pregnancy | 639 | 42.1 (6.9) | 668 | 42.2 (7.0) | 42.0 (0.3) | 42.2 (0.3) | |||||
| 6 months postpartum | 639 | 40.2 (6.3) | 668 | 39.8 (6.7) | 40.1 (0.3) | 39.8 (0.3) | |||||
| Mean change (95% CI) | −1.8 (−2.5, −1.1) | −2.3 (−3.0, −1.6) | 0.4 (−0.3, 1.1) | 1,083 | −2.0 (−2.6, −1.3) | 1,187 | −2.4 (−3.1, −1.8) | 0.3 (−0.4, 1.1) | |||
*Adjusted for baseline values.
In exploratory analyses, the instrumental variable estimates of the absolute differences between conditions among compliers (defined as completing one or more telephone sessions) were almost double the absolute differences observed in the primary analysis: estimates for proportions meeting weight goals were 6.2% at 6 weeks, 13.3% at 6 months, and 9.9% at 12 months; estimates for differences in mean weight change were −0.62 kg at 6 weeks, −1.27 kg at 6 months, and −0.86 kg at 12 months postpartum.
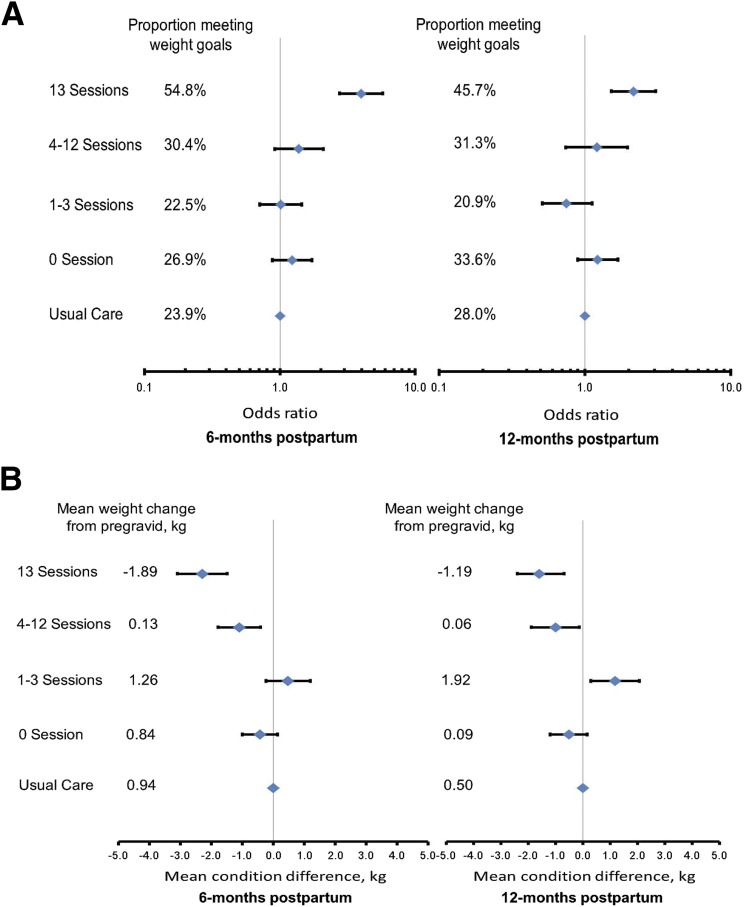
In additional exploratory analyses, women who completed all 13 sessions had significantly higher odds of meeting the postpartum weight goals than women in usual care at 6 months (3.97 [2.75, 5.72]) and 12 months postpartum (2.16 [1.52, 3.07]) (Fig. 2A). In analyses examining weight change from pregravid to postpartum (Fig. 2B), women who completed 4–12 sessions retained less weight than women in usual care at 6 months postpartum (mean 0.13 kg [SD 5.7] vs. 0.94 kg [5.5], adjusted mean condition difference −1.1 kg [95% CI −1.8, −0.41]) and at 12 months postpartum (0.06 kg [5.6] vs. 0.50 kg [5.4]; −1.0 kg [−1.9, −0.13]). Women who completed all 13 sessions lost more weight than women in usual care at 6 months (mean −1.89 kg [SD 5.5] vs. 0.94 kg [5.5], adjusted mean condition difference −2.3 kg [95% CI −3.1, −1.5]) and 12 months postpartum (−1.19 kg [6.1] vs. 0.50 kg [5.4]; −1.6 kg [−2.4, −0.68]).
Figure 2.
OR (95% CI) estimating the condition difference in meeting the postpartum weight goals (A) and adjusted mean condition difference (95% CI) (B) for weight change from pregravid by number of intervention sessions completed and time since delivery: the GEM trial. At 6 months postpartum, the number of women by number of completed telephone sessions were as follows: 338 for 0 sessions, 151 for 1–3 sessions, 171 for 4–12 sessions, and 104 for 13 sessions. At 12 months, the number of women by number of completed telephone sessions were as follows: 301 for 0 sessions, 134 for 1–3 sessions, 112 for 4–12 sessions, and 129 for 13 sessions.
Secondary Outcomes
At 6 months postpartum, women in both conditions increased minutes per week spent in vigorous-intensity activity; however, women in the intervention facilities showed a significantly greater increase (adjusted mean condition difference 15.4 min/week [95% CI 4.9, 25.8]) (Table 3). No condition differences were observed for time spent in moderate-intensity activity or total volume of activity (i.e., MET minutes per week). Women in intervention facilities and usual care facilities reported similar reductions in daily total energy intake and percent of calories from fat, with no condition differences (Table 3). Similar results for diet and physical activity were observed in sensitivity analyses that included imputed values if survey data were missing (Table 3). No condition differences were observed for hypertension or depression (Supplementary Table 3).
Exploratory Outcomes
After excluding women with possible pregestational diabetes (21 in the intervention and 29 in usual care), 873 (83.5%) women in the intervention and 974 (85.4%) in usual care completed postpartum diabetes screening. Fewer women in the intervention facilities developed prediabetes or diabetes than in usual care facilities (310 [29.7%] and 37 [3.5%] in intervention facilities and 370 [32.4%] and 50 [4.4%] in usual care facilities, respectively). However, the risk estimate for prediabetes/diabetes combined did not reach statistical significance (33.2 vs. 36.8%; hazard ratio 0.90 [95% CI 0.78, 1.04]) (Supplementary Fig. 1).
Intervention Process Measures and Economic Cost
The mean number of call attempts to reach women and complete telephone sessions was 2.2 (SD 1.8). The first session lasted a mean of 28.2 min (8.6); subsequent sessions lasted a mean of 18.8 min (7.4). Direct intervention costs per person among all 1,087 women in the intervention were $78.00 ($19.00 for printing and mailing and $59.00 for telephone sessions); costs increased to $121.00 ($19.00 for printing and mailing and $102.00 for telephone sessions) if the cost of telephone sessions was calculated among women who completed one or more telephone sessions.
Adverse Events
During the 12 months postpartum, there were no significant differences between intervention and usual care in the proportion of women who had an emergency room visit for sprains/strains (n = 9 [0.8%] vs. n = 16 [1.3%]; P = 0.24) or fractures (n = 2 [0.2%] vs. 6 [0.5%]; P = 0.29). The proportion of women in the intervention and usual care who were hospitalized or had an emergency room visit was 2.1 vs. 1.8% (P = 0.64) and 7.8 vs. 10.1% (P = 0.05), respectively. No condition differences were observed in underlying medical diagnosis except that fewer women had emergency room visits due to gallbladder disorders in the intervention than usual care (n = 3 [0.3%] vs. n = 15 [1.2%]; P = 0.008).
Conclusions
This cluster randomized trial demonstrated that a health system–based print/telephone lifestyle intervention derived from the DPP modestly reduced postpartum weight retention and increased vigorous-intensity physical activity among women with GDM. Postpartum weight retention and weight gain are associated with increased risk for diabetes (7), recurrent GDM (26), and abnormal cardiometabolic profile (27). The reductions in postpartum weight retention and the suggestion of reductions in prediabetes/diabetes incidence, for which GEM was not powered, might be explained by the significant increase in vigorous-intensity activity among women in the intervention facilities, since no condition differences were observed for changes in daily total energy intake and percent of calories from fat. It has been reported that vigorous-intensity activity is associated with decreased risk of diabetes among women with a history of GDM (28) and greater sports participation is associated with reduced postpartum weight retention (27).
Condition differences in weight observed in GEM were similar to that observed in other cluster randomized trials. In the Tianjin Gestational Diabetes Prevention Program cluster randomized trial (29), women in a lifestyle intervention weighed ∼1 kg less than women in the control condition at 12 months postpartum. The GEM condition difference was also similar to that observed at 12 months in the Da Qing cluster randomized trial (3) of adults with prediabetes, in which lifestyle intervention led to 40 and 60% reductions in diabetes risk at 6 years (3) and 20 years (30), respectively, suggesting potential long-term beneficial effects of modest weight loss. In GEM, condition differences in the proportion meeting postpartum weight goals were statistically significant, on average, over the entire 12-month postpartum period, and at 6 weeks and 6 months postpartum. Although condition differences at the 12-month postpartum time point were not statistically significant, they were similar in magnitude to those at 6 months postpartum, when the intervention ended.
It is noteworthy that intervention effects on postpartum weight retention among women with GDM are greater in trials randomized at the individual level (31,32) than cluster randomized trials (29), since only a select group of motivated volunteers participate in the former. Although in cluster randomized trials such as GEM only a portion of eligible women participate, it should be noted that the effect sizes reported are for all women in the facilities assigned to the intervention. Instrumental variable analysis suggested that the intervention effect would be at least twofold greater if all women had completed one or more sessions, suggesting a potential for great impact if we can improve patient engagement in such lifestyle interventions. To illustrate, in the pilot study that preceded GEM, with randomization at the individual level, more women in the intervention met the postpartum weight goals, with a 16% absolute difference between conditions by 12 months postpartum (31). A trial among 75 women with GDM found women in a web-based lifestyle intervention were closer to their pregravid weight than control subjects at 12 months postpartum (−0.7 vs. +4.0 kg) (32).
Limitations of the GEM trial include missing clinic-measured weight for 26.4% of women at 6 months and 32.7% at 12 months postpartum. These missing data are due to the pragmatic nature of the GEM trial; participants did not volunteer for a study with 12 months of longitudinal follow-up on body weight measurements. We obtained women’s weights from the EHR, although this required women to visit their medical facility. Still, 97.4% of women contributed to the analysis of measured weight across the entire 12-month postpartum period. Furthermore, results based on clinic-measured postpartum weight were comparable to those of sensitivity analyses. Observed differences in postpartum weight retention remained significant in a sensitivity analysis that included clinic-measured weight or self-reported weight (with only 13.7 and 18.6% still missing at 6 and 12 months, respectively) and a sensitivity analysis that imputed missing clinic-measured weight. Given the robustness of our results, it is unlikely that missing clinic-measured weight data created bias.
Strengths of this cluster randomized trial include the ability to randomize all medical facilities in the KPNC region; the large number of facilities and women, and their racial/ethnic diversity; intent-to-treat analyses including all facilities and women; blinding of investigators, data collectors, and health care providers; and follow-up extending 6 months beyond the end of the intervention. A unique strength was that the primary analysis was based on clinic-measured weight obtained via EHR, regardless of women’s participation in the surveys or intervention. These features provide generalizable, real-world findings.
GEM features several aspects of pragmatic trials, which prioritize generalizability at the potential expense of effect size given implicit sample heterogeneity and lower intervention adherence (33). In contrast to traditional clinical trials, GEM had minimal exclusion criteria and the intervention was delivered at the health system level as optional routine care to an unselected population (i.e., regardless of motivation, perinatal complications, or comorbidities), which likely contributed to the 50.3% intervention uptake. Although this uptake is high for a pragmatic trial (34,35)—and considerably higher than, for example, the recently reported uptake of 13% in the health system–based Veterans Health Administration MOVE! lifestyle change program, the largest such program in the U.S. (36)—uptake likely impacted the condition differences observed and shows how challenging it is to engage patients in prevention programs. In GEM, intervention effectiveness increased with the number of sessions completed. Although prespecified categories for the number of completed sessions were used, women were not randomized to these categories, and those who completed more sessions may have lost more weight due to increased motivation for a healthy lifestyle. Nevertheless, these dose-response analyses (37) suggest that to increase the effectiveness of lifestyle interventions, strategies are needed to increase and sustain patient engagement. If health systems were to adopt postpartum interventions for women with GDM, clinician referrals and other strategies to increase patient engagement might increase uptake and effectiveness (38). Finally, the cost per woman of the GEM intervention was relatively modest, and the printed/telephone modality is easily translatable to other settings.
In conclusion, this cluster randomized trial of diabetes prevention strategies delivered at the health system level to women with GDM demonstrated that a DPP-derived, print/telephone-based lifestyle intervention was superior to usual care in reducing postpartum weight retention, a risk factor for diabetes, and increasing physical activity. Although condition differences were modest, differences of similar magnitude have been shown to reduce long-term diabetes incidence in at-risk adults (30). These findings, from a trial embedded in real-world practice, may encourage health systems to adopt DPP-derived postpartum interventions to help women with GDM manage their weight and increase physical activity, thereby potentially preventing or delaying the onset of diabetes.
Supplementary Material
Article Information
Acknowledgments. The authors thank the members of KPNC who participated in the trial. The authors also thank members of the trial Data and Safety Monitoring Board (Dr. Dennis M. Black [University of California, San Francisco], Dr. Thomas A. Buchanan [University of Southern California], and Dr. Bess Marcus [University of California, San Diego]) for their helpful comments and suggestions.
Funding. This research was supported by the Agency for Healthcare Research and Quality (grant R01-HS-019367) and the National Institute of Diabetes and Digestive and Kidney Diseases (grant R18-DK-067334). A.F. also received support from grant P30-DK-092924 from the National Institute of Diabetes and Digestive and Kidney Diseases. S.D.B. also received support from grant K01-DK-099404 from the National Institute of Diabetes and Digestive and Kidney Diseases.
The authors collected, analyzed, and interpreted the data and drafted the manuscript independently from the sponsors.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.F., M.M.H., S.D.B., and C.P.Q. conceived and designed the study; acquired, analyzed, and interpreted data; and drafted and critically revised the manuscript for important intellectual content. C.L.A. conceived and designed the study and critically revised the manuscript for important intellectual content. S.F.E. acquired, analyzed, and interpreted data and drafted and critically revised the manuscript for important intellectual content. A.-L.T. analyzed and interpreted data. B.J.C., B.S., N.P.G., J.A.S., E.P.G., A.A.M., W.H.H., and J.C. acquired data. Y.C. conceived and designed the study, acquired data, and critically revised the manuscript for important intellectual content. A.F. is the guarantor of this work and, as such, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Prior Presentation. Some of these data were presented at the 74th Scientific Sessions of the American Diabetes Association, San Francisco, CA, 13–17 June 2014, and at the Obesity Society’s Annual Scientific Meeting, Boston, MA, 2–7 November 2014.
Footnotes
Clinical trial reg. no. NCT01344278, clinicaltrials.gov.
This article contains Supplementary Data online at http://care.diabetesjournals.org/lookup/suppl/doi:10.2337/dc15-1254/-/DC1.
References
- 1.Knowler WC, Barrett-Connor E, Fowler SE, et al.; Diabetes Prevention Program Research Group . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002;346:393–403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tuomilehto J, Lindstrom J, Eriksson JG, et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. N Engl J Med 2001;344:1343–1350 [DOI] [PubMed] [Google Scholar]
- 3.Pan XR, Li GW, Hu YH, et al. . Effects of diet and exercise in preventing NIDDM in people with impaired glucose tolerance. The Da Qing IGT and Diabetes Study. Diabetes Care 1997;20:537–544 [DOI] [PubMed] [Google Scholar]
- 4.Ratner RE, Christophi CA, Metzger BE, et al.; Diabetes Prevention Program Research Group . Prevention of diabetes in women with a history of gestational diabetes: effects of metformin and lifestyle interventions. J Clin Endocrinol Metab 2008;93:4774–4779 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ferrara A, Kahn HS, Quesenberry CP, Riley C, Hedderson MM. An increase in the incidence of gestational diabetes mellitus: Northern California, 1991-2000. Obstet Gynecol 2004;103:526–533 [DOI] [PubMed] [Google Scholar]
- 6.Bellamy L, Casas JP, Hingorani AD, Williams D. Type 2 diabetes mellitus after gestational diabetes: a systematic review and meta-analysis. Lancet 2009;373:1773–1779 [DOI] [PubMed] [Google Scholar]
- 7.Peters RK, Kjos SL, Xiang A, Buchanan TA. Long-term diabetogenic effect of single pregnancy in women with previous gestational diabetes mellitus. Lancet 1996;347:227–230 [DOI] [PubMed] [Google Scholar]
- 8.American Diabetes Association Gestational diabetes mellitus. Diabetes Care 2004;27(Suppl. 1):S88–S90 [DOI] [PubMed] [Google Scholar]
- 9.Tunis SR, Stryer DB, Clancy CM. Practical clinical trials: increasing the value of clinical research for decision making in clinical and health policy. JAMA 2003;290:1624–1632 [DOI] [PubMed] [Google Scholar]
- 10.Ferrara A, Hedderson MM, Ching J, Kim C, Peng T, Crites YM. Referral to telephonic nurse management improves outcomes in women with gestational diabetes. Am J Obstet Gynecol 2012;206:491.e1–491.e5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ferrara A, Hedderson MM, Albright CL, et al. . A pragmatic cluster randomized clinical trial of diabetes prevention strategies for women with gestational diabetes: design and rationale of the Gestational Diabetes’ Effects on Moms (GEM) study. BMC Pregnancy Childbirth 2014;14:21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Go AS, Hylek EM, Phillips KA, et al. . Prevalence of diagnosed atrial fibrillation in adults: national implications for rhythm management and stroke prevention: the AnTicoagulation and Risk Factors in Atrial Fibrillation (ATRIA) Study. JAMA 2001;285:2370–2375 [DOI] [PubMed] [Google Scholar]
- 13.Hayes RJ, Moulton LH. Cluster Randomzed Trial. Boca Raton, Chapman & Hall/CRC, 2009 [Google Scholar]
- 14.Committee opinion no. 504: Screening and diagnosis of gestational diabetes mellitus [retracted in: Obstet Gynecol 2013;122:405]. Obstet Gynecol 2011;118:751–753 [DOI] [PubMed] [Google Scholar]
- 15.Bandura A. Social Foundations of Thought and Action: A social cognitive theory. Englewood Cliffs, NJ, Prentice Hall, 1986 [Google Scholar]
- 16.Prochaska JO, DiClemente CC. Common processes of self-change in smoking, weight control and psychological distress. In Coping and Substance Use. Shiffman S, Wills T, Eds. New York, Academic Press, 1985, p. 345–363 [Google Scholar]
- 17.U.S. Department of Labor. Bureau of Labor and Statistics occupational outlook handbook. Available from www.bls.gov/ooh/healthcare/dietitians-and-nutritionists.htm. Accessed 25 January 2015
- 18.Block G, Hartman AM, Dresser CM, Carroll MD, Gannon J, Gardner L. A data-based approach to diet questionnaire design and testing. Am J Epidemiol 1986;124:453–469 [DOI] [PubMed] [Google Scholar]
- 19.Chasan-Taber L, Schmidt MD, Roberts DE, Hosmer D, Markenson G, Freedson PS. Development and validation of a Pregnancy Physical Activity Questionnaire. Med Sci Sports Exerc 2004;36:1750–1760 [DOI] [PubMed] [Google Scholar]
- 20.Kroenke K, Spitzer RL, Williams JB. The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001;16:606–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kroenke K, Strine TW, Spitzer RL, Williams JB, Berry JT, Mokdad AH. The PHQ-8 as a measure of current depression in the general population. J Affect Disord 2009;114:163–173 [DOI] [PubMed] [Google Scholar]
- 22.American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 2015;38(Suppl. 1):S8–S16 [DOI] [PubMed] [Google Scholar]
- 23.Rubin DB. Inference and missing data. Biometrika 1976;63:581–592 [Google Scholar]
- 24.Little RJ, Long Q, Lin X. A comparison of methods for estimating the causal effect of a treatment in randomized clinical trials subject to noncompliance. Biometrics 2009;65:640–649 [DOI] [PubMed] [Google Scholar]
- 25.Baker SG. Compliance, all-or-none. In The Encyclopedia of Statistical Science. Vol. 1. New York, John Wiley and Sons, 1997, p. 134–138 [Google Scholar]
- 26.Ehrlich SF, Hedderson MM, Feng J, Davenport ER, Gunderson EP, Ferrara A. Change in body mass index between pregnancies and the risk of gestational diabetes in a second pregnancy. Obstet Gynecol 2011;117:1323–1330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kew S, Ye C, Hanley AJ, et al. . Cardiometabolic implications of postpartum weight changes in the first year after delivery. Diabetes Care 2014;37:1998–2006 [DOI] [PubMed] [Google Scholar]
- 28.Bao W, Tobias DK, Bowers K, et al. . Physical activity and sedentary behaviors associated with risk of progression from gestational diabetes mellitus to type 2 diabetes mellitus: a prospective cohort study. JAMA Intern Med 2014;174:1047–1055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu G, Tian H, Zhang F, et al. . Tianjin Gestational Diabetes Mellitus Prevention Program: study design, methods, and 1-year interim report on the feasibility of lifestyle intervention program. Diabetes Res Clin Pract 2012;98:508–517 [DOI] [PubMed] [Google Scholar]
- 30.Li G, Zhang P, Wang J, et al. . The long-term effect of lifestyle interventions to prevent diabetes in the China Da Qing Diabetes Prevention Study: a 20-year follow-up study. Lancet 2008;371:1783–1789 [DOI] [PubMed] [Google Scholar]
- 31.Ferrara A, Hedderson M, Albright CL, et al. . A pregnancy and postpartum lifestyle intervention in women with gestational diabetes mellitus reduces diabetes risk factors: a feasibility randomized control trial. Diabetes Care 2011;34:1519–1525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nicklas JM, Zera CA, England LJ, et al. . A web-based lifestyle intervention for women with recent gestational diabetes mellitus: a randomized controlled trial. Obstet Gynecol 2014;124:563–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ware JH, Hamel MB. Pragmatic trials--guides to better patient care? N Engl J Med 2011;364:1685–1687 [DOI] [PubMed] [Google Scholar]
- 34.Petter J, Reitsma-van Rooijen MM, Korevaar JC, Nielen MM. Willingness to participate in prevention programs for cardiometabolic diseases. BMC Public Health 2015;15:44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Robroek SJ, van Lenthe FJ, van Empelen P, Burdorf A. Determinants of participation in worksite health promotion programmes: a systematic review. Int J Behav Nutr Phys Act 2009;6:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jackson SL, Long Q, Rhee MK, et al. . Weight loss and incidence of diabetes with the Veterans Health Administration MOVE! lifestyle change programme: an observational study. Lancet Diabetes Endocrinol 2015;3:173–180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ali MK, Echouffo-Tcheugui J, Williamson DF. How effective were lifestyle interventions in real-world settings that were modeled on the Diabetes Prevention Program? Health Aff (Millwood) 2012;31:67–75 [DOI] [PubMed] [Google Scholar]
- 38.Appel LJ, Clark JM, Yeh HC, et al. . Comparative effectiveness of weight-loss interventions in clinical practice. N Engl J Med 2011;365:1959–1968 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.