Abstract
There is a pressing need to develop novel antimicrobials to circumvent the scourge of antimicrobial resistance. The objective of this study is to identify non-antibiotic drugs with potent antimicrobial activity, within an applicable clinical range. A library, containing 727 FDA approved drugs and small molecules, was screened against ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter cloacae). Drugs that showed antimicrobial activity in an applicable clinical range were further tested in vitro and in vivo in an infected mouse model. The initial screening identified 24 non-antibiotic drugs and clinical molecules active against Gram-positive pathogens including methicillin-resistant S. aureus (MRSA) and vancomycin-resistant enterococcus (VRE) isolates. Two non-antibiotic drugs showed activity against Gram-negative pathogens. Among the active non-antibiotic drugs, only ebselen (EB) and 5-fluoro-2′-deoxyuridine (FdUrd), showed bactericidal activity, in an applicable clinical range, against multi-drug-resistant Staphylococcus isolates including MRSA, vancomycin-resistant S. aureus (VRSA), and vancomycin-intermediate S. aureus (VISA). The minimum inhibitory concentration at which 90% of clinical isolates of S. aureus were inhibited (MIC90) was found to be 0.25 and 0.0039 mg/L for EB and FdUrd, respectively. Treatment with EB orally significantly increased mice survival in a lethal model of septicemic MRSA-infection by (60%) compared to that of control. FdUrd oral and intraperitoneal treatment significantly enhanced mouse survival by 60% and 100%, respectively. These data encourage screening and repurposing of non-antibiotic drugs and clinical molecules to treat multidrug-resistant bacterial infections.
Keywords: repurposing, antibiotics, MRSA, antimicrobial, repositioning
1. Introduction
Bacterial infections have become a serious threat to the global public health due to the dearth of effective antimicrobials. Moreover, the development of new antibiotics is becoming increasingly difficult and is unable to keep pace with the rapid emergence of resistant pathogens. Hence, novel drugs and new approaches are urgently needed. Both de novo drug discovery and drug repurposing have been used in the search for effective antibiotics. Unlike the lengthy and costly process of de novo drug discovery, drug repurposing can reduce the time, cost and risk associated with drug innovation [1]. Drug repurposing has already resulted in successes in a number of disease areas, including infectious diseases [2]. Though antibiotics have been repurposed for other clinical indications, to date, not a single non-antibiotic drug has been repurposed and approved for use as an antibacterial. Given the crucial problem posed by multidrug resistant pathogens, especially ESKAPE pathogens (Enterococcus faecium, Staphylococcus aureus Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp), additional effort needs to be focused on using drug repurposing to uncover new treatment options.
Several non-antibiotic drugs have been found to exhibit bactericidal activity; however, they possess high minimum inhibitory concentration (MIC) values that cannot be achieved clinically. In an intensive search for antimicrobial activity in an applicable clinical range among non-antimicrobial drugs, we identified two drugs, ebselen (EB) and 5-fluoro-2′-deoxyuridine (FdUrd), with potent antibacterial activities against Gram-positive pathogens, including highly multidrug-resistant clinical isolates of S. aureus. These two drugs showed antibacterial activity against clinical isolates of methicillin-resistant S. aureus (MRSA), vancomycin-resistant S. aureus (VRSA), and vancomycin-intermediate S. aureus (VISA) with MIC values in submicromolar concentrations. Additionally, both drugs significantly increased mice survival in a lethal model of septicemic MRSA-infection.
2. Materials and methods
2.1. Bacterial strains
ESKAPE pathogens vancomycin resistant E. faecium ATCC 700221 (VRE), MRSA USA300, carbapenemase (KPC)-producing blaKPC+ K. pneumoniae ATCC BAA-1705, multi-drug-resistant A. baumannii ATCC BAA-1605, P. aeruginosa ATCC 15442, and E. cloacae ATCC BAA-1143 were used for the initial screening. Clinical isolates of methicillin-sensitive S. aureus (MSSA), MRSA, VRSA, VISA, and S. epidermidis are described in Table 1.
Table 1.
The MIC and MBC of Ebselen and FdUrd against Gram-positive Pathogens
| Strains type | Strain ID | Isolation | Phenotypic properties | MIC/MBC (mg/L) | ||
|---|---|---|---|---|---|---|
| Origin | year | Ebselen | FdUrd | |||
| Vancomycin-resistant S. aureus (VRSA) | VRSA1 | United States | 2002 | Resistant to erythromycin and spectinomycin as well as being multiresistant to other commonly used therapeutic agents | 0.25/0.25 | 0.0078/0.0078 |
| VRSA2 | United States | 2002 | Resistant to erythromycin and spectinomycin as well as being multiresistant to other commonly used therapeutic agents | 0.25/0.25 | 0.0039/0.0156 | |
| VRSA3a | United States | 2004 | Resistance to tetracycline, macrolides, lincosamides and aminoglycoside | 0.25/0.25 | 0.000975/0.000975 | |
| VRSA3b | United States | - | - | 0.25/1 | 0.00049/0.00049 | |
| VRSA4 | United States | 2005 | Resistant to erythromycin and spectinomycin | 0.125/0.125 | 0.00195/0.0039 | |
| VRSA5 | United States | 2005 | Resistant to erythromycin and spectinomycin | 0.25/0.25 | 0.00195/0.00195 | |
| VRSA6 | United States | - | Resistant to vancomycin | 0.25/0.5 | 0.00049/0.00195 | |
| VRSA7 | United States | 2006 | Resistant to β-lactams, erythromycin and spectinomycin | 0.5/0.5 | 0.00195/0.0039 | |
| VRSA8 | United States | 2007 | Resistant to erythromycin and spectinomycin | 0.125/0.125 | 0.00049/0.000975 | |
| VRSA9 | United States | 2007 | Resistant to erythromycin and spectinomycin | 0.25/0.5 | 0.000975/0.00195 | |
| VRSA10 | United States (Michigan) | 2009 | Resistant to ciprofloxacin, clindamycin, erythromycin, gentamicin, and vancomycin | 0.25/0.25 | 0.000975/0.000975 | |
| VRSA11a | United States | 2010 | Resistant to erythromycin and spectinomycin | 0.125/0.25 | 0.000975/0.0156 | |
| VRSA11b | United States | 2010 | Resistant to erythromycin and spectinomycin | 0.25/0.25 | 0.000975/0.0039 | |
| VRSA12 | United States | - | Resistant to vancomycin | 0.25/0.5 | 0.00195/0.0039 | |
| VRSA13 | United States | - | Resistant to vancomycin | 0.25/0.25 | 0.00195/0.0156 | |
| Methicillin resistant S. aureus (MRSA) | NRS382 | United States (Ohio) | - | Resistant to ciprofloxacin, clindamycin, erythromycin, and methicillin | 0.125/0.125 | 0.000975/0.0078 |
| NRS383 | United States (NorthCarolina | - | Resistant to ciprofloxacin, clindamycin, erythromycin, gentamicin, and methicillin | 0.125/0.125 | 0.0078/0.0078 | |
| NRS 384 | United States (Mississippi) | - | Resistant to erythromycin, methicillin, and tetracycline | 0.125/0.125 | 0.00195/0.00195 | |
| NRS 385 | United States (Connecticut) | - | Resistant to ciprofloxacin, clindamycin, erythromycin, gentamicin, methicillin, tetracycline, and trimethoprim | 0.5/1 | 0.0315/0.0315 | |
| NRS 386 | United States (Louisiana) | - | Resistant to erythromycin and methicillin | 0.125/1 | 0.00195/0.0078 | |
| NRS 387 | United States (Washington) | - | Resistant to methicillin | 0.125/0.25 | 0.0039/0.0156 | |
| NRS 483 | United States (Vermont) | - | Resistant to erythromycin and methicillin | 0.125/0.5 | 0.00195/0.0078 | |
| NRS 484 | United States (Alaska) | Resistant to methicillin | 0.25/0.25 | 0.000975/0.0039 | ||
| NRS70 | Japan | 1982 | Resistant to clindamycin, erythromycin and spectinomycin | 0.25/0.25 | 0.0039/0.00315 | |
| NRS71 | United Kingdom | - | Resistant to tetracycline and methicillin | 0.25/0.25 | 0.00195/0.00195 | |
| NRS100 | United Kingdom | - | Resistant to tetracycline | 0.25/0.5 | 0.000975/0.000975 | |
| NRS108 | France | - | Resistant to gentamicin | 0.25/0.25 | 0.0078/0.0156 | |
| NRS119 | United States (Massachuset) | 2001 | Resistant to linezolid | 0.125/0.25 | 0.0039/0.0156 | |
| NRS123 | United States | 1998 | Resistant to methicillin; susceptible to nonbeta-lactam antibiotics | 0.25/0.5 | 0.000975/0.0039 | |
| NRS194 | United States (North Dakota) | 1999 | Resistant to methicillin | 0.25/1 | 0.0039/0.0625 | |
| Methicillinsensitive S. aureus (MSSA | NRS72 | United Kingdom | - | Resistant to penicillin | 0.25/0.5 | 0.000975/0.00195 |
| NRS77 | United Kingdom | 1960 | Used for typing phage 47 and is considered to be the original strain for most S. aureus genetic research. | 0.25/1 | 0.00049/0.00049 | |
| NRS846 | - | - | - | 0.125/>64 | 0.0039/0.0039 | |
| NRS860 | - | - | - | 0.25/0.25 | 0.00195/0.00195 | |
| Vancomycinintermediate S. aureus (VISA | NRS 1 | Japan | 1996 | Resistant to aminoglycosides and tetracycline (minocycline)Glycopeptide-intermediate S. aureus | 0.125/0.125 | 0.00195/0.00195 |
| NRS19 | United States (Illinois) | 1999 | Glycopeptide-intermediate S. aureus | 0.25/0.025 | 0.00049/0.00195 | |
| NRS37 | France | 1995 | Glycopeptide-intermediate S. aureus | 0.125/0.125 | 0.00195/0.0078 | |
| S. epidermidis | NRS 101 | United States | - | Prototype biofilm producer, Resistant to methicillin and gentamicin | 0.5/0.5 | 0.0078/0.0078 |
| Enterococcus species | E. fecalis ATCC 51229 (VRE) | Peritoneal fluid, St. Louis, MO | - | Low-level vancomycin-resistant, VanB Resistant to Vancomycin. Sensitive to Teichoplanin. |
0.5/0.5 | 0.125/0.125 |
| E. faecium ATCC 700221 (VRE) | Human feces, Connecticut1 | - | Resistant to Vancomycin and Teicoplanin | 0.5/1 | 0.0625/0.125 | |
2.2. Compounds and library
The NIH Clinical Collections 1 and 2 (http://www.nihclinicalcollection.com) containing 727 FDA approved drugs and small molecules previously used in human clinical trials with known safety profiles [3] were screened against ESKAPE pathogens. The library was initially screened at a single concentration of 16 μM to identify “hit” non-antibacterial drugs. Once the antimicrobial activity of the drugs was confirmed, we determined their MICs and minimum bactericidal concentrations (MBCs) according to the Clinical and Laboratory Standards Institute (CLSI) [4]. We selected drugs that showed potent antimicrobial activity in an applicable clinical range for further screening and testing in vitro and in vivo in an infected mouse model.
2.3. Animals
Animal procedures were approved by the Purdue University Animal Care and Use Committee (PACUC) (protocol no. 1311000988). Eight-week-old female BALB/c mice (Harlan Laboratories, Indianapolis, IN) were used for this study. Mice were rendered neutropenic by treatment with cyclophosphamide intraperitoneally (IP) 150 and 100 mg/kg at four days and one day before infection, respectively. Neutropenic mice were inoculated IP with 8×108 MRSA USA200. At one hour after infection, the mice were divided into five groups (n = 10 per group), and two groups were treated orally with either EB 30 mg/kg or FdUrd 25 mg/kg. Two groups were treated IP with either EB 30 mg/kg or FdUrd 25 mg/kg. One group was used as a control with no treatment. Treatment was continued once daily for two more days (animals received three doses total). Mortality was monitored four times daily for six days, and the cumulative percent survival was determined. The data were subjected to statistical analysis using Kaplan-Meier survival curves. A log rank test was performed for 95% confidence intervals by GraphPad Prism 6.0 (GraphPad Software, La Jolla, CA).
3. Results
3.1. In vitro antimicrobial activity
Our initial screening identified 24 non-antibiotic drugs and clinical molecules active against Gram-positive pathogens MRSA and VRE (Table 2). Among the active non-antimicrobial drugs against MRSA identified in the NIH Clinical Collections, EB and FdUrd showed potent bactericidal activity in a nano-molar (clinically achievable) range. Both drugs demonstrated potent bactericidal activity against clinical multi-drug-resistant Staphylococcus isolates. The MIC at which 90% of S. aureus isolates were inhibited (MIC90) was found to be 0.25 and 0.0039 mg/L for EB and FdUrd, respectively (Table 1). Only two non-antibiotic drugs showed activity against Gram-negative pathogens (Table 2).
Table 2.
MIC and MBC of non-antibacterial drugs against Gram-positive and negative pathogens
| N | NCC structure | Name | Description | MIC/MBC μM | |
|---|---|---|---|---|---|
| MRSA | VRE | ||||
| 1 | CPD000466308 | Epirubicin | Antineoplastic | 16/64 | 8 |
| 2 | CPD000469213 | Toremifene | Antineoplastic | 16/16 | 16 |
| 3 | CPD000058267 | Isoproterenol | Bronchodilator | 16/64 | >16 |
| 4 | CPD001491671 | Tamoxifen | Antineoplastic | 16/16 | >16 |
| 5 | CPD001317855 | Clomid | Treat infertility in women | 16/32 | 16 |
| 6 | CPD001906781 | Daunorubicin | Antineoplastic | 16/16 | 8 |
| 7 | CPD000469293 | Oxiconazole | Antifungal | 2/32 | >16 |
| 8 | CPD000466357 | Triclabendazole | Anthelmintic | 8/32 | >16 |
| 9 | CPD000466363 | Carmofur | Antineoplastic | 2/4 | >16 |
| 10 | CPD000466355 | Idarubicin | Antineoplastic | 4/4 | 4 |
| 11 | CPD000466278 | MK-886 | Leukotriene antagonist | 8/16 | 8 |
| 12 | CPD000058970 | Bifonazole | Antifungal | 8/>64 | >16 |
| 13 | CPD000058306 | Clotrimazole | Antifungal | 4/64 | >16 |
| 14 | CPD000466300 | 5-Nonyloxytryptamine | Serotonin receptor agonist | 4/8 | 8 |
| 15 | CPD000058445 | Ebselen | Mimic glutathione peroxidase | <0.5/0.5 | 0.5/1 |
| 16 | CPD001370749 | Econazole | Antifungal | 4/32 | >16 |
| 17 | CPD000058733 | Miconazole | Antifungal | 4/8 | >16 |
| 18 | CPD000038082 | 5-Fluorouracil | Antineoplastic | 4/16 | >16 |
| 19 | CPD001496941 | FdUrd | Antineoplastic | <0.5/0.5 | <0.5/0.5 |
| 20 | CPD000059106 | Ftorafur | Antineoplastic | >16 | 16 |
| 21 | CPD000469217 | Raltitrexed | Antineoplastic | >16 | 2 |
| 22 | CPD000469227 | Dactinomycin | Antineoplastic | >16 | 0.5 |
| 23 | CPD000058394 | Acetazolamide | Antiglaucoma | >16 | 1 |
| 24 | CPD000058202 | Furosemide | Diuretic | >16 | 4 |
| N | Drug | MIC/MBC μM | |||
|---|---|---|---|---|---|
| K. pneumoniae | A. baumannii | E. cloacae | P. aeruginosa | ||
| 1 | 5-Nonyloxytryptamine | >16 | 16 | 16 | >16 |
| 2 | FdUrd | 8 | >16 | >16 | >16 |
3.2. Protection of mice against MRSA infection
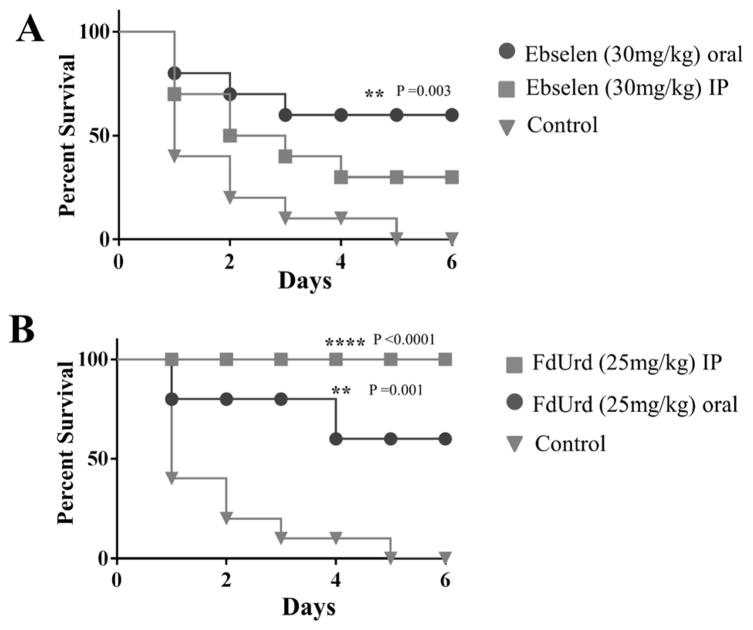
EB oral treatment, but not IP, significantly increased mice survival (60%) compared to that of control (Figure 1A). FdUrd oral and IP treatment significantly enhanced mouse survival by 60% and 100%, respectively, compared to that of control (Figure 1B).
Figure 1.
Efficacy of treatment of MRSA septicemic infection with ebselen (A) and FdUrd (B). Mice received one treatment daily for three days. Mice were monitored for six days and the cumulative percent survival was determined. The data were subjected to statistical analysis using Kaplan-Meier survival curves. A log rank test was performed for 95% confidence intervals by GraphPad Prism 6.0.
4. Discussion
It is well established that currently approved antimicrobials are losing the battle in the fight against multidrug-resistant pathogens. Without a doubt, novel antimicrobials and novel approaches to develop them are urgently needed; however, new antimicrobials are becoming increasingly difficult to develop. Repurposing FDA-approved drugs, with well-characterized toxicology and pharmacology, to find new applications outside the scope of the original medical indication is a novel way to reduce both the time and cost associated with antimicrobial innovation.
Whole-cell screening assays of non-antibiotic drugs and clinical safe molecules in the NIH Clinical Collections against ESKAPE pathogens revealed several non-antibiotic drugs with antimicrobial activity (Table 2). Although some of these drugs were known to have antibacterial activity, many were unexpected because their clinical indications are unrelated to treatment of microbial infections.
Among the active non-antimicrobial drugs against MRSA, EB and FdUrd showed potent bactericidal activity in a nano-molar (clinically achievable) range. Both drugs demonstrated potent bactericidal activity against clinical multi-drug-resistant Staphylococcus isolates, including linezolid-resistant S. aureus, VRSA, VISA, and MRSA. Additionally, the activities of EB and FdUrd exceeded those determined for vancomycin and linezolid. Furthermore, they exhibited strong antimicrobial activity against other important Gram-positive pathogens, including VRE and methicillin-resistant S. epidermidis.
EB, an organoselenium compound, is considered clinically safe but without proven use [3]. It has been widely studied for its anti-inflammatory, anti-atherosclerotic, and antioxidative properties [5]. Independent of its anti-inflammatory effect, EB also has been shown to exhibit antimicrobial activity in vitro [6–8]. It inhibits the thioredoxin reductase enzyme in Escherichia coli, and it inhibits the antigen 85 (Ag85) complex in Mycobacterium tuberculosis [7, 8]. However, clinical applications and the underlying mechanism of action for its antibacterial activity against S. aureus still remain unclear. Treatment with EB orally, but not IP, significantly increased mice survival (60%) in a lethal model of septicemic MRSA-infection compared to that of control (Figure 1A). EB is rapidly absorbed from the gastrointestinal tract, and it maintains a plasma concentration of 2.91 mg/L with 30mg/kg dose (according to increase in selenium concentration in the blood of 0.84±0.1 mg/L−1) [9], several-fold higher than MIC90. Higher doses or multiple doses might be required to achieve 100% protection. Furthermore, the recognized anti-inflammatory response [5] of EB, combined with the potent antimicrobial activity, make it an excellent candidate for topical treatment of MRSA skin infections.
FdUrd is an anticancer drug used to treat colorectal cancers [10]. FdUrd inhibits thymidylate synthase (a key enzyme in DNA synthesis) and acts as a nucleoside analog that impairs the metabolism and structure of nucleic acids [10]. The antimicrobial activity of nucleoside analog against S. aureus has been demonstrated in vitro [11]. FdUrd oral and IP treatment significantly enhanced mouse survival in a lethal model of septicemic MRSA-infection by 60% and 100%, respectively, compared to that of control (Figure 1B). The bioavailability of oral FdUrd is unpredictable, which probably contributed to a lower protection rate compared to an IP route [12]. While FdUrd shows preclinical promise as a medication for the treatment of MRSA infections, the therapeutic dose can be reduced significantly to avoid drug-associated toxicity. The achieved plasma concentration of the drug after receiving a therapeutic dose is estimated to be more than a thousand-folds higher than the required MIC90 against MRSA [13–15].
Although the direct antimicrobial activity of several non-antimicrobial drugs is well documented, to date, no FDA-approved drug has been repurposed to treat bacterial infections. The non-antibiotic drugs that showed antimicrobial activity serve as untapped reservoir for new antibiotic leads that could lead to identification of new targets which will guide the future development of improved antimicrobial agents. The library we screened represents only 7% of the total drugs known to clinical medicine [1]. Finding two hits in a clinical range illustrates the potential to identify more drugs with potent antimicrobial activity and encourage the screening of all clinical compounds to complement current antibiotics. The fact that auranofin, a FDA-approved gold compound used for treating rheumatoid arthritis, recently has been granted orphan-drug status from the FDA for treatment of human amebiasis, further validates antimicrobial repurposing approach.[2]
Acknowledgments
The NIH Clinical Collections 1 & 2 were provided by the National Institutes of Health (NIH). The bacterial strains used in this study were provided by the Network on Antimicrobial Resistance in Staphylococcus aureus (NARSA) for distribution by BEI Resources.
FUNDING
This work was supported by internal funding from Purdue University. Waleed Younis is supported by a scholarship from the Egyptian Cultural and Educational Bureau (ECEB) in Washington DC, USA.
References
- 1.Chong CR, Sullivan DJ. New uses for old drugs. Nature. 2007;448:645–6. doi: 10.1038/448645a. [DOI] [PubMed] [Google Scholar]
- 2.Debnath A, Parsonage D, Andrade RM, He C, Cobo ER, Hirata K, et al. A high-throughput drug screen for Entamoeba histolytica identifies a new lead and target. Nature medicine. 2012;18:956–60. doi: 10.1038/nm.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Austin CP, Brady LS, Insel TR, Collins FS. NIH Molecular Libraries Initiative. Science. 2004;306:1138–9. doi: 10.1126/science.1105511. [DOI] [PubMed] [Google Scholar]
- 4.CLSI. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard M7-A7. CLSI; Wayne, PA: 2007. [Google Scholar]
- 5.Schewe T. Molecular actions of ebselen--an antiinflammatory antioxidant. General pharmacology. 1995;26:1153–69. doi: 10.1016/0306-3623(95)00003-j. [DOI] [PubMed] [Google Scholar]
- 6.Nozawa R, Yokota T, Fujimoto T. Susceptibility of methicillin-resistant Staphylococcus aureus to the selenium-containing compound 2-phenyl-1,2-benzoisoselenazol-3(2H)-one (PZ51) Antimicrobial agents and chemotherapy. 1989;33:1388–90. doi: 10.1128/aac.33.8.1388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lu J, Vlamis-Gardikas A, Kandasamy K, Zhao R, Gustafsson TN, Engstrand L, et al. Inhibition of bacterial thioredoxin reductase: an antibiotic mechanism targeting bacteria lacking glutathione. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:1394–403. doi: 10.1096/fj.12-223305. [DOI] [PubMed] [Google Scholar]
- 8.Favrot L, Grzegorzewicz AE, Lajiness DH, Marvin RK, Boucau J, Isailovic D, et al. Mechanism of inhibition of Mycobacterium tuberculosis antigen 85 by ebselen. Nature communications. 2013;4:2748. doi: 10.1038/ncomms3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Imai H, Masayasu H, Dewar D, Graham DI, Macrae IM. Ebselen protects both gray and white matter in a rodent model of focal cerebral ischemia. Stroke; a journal of cerebral circulation. 2001;32:2149–54. doi: 10.1161/hs0901.095725. [DOI] [PubMed] [Google Scholar]
- 10.van Laar JA, van der Wilt CL, Rustum YM, Noordhuis P, Smid K, Pinedo HM, et al. Therapeutic efficacy of fluoropyrimidines depends on the duration of thymidylate synthase inhibition in the murine colon 26-B carcinoma tumor model. Clinical cancer research : an official journal of the American Association for Cancer Research. 1996;2:1327–33. [PubMed] [Google Scholar]
- 11.Sandrini MP, Clausen AR, On SL, Aarestrup FM, Munch-Petersen B, Piskur J. Nucleoside analogues are activated by bacterial deoxyribonucleoside kinases in a species-specific manner. The Journal of antimicrobial chemotherapy. 2007;60:510–20. doi: 10.1093/jac/dkm240. [DOI] [PubMed] [Google Scholar]
- 12.Malet-Martino M, Martino R. Clinical studies of three oral prodrugs of 5-fluorouracil (capecitabine, UFT, S-1): a review. The oncologist. 2002;7:288–323. doi: 10.1634/theoncologist.7-4-288. [DOI] [PubMed] [Google Scholar]
- 13.Israel VK, Jiang C, Muggia FM, Tulpule A, Jeffers S, Leichman L, et al. Intraperitoneal 5-fluoro-2′-deoxyuridine (FUDR) and (S)-leucovorin for disease predominantly confined to the peritoneal cavity: a pharmacokinetic and toxicity study. Cancer chemotherapy and pharmacology. 1995;37:32–8. doi: 10.1007/BF00685626. [DOI] [PubMed] [Google Scholar]
- 14.Muggia FM, Chan KK, Russell C, Colombo N, Speyer JL, Sehgal K, et al. Phase I and pharmacologic evaluation of intraperitoneal 5-fluoro-2′-deoxyuridine. Cancer chemotherapy and pharmacology. 1991;28:241–50. doi: 10.1007/BF00685529. [DOI] [PubMed] [Google Scholar]
- 15.Muggia FM, Tulpule A, Retzios A, Chen F, Jeffers S, Leichman CG, et al. Intraperitoneal 5-fluoro-2′-deoxyuridine with escalating doses of leucovorin: pharmacology and clinical tolerance. Investigational new drugs. 1994;12:197–206. doi: 10.1007/BF00873960. [DOI] [PubMed] [Google Scholar]