Abstract
Methicillin- and vancomycin-resistant Staphylococcus aureus (MRSA and VRSA) have emerged as a global health concern. A new class of compounds featuring an aryl isonitrile moiety has been discovered that exhibits potent inhibitory activity against several clinically-relevant MRSA and VRSA isolates. Structure-activity relationship studies have been conducted to identify the aryl isonitrile group as the key functional group responsible for the observed antibacterial activity. The most potent antibacterial aryl isonitrile analogs (MIC 2 µM) did not show any toxicity against mammalian cells up to a concentration of 64 µM.
Keywords: Antibiotic, Drug Resistance, MRSA, VRSA, Isonitrile
INTRODUCTION
Multidrug-resistant bacterial infections pose a significant global health challenge afflicting more than 2 million people each year in the United States alone, resulting in over 23,000 fatalities.1 Nearly half of these casualties are due to infections caused by a single pathogen, methicillin-resistant Staphylococcus aureus (MRSA). Currently prevalent in the community setting, MRSA is responsible for a wide spectrum of illnesses from superficial skin infections to invasive diseases including pneumonia, osteomyelitis, and bloodstream infections.2–5 While a robust arsenal of antibiotics was once capable of treating MRSA infections, strains of this pathogen have emerged that exhibit resistance to nearly every class of antibiotics, including agents of last resort such as vancomycin and linezolid.6–11 This underscores the urgent need for the identification and development of novel therapeutic options capable of treating infections due to MRSA.12
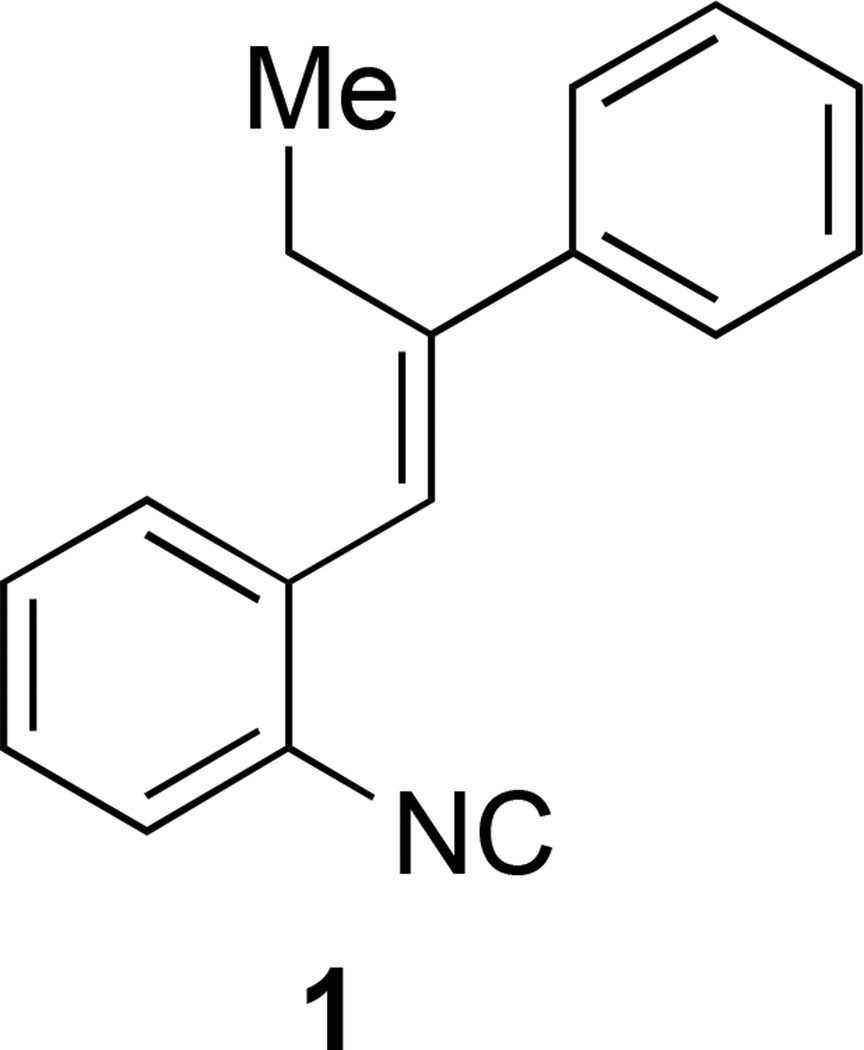
Recently, we have conducted a whole-cell screening of a small number of in house generated small molecules (about 250 molecules) against MRSA USA300 with the aim to identify compounds with novel skeletons to target antibiotic drug resistance. To our delights, among several hit molecules revealed by this screening effort, compound 1 with an isonitrile group attached to a stilbene system was shown to be capable of inhibiting bacterial growth at a concentration of 32 µM (Figure 1). Further analysis revealed this compound is bacteriostatic (the minimum bactericidal concentration exceeded 128 µM). The presence of an isonitrile moiety in this compound is quite unique given that few antimicrobial compounds possessing the isonitrile moiety in their core structure have been described in literature and all of them are complex natural products and are difficult to access.13–19 Natural terpene isonitrile-containing molecules and simplified analogs have been reported to show antimalarial activity as well.20–22 The novel structural skeleton of compound 1 as an antibacterial compound against drug resistant strains prompted us to further study of this type of isonitrile compounds. Herein, we report our chemical synthesis, structure-activity relationship study, and evaluation of the antibacterial performance of compound 1 and closely related analogs against several clinically-relevant MRSA and VRSA strains. These efforts have led to the identification of more potent compounds with MIC as low as 2 µM but do not show any cytotoxicity against mammalian cells up to a concentration of 64 µM. Physiochemical analysis of this potent lead compound has been described to guide the next stage of developing these promising compounds into the antibiotic drug pipeline.
FIGURE 1.
Structure of hit compound 1.
CHEMICAL SYNTHESIS
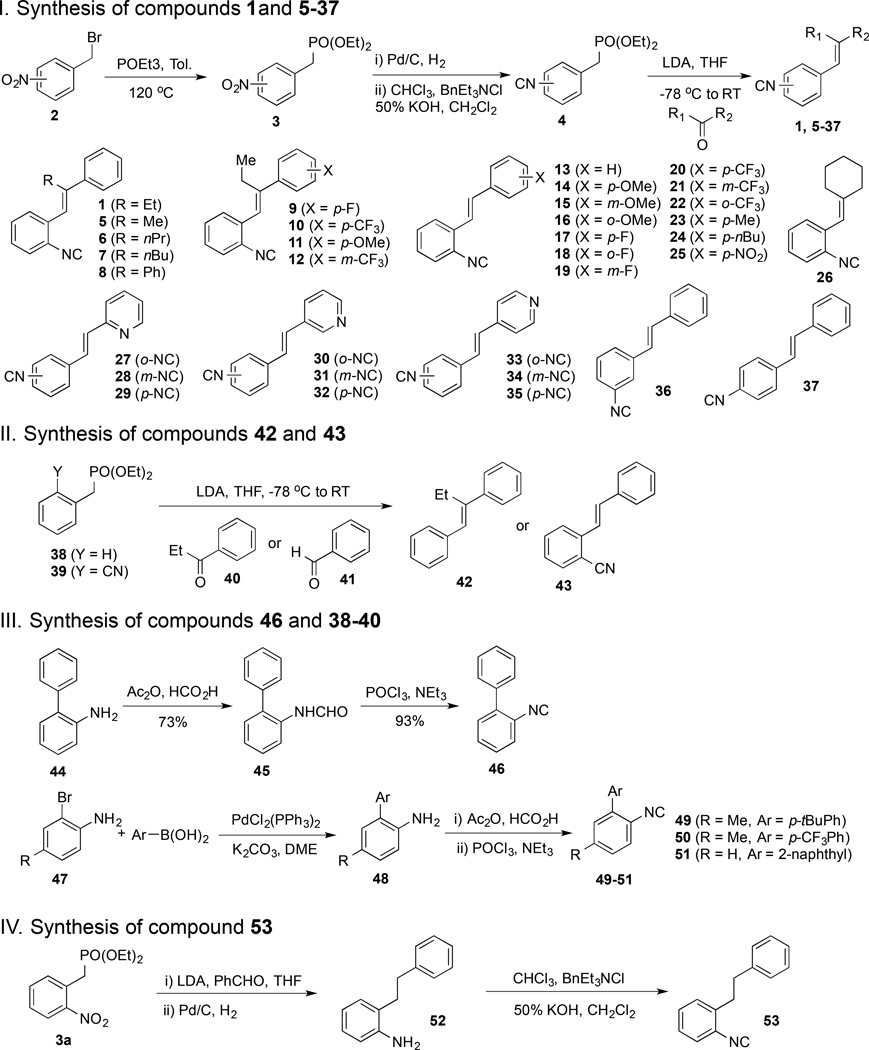
In general, the stilbene isonitrile analogs were prepared from benzylic bromide 2, which was converted to phosphonate 3 by Michaelis-Arbuzov reaction.23 The nitro group of 3 was then converted to an isonitrile group upon a sequence of hydrogenation and Hofmann isonitrile synthesis using dichlorocarbene.24 Compound 4 then served as a divergent point to synthesize a collection of analogs with a Horner-Wadsworth-Emmons reaction.25 By treating various ketones and aldehydes with stabilized phosphonate carbanions derived from phosphonates 4, we obtained thirty-three stilbene isonitrile analogs (1, 5–25, and 27–37) and one styrene isonitrile analog (26). This collection also includes compounds with the isonitrile group at different positions on the aromatic ring as well as pyridine containing analogs. In order to investigate the importance of the isonitrile group for the observed biological activity, compound containing a hydrogen atom (42) or a nitrile group (43) at the isonitrile-substitution position was prepared as well using the Horner-Wadsworth-Emmons reaction. Additionally, four biary isonitrile analogs (46 and 49–51) were prepared.26 Compound 45 was prepared from commercially available amine 33 via formamide formation followed by dehydration. Compounds 49–51 were synthesized from 2-bromoaniline derivatives (47) and arylboronic acids. Suzuki cross-coupling converted 47 to biaryl amines 48 smoothly. The latter was then converted to 49–51 via the aforementioned formamide formation and dehydration sequence. Lastly, we prepared compound 53 with a saturated two-carbon chain to investigate the importance of the double bond linker between the two aromatic moieties. All the newly synthesized compounds were purified using flash chromatography before entering biological evaluations.
BIOLOGICAL RESULTS AND DISCUSSION
Antimicrobial susceptibility analysis of the isonitrile compounds against clinically-relevant isolates of MRSA and VRSA
The bacterial growth inhibiting activity of these synthetic analogs of hit compound 1 were subsequently evaluated (Table 2). When these derivatives were screened against MRSA, via the broth microdilution assay, the results revealed several interesting structural elements that appear to play an important role in the antimicrobial activity of these compounds. Initial inspection of the structural moieties of 1 revealed that the presence of an isonitrile group is essential for its antimicrobial activity. When the isonitrile group of 1 (MIC against MRSA ranging from 8–64 µM) was removed (as in compound 42), a complete loss in the anti-MRSA activity of 42 is observed (MIC > 128 µM). A similar pattern is observed when reviewing the MIC results for compounds 13 and 43. Compound 13, one of the most potent derivatives constructed (with MIC values against MRSA as low as 2 µM), contains the isonitrile group; when the isonitrile group of 13 is replaced with an isosteric nitrile group (resulting in compound 43), complete loss of antimicrobial activity was observed. Similarly, compound 53 with an isonitrile group is active against several strains evaluated particularly MRSA USA100, MRSA USA300, MRSA NRS119, and VISA NRS1, while compound 52 without the isonitrile group lacks antimicrobial activity. These results confirm that the isonitrile group appears necessary for these compounds to possess activity against MRSA and may play an important role in binding to the compound’s molecular target.
Table 2.
Minimum inhibitory concentration (MIC, in µM) of isonitrile compounds, linezolid, and vancomycin against methicillin-sensitive (MSSA) and methicillin-resistant Staphylococcus aureus (MRSA) strains.
| Compound Name |
MSSA1 (NRS72) |
MRSA USA100 |
MRSA USA200 |
MRSA USA300 |
MRSA USA500 |
MRSA USA700 |
MRSA NRS119 |
VISA1 NRS1 |
VRSA2 VRS2 |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 8 | 8 | 16 | 32 | 32 | 8 | 64 | 8 | 32 |
| 4a | >128 | >128 | 128 | >128 | >128 | >128 | >128 | 128 | >128 |
| 5 | 8 | 8 | 64 | >128 | >128 | 16 | >128 | 4 | >128 |
| 6 | 16 | 16 | 16 | 32 | 16 | 16 | 32 | 8 | 32 |
| 7 | 32 | 32 | 32 | >128 | 32 | 16 | >128 | 16 | 64 |
| 8 | 8 | 8 | 8 | 16 | 8 | 4 | 16 | 4 | 16 |
| 9 | 8 | 16 | 16 | 32 | 16 | 8 | 64 | 8 | 32 |
| 10 | 8 | 16 | 16 | 32 | 16 | 16 | 64 | 8 | 16 |
| 11 | 8 | 8 | 8 | 16 | 8 | 4 | >128 | 4 | 8 |
| 12 | 4 | 4 | 8 | >128 | 8 | 2 | 8 | 2 | 8 |
| 13 | 16 | 2 | 4 | 4 | 32 | 4 | 4 | 2 | 32 |
| 14 | 4 | 8 | 2 | 16 | >128 | 8 | 32 | 4 | 32 |
| 15 | 8 | 8 | 4 | 16 | 32 | 16 | 32 | 4 | 32 |
| 16 | 8 | 8 | 8 | 8 | 8 | 8 | 16 | 4 | 16 |
| 17 | 4 | 8 | 2 | 8 | 16 | 8 | 16 | 4 | 32 |
| 18 | 4 | 8 | 2 | 16 | 16 | 8 | 32 | 4 | 32 |
| 19 | 8 | 16 | 16 | 32 | 128 | 16 | 128 | 8 | 128 |
| 20 | 4 | 4 | 2 | 8 | 16 | 2 | 16 | 2 | 16 |
| 21 | 2 | 4 | 4 | 8 | 8 | 4 | 8 | 2 | 8 |
| 22 | 8 | 16 | 16 | 32 | 16 | 16 | >128 | 8 | 32 |
| 23 | 16 | 4 | 4 | 16 | 32 | 8 | 64 | 4 | 32 |
| 24 | >128 | - | >128 | >128 | >128 | - | >128 | - | >128 |
| 25 | 2 | 4 | 4 | 8 | 8 | 8 | 16 | 4 | 8 |
| 26 | 8 | 16 | 64 | >128 | >128 | 16 | >128 | 4 | >128 |
| 27 | 64 | 64 | 64 | 16 | 64 | 32 | 128 | 64 | 32 |
| 28 | 64 | 32 | 64 | 32 | 64 | 64 | 128 | 128 | 128 |
| 29 | 64 | 128 | 128 | 32 | 64 | 128 | 128 | 64 | 64 |
| 30 | 64 | 32 | 32 | 16 | 32 | 32 | 64 | 64 | 32 |
| 31 | 64 | 32 | 64 | 32 | 64 | 64 | 64 | 64 | 64 |
| 32 | 16 | 16 | 32 | 8 | 32 | 16 | 64 | 32 | 8 |
| 33 | 64 | 32 | 32 | 32 | 32 | 32 | 64 | 64 | 64 |
| 34 | 64 | 32 | 64 | 32 | 64 | 64 | 32 | 32 | 64 |
| 35 | 2 | 8 | 8 | 4 | 4 | 8 | 4 | 8 | 4 |
| 36 | 16 | 32 | 32 | 16 | >128 | 16 | 64 | 32 | 32 |
| 37 | 4 | 4 | 32 | 4 | 16 | 64 | >128 | >128 | >128 |
| 42 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 |
| 43 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 |
| 46 | >128 | 16 | 16 | >128 | >128 | 16 | >128 | 8 | >128 |
| 49 | >128 | 16 | 16 | >128 | >128 | 16 | 64 | 4 | >128 |
| 50 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 | >128 |
| 51 | 16 | 16 | 64 | 64 | 32 | 16 | 32 | 4 | 64 |
| 52 | >128 | >128 | 32 | >128 | >64 | >64 | >128 | 8 | >64 |
| 53 | 64 | 4 | 16 | 4 | 128 | 32 | 2 | 2 | 64 |
| Linezolid | 2 | <1 | 2 | 2 | <1 | 2 | 64 | <1 | <1 |
| Vancomycin | <1 | 2 | - | - | 4 | - | - | 8 | 128 |
VISA = Vancomycin-intermediate Staphylococcus aureus.
VRSA = Vancomycin-resistant Staphylococcus aureus.
The presence of a second aromatic substituent (connected to the isonitrile-phenyl group) also appears critical to the biological activity observed; replacement of this moiety in 1 with a diethyl phosphonate (as in analog 4a with an ortho-isonitrile group) results in complete loss of activity against MRSA (MIC > 128 µM). Likewise, substitution of this second aromatic substituent with a cycloalkane (cf. 26) renders this compound inactive against several MRSA isolates (including MRSA USA300, MRSA USA500, and MRSA NRS119). The presence of an alkene bridge between the two aromatic substituents in 1 also appears to be important. When the alkene bridge between the two aromatic substituents is removed, as in compound 46, this compound lacks activity against three strains of MRSA (USA300, USA500, and NRS119). A similar loss in antimicrobial activity is observed with compounds 49 and 50 indicating that the stilbene isonitrile core of 1 plays an important role in its antimicrobial activity. This notion was further supported by a direct comparison of compounds 13 and 53. Compound 53 is a saturated analog of compound 13 and contains a flexible two-carbon linker between the two aromatic moieties. In general, compound 53 is less potent than compound 13 against all the strains texted except for VISA NRS1.
We then evaluated how substituents on the double bond would affect the antimicrobial activity. Interestingly, removal of the ethyl group of 1 (cf. 13) resulted in a dramatic improvement in antimicrobial activity (a two-to-eight fold reduction in the MIC against MRSA was observed). When the ethyl group was replaced by methyl (5), n-propyl (6), n-butyl (7) and phenyl (8) groups, a noticeable change in the MIC value for these compounds is observed.
We next assessed how substituents on the non-isonitrile-containing aromatic ring would affect the potency against MRSA. Analogs constructed include substitution of methoxy group (14–16), fluoride (17–19), trifluoromethyl group (20–22), methyl group (23), n-butyl group (24), and nitro group (25). Interestingly most of these modifications do not produce a major improvement in the MIC observed against MRSA, when compared to the activity of 13. Additionally the positioning of these groups around the benzene ring do not appear to have an impact on the antimicrobial activity of the compound. While most of these modifications have little effect on improving the antimicrobial activity of these compounds, one substitution had an observed deleterious effect. Compound 24, containing a n-butyl group, lacked activity against most MRSA strains tested (MIC >128 µM); interestingly, 23, with a methyl group is active against all MRSA strains tested albeit at a higher concentration than 13 (MIC of 23 ranges from 4 to 64 µM against MRSA). This would appear to indicate that the presence of an alkyl group (in particular one of increased length) is undesirable and can have a negative effect on the activity of these compounds against MRSA. Analogs containing a pyridine ring were synthesized and tested as well (27, 30, 33) and reduced antimicrobial activities were observed.
All the analogs discussed above contain an ortho-substituted isonitrile group. We wondered how the relative position of the isonitrile group would affect the antimicrobial activity and prepared eight analogs with the isonitrile group in para- and meta-relationship to the double bond (cf. 27, 28, 31, 32, 34–37). Different antimicrobial activity patterns are observed. For the group of 13, 36, and 37, the ortho-substituted compound 13 is still the most potent one against most of the strains tested and slight improvement was observed for the para-substituted compound 37 against MSSA (NRS72) and MRSA USA500. Interestingly, for the group of 33, 34, and 35, the para-substituted compound 35 is much more active against all the strains tested than the ortho- and meta-substituted ones. The groups of 27–29 and 30–31 are less potent than the aforementioned two groups, which indicate that the position of the nitrogen atom in the pyridine ring is important for the observed antimicrobial activity as well.
After completing a preliminary examination of the structure-activity relationship of these compounds, we next moved to assess whether these compounds would retain their activity against several of the most challenging strains of MRSA (Table 1 and Table 2). When tested against an array of clinically-relevant MRSA isolates, the most potent compounds (6, 8–18, 20–21, 25, and 37) did retain their antimicrobial activity. Indeed these compounds possess potent activity against MRSA isolates prevalent in the healthcare-setting such as MRSA USA100 (responsible for invasive diseases in infected hospitalized patients),27 and MRSA USA200 (associated with more severe morbidity in affected patients due to the production of toxins that can lead to toxic shock syndrome).28 In addition to this, these compounds exhibit potent activity against MRSA USA300, a strain that has been linked to the majority of MRSA skin and soft tissue infections present in the community setting.10,29 Furthermore, these compounds demonstrate strong antimicrobial activity against MRSA strains exhibiting resistance to numerous antibiotic classes including penicillins, aminoglycosides (NRS1, USA200, and USA500), macrolides (USA100, USA200, USA300, USA500, and USA700), lincosamides (USA100, USA200, USA500), tetracyclines (NRS1, USA300, and USA500), and fluoroquinolones (USA100 and USA500). Additionally, compounds 10, 11, 12, 21, 25, 32, and 35 exhibit potent antimicrobial activity (MIC between 4 and 16 µM) against clinical isolates of S. aureus exhibiting resistance to antibiotics deemed agents of last resort, namely vancomycin (VRS2). These results indicate cross-resistance between these antibiotics and the aryl isonitrile compounds is unlikely; this lends further credence to the notion that the aryl isonitrile compounds have potential to be developed as future alternatives to these antibiotics.
Table 1.
Strains of Staphylococcus aureus utilized in this study.
| Strain Name | Isolation | Molecular Typing | Antimicrobial Resistance Phenotype | |||
|---|---|---|---|---|---|---|
| NARSA ID1 |
Alternate Designation |
Origin | Source | SCCmec type |
spa type | |
| NRS1 | ATCC700699 VISA | Japan | - | II | TJMBMDMGMK | Resistant to aminoglycosides and tetracycline (minocycline) Glycopeptide-intermediate S. aureus |
| NRS722 | MSSA 476 | - | - | - | UKJFKBPE | None |
| NRS119 | SA LinR #12 | United States (Massachusetts) | Dialysis-associated peritonitis | IV | YHGCMBQBLO | Resistant to linezolid |
| NRS382 | USA100 | United States (Ohio) | Bloodstream | II | TJMBMDMGMK | Resistant to erythromycin, clindamycin and levofloxacin |
| NRS383 | USA200 | United States (North Carolina) | Bloodstream | II | WGKAKAOMQQQ | Resistant to erythromycin, clindamycin and gentamicin |
| NRS384 | USA300-0114 | United States (Mississippi) | Wound | IV | YHGFMBQBLO | Resistant to erythromycin, methicillin, and tetracycline |
| NRS385 | USA500 | United States (Connecticut) | Bloodstream | IV | YHGCMBQBLO | Resistant to erythromycin, clindamycin, trimethoprim/sulfamethoxazole, levofloxacin, gentamicin and tetracycline |
| NRS386 | USA700 | United States (Louisiana) | Bloodstream | IV | UJGFMGGM | Resistant to erythromycin and methicillin |
| VRS2 | VRSA | United States (Pennsylvania) | Plantar ulcer | II | TJMBMDMGMK | Resistant to vancomycin |
NARSA = Network on Antimicrobial Resistance in Staphylococcus aureus.
NRS72 = Methicillin-sensitive Staphylococcus aureus (MSSA).
Toxicity analysis of most potent aryl isonitrile compounds against mammalian cells
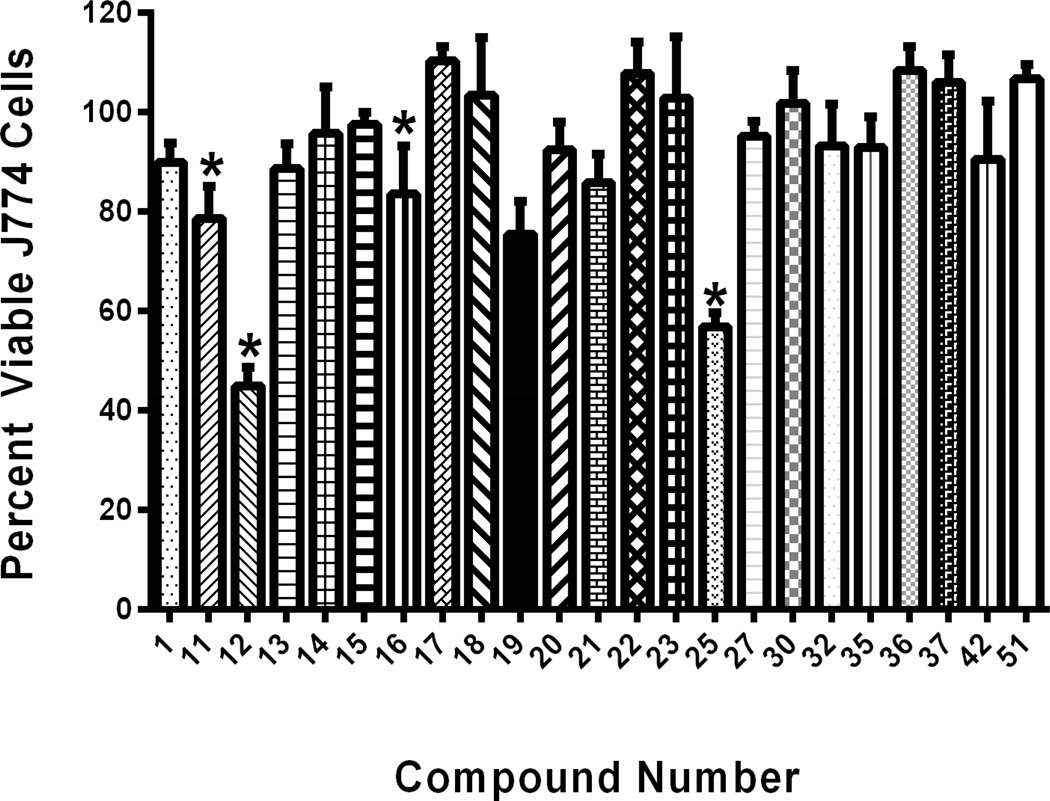
Identification of compounds exhibiting potent antimicrobial activity is the first step in a lengthy process for drug development. Many compounds with promising antimicrobial activity fail to advance further in this process due to concerns about toxicity to mammalian tissues. Selective toxicity is a critical feature novel antimicrobial compounds must possess. The ability for antimicrobial agents to exhibit their activity on the target microorganism while not causing harm to host (mammalian) tissues is important to ascertain early in the drug discovery process. To determine if compound 1 and its most potent derivatives against MRSA exhibited toxicity to mammalian tissues, these compounds were screened against a murine macrophage (J774) cell line utilizing the MTS assay (Figure 2). Initial inspection of the structure-activity relationship revealed that the isonitrile moiety appeared to be a vital component in the antimicrobial activity of these compounds. This was a point of concern given the isonitrile group has been associated with a high degree of toxicity in certain compounds present in nature.30 However, when the most potent compound, 13 (containing the isonitrile moiety), and its analog 42 (lacking the isonitrile moiety) were tested against J774 cells, they produced identical results (neither compound was toxic up to a concentration of 64 µM). This would indicate that the isonitrile group in these compounds does not contribute to undesirable toxicity to mammalian cells. This result is similar to a study conducted at Bayer AG that found compounds, in their discovery pipeline, containing the isonitrile moiety were not toxic to mice when administered orally or subcutaneously (even at concentrations in excess of 500 mg/kg).31 In addition to this, at a concentration of 32 µM, all of the compounds tested, with the exception of 25 with a nitro group, were not toxic. When the compounds were tested at a concentration of 64 µM, nineteen out of twenty-three compounds were found to not be toxic to J774 cells (Figure 2). Compounds 11, 12, 19, and 25 were found to be toxic at 64 µM. When the compounds were tested at 128 µM, all compounds were found to be toxic with the exception of compounds 15, 30, and 37 (data not presented). For the most active compounds (such as 13), a 16-to-32 fold difference exists between the concentration at which the compounds exhibit anti-MRSA activity (MIC) compared to the concentration where toxicity is observed.
Figure 2.
Percent viable mammalian cells (measured as average absorbance ratio (test agent relative to DMSO)) for cytotoxicity analysis of compounds 1, 11–23, 25, 27, 30, 32, 35, 36, 37, 42, and 51 at 64 µM. Compounds were tested against J774 cells using the MTS 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium) assay. DMSO was used as a negative control to determine a baseline measurement for the toxic impact of each compound. The values represent an average of three samples analyzed for each compound. Error bars represent standard deviation values for the absorbance values. Asterisks (*) indicate a statistical difference between the values obtained for the compound relative to the cells treated with DMSO (P < 0.05).
Preliminary study of physicochemical properties of the isonitrole compounds using kinetic solubility analysis and Caco-2 permeability assay
After confirming that most of the isonitrile compounds exhibited strong antimicrobial activity against MRSA were not toxic to mammalian cells up to a concentration of 64 µM, we next moved to analyze the physicochemical properties of the most promising compound 13. These properties play an important role in determining the appropriate route of administration (i.e. systemic vs. local) by which compounds with biological activity can be delivered to the host.32 Additionally, the physicochemical properties of a compound will have a direct impact on its pharmacokinetic profile (in particular absorption and metabolism), and ability to be translated into a viable drug candidate. Indeed, one study found that 40% of new drug candidates were withdrawn due to issues pertaining to significant pharmacokinetic problems.33 Compounds possessing a limited physicochemical profile can have issues pertaining to solubility and permeability which can hinder a compound’s ability to cross biological membranes, reach the bloodstream, and arrive at the target site of an infection (thus limiting their use systemically).34
A kinetic solubility screen (using phosphate-buffered saline) and Caco-2 permeability analysis was performed with compound 13. The solubility screen determined the highest concentration 13 and three control drugs were capable of being fully dissolved in an aqueous solvent (PBS). As presented in Table 3, this experiment revealed that compound 13 possessed partial aqueous solubility (soluble up to 15.6 µM), identical to the control drugs reserpine and tamoxifen.
Table 3.
Kinetic solubility assessment of compound 13, reserpine, tamoxifen, and verapamil in phosphate-buffered saline (PBS).
| Compound Tested | Solubility Limit (µM)1 | Solubility Analysis |
|---|---|---|
| 13 | 15.6 | Low solubility |
| Reserpine | 15.6 | Low solubility |
| Tamoxifen | 15.6 | Low solubility |
| Verapamil | >500 | High solubility |
Solubility limit corresponds to the highest concentration of test compound where no precipitate was detected.
The Caco-2 permeability assay revealed that compound 13 was not able to permeate across the Caco-2 bilayer. As presented in Table 4, this compound was unable to cross from the apical (A) to basolateral (B) surface of the membrane (apparent permeability, Papp = 0.0 cm/sec). A similar pattern is observed in the basolateral to apical direction with Papp = 0.0 cm/sec (indicating this compound is unlikely a substrate for an efflux transporter, like talinolol, which would be one plausible explanation for the inability of this compound to traverse the membrane). This is in stark contrast to the control drug warfarin, which is able to effectively permeate across the membrane from the basolateral to apical surface (Papp = 27.0 × 10−6 cm/sec). This result is a bit surprising given the size, structure, and calculated partition coefficient (clog P = 4.107) for 13. Thus, in addition to possessing only partial aqueous solubility, 13 also possesses a poor permeability profile, indicating that, in its present state, this compound would not be suitable for use systemically.
Table 4.
Permeability analysis of compound 13, ranitidine, warfarin, and talinolol via the Caco-2 permeability assay.
| Compound/Drug Tested |
Mean A → B1 Papp (10−6 cm/sec) |
Mean B → A2 Papp (10−6 cm/sec) |
Efflux Ratio3 | Permeability Analysis |
|---|---|---|---|---|
| 13 | 0.04 | 0.0 | N/A5 | Not permeable |
| Ranitidine | 0.23 | 3.1 | 13.5 | Low permeability |
| Warfarin | 27.0 | 7.2 | 0.3 | High permeability |
| Talinolol | 0.05 | 8.9 | 178 | P-gp6 efflux control |
Mean A → B Papp = mean apparent permeability of test compound from apical to basolateral surface
Mean B → A Papp = mean apparent permeability of test compound from basolateral to apical surface
Compound not detected in receiver compartment
N/A, not applicable
P-gp, P--glycoprotein
The result from the Caco-2 permeability analysis is in agreement with the overall result obtained from the kinetic solubility screen indicating that 13, though a promising antimicrobial candidate, needs to undergo further structural modifications to enhance its physicochemical profile (in order for it to be used systemically). In addition to modifying the structure of this compound, formulation technology can be utilized to overcome this compound’s current limitations. This technology has been used to improve the drug-like properties of promising compounds with similar kinetic profiles to 13 in order to propel these compounds into further stages of drug development. By using a spray drying dispersion technique,35 the antisolvent crystallization method,36 or combining the active compound with an excipient (to create an amorphous solid dispersion),37 the aqueous solubility, permeability and bioavailability profile of this compound can be significantly improved. Identifying that 13 has a problematic physicochemical profile early in the drug discovery process will permit medicinal chemists and formulation scientists to invest time and effort to enhancing both the physiochemical and pharmacokinetic profiles of this promising new antimicrobial compound.
Metabolic stability analysis of 13 via microsomal stability analysis
In addition to studying the solubility and permeability profile of compound 13, the stability of this compound to metabolic processes present in the liver was investigated using human liver microsomes (Table 5). Drugs administered systemically often are subject to various metabolic processes that can convert the active compound to inactive metabolites. Pharmaceutical compounds that are slow to be metabolized have multiple advantages including an improved pharmacokinetic profile, reduced frequency of doses that need to be given to patients (leading to better patient compliance), while also ensuring the active drug circulates within the patient’s system to assist with treating and clearing an infection. As the liver is the primary organ for metabolism of drugs administered systemically in the body, incubating compounds with liver microsomes can shed valuable insight into the stability of these compounds to metabolic processes.32
Table 5.
Evaluation of metabolic stability of compound 13, verapamil, and warfarin in human liver microsomes.
| Compound/Drug Tested |
Average remaining after 60 min (%), with NADPH |
Average remaining after 60 min (%), without NADPH |
Notes |
|---|---|---|---|
| 13 | 24 | 51 | - |
| Verapamil | 13 | 94 | High metabolism control |
| Warfarin | 93 | 94 | Low metabolism control |
When 13 was incubated with human liver microsomes, it was found to be rapidly metabolized (only 24% of the parent compound remained after one hour) similar to the highly metabolized control drug, verapamil (13% remained after one hour incubation with liver microsomes) (Table 5). While verapamil appeared to be metabolized via a NADPH-mediated process (as 94% of the drug remained after one hour when the co-factor NADPH was removed from the reaction mixture), 13 does not appear to mimic this result as only 51% of the parent compound remained after one hour when NADPH was not present. This would appear to suggest that 13 is metabolized by more than one enzyme system/reaction (one dependent on the co-factor NADPH (most likely the cytochrome P450 system), and one independent of NADPH). The metabolic stability analysis performed lends further credence to the argument that in their present state, 13, would not be suitable for use in systemic applications to treat MRSA infections.
CONCLUSION
In summary, we have discovered a novel class of aryl isonitrile compounds as promising and potent antimicrobial compounds without apparent toxicity against mammalian cells up to a concentration of 64 µM. Physicochemical profiling, including solubility, membrane permeability, and metabolic stability of one of the most potent compounds, 13, has been conducted as well. These results indicates that modification of the physical structure of compound 13 is needed to enhance its physicochemical and pharmacokinetic profile so that it can be developed for systemic use against MRSA infections. In addition, identifying other routes of administration (such as topical/local administration) is another avenue to pursue to further develop this promising compound as a novel antimicrobial candidate. Given that S. aureus and its resistant strains (including MRSA) are a leading cause of uncomplicated skin infections (such as abscesses, impetigo, and cellulitis),2,10 it is logical to assess if compound 13, and its analogs, can be used as topical antimicrobial agents for treatment of MRSA skin infections. Topical agents avoid many concerns relating to solubility, permeability, and systemic toxicity associated with drugs administered orally or intravenously. Future work with these compounds will look to pursue two avenues – determining if these compounds can be used as topical antimicrobial agents for MRSA skin infections and improving the physicochemical profile of these compounds (via structural modifications or using formulation technology) so they can potentially be used for treatment of more invasive MRSA infections.
Supplementary Material
SCHEME 1.
Synthesis of Analogs of Lead Compound 1.
Highlights.
Evaluated over forty aryl isonitrile compounds against MRSA and VRSA strains.
Identified compounds with MIC as low as 2 µM but no cytotoxicity at 64 µM.
Established SAR of these novel isonitrile compounds.
Profiled the most potent compound’s physicochemical properties.
ACKNOWLEDGMENT
The authors would like to thank the Network of Antimicrobial Resistance in Staphylococcus aureus (NARSA) program supported under NIAID/NIH Contract # HHSN272200700055C for providing the MRSA, VISA, and VRSA strains used in this study and the NIH P30CA023168 for supporting shared NMR resources to Purdue Center for Cancer Research. M.D. thanks Purdue University for startup support.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production rocess errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Appendix A. Supplementary data
Supplementary data contain the detailed synthetic procedures, compound characterization data, and biological test procedures.
REFERENCES
- 1.Centers for Disease Control and Prevention. Antibiotic Resistance Threats in the United States, 2013. http://www.cdc.gov/drugresistance/threat-report-2013/.
- 2.Giordano P, Weber K, Gesin G, Kubert J. Skin and skin structure infections: treatment with newer generation fluoroquinolones. Ther. Clin. Risk Manag. 2007;3:309–317. doi: 10.2147/tcrm.2007.3.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gillet Y, Issartel B, Vanhems P, Fournet JC, Lina G, Bes M, Vandenesch F, Piémont Y, Brousse N, Floret D, Etienne J. Association between Staphylococcus aureus strains carrying gene for Panton-Valentine leukocidin and highly lethal necrotising pneumonia in young immunocompetent patients. Lancet. 2002;359:753–759. doi: 10.1016/S0140-6736(02)07877-7. [DOI] [PubMed] [Google Scholar]
- 4.Bocchini CE, Hulten KG, Mason EO, Gonzalez BE, Hammerman WA, Kaplan SL. Panton-Valentine leukocidin genes are associated with enhanced inflammatory response and local disease in acute hematogenous Staphylococcus aureus osteomyelitis in children. Pediatrics. 2006;117:433–440. doi: 10.1542/peds.2005-0566. [DOI] [PubMed] [Google Scholar]
- 5.David MZ, Daum RS. Community-associated methicillin-resistant Staphylococcus aureus: epidemiology and clinical consequences of an emerging epidemic. Clin. Microbiol. Rev. 2010;23:616–687. doi: 10.1128/CMR.00081-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chambers HF. Community-associated MRSA-resistance and virulence converge. N. Engl. J. Med. 2005;352:1485–1487. doi: 10.1056/NEJMe058023. [DOI] [PubMed] [Google Scholar]
- 7.Frazee BW, Lynn J, Charlebois ED, Lambert L, Lowery D, Perdreau-Remington F. High prevalence of methicillin-resistant Staphylococcus aureus in emergency department skin and soft tissue infections. Ann. Emerg. Med. 2005;45:311–320. doi: 10.1016/j.annemergmed.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 8.Han LL, McDougal LK, Gorwitz RJ, Mayer KH, Patel JB, Sennott JM, Fontana JL. High frequencies of clindamycin and tetracycline resistance in methicillin-resistant Staphylococcus aureus pulsed-field type USA300 isolates collected at a Boston ambulatory health center. J. Clin. microbiol. 2007;45:1350–1352. doi: 10.1128/JCM.02274-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hiramatsu K. Vancomycin-resistant Staphylococcus aureus: a new model of antibiotic resistance. Lancet Infect. Dis. 2001;1:147–155. doi: 10.1016/S1473-3099(01)00091-3. [DOI] [PubMed] [Google Scholar]
- 10.Moran GJ, Krishnadasan A, Gorwitz RJ, Fosheim GE, McDougal LK, Carey RB, Talan DA. Methicillin-resistant S. aureus infections among patients in the emergency department. N. Engl. J. Med. 2006;355:666–674. doi: 10.1056/NEJMoa055356. [DOI] [PubMed] [Google Scholar]
- 11.Wilson P, Andrews JA, Charlesworth R, Walesby R, Singer M, Farrell DJ, Robbins M. Linezolid resistance in clinical isolates of Staphylococcus aureus. J. Antimicrob. Chemother. 2003;51:186–187. doi: 10.1093/jac/dkg104. [DOI] [PubMed] [Google Scholar]
- 12.Podoll JD, Liu Y, Chang L, Walls S, Wang W, Wang X. Bio-inspired synthesis yields a tricyclic indoline that selectively resensitizes methicillin-resistant Staphylococcus aureus (MRSA) to β-lactam antibiotics. Proc. Natl. Acad. Sci. USA. 2013;110:15573–15578. doi: 10.1073/pnas.1310459110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marconi GG, Molloy BB, Nagarajan R, Martin JW, Deeter JB, Occolowitz JL. A32390A, a new biologically active metabolite. II. Isolation and structure. J. Antibiot. 1978;31:27–32. doi: 10.7164/antibiotics.31.27. [DOI] [PubMed] [Google Scholar]
- 14.Mo S, Krunic A, Chlipala G, Orjala J. Antimicrobial ambiguine isonitriles from the cyanobacterium Fischerella ambigua. J. Nat. Prod. 2009;72:894–899. doi: 10.1021/np800751j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Raveh A, Carmeli S. Antimicrobial ambiguines from the cyanobacterium Fischerella sp. collected in Israel. J. Nat. Prod. 2007;70:196–201. doi: 10.1021/np060495r. [DOI] [PubMed] [Google Scholar]
- 16.Sugawara T, Tanaka A, Imai H, Nagai K, Suzuki K. YM-47515, a novel isonitrile antibiotic from Micromonospora echinospora subsp echinospora. J. Antibiot. 1997;50:944–948. doi: 10.7164/antibiotics.50.944. [DOI] [PubMed] [Google Scholar]
- 17.Fujiwara A, Okuda T, Masuda S, Shiomi Y, Miyamoto C, Sekine Y, Tazoe M, Fujiwara M. Fermentation, isolation and characterization of isonitrile antibiotics. Agric. Biol. Chem. 1982;46:1803–1809. [Google Scholar]
- 18.Marquez JA, Horan AC, Kalyanpur M, Lee BK, Loebenberg D, Miller GH, Patel M, Waitz JA. The hazimicins, a new class of antibiotics taxonomy, fermentation, isolation, characterization, and biological properties. J. Antibiot. 1983;36:1101–1108. doi: 10.7164/antibiotics.36.1101. [DOI] [PubMed] [Google Scholar]
- 19.Bettley FR. Xanthocillin cream for local treatment. Br. Med. J. 1959;1:1226–1227. doi: 10.1136/bmj.1.5131.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wright AD, Wang H, Gurrath G, König GM, Kocak G, Neumann G, Loria P, Foley M, Tilley L. Inhibition of heme detoxification processes underlies the antimalarial activity of terpene isonitrile compounds from marine sponges. J. Med. Chem. 2001;44:873–885. doi: 10.1021/jm0010724. [DOI] [PubMed] [Google Scholar]
- 21.Schwarz O, Brun R, Bats JW, Schmalz H. Synthesis and biological evaluation of new antimalarial isonitriles related to marine diterpenoids. Tetrahedron Lett. 2002;43:1009–1013. [Google Scholar]
- 22.Singh C, Srivastav NC, Puri SK. In vivo active antimalarial isonitriles. Bioorg. Med. Chem. Lett. 2002;12:2277–2279. doi: 10.1016/s0960-894x(02)00457-2. [DOI] [PubMed] [Google Scholar]
- 23.Arbuzov BA. Michaelis–Arbusow- und Perkow-Reaktionen. Pure. Appl. Chem. 1964;9:307–353. [Google Scholar]
- 24.Weber WP, Gokel GW. An improved procedure for the Hofmann carbylamine synthesis of isonitriles. Tetrahedron Lett. 1972:1637–1640. [Google Scholar]
- 25.Zhang B, Studer A. 2-Trifluoromethylated indoles via radical trifluoromethylation of isonitriles. Org. Lett. 2014;16:1216–1219. doi: 10.1021/ol5001395. [DOI] [PubMed] [Google Scholar]
- 26.Zhang B, Mück-Lichtenfeld C, Daniliuc CG, Studer A. 6-Trifluoromethyl-phenanthridines through radical trifluoromethylation of isonitriles. Angew. Chem. Int. Ed. 2013;52:10792–10795. doi: 10.1002/anie.201306082. [DOI] [PubMed] [Google Scholar]
- 27.Klevens RM, Morrison MA, Nadle J, Petit S, Gershman K, Ray S, Harrison LH, Lynfield R, Dumyati G, Townes JM, Craig AS, Zell ER, Fosheim GE, McDougal LK, Carey RB, Fridkin SK. Invasive methicillin-resistant Staphylococcus aureus infections in the United States. JAMA. 2007;298:1763–1771. doi: 10.1001/jama.298.15.1763. [DOI] [PubMed] [Google Scholar]
- 28.Lin YC, Anderson MJ, Kohler PL, Strandberg KL, Olson ME, Horswill AR, Schlievert PM, Peterson ML. Proinflammatory exoprotein characterization of toxic shock syndrome Staphylococcus aureus. Biochemistry. 2011;50:7157–7167. doi: 10.1021/bi200435n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McDougal LK, Steward CD, Killgore GE, Chaitram JM, McAllister SK, Tenover FC. Pulsed-field gel electrophoresis typing of oxacillin-resistant Staphylococcus aureus isolates from the United States: Establishing a national database. J. Clin. Microbiol. 2003;41:5113–5120. doi: 10.1128/JCM.41.11.5113-5120.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goda M, Hashimoto Y, Shimizu S, Kobayashi M. Discovery of a novel enzyme, isonitrile hydratase, involved in nitrogen-carbon triple bond cleavage. J. Biol. Chem. 2001;276:23480–23485. doi: 10.1074/jbc.M007856200. [DOI] [PubMed] [Google Scholar]
- 31.Ugi I, Fetzer U, Eholzer U, Knupfer H, Offerman K. Isonitrile Syntheses. Angew. Chem. Int. Ed. 1965;4:472–484. [Google Scholar]
- 32.Kerns EH, Di L. Drug-like properties: concepts, structure design and methods- from ADME to toxicity optimization. Amsterdam; Boston: Academic Press; 2008. [Google Scholar]
- 33.Prentis RA, Lis Y, Walker SR. Pharmaceutical innovation by the seven UK-owned pharmaceutical companies (1964–1985) Br. J. Clin. Pharmacol. 1988;25:387–396. doi: 10.1111/j.1365-2125.1988.tb03318.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lin JH, Lu AY. Role of pharmacokinetics and metabolism in drug discovery and development. Pharmacol. Rev. 1997;49:403–449. [PubMed] [Google Scholar]
- 35.Kwong AD, Kauffman RS, Hurter P, Mueller P. Discovery and development of telaprevir: an NS3-4A protease inhibitor for treating genotype 1 chronic hepatitis C virus. Nat. Biotechnol. 2011;29:993–1003. doi: 10.1038/nbt.2020. [DOI] [PubMed] [Google Scholar]
- 36.Lonare AA, Patel SR. Antisolvent Crystallization of Poorly Water Soluble Drugs. Int. J. Chem. Eng. Appl. 2013;4:337–341. [Google Scholar]
- 37.Van den Mooter G. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discov. Today. Technol. 2012;9:e71–e174. doi: 10.1016/j.ddtec.2011.10.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.