Abstract
The International Knockout Mouse Consortium (IKMC) introduces its targeted constructs into C57BL/6N embryonic stem cells. However, breeding with a Cre-recombinase and/or Flp-recombinase mouse is required for the generation of a null allele with the IKMC cassette. Many recombinase strains are in the C57BL/6J background, resulting in knockout animals on a mixed strain background. This can lead to variability in metabolic data and the use of improper control groups. While C57BL/6N and C57BL/6J are derived from the same parental C57BL/6 strain, there are key genotypic and phenotypic differences between these substrains. Many researchers may not even be aware of these differences, as the shorthand C57BL/6 is often used to describe both substrains. We found that 58% of articles involving genetically modified mouse models did not completely address background strain. This review will describe these two substrains and highlight the importance of separate consideration in mouse model development. Our aim is to increase awareness of this issue in the diabetes research community and to provide practical strategies to enable researchers to avoid mixed strain animals when using IKMC knockout mice.
Introduction
The study of diabetes and metabolism requires the use of mouse models to examine whole animal physiology. For study of the role of specific genes, knockout mouse technology is often used. One of the most widely used laboratory mouse strains is the C57BL/6 mouse. These mice are commonly referred to as “Black 6,” “B6,” or “C57 Black.”
There are many substrains of the C57BL/6 mouse (1). This review will discuss two of the most commonly used substrains, C57BL/6J and C57BL/6N. We refer to these as 6J and 6N, respectively, throughout the review when discussing the general substrain. This review will highlight why these substrains should be considered distinct. The 6J substrain has been widely used in metabolic research, as these mice are susceptible to diabetes and diet-induced obesity (DIO). It is the background strain for the commonly used ob/ob mouse (2). The 6J was the first and most extensively sequenced mouse genome (3). Therefore, large amounts of genetic and metabolic data have been generated using 6J. However, this substrain was not used for generation of knockout animals, as the 6J embryonic stem (ES) cells have low rates of germline transmission. Recently, an ES cell line was developed from the 6N substrain and was selected for generation of targeted alleles in the International Knockout Mouse Consortium (IKMC) (4). As a result, knockout mice can be on a mixed background if 6N ES cells are used with 6J animals. There are key genetic and phenotypic differences between these substrains, including a mutation in the nicotinamide nucleotide transhydrogenase (Nnt) gene in the 6J mouse (5,6). Improper use of control groups can lead to misinterpretation of phenotypic results. In many publications, including much of the literature related to the IKMC, mice are described only by their parent strain, C57BL/6 (7,8). Incomplete description of background strain can lead to confusion and error. This review aims to increase awareness and encourage publication of detailed and complete information on background strain, breeding practice, and control groups. Journal editors may consider requiring this information, similar to policies requiring the sex of experimental animals.
IKMC Knockout Mice
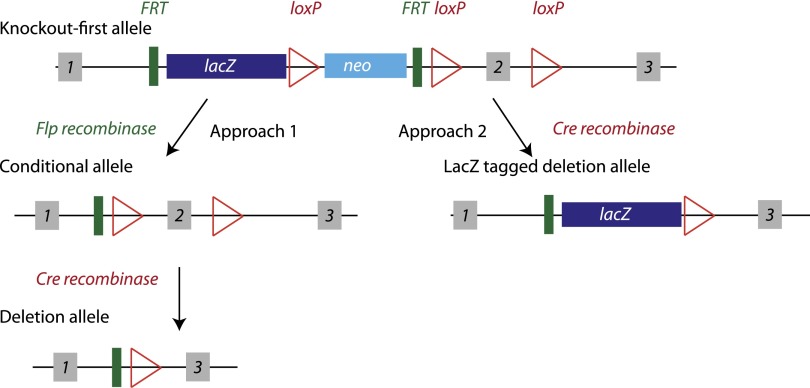
The IKMC had the goal of creating mouse ES cell lines that collectively lack every protein-coding gene in the mouse genome (7). With use of a high-throughput gene-targeting approach (9), many of the genes have been targeted in multiple ways, giving options for conditional as well as germline knockouts. While many different targeted and trapped alleles are available from IKMC, the most common is the “knockout-first” allele, shown schematically in Fig. 1 (8).
Figure 1.
Schematic representation of the commonly used “knockout-first” allele from IKMC. Adapted from Skarnes et al. (8). The targeting allele contains a cassette with the lacZ gene and a promoter-driven neomycin resistance gene (neo), flanked by Flp recombinase target (FRT) sites. This cassette is inserted into an intron of the targeted gene to disrupt endogenous gene expression and allow lacZ reporter expression under control of the endogenous gene promoter. A critical exon of the targeted gene is also flanked by loxP sites. There is also a loxP site between lacZ and neo. There are two recommended approaches for generating animals. 1) Breed first with a Flp-recombinase mouse, removing both the lacZ and neo between the FRT sites. This conditional allele can then be bred with a Cre-recombinase mouse to remove the flanked exon between the loxP sites, creating a deletion allele with no reporter. 2) Breed with a Cre-recombinase mouse first, removing both the neo and the loxP-flanked exon, generating a reporter-tagged deletion allele. The first approach is typically followed in generation of knockout animals, necessitating two generations of breeding with recombinase mice. Breeding with Flp-recombinase or Cre-recombinase mice in the C57BL/6N background will produce a deletion allele in an isogenic background. Breeding with other strains will result in a knockout mouse on a mixed background.
Due to the prevalent use of C57BL/6 mice in a wide variety of fields, participants of a 2005 National Institutes of Health (NIH) workshop strongly supported the choice of C57BL/6 ES cells for use in the IKMC (7). Pettitt et al. (4) created ES cells from C57BL/6N with reliable and robust germline transmission. Therefore, all of the targeted ES cells used by the IKMC are in the 6N background.
These ES cells were further manipulated to repair the mutation in the Agouti locus that gives C57BL/6 strains their black coat color. The result is these 6N ES cells are heterozygous for functional Agouti (A/a) and contribute an agouti, or brownish, coat color in chimeras. If germline transmission is achieved after mating the chimeras with homozygous nonagouti (a/a) 6J or 6N mice, a mixture of black (a/a) and agouti (A/a) offspring may result. Importantly, the coat color distribution seen in a colony does not help to determine whether the knockout is predominantly in a 6N or 6J background, as only the ES cells carry the corrected Agouti allele, and both 6N and 6J breeding partners will have black coat color.
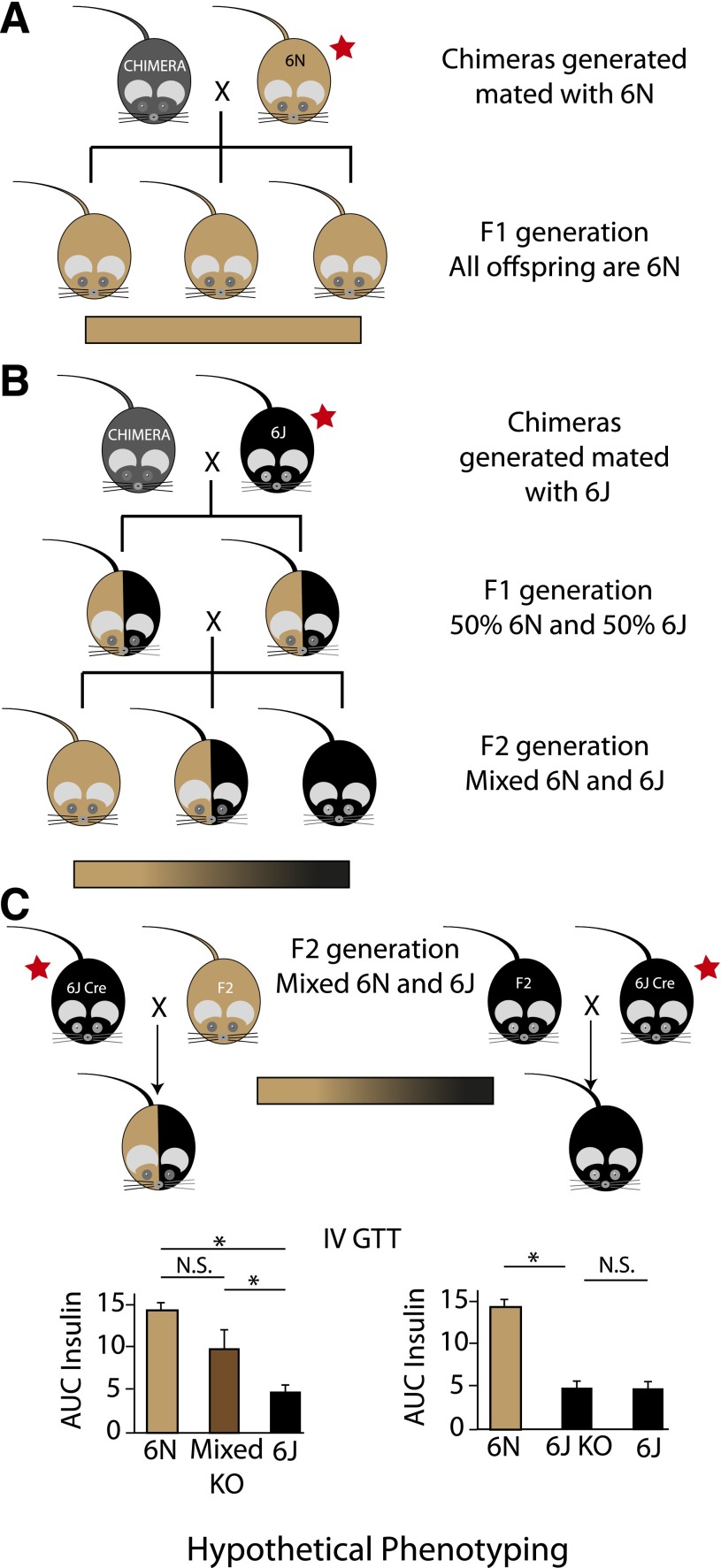
ES cells from the IKMC can be used to generate knockout mice by injection into a host blastocyst to obtain chimeric mice. The first breeding step where strain selection becomes critical is in mating of these chimeric mice to achieve germline transmission of the targeted allele (Fig. 2A). If the chimeras are bred with a substrain other than 6N, the resulting germline founders will already be on a mixed background. Further sibling matings to generate homozygosity at the knockout allele will result in an F2 generation with genetic diversity across the two background strains (Fig. 2B).
Figure 2.
Results of differing breeding strategies using 6N ES cells from IKMC to generate knockout mice. A: 6N ES cells are microinjected into a nonisogenic blastocyst and implanted into a female surrogate. The resulting chimeras (represented as gray) are then mated with a 6N mouse (represented as light brown) to generate isogenic 6N offspring with germline transmission of the targeted allele. B: In this example, the chimeras generated with 6N ES cells are instead mated to a 6J (represented as black), giving rise to an F1 generation with germline transmission on a mixed background that is 50% 6J and 50% 6N (heterozygous at all alleles that differ between these two substrains). If sibling or nonlittermate pairs from the F1 generation are mated together, the F2 generation is a mixed 6J/6N background, with some animals inheriting primarily 6J alleles and others inheriting predominantly 6N alleles. The large variability in the background of this generation is depicted as a gradient bar. C: This schematic shows some of the problems that can arise with mixed backgrounds and breeding with nonisogenic recombinase strains (Cre) to produce knockout (KO) animals. Littermates selected from the F2 generation in B for breeding represent the extremes of the genetic variation in this generation, with predominantly 6N or 6J alleles. If these F2 littermates are selected as mating partners with a Cre mouse on the 6J background, the offspring from each mating pair will have very different genetic backgrounds. The predominantly 6N mouse will produce pups that are on a highly heterozygous background for 6N/6J alleles (left scenario). However, the predominantly 6J mouse will produce offspring with nearly 100% homozygosity for 6J alleles (right scenario). When phenotyping for a metabolic trait that differs between the 6N and 6J background strains, the analysis can be confounded. We provide representative graphs of hypothetical area under the curve (AUC) insulin data during an intravenous (IV) GTT. An asterisk indicates a statistically significant difference. The magnitude of difference between the 6N and 6J strains is based on actual data (35). Differing conclusions can be drawn depending on what control group is used. The red stars indicate breeding steps where use of the correct substrain is critical to avoid mixed backgrounds. Of note, the colors selected for representation are not intended to depict the predicted coat colors of these mice. N.S., not significant.
As noted in Fig. 1, generation of knockout alleles requires breeding with a Flp-recombinase and/or Cre-recombinase mouse. This breeding step can further introduce a mixed background if these recombinase strains are not on a C57BL/6N background. These scenarios are illustrated in Fig. 2C. Currently, efforts are under way to generate and phenotype live mice from the IKMC ES cells. Part of this work is being done at The Jackson Laboratory (JAX), where their breeding strategy will create isogenic 6N knockout lines. Of note, individual researchers who purchase these mice will need to breed only with 6N recombinase lines in order to maintain an isogenic strain background in their knockout animals. Some recombinase mice are available in both 6J and 6N backgrounds. However, many of the tissue-specific Cre lines commonly used in metabolic research are not available on a pure 6N background.
History of the Black 6 Strain
An inbred strain is defined as any set of brother-sister matings that occurs for at least 20 consecutive generations. A substrain is a branch of the inbred strain that has significantly diverged from the original founding strain. Genetic drift occurs when natural mutations are maintained within an isolated breeding population. This can lead to distinct substrains at different breeding facilities over time.
In the early 1920s, C.C. Little established a colony of C57BL mice that later gave rise to the C57BL/6 strain in the 1930s. Beginning in 1948, these mice were maintained at JAX and henceforth known as C57BL/6J, with “J” for Jackson (10,11). In 1951, at generation F32, the C57BL/6J strain was sent from JAX to the NIH (5). These animals were maintained at the NIH for decades as an inbred colony via sibling matings, resulting in a separate substrain known as C57BL/6N, with “N” for NIH. Therefore, the 6J and 6N colonies have been separated since 1951, allowing significant genetic variation to develop between these substrains.
The 6N mice have been sent to commercial vendors for wider distribution to researchers. Live animals were sent from the NIH to Charles River Laboratories in 1974 (C57BL/6NCrl) and to Harlan Laboratories in 1988 (C57BL/6NHsd). In 2005, JAX received C57BL/6N cryopreserved embryos derived from a father-daughter backcross of a 1984 freeze at approximately F126 (now called C57BL/6NJ). In 1991, animals at generation F151 were sent from NIH to Taconic (C57BL/6NTac). Single nucleotide polymorphism (SNP) genotyping panels found no genetic differences between the 6N substrains from Harlan, Taconic, or Charles River (12,13). However, multiple generations separate these 6N colonies, and genetic variation may still be present that is not detected by these limited genotyping panels. Therefore, it is ideal to use 6N mice from the same vendor. Of note, the ES cells used for the IKMC were derived from C57BL/6NTac mice (4).
6J mice can also be purchased from multiple commercial vendors. The gold standard 6J mice continue to be commercially available from JAX. In Europe and Japan, 6J mice are available from Charles River. Charles River frequently reintroduces breeding stock directly from the JAX colony so as to avoid genetic drift. However, other vendors have maintained completely separate 6J colonies over time. Notably, Harlan Laboratories offers two “6J” substrains separated from JAX in 1973–1974 and maintained at other laboratories. These substrains, C57BL/6JOlaHsd and C57BL/6JRccHsd, do not have the mutation in Nnt found in other 6J substrains and therefore are not comparable controls for current C57BL/6J animals (13). In 1987, animals were sent from JAX to Japanese vendors, now commercially distributed as C57BL/6JJcl and C57BL/6JJmsSlc. These Japanese 6J substrains contain the Nnt mutation (5), suggesting it occurred in the JAX colony sometime between 1974 and 1987. Janvier Laboratories in France provides the C57BL/6JRj mice, which have also been bred separately since at least 1993. C57BL/6JRj have known genetic differences from the JAX 6J, despite sharing the Nnt mutation (14,15). Because of these known genetic differences, attention to mouse vendor is even more important when using 6J mice.
Expression of two obesity mutations, the leptin (Lepob) mutation and the leptin receptor (Leprdb) mutation, provides a relevant example of the impact of strain background on metabolic phenotype (2). The db/db mutation is commonly provided on a C57BLKS/J background, a substrain that contains only 71% 6J genome due to breeding contamination of 6J stock in the 1940s (16). Obese ob/ob and db/db mutant mice on the C57BLKS/J background both develop severe hyperglycemia with islet atrophy, whereas the same mutations expressed on the C57BL/6J background produce only mild diabetes, well compensated by islet hypertrophy and hyperplasia (2,17). As the ob/ob and db/db models are frequently used to examine the impact of transgenic overexpression or knockout alleles on obesity, diabetes, and islet biology, attention to background strain in developing and analyzing these models is critical. The impact of background strain on the phenotype of the Lepob and Leprdb mutations also highlights the utility of analyzing mutant phenotypes on more than one background, as the results may differ dramatically.
Genetic Differences
It has become clear over the past decade that there are multiple genetic differences between the 6N and 6J substrains. The most widely known is the mutation in the Nnt gene. A spontaneous in-frame five-exon deletion in Nnt was identified in the 6J mouse in 2005 (6). This deletion of exons 7–11 results in a complete absence of NNT protein. In 2009–2010, several SNPs were identified using SNP genotyping panels that distinguished 6N from 6J substrains (4,5,12). However, in 2013 an extensive whole-genome comparison between 6N and 6J carefully validated many more genetic variants between the substrains. In total, Simon et al. (18) identified 34 coding SNPs (leading to amino acid substitutions in the encoded protein), 2 coding small indels (insertions or deletions), 146 noncoding SNPs, 54 noncoding small indels, and 43 structural variants (including the Nnt mutation). Structural variation affected the coding region of two other genes: Vmn2r65 (vomeronasal 2, receptor 65) and Cyp2a22 (cytochrome P450 family 2, subfamily a, polypeptide 22). The function of these genes in metabolism is unknown. Notably, a retrotransposon insertion into an intron of Rptor (Raptor) was found in the 6J substrain, but it is unknown whether Rptor expression or function is affected (18). Raptor is a key protein in the regulation of the frequently studied mammalian target of rapamycin complex 1 (mTORC1) signaling pathways (19). Among the 34 coding SNPs were some notable genes such as Plk1 (important in cell cycle), Herpud2 (involved in endoplasmic reticulum protein processing), Crb1 (mutation leads to retinal degeneration, relevant for studies on diabetic retinopathy [20]), and Cyfip2M1N (may play a role in neuronal function and actin polymerization [21]). Additionally, a copy number variant was found within the 6J genome resulting in increased expression of insulin-degrading enzyme (Ide) and fibroblast growth factor binding protein 3 (Fgfbp3). This copy number variant arose in the 6J colony after 1994 and is therefore unlikely to be present in the 6N colony (22).
Phenotypic Differences
The 6J mouse is frequently used as a model of DIO (23) and exhibits glucose intolerance and impaired glucose-stimulated insulin secretion independent of obesity (6,18,24–28). The 6J substrain exhibits these phenotypes in comparison with many other commonly used strains, such as C57BLKS/J (29,30), DBA (31–33), FVB/N (33), and 129T2 (32), and has therefore been widely used in metabolic research.
There are notable differences in the metabolic phenotypes of the 6J and the 6N substrains when directly compared. In lean animals, 6J have higher glucose levels than 6N after both an intraperitoneal and an intravenous glucose tolerance test (GTT) (18,34). In high-fat diet (HFD) studies, both 6N and 6J mice develop glucose intolerance, but glucose levels are higher in the 6J substrain (25). These elevated glucose levels correlate with significantly lower insulin secretion in the 6J mice both after intravenous GTT and in hyperglycemic clamp experiments (35). Notably, despite lower insulin secretion there is not a difference in β-cell mass or a difference in insulin sensitivity in lean animals (35). Therefore, an insulin secretion defect is present in the 6J substrain, possibly leading to confounding results if experiments are not controlled with the proper substrain (Fig. 2C).
We found two studies reporting no difference in glucose tolerance or insulin secretion when comparing 6J and 6N (36,37). However, one of these studies used 6J animals separated from JAX since 1989, so these animals may have diverged in both genotype and phenotype (36). The other study found highly variable insulin secretion after glucose administration in the 6J mice that on average did not differ from that in 6N; they also did not see a difference after intravenous GTT in lean mice (37). Possible explanations for this include different 6N vendors and different measurement methodology. Notably, Simon et al. (18) found worse glucose tolerance in 6J across four different phenotyping centers, suggesting the phenotypic difference was robust. Although it is possible the differences between 6N and 6J depend on measurement methodology or vendor, the concern remains for erroneous conclusions when one substrain is used as a control for the other.
Differences have also been identified in weight regulation between 6N and 6J animals. On an HFD, C57BL/6J gains more weight compared with C57BL/6NJ (25). Lean 6J mice have reduced energy expenditure and O2 consumption, while the data on activity level, fat mass, and food intake in lean mice are conflicting in different reports (18,35,38). Again, these differences in weight gain are not consistently supported in the literature, perhaps due to differences in vendor. In one report, the C57BL/6JRj had less weight gain than the C57BL/6NTac; however, C57BL/6JRj are genetically distinct from the JAX 6J (15). Also, C57BL/6NTac appear to gain more weight on an HFD than C57BL/6NJ, suggesting that even 6N strains from different vendors may differ in body weight regulation (39). The gut microbiome, which can be related to vendor, may also lead to different outcomes in HFD studies comparing the two substrains (38,39). Overall, consistent use of substrain controls from the same vendor is recommended for studies related to body weight regulation.
Key differences between 6N and 6J have been identified that may impact nonmetabolic parameters. C57BL/6 substrains have differential responses to alcohol (40,41), susceptibility to tumor formation (42), and susceptibility to chronic pancreatitis (43). There are differences in eye development and vision between the substrains (18,44), including cataract development and pupillary light response, which may explain sleep fragmentation and differences in circadian rhythms (20). Systolic arterial pressure, grip strength, behavior, and motor ability also differ (18,45). Differences in immune response include a more robust proinflammatory response in 6N and increased interleukin-mediated activation of natural killer cells in 6J (18). These differences may impact inflammatory responses in obesity or models of type 1 diabetes.
In addition to experimental phenotypes, substrain differences may affect mouse colony survival as well. Sperm survival rate after cryopreservation is lower in 6J, likely due to mitochondrial oxidative stress (46). Sperm survival rate after cryopreservation can be increased with pharmacological means (46) if the sperm substrain is identified to properly preserve the samples.
The mutation in Nnt may play a key role in some of the phenotypic differences between 6N and 6J substrains. In mammalian cells, NNT is an integral protein of the inner mitochondrial membrane and catalyzes the reversible transfer of hydrogen between NAD+ and NADP+. The loss of NNT in the 6J mouse leads to significant impairment in mitochondrial function (47). In diabetes research, mitochondrial function is central to many of the phenotypes studied, including insulin secretion (48–50). NNT is also important in regulation of redox in the mitochondria, including roles in the glutathione and thioredoxin/peroxiredoxin pathways (51). 6J mice have differential sensitization to genetic deletion of antioxidant proteins, believed to be a direct result of the loss of NNT (52,53).
The mutation in Nnt was mapped as a critical locus explaining the impaired glucose tolerance and reduced insulin secretion in 6J mice (6,26). When NNT function is restored in 6J mice by transgenic overexpression, glucose tolerance and insulin secretion normalize (26).
NNT may also have other functions in metabolic tissues. Interestingly, the NNT gene in humans has been associated with familial glucocorticoid deficiency, yet adrenal function has not been studied in mouse models of the Nnt mutation (54,55). NNT is also highly expressed in the brain. Although no difference was identified in the sensing of glucose in the brain and central control of insulin secretion between the two substrains, it is possible NNT has central effects on appetite regulation or metabolism (35).
However, the Nnt mutation cannot explain all of the phenotypic differences between 6J and 6N substrains. For example, in studies on DIO, the Nnt genotype did not correlate with weight gain (15,39). While a direct phenotypic effect of the other genetic variants remains unknown, each of these loci could contribute a modifier effect when combined with a knockout allele in a particular strain. Therefore, a knockout in the 6N background may have a different phenotype than in the 6J background. For example, while the increased copy number of Ide (22) does not result in a detectable difference in insulin clearance between the parental 6N and 6J substrains (35), it is possible this genetic difference could have a unique modifier effect in 6J mouse models where insulin clearance is central to the phenotype.
Recommended Approaches
Understanding and documenting the exact background substrain of a knockout mouse are critical to the accurate interpretation of experimental data. Failure to do so can result in several potential problems. First, as highlighted in Fig. 2B, littermate controls generated by breeding animals on a mixed background can have large genetic and phenotypic variation. Therefore, one littermate may be mainly on a 6N background, while another is mainly on a 6J background. If the phenotype examined differs between these substrains, there will be increased variability in the measurement. A phenotype related to the knockout gene may be masked by large variability in the mixed background and control groups. The use of mixed backgrounds therefore may require larger experimental numbers to avoid a type II error. If breeders are chosen from a mixed background colony, one breeding pair may contain more 6N traits than another, creating separate subcolonies within the knockout line. Many researchers implement a null/null × null/null breeding strategy to increase the number of knockout animals obtained. However, this method does not generate wild-type or recombinase-expressing controls within the same litters. Therefore, control animals are often drawn from a different colony. For example, a 6J Cre may be used as a control for a knockout on a mixed 6N/6J background or even on a full 6N background. In this case, researchers can mistakenly attribute a phenotype to the knockout gene when it is in fact related to genetic differences between the background strains of the experimental and control animals (Fig. 2C). Completely opposite conclusions can be drawn depending on which C57BL/6 substrain served as the control group (56). In order for experiments to be repeatable between laboratories, the exact strain of mouse must be made clear so future studies can be properly controlled (57).
Investigators who would like to maintain their 6N-derived knockouts on a full 6J background have several options. The most commonly accepted backcrossing strategy is to mate with 6J for 10 consecutive generations. However, this is time-consuming, and given the relatively small genetic variation between the substrains a shorter backcross is likely to be adequate. This can be achieved through directed backcrossing using a number of known SNPs between the substrains (5,12). We have used a SNP genotyping system to facilitate the identification of offspring with the most 6J traits for breeding in the next generation. Tetra-primer ARMS PCR (PCR) (58,59) uses two primer pairs to amplify the two different alleles containing the SNP in a single PCR reaction. The resulting PCR products differ in size depending on which allele is present. We provide a list of 10 different SNP primer pairs and their predicted PCR product sizes, which can be used to identify 6N or 6J alleles (60) (Table 1). The Nnt allele should also be genotyped, and this can be done using previously published methods (52). Alternatively, JAX and Charles River offer a genotyping panel across 128–150 SNPs between the 6J and 6N substrains. These genotyping strategies can also be used to clarify the background of your current mouse line.
Table 1.
SNP genotyping primers for alleles that distinguish between C57BL/6J and C57BL/6N substrains
| Ch. | SNP | Temp (°C) | 6N size | 6J size | Outer size | Forward inner primer (5′-3′) | Reverse inner primer (5′-3′) | Forward outer primer (5′-3′) | Reverse outer primer (5′-3′) |
|---|---|---|---|---|---|---|---|---|---|
| 3 | rs13477019 | 55 | A-204 | T-137 | 287 | AAATGTGCATGCAGTTCTAAACACTA | AACAATATCAATACTAGTTGGATCTGTA | AAATAACTTCAGATTTTCTTCTATAGGAAA | ACTTTACATGATAATTTACCATTGACCT |
| 6 | rs13478783 | 61 | G-272 | A-201 | 418 | CACTCTGAAAAGGCCCAGGACCAAAA | AGATTGGCCCAGGCCTACCTTTCTTAGTC | CTAGGCCCAAAGAAGAAGAAATGTGGGC | AAGCAGGCGAAGGAGCAAGAGCTAGACT |
| 7 | rs13479522 | 65 | G-207 | A-253 | 407 | CTGTTGAGAAGCAGGTGCCGGACAAA | TGTGCACTCAGCATTGACGAGAACCAC | CCATCCCATGTGGGAGAGCAAACACTT | CCATGCTTCCAGCCATGATGATAGTGGA |
| 9 | rs13480122 | 65 | C-193 | T-256 | 393 | AAAGCAGAGAGAGGCTGTACATGCATGTCC | CCAGACCTCGGTGAGGTTGTGGGTTA | CATGTATTCCTGAGGAGAGAGAAGCGGGA | GACTTCAACAGAAACGCCTTTGGAACCA |
| 10 | rs13480619 | 57 | C-217 | T-259 | 421 | GCTTCCTACTCTTTTGTTTTGTTTTTC | CTAGTTTGAAAAGTCAAACCCAGATTAA | TGCAAGACAAACTCAACTCATACTTTAA | GATGATTCACTACAAGAACAGATCTCAA |
| 10 | rs13480759 | 57 | T-268 | C-199 | 414 | GGCTTTTGTCTTCTGTAATGTTTCAC | ATTAGAAGACACTTCAGGTTAAGGGAA | ATGTGAAGTCATCGTACACATTTTAGATT | GGTTTATTTTCATTTTCTTGTGTTCTCA |
| 10 | rs29359333 | 65 | A-263 | G-226 | 433 | AAGGTGGACTACAGTCACAAACAGATTTG | GCATTGTATGTGTGCAATTACACAGGT | ATATGGGGGATGGCTTAGTCAGTAAAAC | TCTAACCCTGTCACATCACAAATTGCTA |
| 11 | rs13481014 | 61 | C-252 | T-183 | 379 | ATAATCTCCAGTGCATAATGTAGGTGTGTT | GTTAGTCCATTACCCTCTATTTGCGG | TAAAAAATATGCCCTCCTGATTATTCCA | AGTATGTATGTTTCTCTGAGCAGGTTGC |
| 13 | rs13481734 | 61 | G-206 | A-268 | 418 | AAAGGCAAACACTTGGATCCCATATG | TTTCAAAAAGAATTGATCCCAGAACTGAAT | TGACATTCAGATGCAAAGTGAGTACATGA | CAGAGGGCTAATATCCAAAATGTGTGTG |
| 14 | rs31233932 | 63 | T-200 | C-298 | 438 | CCCTAATGCTACTTTTTTTCTATTGAGGTT | GTGATAATGAAGAATCGACAAAATTACCTG | GAGAATATGGGCCTCTAGGAAGTTAACA | ACAGAAAGAGAATGAGAATCAAAAGTGC |
| 16 | rs4165065 | 55 | C-208 | T-179 | 334 | CTACAAACACCCTGAATGCTCATTTT | ATGGAAATATGCCATTTTATTTAATGG | TATTTAACCCTTTGATAGAAAAAGCAGC | AGGTAGGGAAGAAGGTAGGTTTGAGTAG |
| 17 | rs13483055 | 65 | C-250 | T-213 | 407 | CAGAAAGATCTAATCATTGCCAGGCCAC | TCTGGACCTCCTCTTCTGATAAGGTGCA | GCAGCTTTGGAATTCAGTGACTTTGACAA | GCACAACAGAAACTAAAGCAATCACCAGC |
Primers were designed using the Tetra-Primer ARMS PCR method (58,59). In this method, outer primers amplify the region of the SNP and will give a PCR product of the same size (outer size) in both substrains. The forward and reverse inner primers are designed to recognize the different alleles of the SNP and will generate PCR products that differ in size from one another. The table lists the nucleotide present in each substrain at each SNP and the predicted size in base pairs of the PCR product for that substrain (6N size or 6J size). For example, for SNP rs13477019, PCR products of 204 and 287 base pairs would be seen for a mouse that was homozygous for the 6N allele at this SNP, whereas products of 204, 137, and 287 base pairs would be seen if the mouse was heterozygous 6N/6J at this SNP. PCR products were run on a 2% agarose gel and visualized with ethidium bromide. The melting temperatures (temp) used for each four primer reaction are indicated. Ch., chromosome location of the SNP.
Even with proper backcrossing, genes from the origin donor strain surrounding the targeted gene, known as the passenger genome (61), often remain due to a decrease in genetic recombination near the targeted locus. Any passenger mutations may influence phenotypic outcomes and should be considered when using backcrossed mice. With the advent of clustered, regularly interspaced, short palindromic repeat (CRISPR) technology, it is possible to perform direct allele manipulation in any mouse strain (62). However, the ability to obtain live mice with conditional deletion alleles from the IKMC means that many groups will continue to use more traditional knockout mouse technology.
We recommend that researchers deposit their genetically modified mice in an approved repository, such as JAX. By allowing the vendor to maintain the mouse colony and conduct any necessary backcrossing, other researchers can purchase modified mice and have confidence the background strain is properly maintained. It is common to obtain animals from collaborators, often without knowing how the colony has been maintained throughout its breeding history. Much of this uncertainty can be alleviated when purchasing from an approved vendor. We encourage all researchers to include full background substrain information (C57BL/6NTac, for example) and carefully describe any breeding to nonisogenic strains in all publications.
Current Status of Complete Background Strain Information in the Literature
The past decade has seen an increase in awareness of strain and substrain differences, specifically between the 6N and 6J. Despite this, we found that many articles still do not state the specific substrain used in their experiments. We conducted a review of all articles published in Diabetes in 2010–2014 (Table 2). We identified articles describing experiments using genetically modified mice, and each article was scored to determine whether it clearly stated the substrain of mouse. The “Research Design and Methods” sections were carefully reviewed, including checking cited references for established mouse lines. If there was a clear explanation of the exact strain/substrain and if there was a clear discussion of a breeding scheme or backcrossing, then an article was scored as “complete.” An example of a complete description included receipt of genetically modified animals from another group, backcrossing to C57BL/6J for 10 generations, and then mating with the Cre mouse on the C57BL/6J background. Incomplete explanations would include, “we used a null-allele mouse and mated with a recombinase mouse,” without any mention of the background strain. Other incomplete examples included description of animals on a “mixed background” without indication of what strains were used to create the animals, using a control colony from a different background, or having a genetically modified mouse on one background and yet breeding with a recombinase animal from a different strain without mention of how appropriate control groups were obtained. The majority (63%) of articles scored “incomplete” received that score because they only provided C57BL/6 as the strain, without specifying a substrain. Overall, we found 58.5% of publications in the past 4 years had incomplete explanations of the background substrain. We also found no improvement in this percentage over time, despite increasing awareness in the literature.
Table 2.
Assessment of completeness of background strain discussion in recent publications
| Background strain notation | 2010 | 2011 | 2012 | 2013 | 2014 | Combined totals or averages |
|---|---|---|---|---|---|---|
| Completely addressed | 54 | 56 | 49 | 52 | 43 | Combined total: 254 |
| Incompletely addressed | 77 | 64 | 55 | 86 | 80 | Combined total: 362 |
| Incomplete due to C57BL/6 | 43 | 43 | 40 | 52 | 48 | Combined total: 226 |
| Total number of articles | 126 | 123 | 105 | 138 | 124 | Combined total: 616 |
| % incomplete total | 61 | 52 | 52 | 62 | 64.5 | Average: 58.5 |
| % of incompletes owing to C57BL/6 | 56 | 67 | 73 | 60.5 | 60 | Average: 63 |
Articles published in Diabetes in 2010–2014 containing data from genetically modified mice were examined for completeness in the description of background strain and backcrossing or breeding strategy. The number of articles with either a complete or an incomplete description of background strain is listed by individual year and as a combined total over the 5-year period. Also, the number of articles scored as “incomplete due to C57BL/6” is given. These articles were only marked incomplete because they simply mention the parental strain C57BL/6, with no mention of substrain. At the bottom of the table is the total percentage of articles with incomplete discussion of background strain (incompletely addressed/total number of articles) and the percentage of these incomplete articles that were scored as such for only mentioning the C57BL/6 parent strain (incomplete due to C57BL/6/incompletely addressed).
We separately scored articles using animals without genetic modification. Of these, 25% did not completely specify the substrain. As we have discussed, there are many metabolic differences between the 6N and 6J substrains, and therefore studies, such as on HFD, can lead to different results depending on the substrain.
Conclusions
The different substrains of the C57BL/6 mouse have been separated from one another for many decades, leading to a number of differences in genotype and phenotype that can have direct impact on research in diabetes and metabolism. The IKMC has generated targeted ES cells and knockout mice in 6N, while many of the Cre recombinase lines are in 6J. Therefore, careful attention to background substrain and/or backcrossing will often be necessary to ensure valid experimental data. We hope journals will adopt policies requiring complete discussion of background substrain and breeding strategies to help improve data interpretation and reproducibility and that researchers will consider the impact of background substrain in their experimental design and analysis.
Article Information
Acknowledgments. The authors acknowledge editorial assistance from Alan Attie, Carly Kibbe, Justin Bushkofsky, Mieke Baan, and Amelia Linnemann (all at University of Wisconsin–Madison, Madison, WI).
Funding. D.A.F. is supported by a training grant from the University of Wisconsin–Madison (UW–Madison) Institute on Aging (from the National Institute on Aging [T32 AG000213]) and has been supported by the UW–Madison Science and Medicine Graduate Research Scholars program. D.B.D. is supported by the U.S. Department of Veterans Affairs (1I01BX001880) and the UW–Madison Department of Medicine.
The contents of this article do not represent the views of the Department of Veterans Affairs or the U.S. government.
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. D.A.F. and D.B.D. reviewed the literature and wrote the manuscript. D.A.F. performed the experiments to develop genotyping primers in Table 1.
References
- 1.C57BL/6 Mice [Internet], 2011. Charles River Laboratories International, Inc. Available from http://www.criver.com/files/pdfs/rms/c57bl6/rm_rm_d_c57bl6n_mouse.aspx. Accessed 1 May 2015
- 2.Coleman DL, Hummel KP. The influence of genetic background on the expression of the obese (Ob) gene in the mouse. Diabetologia 1973;9:287–293 [DOI] [PubMed] [Google Scholar]
- 3.Waterston RH, Lindblad-Toh K, Birney E, et al.; Mouse Genome Sequencing Consortium . Initial sequencing and comparative analysis of the mouse genome. Nature 2002;420:520–562 [DOI] [PubMed] [Google Scholar]
- 4.Pettitt SJ, Liang Q, Rairdan XY, et al. Agouti C57BL/6N embryonic stem cells for mouse genetic resources. Nat Methods 2009;6:493–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mekada K, Abe K, Murakami A, et al. Genetic differences among C57BL/6 substrains. Exp Anim 2009;58:141–149 [DOI] [PubMed] [Google Scholar]
- 6.Toye AA, Lippiat JD, Proks P, et al. A genetic and physiological study of impaired glucose homeostasis control in C57BL/6J mice. Diabetologia 2005;48:675–686 [DOI] [PubMed] [Google Scholar]
- 7.Collins FS, Rossant J, Wurst W; International Mouse Knockout Consortium . A mouse for all reasons. Cell 2007;128:9–13 [DOI] [PubMed] [Google Scholar]
- 8.Skarnes WC, Rosen B, West AP, et al. A conditional knockout resource for the genome-wide study of mouse gene function. Nature 2011;474:337–342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Collins FS, Finnell RH, Rossant J, Wurst W. A new partner for the international knockout mouse consortium. Cell 2007;129:235. [DOI] [PubMed] [Google Scholar]
- 10.Inbred and Genetically Defined Strains of Laboratory Animals: Mouse and Rat. Altman PL, Katz DD, Eds. Bethesda, MD, Federation of American Societies for Experimental Biology, 1979 [Google Scholar]
- 11.Origins of Inbred Mice. Morse HC III, Ed. New York, Academic Press, 1978 [Google Scholar]
- 12.Zurita E, Chagoyen M, Cantero M, et al. Genetic polymorphisms among C57BL/6 mouse inbred strains. Transgenic Res 2011;20:481–489 [DOI] [PubMed] [Google Scholar]
- 13.C57BL/6 substrain information [Internet], Envigo (previously Harlan Laboratories), Indianapolis, IN. Available from http://www.envigo.com/resources/data-sheets/envigo-68-c57bl6-enhanced-technical-data-sheet_screen.pdf. Accessed 1 May 2015
- 14.C57BL/6NRj mouse [Internet], 2013. Janvier Laboratories. Available from http://www.janvier-labs.com/rodent-research-models-services/research-models/per-species/inbred-mice/product/c57bl6nrj.html. Accessed 1 May 2015
- 15.Kern M, Knigge A, Heiker JT, et al. C57BL/6JRj mice are protected against diet induced obesity (DIO). Biochem Biophys Res Commun 2012;417:717–720 [DOI] [PubMed] [Google Scholar]
- 16.Mao HZ, Roussos ET, Péterfy M. Genetic analysis of the diabetes-prone C57BLKS/J mouse strain reveals genetic contribution from multiple strains. Biochim Biophys Acta 2006;1762:440–446 [DOI] [PubMed] [Google Scholar]
- 17.Hummel KP, Coleman DL, Lane PW. The influence of genetic background on expression of mutations at the diabetes locus in the mouse. I. C57BL-KsJ and C57BL-6J strains. Biochem Genet 1972;7:1–13 [DOI] [PubMed] [Google Scholar]
- 18.Simon MM, Greenaway S, White JK, et al. A comparative phenotypic and genomic analysis of C57BL/6J and C57BL/6N mouse strains. Genome Biol 2013;14:R82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lamming DW, Ye L, Sabatini DM, Baur JA. Rapalogs and mTOR inhibitors as anti-aging therapeutics. J Clin Invest 2013;123:980–989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Banks G, Heise I, Starbuck B, et al. Genetic background influences age-related decline in visual and nonvisual retinal responses, circadian rhythms, and sleep. Neurobiol Aging 2015;36:380–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kumar V, Kim K, Joseph C, et al. C57BL/6N mutation in cytoplasmic FMRP interacting protein 2 regulates cocaine response. Science 2013;342:1508–1512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Watkins-Chow DE, Pavan WJ. Genomic copy number and expression variation within the C57BL/6J inbred mouse strain. Genome Res 2008;18:60–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Surwit RS, Kuhn CM, Cochrane C, McCubbin JA, Feinglos MN. Diet-induced type II diabetes in C57BL/6J mice. Diabetes 1988;37:1163–1167 [DOI] [PubMed] [Google Scholar]
- 24.Kaku K, Fiedorek FT Jr, Province M, Permutt MA. Genetic analysis of glucose tolerance in inbred mouse strains. Evidence for polygenic control. Diabetes 1988;37:707–713 [DOI] [PubMed] [Google Scholar]
- 25.Nicholson A, Reifsnyder PC, Malcolm RD, et al. Diet-induced obesity in two C57BL/6 substrains with intact or mutant nicotinamide nucleotide transhydrogenase (Nnt) gene. Obesity (Silver Spring) 2010;18:1902–1905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Freeman HC, Hugill A, Dear NT, Ashcroft FM, Cox RD. Deletion of nicotinamide nucleotide transhydrogenase: a new quantitive trait locus accounting for glucose intolerance in C57BL/6J mice. Diabetes 2006;55:2153–2156 [DOI] [PubMed] [Google Scholar]
- 27.Surwit RS, Seldin MF, Kuhn CM, Cochrane C, Feinglos MN. Control of expression of insulin resistance and hyperglycemia by different genetic factors in diabetic C57BL/6J mice. Diabetes 1991;40:82–87 [DOI] [PubMed] [Google Scholar]
- 28.Lee SK, Opara EC, Surwit RS, Feinglos MN, Akwari OE. Defective glucose-stimulated insulin release from perifused islets of C57BL/6J mice. Pancreas 1995;11:206–211 [DOI] [PubMed] [Google Scholar]
- 29.Anderson AA, Helmering J, Juan T, et al. Pancreatic islet expression profiling in diabetes-prone C57BLKS/J mice reveals transcriptional differences contributed by DBA loci, including Plagl1 and Nnt. PathoGenetics 2009;2:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wencel HE, Smothers C, Opara EC, Kuhn CM, Feinglos MN, Surwit RS. Impaired second phase insulin response of diabetes-prone C57BL/6J mouse islets. Physiol Behav 1995;57:1215–1220 [DOI] [PubMed] [Google Scholar]
- 31.Aston-Mourney K, Wong N, Kebede M, et al. Increased nicotinamide nucleotide transhydrogenase levels predispose to insulin hypersecretion in a mouse strain susceptible to diabetes. Diabetologia 2007;50:2476–2485 [DOI] [PubMed] [Google Scholar]
- 32.Andrikopoulos S, Massa CM, Aston-Mourney K, et al. Differential effect of inbred mouse strain (C57BL/6, DBA/2, 129T2) on insulin secretory function in response to a high fat diet. J Endocrinol 2005;187:45–53 [DOI] [PubMed] [Google Scholar]
- 33.Berglund ED, Li CY, Poffenberger G, et al. Glucose metabolism in vivo in four commonly used inbred mouse strains. Diabetes 2008;57:1790–1799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fisher-Wellman KH, Lin CT, Ryan TE, et al. Pyruvate dehydrogenase complex and nicotinamide nucleotide transhydrogenase constitute an energy-consuming redox circuit. Biochem J 2015;467:271–280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fergusson G, Éthier M, Guévremont M, et al. Defective insulin secretory response to intravenous glucose in C57Bl/6J compared to C57Bl/6N mice. Mol Metab 2014;3:848–854 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong N, Blair AR, Morahan G, Andrikopoulos S. The deletion variant of nicotinamide nucleotide transhydrogenase (Nnt) does not affect insulin secretion or glucose tolerance. Endocrinology 2010;151:96–102 [DOI] [PubMed] [Google Scholar]
- 37.Alonso LC, Watanabe Y, Stefanovski D, et al. Simultaneous measurement of insulin sensitivity, insulin secretion, and the disposition index in conscious unhandled mice. Obesity (Silver Spring) 2012;20:1403–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Walker A, Pfitzner B, Neschen S, et al. Distinct signatures of host-microbial meta-metabolome and gut microbiome in two C57BL/6 strains under high-fat diet. ISME J 2014;8:2380–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harley ITW, Giles DA, Pfluger PT, et al. Differential colonization with segmented filamentous bacteria and Lactobacillus murinus do not drive divergent development of diet-induced obesity in C57BL/6 mice. Mol Metab 2013;2:171–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Khisti RT, Wolstenholme J, Shelton KL, Miles MF. Characterization of the ethanol-deprivation effect in substrains of C57BL/6 mice. Alcohol 2006;40:119–126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Green ML, Singh AV, Zhang Y, Nemeth KA, Sulik KK, Knudsen TB. Reprogramming of genetic networks during initiation of the Fetal Alcohol Syndrome. Dev Dyn 2007;236:613–631 [DOI] [PubMed] [Google Scholar]
- 42.Diwan BA, Blackman KE. Differential susceptibility of 3 sublines of C57BL/6 mice to the induction of colorectal tumors by 1,2-dimethylhydrazine. Cancer Lett 1980;9:111–115 [DOI] [PubMed] [Google Scholar]
- 43.Ulmasov B, Oshima K, Rodriguez MG, Cox RD, Neuschwander-Tetri BA. Differences in the degree of cerulein-induced chronic pancreatitis in C57BL/6 mouse substrains lead to new insights in identification of potential risk factors in the development of chronic pancreatitis. Am J Pathol 2013;183:692–708 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci 2004;45:4611–4616 [DOI] [PubMed] [Google Scholar]
- 45.Matsuo N, Takao K, Nakanishi K, Yamasaki N, Tanda K, Miyakawa T. Behavioral profiles of three C57BL/6 substrains. Front Behav Neurosci 2010;4:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gray JE, Starmer J, Lin VS, Dickinson BC, Magnuson T. Mitochondrial hydrogen peroxide and defective cholesterol efflux prevent in vitro fertilization by cryopreserved inbred mouse sperm. Biol Reprod 2013;89:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ronchi JA, Figueira TR, Ravagnani FG, Oliveira HCF, Vercesi AE, Castilho RF. A spontaneous mutation in the nicotinamide nucleotide transhydrogenase gene of C57BL/6J mice results in mitochondrial redox abnormalities. Free Radic Biol Med 2013;63:446–456 [DOI] [PubMed] [Google Scholar]
- 48.Erecińska M, Bryła J, Michalik M, Meglasson MD, Nelson D. Energy metabolism in islets of Langerhans. Biochim Biophys Acta 1992;1101:273–295 [DOI] [PubMed] [Google Scholar]
- 49.Maechler P, Wollheim CB. Mitochondrial function in normal and diabetic beta-cells. Nature 2001;414:807–812 [DOI] [PubMed] [Google Scholar]
- 50.Wallace DC. Mouse models for mitochondrial disease. Am J Med Genet 2001;106:71–93 [DOI] [PubMed] [Google Scholar]
- 51.Hoek JB, Rydström J. Physiological roles of nicotinamide nucleotide transhydrogenase. Biochem J 1988;254:1–10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang T-T, Naeemuddin M, Elchuri S, et al. Genetic modifiers of the phenotype of mice deficient in mitochondrial superoxide dismutase. Hum Mol Genet 2006;15:1187–1194 [DOI] [PubMed] [Google Scholar]
- 53.Huang Y-C, Hwang T-L, Yang Y-L, et al. Acetogenin and prenylated flavonoids from Helminthostachys zeylanica with inhibitory activity on superoxide generation and elastase release by neutrophils. Planta Med 2010;76:447–453 [DOI] [PubMed] [Google Scholar]
- 54.Hasselmann C, Deladoëy J, Vuissoz JM, et al. . Expanding the phenotypic spectrum of nicotinamide nucleotide transhydrogenase (NNT) mutations and using whole exome sequencing to discover potential disease modifiers. Journal of Genomes and Exomes 2013;1:19–30 [Google Scholar]
- 55.Weinberg-Shukron A, Abu-Libdeh A, Zhadeh F, et al. Combined mineralocorticoid and glucocorticoid deficiency is caused by a novel founder nicotinamide nucleotide transhydrogenase mutation that alters mitochondrial morphology and increases oxidative stress. J Med Genet 2015;52:636–641 [DOI] [PubMed] [Google Scholar]
- 56.Bourdi M, Davies JS, Pohl LR. Mispairing C57BL/6 substrains of genetically engineered mice and wild-type controls can lead to confounding results as it did in studies of JNK2 in acetaminophen and concanavalin A liver injury. Chem Res Toxicol 2011;24:794–796 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Taft RA, Davisson M, Wiles MV. Know thy mouse. Trends Genet 2006;22:649–653 [DOI] [PubMed] [Google Scholar]
- 58.Ye S, Dhillon S, Ke X, Collins AR, Day IN. An efficient procedure for genotyping single nucleotide polymorphisms. Nucleic Acids Res 2001;29:E88-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Collins A, Ke X. Primer1: primer design web service for tetra-primer ARMS-PCR. Open Bioinform J 2012;6:55–58 [Google Scholar]
- 60.Mekada K, Hirose M, Murakami A, Yoshiki A. Development of SNP markers for C57BL/6N-derived mouse inbred strains. Exp Anim 2015;64:91–100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Vanden Berghe T, Hulpiau P, Martens L, et al. Passenger Mutations Confound Interpretation of All Genetically Modified Congenic Mice. Immunity 2015;43:200–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sander JD, Joung JK. CRISPR-Cas systems for editing, regulating and targeting genomes. Nat Biotechnol 2014;32:347–355 [DOI] [PMC free article] [PubMed] [Google Scholar]