Abstract
Studies have shown associations between exposure to hypoglycemia and increased mortality, raising the possibility that hypoglycemia has adverse cardiovascular effects. In this study, we determined the acute effects of hypoglycemia on cardiovascular autonomic control. Seventeen healthy volunteers were exposed to experimental hypoglycemia (2.8 mmol/L) for 120 min. Cardiac vagal baroreflex function was assessed using the modified Oxford method before the initiation of the hypoglycemic-hyperinsulinemic clamp protocol and during the last 30 min of hypoglycemia. During hypoglycemia, compared with baseline euglycemic conditions, 1) baroreflex sensitivity decreases significantly (19.2 ± 7.5 vs. 32.9 ± 16.6 ms/mmHg, P < 0.005), 2) the systolic blood pressure threshold for baroreflex activation increases significantly (the baroreflex function shifts to the right; 120 ± 14 vs. 112 ± 12 mmHg, P < 0.005), and 3) the maximum R-R interval response (1,088 ± 132 vs. 1,496 ± 194 ms, P < 0.001) and maximal range of the R-R interval response (414 ± 128 vs. 817 ± 183 ms, P < 0.001) decrease significantly. These findings indicate reduced vagal control and impaired cardiovascular homeostasis during hypoglycemia.
Introduction
Rigorous glycemic control is the cornerstone of diabetes management; however, this results in increased episodes of iatrogenic hypoglycemia (1). Multiple community- and hospital-based studies have shown an association between exposure to hypoglycemia and increased mortality (2,3). Furthermore, results of several large-scale clinical trials investigating the outcome of intensive glycemic control have shown increased cardiovascular mortality or a lack of cardiovascular benefit (4–7). These results raise the possibility that exposure to hypoglycemia has adverse and unknown effects that persist after hypoglycemia resolves and may oppose cardiovascular benefits of improved glycemic control (8,9).
Prior exposure to hypoglycemia impairs the hormonal and muscle sympathetic nerve activity (MSNA) response to subsequent hypoglycemia (10), the hormonal responses to subsequent exercise (11), and autonomic control of cardiovascular function (12). After exposure to hypoglycemia, induced by the hypoglycemic-hyperinsulinemic clamp protocol, individuals exhibit decreased baroreflex sensitivity (BRS), decreased MSNA response to transient hypotension, and decreased norepinephrine response to orthostatic stress compared with prior exposure to euglycemic-hyperinsulinemic conditions. These changes in autonomic cardiovascular control are present in the euglycemic state 16 h after hypoglycemic exposure (12). The time of onset for these changes is not established.
The baroreflex plays a central role in maintaining cardiovascular homeostasis. Impaired baroreflex function is associated with increased mortality in patients with diabetes, hypertension, and cardiovascular disease (13–15). Thus, hypoglycemia-induced changes in autonomic cardiovascular control could contribute to the mortality associated with hypoglycemia (7,8,16).
The modified Oxford test is the gold standard for characterizing the baroreflex. By assessing baroreflex function across a wide range of clinically relevant blood pressures, this test defines the full hemodynamic range of baroreflex engagement. The modified Oxford method has not been used to assess BRS during hypoglycemia. The purpose of this study was to test the hypothesis that impairment in baroreflex cardiovascular control begins during hypoglycemia. We therefore assessed baroreflex function using the modified Oxford method before and during a hypoglycemic-hyperinsulinemic clamp protocol in healthy participants.
Research Design and Methods
Study Population
Healthy men and women, aged 18 to 40 years, were recruited from the greater Boston area. Exclusion criteria included evidence of any medical illness on history or physical examination, BMI >30 kg/m2, tobacco use, substance abuse, pregnancy, lactation, menopause, abnormalities on electrocardiogram, or abnormal blood electrolyte, liver function test, complete blood count, or urinalysis values. The study protocol (NCT01394627) was approved by the Partners Institutional Review Board, and all subjects provided written informed consent.
Diet and Activity
Participants stopped over-the-counter medications 2 weeks before admission and refrained from vigorous exercise from 1 week before admission to the Center for Clinical Investigation at Brigham and Women’s Hospital. Participants consumed an isocaloric diet (125 mmol/day Na+, 125 mmol/day K+, 20 mmol/day Ca2+) lacking foods high in monoamines beginning 4 days before and continuing throughout the admission. A hypoglycemic-hyperinsulinemic clamp procedure was performed in the morning, with participants having been supine and fasting since midnight.
Hypoglycemic-Hyperinsulinemic Clamp Protocol
As previously described, participants received a primed continuous intravenous infusion of regular insulin at 80 mU/m2 body surface area/min for ∼150 min (all participants received Humulin R [Eli Lilly, Indianapolis, IN], except one who received Novolin R [Novo Nordisk, Princeton, NJ]). Twenty percent dextrose was infused intravenously to achieve blood glucose of 2.8 mmol/L (50 mg/dL) for 120 min (17). Blood samples were withdrawn through an indwelling intravenous catheter placed in a retrograde fashion in the participant’s wrist/hand that rested in a warm box (150°F) throughout the procedure. Plasma glucose was assessed every 5 min using a bedside glucose analyzer (YSI 2300 STAT Plus Glucose & Lactate Analyzer; YSI, Yellow Springs, OH). Blood was collected for analysis of insulin at baseline and during the insulin infusion.
Baroreflex Assessment
Cardiac vagal baroreflex function was assessed using the modified Oxford method (18) at baseline and during the last 30 min of the hypoglycemic clamp (Fig. 1). After a baseline measurement of blood pressure and heart rate using an automated oscillometric blood pressure monitor (Dinamap; Critikon, Inc., Tampa, FL), participants received intravenous bolus injections of 100 µg sodium nitroprusside, followed 60 s later by 150 µg phenylephrine hydrochloride as previously described (12). This procedure causes a decrease in systolic blood pressure (SBP) of ∼15 mmHg below baseline SBP, followed by an increase of ∼15 mmHg above baseline. R-R interval and beat-to-beat blood pressure were measured using the Finometer (FMS, Amsterdam, the Netherlands). Baroreflex assessments were performed in duplicate. The minimum and maximum R-R intervals were used to define the range of heart rate response during the baroreflex function assessment. BRS was determined by the slope of the relation between the R-R interval and SBP. The threshold of baroreflex function was defined as the SBP at which the R-R interval started to increase after reaching its minimum value during the modified Oxford test. The saturation point of baroreflex function was defined as the SBP at which the R-R interval reached its maximum value (Figs. 2 and 3).
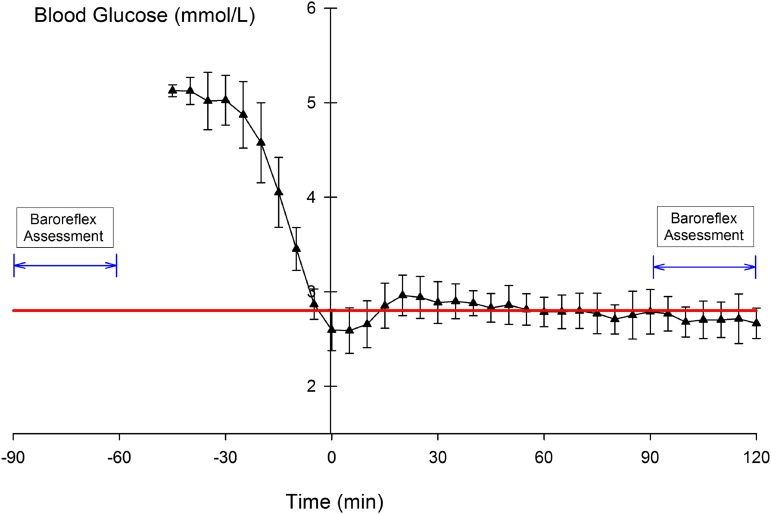
Figure 1.
Timing of the baroreflex assessment (modified Oxford) relative to the plasma glucose levels (mean ± SD) during the hypoglycemic-hyperinsulinemic clamp protocol. The straight line represents the target glucose level (2.8 mmol/L).
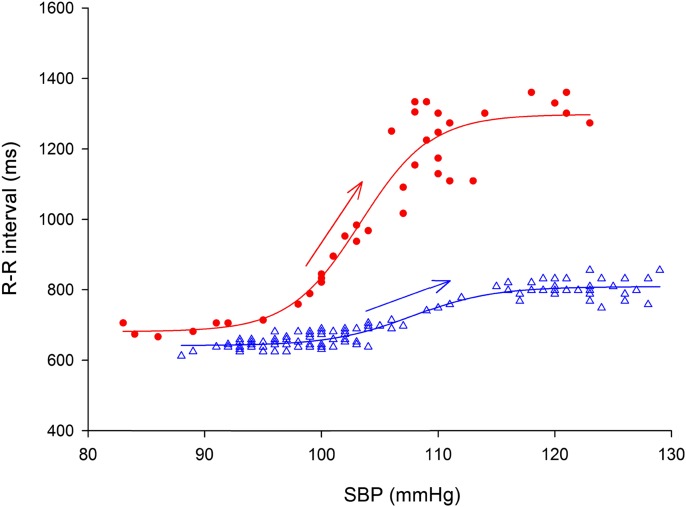
Figure 2.
Baroreflex function from a representative subject measured by the modified Oxford method during baseline euglycemia (circles) and hypoglycemia (triangles). Data points represent the corresponding values of SBP and cardiac interval (R-R) during the pharmacologically induced blood pressure change. For baroreflex assessment, R-R is plotted as a function of SBP between the lowest and the highest SBP values. The linear portion (arrows) of the sigmoid function describes BRS (see research design and methods). During hypoglycemia, BRS is decreased, maximal R-R interval is decreased, and the sigmoidal baroreflex function curve shifts to the right.
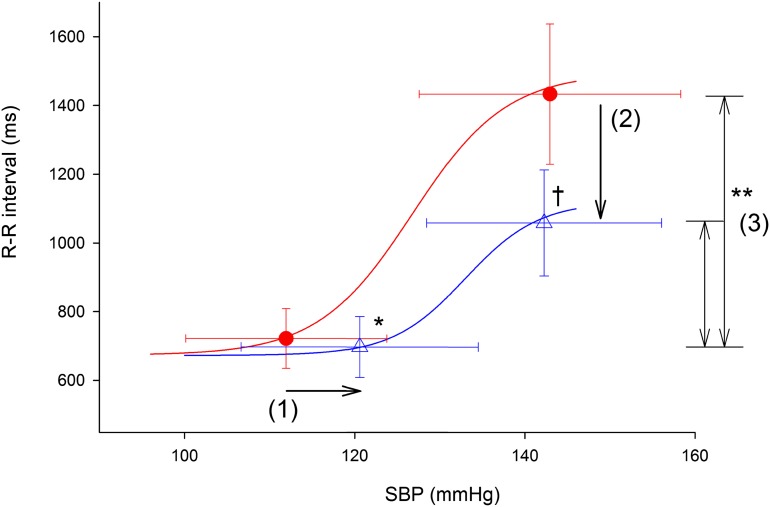
Figure 3.
Baroreflex function measured by the modified Oxford method during baseline euglycemia (circles) and hypoglycemia (triangles). Group average values for the threshold and saturation blood pressures and corresponding cardiac intervals (R-R) are displayed for each glycemic condition. A sigmoidal curve is fitted to these group data for both glycemic conditions. Horizontal error bars denote the SD of blood pressure, and the vertical error bars denote the SD of the R-R interval. During hypoglycemia, the baroreflex threshold shifts to higher blood pressures (arrow 1), the R-R interval at the blood pressure saturation point decreases (arrow 2), and the R-R interval range is lower (double-headed arrows 3). Data for BRS (the slope of the linear portion of the baroreflex sigmoid curve) showing a significant decrease with hypoglycemia are reported in Table 1. *P < 0.005 for the difference in the baroreflex blood pressure threshold, hypoglycemia vs. baseline. †P < 0.001 for the difference in maximum R-R interval at the blood pressure saturation point, hypoglycemia vs. baseline. **P < 0.001 for the difference in R-R interval ranges, hypoglycemia vs. baseline.
Statistical Analysis
Data were analyzed using the Student two-tailed t test and ANOVA with repeated measures (general linear model, SPSS). Categorical variables were compared using the Fisher exact test. Data are expressed as mean ± SD unless specified otherwise. A P value <0.05 was considered statistically significant. Statistical analysis was performed with SAS 9.3 (SAS Institute, Inc., Cary, NC), JMP Pro 10.0 (SAS Institute, Inc.), or SPSS software (IBM Corp., Armonk, NY).
Results
Subject Demographics and Clamp Data
The analysis included 17 participants (age 26 ± 6 years, 76% male, 59% Caucasian, BMI 23.2 ± 3.6 kg/m2). Four additional participants were excluded due to incomplete autonomic testing data (n = 3) or average blood glucose >3.0 mmol/L during the final 120 min of the clamp protocol (n = 1).
All 17 participants were insulin sensitive, with an average homeostatic model assessment of 1.05 ± 0.42 and fasting glucose of 5.0 ± 0.3 mmol/L. Insulin infusion increased serum insulin from baseline levels of 29 ± 11 pmol/L to 731 ± 290 pmol/L. Figure 1 shows plasma glucose levels during the clamp procedure and timing of the modified Oxford test. The average plasma glucose during the 120 min of hypoglycemia was 2.8 ± 0.1 mmol/L.
Baroreflex Assessment Before and During Hypoglycemia
Blood pressure and heart rate were assessed before the modified Oxford procedures at baseline, when subjects were euglycemic, and during the hypoglycemic clamp (Fig. 1). SBPs were similar, whereas diastolic blood pressure (DBP) was significantly lower, under hypoglycemic compared with baseline euglycemic conditions (SBPbaseline 116 ± 11 mmHg vs. SBPhypoglycemia 119 ± 14 mmHg, P = NS; DBPbaseline 67 ± 7 mmHg vs. DBPhypoglycemia 61 ± 7 mmHg, P < 0.001). Heart rate increased significantly during hypoglycemia compared with baseline euglycemia (baseline heart rate 60 ± 8 bpm vs. 71 ± 8 bpm; P < 0.001).
The minimum and maximum (peak and trough) SBPs during the modified Oxford procedure were similar under hypoglycemic and baseline euglycemic conditions (Table 1). Analysis of baroreflex function revealed significant differences between baseline and hypoglycemic conditions (see Fig. 2 for a representative subject and Fig. 3 and Table 1 for group data). BRS decreased during hypoglycemia compared with baseline as shown by the decrease in the slope of the linear portion of the sigmoidal baroreflex function plot (19.2 ± 7.5 ms/mmHg during hypoglycemia vs. 32.9 ± 16.6 ms/mmHg at baseline, P < 0.005).
Table 1.
Characteristic points of the modified Oxford baroreflex function for all subjects during baseline euglycemia and hypoglycemia
| Baseline | Hypoglycemia | |
|---|---|---|
| SBP (mmHg) | ||
| Minimum | 98 ± 13 | 102 ± 15 |
| Threshold | 112 ± 12 | 120 ± 14* |
| Saturation | 143 ± 15 | 142 ± 14 |
| Maximum | 146 ± 1 4 | 145 ± 13 |
| R-R interval (ms) | ||
| Minimum | 721 ± 86 | 697 ± 88 |
| Maximum | 1,496 ± 194 | 1,088 ± 132** |
| Range | 817 ± 183 | 414 ± 128** |
| BRS (ms/mmHg) | 32.9 ± 16.6 | 19.2 ± 7.5* |
Data are mean ± SD. *P < 0.005 hypoglycemia vs. baseline.
**P < 0.001 hypoglycemia vs. baseline.
The SBP threshold for activation of the baroreflex increased significantly (a shift to the right of the baroreflex plot) during hypoglycemia compared with baseline euglycemia (Fig. 2, Fig. 3, and Table 1), indicating higher blood pressures were required for baroreflex activation. The saturation point of the baroreflex curve (the point at which blood pressure increases elicited no further increases in the R-R interval) was similar during baseline euglycemia compared with hypoglycemia (Fig. 2, Fig. 3, and Table 1).
The maximum R-R interval response elicited by the increase in blood pressure during the modified Oxford procedure decreased significantly (a downward shift of the baroreflex plot) during hypoglycemia compared with baseline (Fig. 2, Fig. 3, and Table 1). Further, the range between the maximum and minimum R-R intervals was significantly decreased during hypoglycemia versus baseline, indicating a blunted heart rate response to pharmacologically induced changes in blood pressure (Fig. 2, Fig. 3, and Table 1).
Discussion
Our data demonstrate that during insulin-induced hypoglycemia 1) BRS is decreased, 2) the blood pressure threshold for baroreflex activation is increased, and 3) the maximum R-R interval response and maximal range of the R-R interval responses are decreased. These findings indicate an inability to maintain optimal vagal control during hypoglycemia. Impaired cardiovascular homeostasis during hypoglycemia could contribute to the adverse cardiac outcomes associated with hypoglycemia. These results extend our previous findings, which showed a decrease in BRS 16 h after exposure to hypoglycemia (12). Taken together, these two studies suggest that hypoglycemia-mediated attenuation of cardiac vagal baroreflex function begins during hypoglycemia and persists for at least 16 h after hypoglycemia has ended.
Patients with diabetes are often exposed to asymptomatic episodes of hypoglycemia. The incidence of severe hypoglycemic episodes (those that require medical assistance) may be as high as 3.2 episodes per patient per year in individuals with type 1 diabetes and 0.1 to 0.7 episodes per patient per year in individuals with type 2 diabetes (19,20). These episodes can last up to 10% of a 24-h period (21). There is an increased interest in the potential adverse effects of hypoglycemia prompted by studies showing associations between severe hypoglycemia and cardiovascular disease and microvascular disease in individuals with type 2 diabetes (9) and by large-scale clinical studies showing an association between intensive glycemic control and adverse cardiovascular events (4–6). Multiple processes associated with cardiovascular injury or dysfunction are induced during hypoglycemia. These include increased activation of the renin-angiotensin-aldosterone system (22); increases in inflammatory cytokines, including interleukin-6, interleukin-8, tumor necrosis factor-α, and endothelin-1 (17,23,24); endothelial dysfunction (25); QT interval prolongation (26); cardiac arrhythmias (16); decrease in the spontaneous baroreflex (27); and increased sympathetic nerve activity (28). All of these factors could have a role in the adverse clinical outcomes associated with hypoglycemia.
The present finding that autonomic control of cardiovascular function is altered during hypoglycemia extends our understanding of the potential adverse cardiovascular effects of hypoglycemia. We used pharmacological provocations to fully characterize the baroreflex across a range of physiologically relevant blood pressures, allowing us to determine the specific changes in baroreflex control induced by hypoglycemia. Our observation that BRS is reduced during hypoglycemia over a wide range of blood pressures extends and supports a prior study of spontaneous cardiac BRS during hypoglycemia (27). In addition, we show directly, for the first time, that the increase in the blood pressure threshold for activation of the baroreflex and the decrease in the range of heart rate responses to blood pressure changes during hypoglycemia both contribute to the altered autonomic heart rate control during hypoglycemia. These mechanisms indicate an inability to maintain optimal vagal control leading to a higher probability of impaired cardiovascular homeostasis and adverse cardiac events during hypoglycemia. The observation that alteration of baroreflex function is present within 90–120 min of exposure to hypoglycemia and with exposure to a relatively moderate degree of hypoglycemia (2.8 mmol/L) increases the relevancy to clinical care.
The baroreflex plays a pivotal role in cardiovascular homeostasis. Decreases in BRS lead to an impaired homeostatic response to hemodynamic stress. Baroreflex dysfunction is also associated with an increased risk of cardiac arrhythmias (29) and is a predictor of mortality in the period after a myocardial infarction (15) and in individuals with type 2 diabetes (13). Thus, hypoglycemia-induced changes in baroreflex function could have significant clinical implications that may be particularly relevant in individuals with diabetes who experience hypoglycemia or have underlying autonomic dysfunction. Although the current studies were performed in healthy subjects, our results are consistent with published studies in individuals with diabetes. In individuals with type 1 diabetes, acute hypoglycemia reduces measures of autonomic and cardiovascular function (30). Further, indices of hypoglycemia derived from continuous glucose monitoring were associated with reduced heart rate variability in individuals with type 1 diabetes (31).
Several studies suggest that activation of carotid body chemoreceptors by hypoxia reduces BRS (32) or shifts the baroreflex stimulus response curves to higher blood pressures and heart rates (33,34). When studied in individuals with type 1 diabetes, acute hypoxia further deteriorates hypoglycemia-evoked decreases in the spontaneous cardiac baroreflex and measures of heart rate variability (35). This may be relevant in patients with sleep apnea, a common condition in individuals with diabetes (36).
Our study has some limitations. We cannot identify the exact mechanisms that underlie the decreased BRS, the increased blood pressure threshold, and the decreased maximum R-R interval response. This requires further studies. However, the changes in the baroreflex are not due to differences in the blood pressure provocation because the blood pressure range and blood pressure saturation point were similar during baseline euglycemia and hypoglycemia. In addition, the current study cannot determine the relative roles played by hyperinsulinemia and hypoglycemia in the observed impairments in the cardiovagal baroreflex. Insulin induces vasodilation and increases sympathetic activity in the presence of euglycemia (37) and, when administered centrally to anesthetized rats, increases baroreflex gain of heart rate and MSNA (38,39). In contrast, in a study in humans, hyperinsulinemia increased the MSNA baroreflex gain (i.e., the MSNA response to spontaneous changes in blood pressure) in the presence of euglycemia but did not modify cardiovagal baroreflex gain (i.e., the heart rate response to spontaneous changes in blood pressure) (40). These findings contrast with our observation that insulin-induced hypoglycemia blunts the cardiovagal baroreflex and support the hypothesis that blunting of the cardiovagal baroreflex is due to hypoglycemia, not hyperinsulinemia. Further studies are necessary to prove this point.
In summary, these data suggest that cardiac vagal baroreflex is impaired in healthy nonobese individuals during acute insulin-induced hypoglycemia. Further studies are needed to determine whether these changes occur in individuals with diabetes (41) and to determine the relevance of hypoglycemia-induced alterations in autonomic control of cardiovascular function to clinical outcomes.
Article Information
Acknowledgments. The authors thank the staff of the clinical research center at Brigham and Women’s Hospital and the volunteers of this study.
Funding. A.D.R. was supported by grants from the National Heart, Lung, and Blood Institute (T32-HL-007609) and the Richard Laylord Evans and Dorothy L. Evans Foundation (Temple University Department of Medicine Faculty Development Research Award) and received research support for this project from the Robert Wood Johnson Foundation. This research was also supported by National Heart, Lung, and Blood Institute grants K24-HL-103845 (G.K.A.) and R01-HL-109634 and RO1-HL-111465 (R.F.).
Duality of Interest. No potential conflicts of interest relevant to this article were reported.
Author Contributions. A.D.R. conceived the idea, recruited participants, conducted the study, interpreted the data, and wrote the manuscript. I.B. interpreted the data and wrote the manuscript. J.D., M.B.-G., and L.K. helped recruit participants and conducted the study. S.B. helped conduct the study. R.F. and G.K.A. conceived the idea, interpreted the data, and wrote the manuscript. All authors contributed to the manuscript and take full responsibility for its originality. A.D.R., R.F., and G.K.A. are the guarantors of this work and, as such, had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.Cryer P. Hypoglycemia in Diabetes: Pathophysiology, Prevalence, and Prevention. Alexandria, VA, American Diabetes Association, 2012 [Google Scholar]
- 2.Turchin A, Matheny ME, Shubina M, Scanlon JV, Greenwood B, Pendergrass ML. Hypoglycemia and clinical outcomes in patients with diabetes hospitalized in the general ward. Diabetes Care 2009;32:1153–1157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Garg R, Hurwitz S, Turchin A, Trivedi A. Hypoglycemia, with or without insulin therapy, is associated with increased mortality among hospitalized patients. Diabetes Care 2013;36:1107–1110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerstein HC, Miller ME, Byington RP, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of intensive glucose lowering in type 2 diabetes. N Engl J Med 2008;358:2545–2559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Patel A, MacMahon S, Chalmers J, et al.; ADVANCE Collaborative Group . Intensive blood glucose control and vascular outcomes in patients with type 2 diabetes. N Engl J Med 2008;358:2560–2572 [DOI] [PubMed] [Google Scholar]
- 6.Duckworth W, Abraira C, Moritz T, et al.; VADT Investigators . Glucose control and vascular complications in veterans with type 2 diabetes. N Engl J Med 2009;360:129–139 [DOI] [PubMed] [Google Scholar]
- 7.Cryer PE. Death during intensive glycemic therapy of diabetes: mechanisms and implications. Am J Med 2011;124:993–996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Frier BM, Schernthaner G, Heller SR. Hypoglycemia and cardiovascular risks. Diabetes Care 2011;34(Suppl. 2):S132–S137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zoungas S, Patel A, Chalmers J, et al.; ADVANCE Collaborative Group . Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010;363:1410–1418 [DOI] [PubMed] [Google Scholar]
- 10.Diedrich L, Sandoval D, Davis SN. Hypoglycemia associated autonomic failure. Clin Auton Res 2002;12:358–365 [DOI] [PubMed] [Google Scholar]
- 11.Galassetti P, Tate D, Neill RA, Morrey S, Wasserman DH, Davis SN. Effect of sex on counterregulatory responses to exercise after antecedent hypoglycemia in type 1 diabetes. Am J Physiol Endocrinol Metab 2004;287:E16–E24 [DOI] [PubMed] [Google Scholar]
- 12.Adler GK, Bonyhay I, Failing H, Waring E, Dotson S, Freeman R. Antecedent hypoglycemia impairs autonomic cardiovascular function: implications for rigorous glycemic control. Diabetes 2009;58:360–366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gerritsen J, Dekker JM, TenVoorde BJ, et al. Impaired autonomic function is associated with increased mortality, especially in subjects with diabetes, hypertension, or a history of cardiovascular disease: the Hoorn Study. Diabetes Care 2001;24:1793–1798 [DOI] [PubMed] [Google Scholar]
- 14.De Ferrari GM, Sanzo A, Bertoletti A, Specchia G, Vanoli E, Schwartz PJ. Baroreflex sensitivity predicts long-term cardiovascular mortality after myocardial infarction even in patients with preserved left ventricular function. J Am Coll Cardiol 2007;50:2285–2290 [DOI] [PubMed] [Google Scholar]
- 15.La Rovere MT, Bigger JT Jr, Marcus FI, Mortara A, Schwartz PJ; ATRAMI (Autonomic Tone and Reflexes After Myocardial Infarction) Investigators . Baroreflex sensitivity and heart-rate variability in prediction of total cardiac mortality after myocardial infarction. Lancet 1998;351:478–484 [DOI] [PubMed] [Google Scholar]
- 16.Chow E, Bernjak A, Williams S, et al. Risk of cardiac arrhythmias during hypoglycemia in patients with type 2 diabetes and cardiovascular risk. Diabetes 2014;63:1738–1747 [DOI] [PubMed] [Google Scholar]
- 17.Dotson S, Freeman R, Failing HJ, Adler GK. Hypoglycemia increases serum interleukin-6 levels in healthy men and women. Diabetes Care 2008;31:1222–1223 [DOI] [PubMed] [Google Scholar]
- 18.Ebert TJ, Morgan BJ, Barney JA, Denahan T, Smith JJ: Effects of aging on baroreflex regulation of sympathetic activity in humans. Am J Physiol 1992;263:H798–H803 [DOI] [PubMed]
- 19.UK Hypoglycaemia Study Group . Risk of hypoglycaemia in types 1 and 2 diabetes: effects of treatment modalities and their duration. Diabetologia 2007;50:1140–1147 [DOI] [PubMed] [Google Scholar]
- 20.Pedersen-Bjergaard U, Pramming S, Heller SR, et al. Severe hypoglycaemia in 1076 adult patients with type 1 diabetes: influence of risk markers and selection. Diabetes Metab Res Rev 2004;20:479–486 [DOI] [PubMed] [Google Scholar]
- 21.Boland E, Monsod T, Delucia M, Brandt CA, Fernando S, Tamborlane WV. Limitations of conventional methods of self-monitoring of blood glucose: lessons learned from 3 days of continuous glucose sensing in pediatric patients with type 1 diabetes. Diabetes Care 2001;24:1858–1862 [DOI] [PubMed] [Google Scholar]
- 22.Adler GK, Bonyhay I, Curren V, Waring E, Freeman R. Hypoglycaemia increases aldosterone in a dose-dependent fashion. Diabet Med 2010;27:1250–1255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wright RJ, Macleod KM, Perros P, Johnston N, Webb DJ, Frier BM. Plasma endothelin response to acute hypoglycaemia in adults with type 1 diabetes. Diabet Med 2007;24:1039–1042 [DOI] [PubMed] [Google Scholar]
- 24.Wright RJ, Newby DE, Stirling D, Ludlam CA, Macdonald IA, Frier BM. Effects of acute insulin-induced hypoglycemia on indices of inflammation: putative mechanism for aggravating vascular disease in diabetes. Diabetes Care 2010;33:1591–1597 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joy NG, Tate DB, Younk LM, Davis SN. Effects of acute and antecedent hypoglycemia on endothelial function and markers of atherothrombotic balance in healthy humans. Diabetes 2015;64:2571–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Robinson RT, Harris ND, Ireland RH, Lee S, Newman C, Heller SR. Mechanisms of abnormal cardiac repolarization during insulin-induced hypoglycemia. Diabetes 2003;52:1469–1474 [DOI] [PubMed] [Google Scholar]
- 27.Limberg JK, Taylor JL, Dube S, et al. Role of the carotid body chemoreceptors in baroreflex control of blood pressure during hypoglycaemia in humans. Exp Physiol 2014;99:640–650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hoffman RP, Sinkey CA, Anderson EA. Hypoglycemia increases muscle sympathetic nerve activity in IDDM and control subjects. Diabetes Care 1994;17:673–680 [DOI] [PubMed] [Google Scholar]
- 29.La Rovere MT, Pinna GD, Hohnloser SH, et al.; ATRAMI Investigators. Autonomic Tone and Reflexes After Myocardial Infarction . Baroreflex sensitivity and heart rate variability in the identification of patients at risk for life-threatening arrhythmias: implications for clinical trials. Circulation 2001;103:2072–2077 [DOI] [PubMed] [Google Scholar]
- 30.Limberg JK, Farni KE, Taylor JL, et al. Autonomic control during acute hypoglycemia in type 1 diabetes mellitus. Clin Auton Res 2014;24:275–283 [DOI] [PubMed]
- 31.Jaiswal M, McKeon K, Comment N, et al. Association between impaired cardiovascular autonomic function and hypoglycemia in patients with type 1 diabetes. Diabetes Care 2014;37:2616–2621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cooper VL, Pearson SB, Bowker CM, Elliott MW, Hainsworth R. Interaction of chemoreceptor and baroreceptor reflexes by hypoxia and hypercapnia - a mechanism for promoting hypertension in obstructive sleep apnoea. J Physiol 2005;568:677–687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halliwill JR, Morgan BJ, Charkoudian N. Peripheral chemoreflex and baroreflex interactions in cardiovascular regulation in humans. J Physiol 2003;552:295–302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Monahan KD, Leuenberger UA, Ray CA. Effect of repetitive hypoxic apnoeas on baroreflex function in humans. J Physiol 2006;574:605–613 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Limberg JK, Dube S, Kuijpers M, et al. Effect of hypoxia on heart rate variability and baroreflex sensitivity during hypoglycemia in type 1 diabetes mellitus. Clin Auton Res 2015;25:243–250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manin G, Pons A, Baltzinger P, et al. Obstructive sleep apnoea in people with type 1 diabetes: prevalence and association with micro- and macrovascular complications. Diabet Med 2015;32:90–96 [DOI] [PubMed]
- 37.Anderson EA, Hoffman RP, Balon TW, Sinkey CA, Mark AL. Hyperinsulinemia produces both sympathetic neural activation and vasodilation in normal humans. J Clin Invest 1991;87:2246–2252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cassaglia PA, Hermes SM, Aicher SA, Brooks VL. Insulin acts in the arcuate nucleus to increase lumbar sympathetic nerve activity and baroreflex function in rats. J Physiol 2011;589:1643–1662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pricher MP, Freeman KL, Brooks VL. Insulin in the brain increases gain of baroreflex control of heart rate and lumbar sympathetic nerve activity. Hypertension 2008;51:514–520 [DOI] [PubMed] [Google Scholar]
- 40.Young CN, Deo SH, Chaudhary K, Thyfault JP, Fadel PJ. Insulin enhances the gain of arterial baroreflex control of muscle sympathetic nerve activity in humans. J Physiol 2010;588:3593–3603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pop-Busui R, Evans GW, Gerstein HC, et al.; Action to Control Cardiovascular Risk in Diabetes Study Group . Effects of cardiac autonomic dysfunction on mortality risk in the Action to Control Cardiovascular Risk in Diabetes (ACCORD) trial. Diabetes Care 2010;33:1578–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]