Abstract
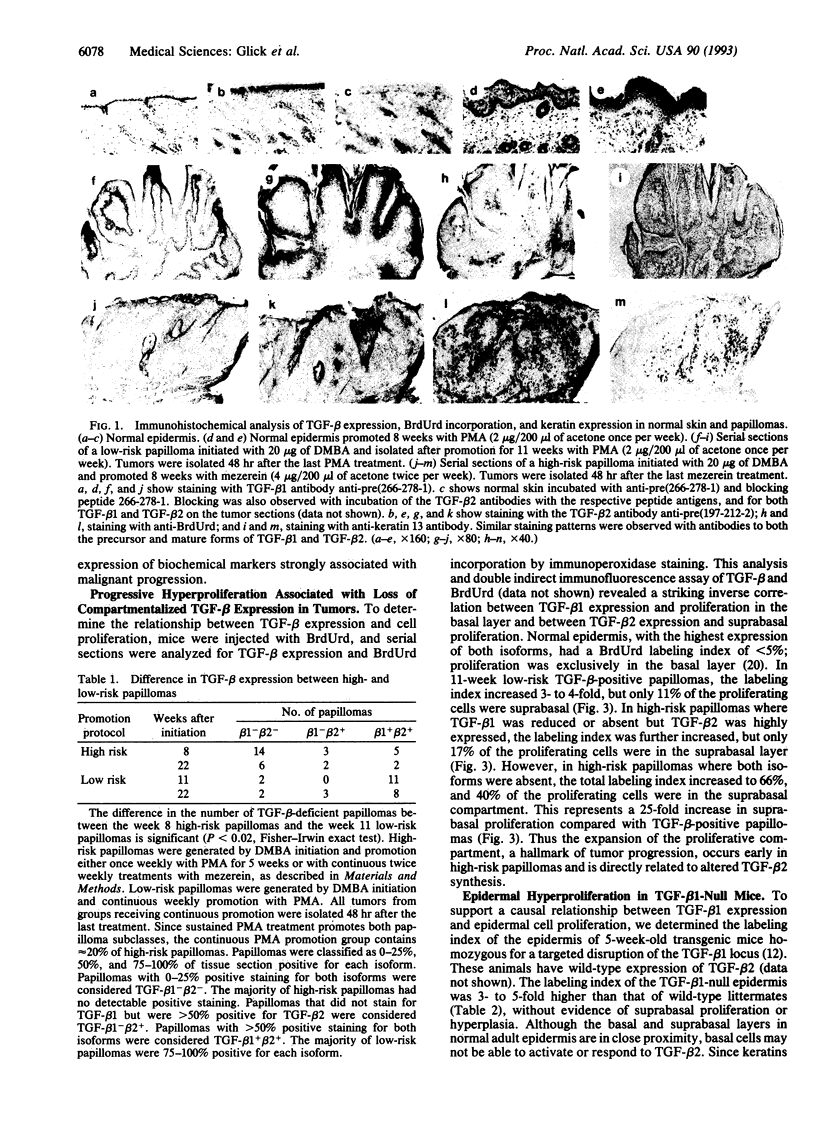
Mouse skin carcinomas arise from a small subpopulation of benign papillomas with an increased risk of malignant conversion. These papillomas arise with limited stimulation by tumor promoters, appear rapidly, and do not regress, suggesting that they differ in growth properties from the majority of benign tumors. The transforming growth factor beta (TGF-beta) proteins are expressed in the epidermis and are growth inhibitors for mouse keratinocytes in vitro; altered TGF-beta expression could influence the growth properties of high-risk papillomas. Normal epidermis, tumor promoter-treated epidermis, and skin papillomas at low risk for malignant conversion express TGF-beta 1 in the basal cell compartment and TGF-beta 2 in the suprabasal strata. In low-risk tumors, 90% of the proliferating cells are confined to the basal compartment. In contrast, the majority of high-risk papillomas are devoid of both TGF-beta 1 and TGF-beta 2 as soon as they arise; these tumors have up to 40% of the proliferating cells in the suprabasal layers. Squamous cell carcinomas are also devoid of TGF-beta, suggesting that they arise from the TGF-beta-deficient high-risk papillomas. In some high-risk papillomas, TGF-beta 1 loss can occur first and correlates with basal cell hyperproliferation, while TGF-beta 2 loss correlates with suprabasal hyperproliferation. Similarly, TGF-beta 1-null transgenic mice, which express wild-type levels of TGF-beta 2 in epidermis but no TGF-beta 1 in the basal layer, have a hyperproliferative basal cell layer without suprabasal proliferation. In tumors, loss of TGF-beta is controlled at the posttranscriptional level and is associated with expression of keratin 13, a documented marker of malignant progression. These results show that TGF-beta expression and function are compartmentalized in epidermis and epidermal tumors and that loss of TGF-beta is an early, biologically relevant risk factor for malignant progression.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akhurst R. J., Fee F., Balmain A. Localized production of TGF-beta mRNA in tumour promoter-stimulated mouse epidermis. Nature. 1988 Jan 28;331(6154):363–365. doi: 10.1038/331363a0. [DOI] [PubMed] [Google Scholar]
- Aldaz C. M., Conti C. J., Klein-Szanto A. J., Slaga T. J. Progressive dysplasia and aneuploidy are hallmarks of mouse skin papillomas: relevance to malignancy. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2029–2032. doi: 10.1073/pnas.84.7.2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aldaz C. M., Trono D., Larcher F., Slaga T. J., Conti C. J. Sequential trisomization of chromosomes 6 and 7 in mouse skin premalignant lesions. Mol Carcinog. 1989;2(1):22–26. doi: 10.1002/mc.2940020104. [DOI] [PubMed] [Google Scholar]
- Balmain A., Pragnell I. B. Mouse skin carcinomas induced in vivo by chemical carcinogens have a transforming Harvey-ras oncogene. Nature. 1983 May 5;303(5912):72–74. doi: 10.1038/303072a0. [DOI] [PubMed] [Google Scholar]
- Balmain A., Ramsden M., Bowden G. T., Smith J. Activation of the mouse cellular Harvey-ras gene in chemically induced benign skin papillomas. Nature. 1984 Feb 16;307(5952):658–660. doi: 10.1038/307658a0. [DOI] [PubMed] [Google Scholar]
- Bremner R., Balmain A. Genetic changes in skin tumor progression: correlation between presence of a mutant ras gene and loss of heterozygosity on mouse chromosome 7. Cell. 1990 May 4;61(3):407–417. doi: 10.1016/0092-8674(90)90523-h. [DOI] [PubMed] [Google Scholar]
- Dotto G. P., O'Connell J., Patskan G., Conti C., Ariza A., Slaga T. J. Malignant progression of papilloma-derived keratinocytes: differential effects of the ras, neu, and p53 oncogenes. Mol Carcinog. 1988;1(3):171–179. doi: 10.1002/mc.2940010305. [DOI] [PubMed] [Google Scholar]
- Fitzpatrick D. R., Denhez F., Kondaiah P., Akhurst R. J. Differential expression of TGF beta isoforms in murine palatogenesis. Development. 1990 Jul;109(3):585–595. doi: 10.1242/dev.109.3.585. [DOI] [PubMed] [Google Scholar]
- Flanders K. C., Cissel D. S., Mullen L. T., Danielpour D., Sporn M. B., Roberts A. B. Antibodies to transforming growth factor-beta 2 peptides: specific detection of TGF-beta 2 in immunoassays. Growth Factors. 1990;3(1):45–52. doi: 10.3109/08977199009037501. [DOI] [PubMed] [Google Scholar]
- Fowlis D. J., Flanders K. C., Duffie E., Balmain A., Akhurst R. J. Discordant transforming growth factor beta 1 RNA and protein localization during chemical carcinogenesis of the skin. Cell Growth Differ. 1992 Feb;3(2):81–91. [PubMed] [Google Scholar]
- Fürstenberger G., Rogers M., Schnapke R., Bauer G., Höfler P., Marks F. Stimulatory role of transforming growth factors in multistage skin carcinogenesis: possible explanation for the tumor-inducing effect of wounding in initiated NMRI mouse skin. Int J Cancer. 1989 May 15;43(5):915–921. doi: 10.1002/ijc.2910430531. [DOI] [PubMed] [Google Scholar]
- Glick A. B., Danielpour D., Morgan D., Sporn M. B., Yuspa S. H. Induction and autocrine receptor binding of transforming growth factor-beta 2 during terminal differentiation of primary mouse keratinocytes. Mol Endocrinol. 1990 Jan;4(1):46–52. doi: 10.1210/mend-4-1-46. [DOI] [PubMed] [Google Scholar]
- Glick A. B., Sporn M. B., Yuspa S. H. Altered regulation of TGF-beta 1 and TGF-alpha in primary keratinocytes and papillomas expressing v-Ha-ras. Mol Carcinog. 1991;4(3):210–219. doi: 10.1002/mc.2940040308. [DOI] [PubMed] [Google Scholar]
- Greenhalgh D. A., Welty D. J., Player A., Yuspa S. H. Two oncogenes, v-fos and v-ras, cooperate to convert normal keratinocytes to squamous cell carcinoma. Proc Natl Acad Sci U S A. 1990 Jan;87(2):643–647. doi: 10.1073/pnas.87.2.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heine U., Munoz E. F., Flanders K. C., Ellingsworth L. R., Lam H. Y., Thompson N. L., Roberts A. B., Sporn M. B. Role of transforming growth factor-beta in the development of the mouse embryo. J Cell Biol. 1987 Dec;105(6 Pt 2):2861–2876. doi: 10.1083/jcb.105.6.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennings H., Shores R., Balaschak M., Yuspa S. H. Sensitivity of subpopulations of mouse skin papillomas to malignant conversion by urethane or 4-nitroquinoline N-oxide. Cancer Res. 1990 Feb 1;50(3):653–657. [PubMed] [Google Scholar]
- Hennings H., Shores R., Mitchell P., Spangler E. F., Yuspa S. H. Induction of papillomas with a high probability of conversion to malignancy. Carcinogenesis. 1985 Nov;6(11):1607–1610. doi: 10.1093/carcin/6.11.1607. [DOI] [PubMed] [Google Scholar]
- Hennings H., Shores R., Wenk M. L., Spangler E. F., Tarone R., Yuspa S. H. Malignant conversion of mouse skin tumours is increased by tumour initiators and unaffected by tumour promoters. Nature. 1983 Jul 7;304(5921):67–69. doi: 10.1038/304067a0. [DOI] [PubMed] [Google Scholar]
- Hennings H., Spangler E. F., Shores R., Mitchell P., Devor D., Shamsuddin A. K., Elgjo K. M., Yuspa S. H. Malignant conversion and metastasis of mouse skin tumors: a comparison of SENCAR and CD-1 mice. Environ Health Perspect. 1986 Sep;68:69–74. doi: 10.1289/ehp.866869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huitfeldt H. S., Heyden A., Clausen O. P., Thrane E. V., Roop D., Yuspa S. H. Altered regulation of growth and expression of differentiation-associated keratins in benign mouse skin tumors. Carcinogenesis. 1991 Nov;12(11):2063–2067. doi: 10.1093/carcin/12.11.2063. [DOI] [PubMed] [Google Scholar]
- Kane C. J., Hebda P. A., Mansbridge J. N., Hanawalt P. C. Direct evidence for spatial and temporal regulation of transforming growth factor beta 1 expression during cutaneous wound healing. J Cell Physiol. 1991 Jul;148(1):157–173. doi: 10.1002/jcp.1041480119. [DOI] [PubMed] [Google Scholar]
- Kim S. J., Park K., Koeller D., Kim K. Y., Wakefield L. M., Sporn M. B., Roberts A. B. Post-transcriptional regulation of the human transforming growth factor-beta 1 gene. J Biol Chem. 1992 Jul 5;267(19):13702–13707. [PubMed] [Google Scholar]
- Kulkarni A. B., Huh C. G., Becker D., Geiser A., Lyght M., Flanders K. C., Roberts A. B., Sporn M. B., Ward J. M., Karlsson S. Transforming growth factor beta 1 null mutation in mice causes excessive inflammatory response and early death. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):770–774. doi: 10.1073/pnas.90.2.770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee E., Punnonen K., Cheng C., Glick A., Dlugosz A., Yuspa S. H. Analysis of phospholipid metabolism in murine keratinocytes transformed by the v-ras oncogene: relationship of phosphatidylinositol turnover and cytokine stimulation to the transformed phenotype. Carcinogenesis. 1992 Dec;13(12):2367–2373. doi: 10.1093/carcin/13.12.2367. [DOI] [PubMed] [Google Scholar]
- Missero C., Ramon y Cajal S., Dotto G. P. Escape from transforming growth factor beta control and oncogene cooperation in skin tumor development. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9613–9617. doi: 10.1073/pnas.88.21.9613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nischt R., Roop D. R., Mehrel T., Yuspa S. H., Rentrop M., Winter H., Schweizer J. Aberrant expression during two-stage mouse skin carcinogenesis of a type I 47-kDa keratin, K13, normally associated with terminal differentiation of internal stratified epithelia. Mol Carcinog. 1988;1(2):96–108. doi: 10.1002/mc.2940010205. [DOI] [PubMed] [Google Scholar]
- Oberhammer F. A., Pavelka M., Sharma S., Tiefenbacher R., Purchio A. F., Bursch W., Schulte-Hermann R. Induction of apoptosis in cultured hepatocytes and in regressing liver by transforming growth factor beta 1. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5408–5412. doi: 10.1073/pnas.89.12.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai S. B., Steele V. E., Nettesheim P. Neoplastic transformation of primary tracheal epithelial cell cultures. Carcinogenesis. 1983;4(4):369–374. doi: 10.1093/carcin/4.4.369. [DOI] [PubMed] [Google Scholar]
- Roop D. R., Krieg T. M., Mehrel T., Cheng C. K., Yuspa S. H. Transcriptional control of high molecular weight keratin gene expression in multistage mouse skin carcinogenesis. Cancer Res. 1988 Jun 1;48(11):3245–3252. [PubMed] [Google Scholar]
- Shipley G. D., Pittelkow M. R., Wille J. J., Jr, Scott R. E., Moses H. L. Reversible inhibition of normal human prokeratinocyte proliferation by type beta transforming growth factor-growth inhibitor in serum-free medium. Cancer Res. 1986 Apr;46(4 Pt 2):2068–2071. [PubMed] [Google Scholar]
- Shivers B. D., Schachter B. S., Pfaff D. W. In situ hybridization for the study of gene expression in the brain. Methods Enzymol. 1986;124:497–510. doi: 10.1016/0076-6879(86)24036-7. [DOI] [PubMed] [Google Scholar]
- Wakefield L. M., Smith D. M., Masui T., Harris C. C., Sporn M. B. Distribution and modulation of the cellular receptor for transforming growth factor-beta. J Cell Biol. 1987 Aug;105(2):965–975. doi: 10.1083/jcb.105.2.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S. P., Theodorescu D., Kerbel R. S., Willson J. K., Mulder K. M., Humphrey L. E., Brattain M. G. TGF-beta 1 is an autocrine-negative growth regulator of human colon carcinoma FET cells in vivo as revealed by transfection of an antisense expression vector. J Cell Biol. 1992 Jan;116(1):187–196. doi: 10.1083/jcb.116.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara K., Tsumuraya M. Transforming growth factor beta 1 induces apoptotic cell death in cultured human gastric carcinoma cells. Cancer Res. 1992 Jul 15;52(14):4042–4045. [PubMed] [Google Scholar]





