ABSTRACT
Purpose: To complete the baseline trachoma map worldwide by conducting population-based surveys in an estimated 1238 suspected endemic districts of 34 countries.
Methods: A series of national and sub-national projects owned, managed and staffed by ministries of health, conduct house-to-house cluster random sample surveys in evaluation units, which generally correspond to “health district” size: populations of 100,000–250,000 people. In each evaluation unit, we invite all residents aged 1 year and older from h households in each of c clusters to be examined for clinical signs of trachoma, where h is the number of households that can be seen by 1 team in 1 day, and the product h × c is calculated to facilitate recruitment of 1019 children aged 1–9 years. In addition to individual-level demographic and clinical data, household-level water, sanitation and hygiene data are entered into the purpose-built LINKS application on Android smartphones, transmitted to the Cloud, and cleaned, analyzed and ministry-of-health-approved via a secure web-based portal. The main outcome measures are the evaluation unit-level prevalence of follicular trachoma in children aged 1–9 years, prevalence of trachomatous trichiasis in adults aged 15 + years, percentage of households using safe methods for disposal of human feces, and percentage of households with proximate access to water for personal hygiene purposes.
Results: In the first year of fieldwork, 347 field teams commenced work in 21 projects in 7 countries.
Conclusion: With an approach that is innovative in design and scale, we aim to complete baseline mapping of trachoma throughout the world in 2015.
Keywords: Blindness, mHealth, prevalence study, trachoma, trichiasis
INTRODUCTION
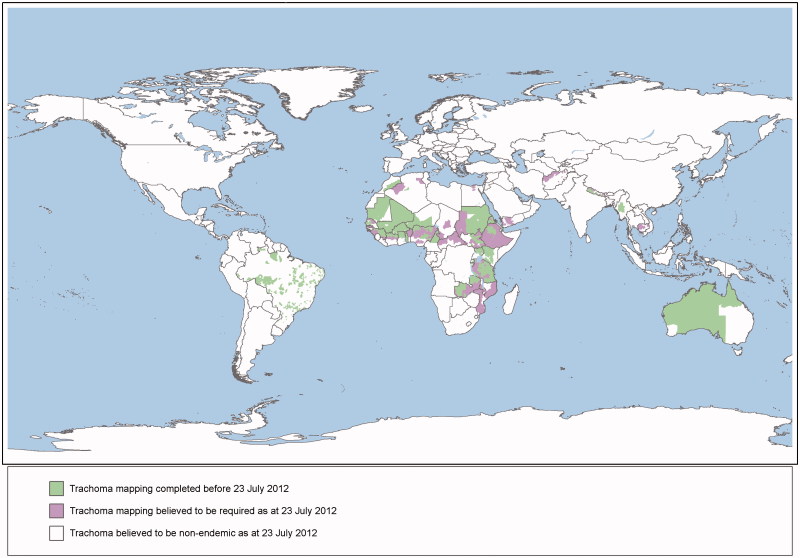
The World Health Assembly has set the year 2020 as the target for global elimination of trachoma as a public health problem.1 Mapping is a critical first stage for the elimination of trachoma because programs determine the need for interventions based on population-level prevalence of disease.2 The geographical burden of trachoma has only been partially assessed3; from 1987 to the beginning of 2012, population-based surveys mapped trachoma in 1115 districts worldwide, with data thought to be required from at least another 1238 suspected endemic districts4 to complete the global picture (Figure 1).
FIGURE 1.
Scale of the Global Trachoma Mapping Project.
At a minimum, trachoma mapping involves generation of prevalence data on the clinical signs follicular trachoma (TF) in children aged 1–9 years, and trachomatous trichiasis (TT) in adults aged 15 + years. In endemic communities, a person is considered to have TF when there are five or more follicles, each at least 0.5 mm in diameter, in the central part of the upper tarsal conjunctiva.5 TF is associated with conjunctival Chlamydia trachomatis infection,6 and is most common in children.7 , 8 In those same communities TT is the presence of at least one eyelash touching the eyeball or evidence of recent removal of in-turned eyelashes;5 it occurs more frequently with increasing age9 , 10 and disproportionately affects women.7 , 10–12 To generate baseline data for program use, the World Health Organization (WHO) recommends cluster random sample surveys, using 20–30 clusters.2 In general, the evaluation unit is the district, which for trachoma control purposes WHO defines as “the normal administrative unit for health care management, consisting of a population unit between 100,000–250,000 persons.”2 In contexts in which trachoma is expected to be highly and widely endemic, evaluation units can be constructed to encompass larger populations than this, in order to allow control programs to commence.13
In populations in which TF prevalence in 1–9-year-olds is ≥ 30%, WHO recommends the SAFE strategy (surgery, antibiotics, facial cleanliness, environmental improvement) for elimination be implemented for at least 5 years before TF prevalence is estimated again.13 Therefore, mapping must be completed by 2015 to allow programs to complete at least one phase of interventions in high prevalence areas before the 2020 elimination target date. The Global Trachoma Mapping Project (GTMP) began formal operations on 23 July 2012 and commenced fieldwork on 17 December 2012, with the aim of mapping all remaining suspected endemic districts by the end of 2015.
This paper describes the process of methodological development, and the methodology itself.
MATERIALS AND METHODS
Advisory Committee and Working Groups
A committee established in March 2012 by the International Trachoma Initiative14 to outline the issues surrounding global trachoma mapping was asked by Sightsavers, the GTMP grant manager, to serve as an Advisory Committee to the GTMP. The four working groups of that committee (Table 1) were requested to continue to develop ideas and provide input to the Advisory Committee. A chief scientist (AWS) was appointed to collate advice from the working groups and make final recommendations to the Advisory Committee on strategies to adopt.
TABLE 1. Working groups for the Global Trachoma Mapping Project.
| Name of working group | Goals |
|---|---|
| Methodologies | (1) To produce guidelines for when to use each of the current protocols for determining the need for intervention against trachoma, i.e., (a) at district level (trachoma rapid assessment,39 population-based prevalence surveys,2 and integrated threshold mapping19). (b) at larger-than-district level (trachoma rapid assessment,39 population-based prevalence surveys,2 and integrated threshold mapping19). (2) To review current indicators and protocols for collecting data on water, sanitation and hygiene (WASH) variables, and see to what extent they could be practically integrated with the protocols considered in (1). |
| Prioritization | (1) Prioritize countries for baseline trachoma mapping. (2) Prioritize (in collaboration with in-country opinion leaders and other stakeholders) districts for baseline trachoma mapping within each country. (3) Identify opportunities to coordinate with other mapping efforts. |
| Tools | (1) Review available systems for data collection, validation, storage, analysis and sharing in projects originating with fieldwork conducted in rural communities of developing countries. (2) Recommend (to the Advisory Committee) the most appropriate systems for use in baseline trachoma mapping, bearing in mind the separate needs of mapping efforts that collect: (a) data relevant only to trachoma control, (b) data relevant to control of trachoma and other endemic diseases, and (c) data relevant to control of one or more endemic diseases and WASH programs. |
| Training | (1) Review current systems and materials for training teams to undertake protocols that estimate the need for intervention against trachoma, including those in which such estimates are collected at the same time as estimates of the prevalence of other endemic diseases and/or WASH variables. (2) Review current systems and materials for training the trainers who will train the teams described in (1), including materials to develop trainers' training skills. (3) Recommend (to the Advisory Committee) the most appropriate training methods for use in baseline trachoma mapping, bearing in mind the separate training needs of mapping efforts that collect: (a) data relevant only to trachoma control, (b) data relevant to control of trachoma and other endemic diseases, and (c) data relevant to control of one or more endemic diseases and WASH programs. |
Project Structure and Ownership
The GTMP is constructed as a series of administratively separate projects with a common methodology, generally operating at the national level. In Ethiopia and Nigeria, where it was believed at the time of project launch that 434 and 209 districts, respectively, required mapping,4 projects are being implemented at regional state level (Ethiopia) and state level (Nigeria).
As the central objective of the GTMP is to generate data for use by trachoma elimination programs, government ownership of mapping is essential. Without this, the data that it generates are less likely to be acted upon locally. This philosophy has influenced a number of key project decisions (Table 2).
TABLE 2. Efforts to maximize government ownership within the Global Trachoma Mapping Project.
| Domain | Approach to facilitate government ownership |
|---|---|
| Stakeholder engagement | Initial engagement with ministry of health is preferably made in conjunction with a non-governmental organization already collaborating with the ministry. |
| Field team composition | Grader and recorder trainees are selected, recruited and contracted by the ministry of health. |
| Field team training | Field team training is conducted under the auspices of the ministry of health, using (as far as is practical) national trainers. |
| Capacity building | Ministry of health staff draft the evaluation unit divisions, cluster list and fieldwork protocol, which is then refined and finalized in discussion with Global Trachoma Mapping Project epidemiologists to ensure international standardization. The ministry of health appoints a lead author to draft a paper presenting the project results for publication in a peer-reviewed journal. |
| Data management | Although (in order to ensure international standardization) data cleaning is undertaken by a single data manager who works across all projects, the adequacy of data collection and data cleaning process must be approved by a designated ministry of health official before analysis. Prevalence estimates must be approved by a designated ministry of health official before they are considered finalized. Only the ministry of health has access to download the full datasets. Only prevalence categories (not the estimates themselves) are released to the Global Atlas of Trachoma, and only after ministry of health approval. The ministry of health is free to share data with its partners at any time. |
Prioritization
Highly-burdened endemic countries need rapid scale-up of trachoma elimination activities and are being prioritized by the GTMP. The level of local preparedness to undertake mapping and to implement SAFE interventions after mapping is also important to the prioritization process; commencing work in countries ready to collect data and use them for programmatic decision making allowed us to make rapid early progress.
Training of Field Teams
We developed standardized training material for trachoma graders and data recorders. We compiled a draft training system (comprising a trainers' manual, a series of slide sets and Microsoft Excel-based kappa score calculators) by assembly and adaptation of a range of materials used previously, plus generation of some entirely new resources, then field-tested it at a developmental training week in Oromia, Ethiopia, from 8–12 October 2012, involving 15 field teams. We incorporated lessons learned into subsequent revisions. We also developed a training-of-trainers package.
GTMP field teams are each composed of one trachoma grader and one data recorder, plus a driver and a local guide. Graders and recorders are recruited (against standard selection requirements, Figure 2) by the local ministry of health, then trained using GTMP materials15 over 5 days. The first 2 training days are an intensive classroom- and field-based training and testing period for prospective graders, labeled the “grader qualifying workshop.” In the fieldwork component, we provide one grader trainer for every four grader trainees to make effective patient-based instruction possible. Only grader trainees who pass slide- and field-based tests of diagnostic accuracy (see below), and those recorder trainees who pass an electronically-marked test on the accuracy of their data capture, graduate to become members of the survey team.
FIGURE 2.
Selection criteria for Global Trachoma Mapping Project (GTMP) field team trainees.15
In an effort to ensure consistency of grading, we established a 3-level grader training and certification cascade:
Each GTMP master grader (initially ABK, AWS, CM and WA) has experience delivering clinical teaching at post-graduate level; experience of previous trachoma surveys as a grader or supervisor; a kappa statistic ≥ 0.80 against the chief scientist for diagnosis of TF in children in live-subject inter-grader agreement (IGA) tests; and participation as a trainer in at least 2 grader qualifying workshops.
To be certified as GTMP grader trainers, individuals need experience delivering clinical teaching at post-graduate level or experience of previous trachoma surveys as a grader or supervisor; a kappa statistic ≥ 0.80 against a GTMP master grader for diagnosis of TF in children in a live-subject IGA test; and participation as a trainee in at least one grader qualifying workshop.
To be certified as GTMP graders, individuals need a medical or nursing background or equivalent experience; a kappa statistic ≥ 0.70 against a GTMP grader trainer for diagnosis of TF in children in a live-subject IGA test; and participation as a trainee in at least one grader qualifying workshop.
The master graders have all been extensively involved in the development and/or delivery of the GTMP under the oversight of the chief scientist, and understand it thoroughly. They train grader trainers using the full training package, incorporating some teaching about teaching, preparing grader trainers who pass the IGA test against a master grader to then go on and deliver training to candidate graders in their own projects. Materials and methods to be used for all training activities are clearly outlined.
Before progressing to a live-subject IGA test, prospective grader trainers and graders must pass a slide-based IGA test using a set of 50 slides, on each of which the presence or absence of TF has been unanimously agreed by five internationally recognized trachoma graders (ABK, AWS, HRT, TML, WA).
Live-subject IGA tests require 50 children aged 1–9 years, of whom ideally a minimum of 15 and a maximum of 35 have TF, as determined by the reference grader for the test. If fewer than 15 or more than 35 subjects have TF, the test is not invalid, but becomes more difficult to pass, since the kappa statistic heavily penalizes mis-calls when the frequency of the outcome of interest is very low or very high in the test sample. Countries with suspected low TF prevalence send candidate grader trainers (and in some cases, candidate graders) to highly endemic countries for training and certification. To avoid incorrect estimation of diagnostic agreement because of between-eye correlation of clinical phenotype,16 only one eye of each child is examined by those taking the IGA test.
Survey Sampling Approach and Sample Size
A reliable survey methodology was essential to the aims of the project. A number of survey methods have previously been used to map trachoma, including trachoma rapid assessment, acceptance sampling trachoma rapid assessment, cluster random sampling and integrated threshold mapping (ITM).17–19 We did not consider the first two methods as they do not provide prevalence estimates. To compare cluster random sampling and ITM, computer-based simulations were undertaken in order to investigate ITM's utility as a possible trachoma mapping approach in areas where mapping of other diseases was also required. Both the simulations20 and multi-country data analyses21 suggest that ITM will systematically under-estimate trachoma prevalence, with the extent of underestimation proportional to the degree of bias introduced by school-based sampling. This results in greater likelihood of misclassification at the evaluation unit level when the true prevalence is close to a treatment decision threshold.20 For each evaluation unit, therefore, the GTMP uses a 2-stage cluster random sample survey.2 Where evaluation units are larger than districts, first-stage clusters are selected in proportion to constituent district population size. Further specifics of cluster sampling depend on local geopolitical divisions and community population structure. GTMP epidemiologists work with ministries of health to construct a bespoke sampling framework for each project, which, to the extent possible, follows the principles of equal probability random sampling.
The survey sample size in each evaluation unit is based on an expected TF prevalence in 1–9-year-olds of 10%, since this is the most critical threshold for programmatic decision making. Evaluation units in which the baseline TF prevalence in 1–9-year-olds is 10% or above qualify for annual single-dose azithromycin treatment of the entire population for at least 3 years, whereas those with a TF prevalence in 1–9-year-olds of < 10% do not.2 According to the single population proportion for precision formula,22 if the expected TF prevalence is 10% and we wish to have 95% confidence of estimating the true prevalence with absolute precision of 3%, 384 children aged 1–9 years selected by simple random sampling would be required. Assuming a cluster size of 50 children, the design effect is estimated at 2.65 (based on previous trachoma prevalence surveys: Beatriz Muñoz, unpublished data), so 1019 children are needed. Inflating this figure by a factor of 1.2 to account for non-response, we sample a sufficient number of households in each evaluation unit for 1222 children aged 1–9 years to be resident therein.
The approach to household selection within clusters varies from country to country, and will be described in the forthcoming series of papers containing country-specific results. In each setting, however, to avoid convenience sampling by field teams anxious to ensure that a minimum number of children are examined in the course of a day's work, we prescribe a fixed number of households to be enrolled per cluster, based on the number of households (generally ≤30) that a team should be able to complete in a day. The number of clusters per evaluation unit for each context is determined by dividing 1222 by the product of the number of households per cluster and the mean number of 1–9-year-olds in each household, with the latter derived from best available census data. The minimum number of clusters per evaluation unit is 20.2
Although we also aim to estimate TT prevalence in ≥15-year-olds, sample sizes have been calculated based only on parameters relating to TF in children; the low prevalence of TT (nearly always < 2% in adults except in the most hyperendemic areas) means that accurately estimating its prevalence requires substantially larger samples. Having determined the number of households required to recruit sufficient children to estimate TF prevalence as above, the sample of ≥15-year-olds used for estimating TT prevalence is set as the adults living in those same households. We accept the loss of precision in the estimate of TT prevalence inherent in this approach.
Because of the link between environmental hygiene parameters and trachoma,23 household-level water, sanitation and hygiene (WASH) variables (Table 3) are included in the GTMP data collection package.
TABLE 3. Water, sanitation and hygiene data collected at each household included in surveys for the Global Trachoma Mapping Project,.
| Question | Possible responses |
|---|---|
| In the dry season, what is the main source of drinking water for members of your household? | 01 = Piped water into dwelling 02 = Piped water to yard/plot 03 = Public tap/standpipe 04 = Tubewell/borehole 05 = Protected dug well 06 = Unprotected dug well 07 = Protected spring 08 = Unprotected spring 09 = Rainwater collection 10 = Water vendor 11 = Surface water (e.g. river, dam, lake, canal) 99 = Other (specify) |
| How long does it take to go there, get water, and come back? | 1 = Water source in the yard 2 = Less than 30 minutes 3 = Between 30 minutes and 1 hour 4 = More than 1 hour |
| In the dry season, what is the main source of water used by your household for washing faces? | 01 = Piped water into dwelling 02 = Piped water to yard/plot 03 = Public tap/standpipe 04 = Tubewell/borehole 05 = Protected dug well 06 = Unprotected dug well 07 = Protected spring 08 = Unprotected spring 09 = Rainwater collection 10 = Water vendor 11 = Surface water (e.g. river, dam, lake, canal) 99 = Other (specify) |
| If you collected water there to bring back to the house, how long would it take to go there, get water, and come back? | 0 = All face washing done at water source 1 = Water source in the yard 2 = Less than 30 minutes 3 = Between 30 minutes and 1 hour 4 = More than 1 hour |
| Where do you and other adults in the household usually defecate? | 1 = Shared or public latrine 2 = Private latrine 3 = No structure, outside near the house 4 = No structure, in the bush or field 9 = Other |
| Ask to see the latrine/toilet. Observation: What kind of toilet facility do the adults in the household use? | 01 = Flush/pour flush to piped sewer system 02 = Flush/pour flush to septic tank 03 = Flush/pour flush to pit latrine 04 = Flush/pour flush to open drains 05 = Flush/pour flush to unknown place 06 = Ventilated improved pit latrine 07 = Pit latrine with slab 08 = Pit latrine without slab/open pit 09 = Composting toilet 10 = Bucket 11 = Hanging toilet/hanging latrine 12 = No facilities or bush or field 99 = Other (specify) |
| Observation: Is there a handwashing facility within 15 m of the latrine/toilet? | 0 = No 1 = Yes 5 = Not applicable (no latrine/toilet) |
| Observation: At the time of the visit, is water available at the handwashing facility? | 0 = No 1 = Yes 5 = Not applicable (no handwashing facility) |
| Observation: At the time of the visit, is soap or ash available at the handwashing facility? | 0 = No 1 = Yes 5 = Not applicable (no handwashing facility) |
Field Methodology
In selected households, we could either invite all resident 1–9-year-olds and all resident ≥15-year-olds to participate in the survey, or invite all residents over the age of 1 year to participate. We are doing the latter because it is considerably easier to explain to communities, and allows for more complete data on TT to be collected.
The head of each household is greeted, and the purpose and conduct of the survey explained in the local language. If the household head consents to participate, global positioning system (GPS) coordinates are collected from outside the front door of the house or compound, and the WASH questions asked of the household head or their proxy. A visual inspection of the household latrine and hand-washing facilities, if present, follows (Table 3).
All data capture is electronic, using a purpose-built Open Data Kit-based Android smartphone application (LINKS, Task Force for Global Health, Atlanta, GA, USA; https://linkssystem.org/ 24). Android-based technology is suitable for large-scale trachoma surveys, and compared to standard paper questionnaires, saves time, is preferred by data recorders and is more accurate.25 GPS data permit verification that field teams have visited randomly selected clusters, even when remote and difficult to access. Data are stored on the smartphone's micro-secure digital (SD) card, allowing retention until a data-enabled mobile network or WiFi signal is available, and recovery in nearly all instances of phone failure or destruction.
Consenting individuals are examined by GTMP-certified graders for the signs TT, TF and intense trachomatous inflammation (TI) of the WHO simplified trachoma grading scheme,5 using 2.5 × magnifying binocular loupes, and sunlight or a torch for illumination.2 To prevent pathogen carry-over between successive subjects, graders clean their hands with alcohol-based hand gel after each examination.
Households in which one or more resident 1–9-year-olds are missing at the time of the survey team's first visit are re-visited wherever possible at the end of the day. The scale of the GTMP does not allow for repeat visits to households with other missing data.
Ethical Considerations
Trachoma mapping is the mandate of ministries of health, who consider it part of routine surveillance activities rather than a research activity. For this reason, and because in the largely non-literate rural populations among whom these surveys are conducted, verbal consent is generally more acceptable than written consent, we request informed verbal consent for eye examination from each resident, or in the case of minors, from their parent or legal guardian. Consent is documented in the LINKS application.
Examination for TT is benign. Eversion of the tarsal conjunctivae to allow examination for TF and TI causes only minimal transient discomfort. Following WHO recommendations,26 young children are held by their mother or a community assistant to ensure that they are able to keep still during the examination. This prevents accidental injury to the eye and ensures the process is as quick and as minimally distressing as possible.
TF is generally most common in pre-school-age children, while the prevalence of TT increases with age. Therefore, it is particularly important to ensure that young children and the elderly are proportionately represented in returned survey data. Because people with disabilities or mental health issues are more likely than others to be socioeconomically disadvantaged, and trachoma is associated with poverty,27 we try to ensure that such individuals are not excluded from participating.
The project was approved by the ethics committee of the London School of Hygiene & Tropical Medicine (reference number 6319), and the appropriate local ethics committee identified by each ministry of health. The work conforms to the guidelines of the Declaration of Helsinki.
Treatment
All individuals with active trachoma identified during the surveys are offered 1% tetracycline eye ointment to apply twice daily for 6 weeks, while those with trichiasis are referred to the nearest health facility designated to provide TT surgery.
Supervision
A supervisor (either an ophthalmologist or senior ophthalmic nurse) is appointed for every 7–10 teams, spending at least 1 day per fortnight in the field with each team to provide in-service feedback and supportive supervision of diagnostic accuracy, as well as other technical, logistic and pastoral assistance as required.
Data Upload, Storage and Access
Data are uploaded (when the phone connects with a cellular or WiFi network) to a Cloud-based server, with 128-bit encryption applied at the transport layer. The connection uses TLS 1.2; is encrypted and authenticated using AES_128_GCM, with DHE_RSA as the key exchange mechanism. Subsequent interactions with data (Figure 3) take place only through a secure website (transport layer security, IP-restricted firewall, and site authentication and authorization), with each project walled off from others and accessible only via password-protected individual login. Personally identifiable data are available only to nominated personnel at the relevant ministry of health and one GTMP-dedicated, Atlanta-based data manager, with only ministry of health nominees having the ability to download complete datasets. To provide standardization of data cleaning across projects, only the data manager is able to make changes to the data, all of which are logged.
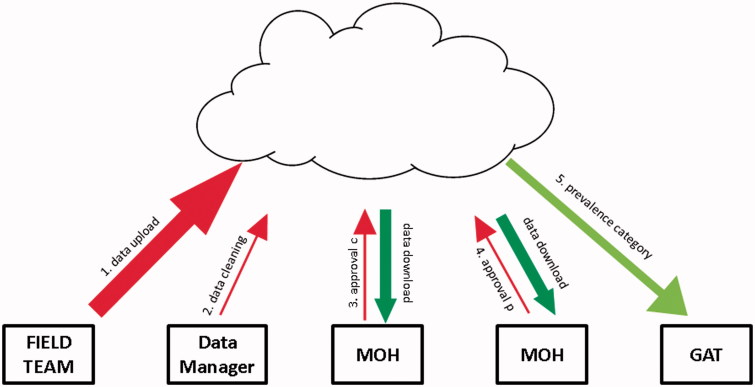
FIGURE 3.
Global Trachoma Mapping Project (GTMP) data handling and flow. (1) The field team upload the raw data, which are available for review and download by designated ministry of health (MOH) personnel. (2) The GTMP Data Manager cleans the raw data by checking for and querying (with the field team) any errors, internal inconsistencies or missing data. (3) A designated MOH official evaluates the cleaned dataset, and then either approves it (“approval C”) or queries it; once approved, analyses to generate evaluation unit-level prevalences are run automatically. (4) A designated MOH official evaluates the prevalence figures and either approves them (“approval P”) or queries them. (5) Once approved, categorical prevalence data are uploaded to the Global Atlas of Trachoma (GAT).
Data Approvals and Analysis
Data are checked and cleaned by the data manager each weekday, with queries being resolved through dialogue with field teams. The cleaned dataset is then available for electronic review by a designated individual at the ministry of health, who is first asked to approve the adequacy of data collection and the appropriateness of any cleaning that has been undertaken (“approval c”, Figure 3).
Data are then analyzed in R (R Foundation for Statistical Computing, Vienna, Austria) and Structured Query Language (SQL) using automated algorithms. For each cluster, the proportion of 1–9-year-olds with TF is adjusted by weighting the proportion of each 1-year age band observed to have TF by the proportion of the local 1–9-year-old population expected to have that age, according to the most recent census. Similarly, for each cluster, the proportion of ≥15-year-olds with TT is adjusted by weighting the proportion of each sex-specific 5-year age band observed to have TT by the proportion of the local ≥15-year-old population expected to have that age and sex. These adjustments are intended to compensate for non-random differences in examination availability between different age groups and sexes, which would otherwise tend to systematically bias prevalence estimates; the very young and very old are less mobile and more likely to have TF and TT respectively. Then, because field teams are instructed to examine all residents of a set number of households in each cluster, to prevent clusters in which greater numbers are examined having greater influence on the calculated prevalence, the mean of the adjusted cluster-level TF proportions is taken as the evaluation unit-level prevalence of TF, and the mean of the adjusted cluster-level TT proportions is taken as the evaluation unit-level prevalence of TT.28
Analyzed data are then approved for release by a designated individual at the ministry of health (“approval p”, Figure 3). This approval results in automatic upload29 of evaluation unit-level prevalence categorization to the open access Trachoma Atlas30 (www.trachomaatlas.org), as well as production of summary reports for the ministry. Data are easily fed into the Trachoma Action Planning process,31 facilitating rapid programmatic decision-making.
RESULTS
Arabic, English, French, Portuguese and Spanish versions of the training package15 (consisting of a trainer's manual, 13 accompanying Microsoft PowerPoint presentations and 3 Microsoft Excel spreadsheets) have been produced. Using these materials, in the first year of fieldwork (17 December 2012–16 December 2013), 347 field teams were trained for and deployed in 21 projects in 7 countries: Ethiopia, Laos, Malawi, Mozambique, Nigeria, Solomon Islands and Yemen.
DISCUSSION
A complete understanding of the geographical distribution and intensity of disease endemicity worldwide is key to the elimination of blinding trachoma. Roll-out of a mapping methodology that is internationally standardized in its epidemiological approach, training cascade and data handling standards, as we are undertaking in the GTMP, permits relatively rapid scale-up of mapping and promotes confidence in the internal comparability of the data. There is little doubt among project partners that Android-based data capture, Cloud-based storage and secure online approval are considerable improvements on the paper-based data collection systems of trachoma surveys completed in years past.
On the analytical side, we believe that our adjustment algorithms lead to more robust estimates of trachoma prevalence than have been reported in the majority of previous surveys. In particular, because TT is more common in the elderly and the elderly are more likely to be found at home than are young adults, age-adjustment means that estimates of the trichiasis “backlog” are significantly reduced (by > 50% in some locations) as a result of adjustment. The practical consequence of this is that elimination programs are less likely to end up searching for TT cases that do not actually exist. We recognize, though, that even with adjustment for recruitment of specific age groups, prevalence estimates can be biased upwards or downwards by differential attendance of those with TF or TT. Our methods for encouraging high survey attendance minimize but do not negate this bias.
A number of decisions on methodologies to be used for the GTMP were controversial, even within the group of experts who provided advice on their development – the authors of this paper. These issues were discussed at length within working groups and the Advisory Committee. First, we are training teams to record data on only three of the five signs of the WHO simplified trachoma grading system,5 having excluded trachomatous scarring (TS) and corneal opacity (CO). We have done so because there is no specific program response to the observation of high prevalence of either of these signs, and this project is focused on providing baseline data for initiation of elimination activities. TI and TT usually have low prevalence, so we do not attempt to demonstrate inter-grader agreement for these signs. We do not currently intend to generate a prevalence estimate for TI, and are collecting data on this sign principally to prevent graders being tempted to flag conjunctival inflammation as “TF” in the absence of five central follicles,5 and, to a lesser extent, as an insurance policy against potential future changes in guidelines for monitoring program impact. The main outcome measure is the prevalence of TF in 1–9-year-olds at evaluation unit-level, which is the current indicator to determine whether A, F and E aspects of the SAFE strategy should be implemented. Second, we are not training teams to record data on the presence or absence of signs of an “unclean face.” We recognize the likely importance of keeping children's faces clean for reducing transmission of ocular C. trachomatis infection,32 but the definition of an unclean face for survey purposes is controversial, and the reproducibility of its observation has been questioned.33–36 Third, an initial goal, obtaining unanimous agreement on the list of household-level WASH questions, proved elusive. There are a variety of established instruments used for assessing WASH coverage, among which there is considerable overlap but also considerable diversity. Our questions are essentially a subset of those from the WHO/UNICEF Joint Monitoring Program (JMP) household questionnaire,37 adapted (with as light a touch as possible) to the specific interests of trachoma control programs. We chose the JMP questionnaire because it is the established metric for measuring progress towards the Millennium Development Goals, and is widely used by national governments to estimate national water and sanitation coverage. However, JMP data do not accurately estimate coverage in sub-national administrative areas and so may be less useful in guiding effective trachoma control. We hope that our data can validate or challenge other estimates of WASH coverage, such as recent work using household survey data and spatial statistics,38 and thereby help determine where particular investment in WASH interventions is needed to hasten trachoma elimination.
Despite these issues that have generated debate, we have a collective confidence in the relevance and robustness of our approach, and with our many partners are generating the momentum necessary to complete the global trachoma map. We look forward to the GTMP's completion, at which point we will be able to understand the true scale of the task required to eliminate trachoma as a public health problem worldwide.
DECLARATION OF INTEREST
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
The GTMP is funded by a grant from the United Kingdom's Department for International Development (DFID)(ARIES: 203145) to Sightsavers, who is leading a consortium of non-governmental organizations and academic institutions to complete the work described. Additional funding for fieldwork is provided by the United States Agency for International Development through its END in Asia grant to FHI 360; and its ENVISION grant to Sightsavers. The Committee established in March 2012 to examine issues surrounding completion of global trachoma mapping was initially supported by the International Trachoma Initiative, who receive funding from Pfizer. AWS was a Wellcome Trust Intermediate Clinical Fellow (098521) and SJB a Wellcome Trust Senior Research Fellow (098045) at the London School of Hygiene & Tropical Medicine. None of the funders had any role in study design, in study implementation or analysis or interpretation of data, in the decisions on where, how or when to publish in the peer reviewed press, or in preparation of the manuscript.
REFERENCES
- World Health Assembly . Global elimination of blinding trachoma. 51st World Health Assembly, Geneva, 16 May 1998, Resolution WHA51.11. Geneva: World Health Organization; 1998. [Google Scholar]
- Solomon AW, Zondervan M, Kuper H, et al. Trachoma control: a guide for program managers. Geneva: World Health Organization; 2006. [Google Scholar]
- Smith JL, Flueckiger RM, Hooper PJ, et al. The geographical distribution and burden of trachoma in Africa. PLoS Negl Trop Dis. 2013;7:e2359. doi: 10.1371/journal.pntd.0002359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- International Coalition for Trachoma Control . The end in sight: 2020 INSight. Atlanta: International Coalition for Trachoma Control; 2011. [Google Scholar]
- Thylefors B, Dawson CR, Jones BR, et al. A simple system for the assessment of trachoma and its complications. Bull World Health Organ. 1987;65:477–483. [PMC free article] [PubMed] [Google Scholar]
- Solomon AW, Peeling RW, Foster A, et al. Diagnosis and assessment of trachoma. Clin Microbiol Rev. 2004;17:982–1011. doi: 10.1128/CMR.17.4.982-1011.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treharne JD. The community epidemiology of trachoma. Rev Infect Dis. 1985;7:760–764. doi: 10.1093/clinids/7.6.760. [DOI] [PubMed] [Google Scholar]
- Bailey R, Duong T, Carpenter R, et al. The duration of human ocular Chlamydia trachomatis infection is age dependent. Epidemiol Infect. 1999;123:479–486. doi: 10.1017/s0950268899003076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munoz B, Aron J, Turner V, et al. Incidence estimates of late stages of trachoma among women in a hyperendemic area of central Tanzania. Trop Med Int Health. 1997;2:1030–1038. doi: 10.1046/j.1365-3156.1997.d01-186.x. [DOI] [PubMed] [Google Scholar]
- Courtright P, Sheppard J, Schachter J, et al. Trachoma and blindness in the Nile Delta: current patterns and projections for the future in the rural Egyptian population. Br J Ophthalmol. 1989;73:536–540. doi: 10.1136/bjo.73.7.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor HR, Velasco FM, Sommer A. The ecology of trachoma: an epidemiological study in southern Mexico. Bull World Health Organ. 1985;63:559–567. [PMC free article] [PubMed] [Google Scholar]
- West SK, Munoz B, Turner VM, et al. The epidemiology of trachoma in central Tanzania. Int J Epidemiol. 1991;20:1088–1092. doi: 10.1093/ije/20.4.1088. [DOI] [PubMed] [Google Scholar]
- World Health Organization . Report of the 3rd global scientific meeting on trachoma, Johns Hopkins University, Baltimore, MA, 19–20 July 2010. Geneva: World Health Organization; 2010. [Google Scholar]
- The founding of the International Trachoma Initiative and the challenges ahead in drug donations for the elimination of blinding trachoma. Organisation Internationale Pour La Lutte Contre Le Trachome Annual General Assembly; 2003; Paris, France: [Google Scholar]
- Courtright P, Gass K, Lewallen S, et al. Global trachoma mapping project: training for mapping of trachoma (version 2) London: International Coalition for Trachoma Control; 2013. http://www.trachomacoalition.org/resources/global-trachoma-mapping-project-training-mapping-trachoma [Google Scholar]
- Schouten HJ. Estimating kappa from binocular data and comparing marginal probabilities. Stat Med. 1993;12:2207–2217. doi: 10.1002/sim.4780122306. [DOI] [PubMed] [Google Scholar]
- Ngondi J, Reacher M, Matthews F, et al. Trachoma survey methods: a literature review. Bull World Health Organ. 2009;87:143–151. doi: 10.2471/BLT.07.046326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelletreau S, Nyaku M, Dembele M, et al. The field-testing of a novel integrated mapping protocol for neglected tropical diseases. PLoS Negl Trop Dis. 2011;5:e1380. doi: 10.1371/journal.pntd.0001380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorkenoo AM, Bronzan RN, Ayena KD, et al. Nationwide integrated mapping of three neglected tropical diseases in Togo: countrywide implementation of a novel approach. Trop Med Int Health. 2012;17:896–903. doi: 10.1111/j.1365-3156.2012.03004.x. [DOI] [PubMed] [Google Scholar]
- Smith JL, Sturrock HJ, Olives C, et al. Comparing the performance of cluster random sampling and integrated threshold mapping for targeting trachoma control, using computer simulation. PLoS Negl Trop Dis. 2013;7:e2389. doi: 10.1371/journal.pntd.0002389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JD, Odermatt P, Utzinger J, et al. Trachoma among children in community surveys from four African countries and implications of using school surveys for evaluating prevalence. Int Health. 2013;5:280–287. doi: 10.1093/inthealth/iht027. [DOI] [PubMed] [Google Scholar]
- Kirkwood BR. Essentials of medical statistics. Oxford: Blackwell Science; 1988. [Google Scholar]
- Stocks ME, Ogden S, Haddad D, et al. Effect of water, sanitation, and hygiene on the prevention of trachoma: a systematic review and meta-analysis. PLoS medicine. 2014;11:e1001605. doi: 10.1371/journal.pmed.1001605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavluck A, Chu B, Mann Flueckiger R, et al. Electronic data capture tools for global health programs: evolution of LINKS, an Android-, web-based system. PLoS Negl Trop Dis. 2014;8:e2654. doi: 10.1371/journal.pntd.0002654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King JD, Buolamwini J, Cromwell EA, et al. A novel electronic data collection system for large-scale surveys of neglected tropical diseases. PloS one. 2013;8:e74570. doi: 10.1371/journal.pone.0074570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Primary health care level management of trachoma (WHO/PBL/93.33). Geneva: World Health Organization; 1993. [Google Scholar]
- Kasi PM, Gilani AI, Ahmad K, et al. Blinding trachoma: a disease of poverty. PLoS medicine. 2004;1:e44. doi: 10.1371/journal.pmed.0010044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ndayishimiye O, Willems J, Manirakiza E, et al. Population-based survey of active trachoma in 11 districts of Burundi. Ophthalmic Epidemiol. 2011;18:146–149. doi: 10.3109/09286586.2011.595039. [DOI] [PubMed] [Google Scholar]
- Anonymous. GIS helps fight world's leading cause of preventable blindness: tracking the global distribution of trachoma. ArcNews 2013;Winter [Google Scholar]
- Smith JL, Haddad D, Polack S, et al. Mapping the global distribution of trachoma: why an updated atlas is needed. PLoS Negl Trop Dis. 2011;5:e973. doi: 10.1371/journal.pntd.0000973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Report of the fifteenth meeting of the WHO Alliance for the elimination of blinding trachoma by 2020. Geneva: World Health Organization; 2011. [Google Scholar]
- West S, Munoz B, Lynch M, et al. Impact of face-washing on trachoma in Kongwa, Tanzania. Lancet. 1995;345(8943):155–158. doi: 10.1016/s0140-6736(95)90167-1. [DOI] [PubMed] [Google Scholar]
- Zack R, Mkocha H, Zack E, et al. Issues in defining and measuring facial cleanliness for national trachoma control programs. Trans R Soc Trop Med Hyg. 2008;102:426–431. doi: 10.1016/j.trstmh.2008.02.001. [DOI] [PubMed] [Google Scholar]
- King JD, Ngondi J, Kasten J, et al. Randomised trial of face-washing to develop a standard definition of a clean face for monitoring trachoma control programmes. Trans R Soc Trop Med Hyg. 2011;105:7–16. doi: 10.1016/j.trstmh.2010.09.008. [DOI] [PubMed] [Google Scholar]
- Emerson PM, Bailey RL, Mahdi OS, et al. Transmission ecology of the fly Musca sorbens, a putative vector of trachoma. Trans R Soc Trop Med Hyg. 2000;94:28–32. doi: 10.1016/s0035-9203(00)90427-9. [DOI] [PubMed] [Google Scholar]
- Emerson PM, Bailey RL, Walraven GE, et al. Human and other faeces as breeding media of the trachoma vector Musca sorbens . Med Vet Entomol. 2001;15:314–320. doi: 10.1046/j.0269-283x.2001.00318.x. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Core questions on drinking-water and sanitation for household surveys. [Available at: http://www.wssinfo.org/fileadmin/user_upload/resources/1268174016-JMP_Core_Questions.pdf [last accessed 1 Sept 2013]. Geneva: World Health Organization/ UNICEF, 2006
- Pullan RL, Freeman MC, Gething PW, et al. Geographical inequalities in use of improved drinking water supply and sanitation across Sub-Saharan Africa: mapping and spatial analysis of cross-sectional survey data. PLoS medicine. 2014;11:e1001626. doi: 10.1371/journal.pmed.1001626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Negrel AD, Taylor HR, West S. Guidelines for the rapid assessment for blinding trachoma (WHO/PBD/GET/00.8). Geneva: World Health Organization; 2001. [Google Scholar]