Abstract
Reconstruction of the bladder by means of both natural and synthetic materials remains a challenge due to severe adverse effects such as mechanical failure. Here we investigate the application of spider major ampullate gland-derived dragline silk from the Nephila edulis spider, a natural biomaterial with outstanding mechanical properties and a slow degradation rate, as a potential scaffold for bladder reconstruction by studying the cellular response of primary bladder cells to this biomaterial. We demonstrate that spider silk without any additional biological coating supports adhesion and growth of primary human urothelial cells (HUCs), which are multipotent bladder cells able to differentiate into the various epithelial layers of the bladder. HUCs cultured on spider silk did not show significant changes in the expression of various epithelial-to-mesenchymal transition and fibrosis associated genes, and demonstrated only slight reduction in the expression of adhesion and cellular differentiation genes. Furthermore, flow cytometric analysis showed that most of the silk-exposed HUCs maintain an undifferentiated immunophenotype. These results demonstrate that spider silk from the Nephila edulis spider supports adhesion, survival and growth of HUCs without significantly altering their cellular properties making this type of material a suitable candidate for being tested in pre-clinical models for bladder reconstruction.
Introduction
Various diseases of the genitourinary system, such as bladder exstrophy and neurogenic bladder, can cause damage to the bladder and often require reconstructive surgery to prevent urinary retention, incontinence or renal damage. To prevent upper tract damage and achieve social continence, augmentation cystoplasty is performed in which the bladder is reconstructed by enlargement of the bladder with a piece of autologous intestine [1,2]. However, serious adverse events are associated with the use of intestine for augmentation cystoplasty such as metabolic disorders, mucus production, bladder rupture and malignancy [3,4].
To avoid the use of intestine in the urinary tract and prevent associated complications, researchers developed a number of strategies to reconstruct the bladder using both natural (e.g. collagen), synthetic (e.g. polyglycolic acid (PGA)) or a combination of both materials (e.g. decellularized submucosa such as small intestine submucosa (SIS) or composite collagen-PGA) as a scaffold. However, these materials are subject to structural, mechanical, functional and biocompatibility failure [5–12]. A highly suitable and promising biomaterial for bladder reconstruction is silk, which has a slow degradation rate as well as high tensile strength and elasticity [13–17]. A very widely studied silk is cocoon silk from the silkworm Bombyx mori (B. mori). Seth et al. already demonstrated the in vivo use of B. Mori silk-based biomaterials for augmentation cystoplasty by means of salt-leaching methods [18]. Although this silk can easily be produced on a large scale, sericin coating surrounding the fibers induces T cell mediated allergic reactions as well as macrophage activation. Furthermore, degumming of the silk to remove the sericin coating makes the silk very susceptible to tensile deformation [15,19–21].
In contrast to B. Mori silk, spider silks generally lack a sericin coating and offers great improvements in tensile strength, toughness and extension [15,17,22–24]. Spider major ampullate gland-derived (MA) dragline silk of Nephila species, being one of the most well studied spider silks, provides this combination of outstanding strength, elasticity, biocompatibility, biodegradability and high temperature resistance [14,25]. In fact Nephila species-derived spider silk has better tensile strength, is less stiff and has a higher % strain at break than degummed B.Mori silk [15]. As spider silk consists of mostly nonpolar amino acids alanine (27%), glycine (20%) and Prolin (13%) as well as the amino acids glutamate, arginine, serine, aspartic acid, isoleucine, lysine, phenylalanine, threonine, tyrosine and valine (1–9%), it is highly biocompatible [26–28]. This makes spider silk a good candidate for reconstruction of a complex organ such as the bladder, which endures high elongation and tensile forces.
In vitro studies show great potential for native spider silk in the reconstruction of various organs [22,29,30]. In vivo studies testing the biocompatibility of spider silk in animal models show a mild to low inflammatory reaction and a degradation rate of approximately six months [22,24,31–33]. Although current drawback of native spider silk for in vivo use is the difficulty of large-scale production, ongoing advances in recombinant spider silk are likely to solve these issues [34].
The promising results using spider silk as a biomaterial in preclinical animal models, encouraged us to investigate native Nephila edulis spider-derived MA dragline silk, as a potential biomaterial for bladder reconstruction. For this purpose, we here studied the cell–material interactions of primary human urothelial cells (HUCs), which are multipotent cells able to form the mucosa of the bladder with spider silk [35–38]. We describe the successful culturing of HUCs on native dragline silk matrices and provide thorough assessment of the effect of spider silk on survival, expansion and differentiation of HUCs in vitro, identifying it as a promising biomaterial for being test for in vivo bladder reconstruction.
Materials and Methods
Breeding and handling of spiders and frame preparation
Nephila edulis spiders were bred at the Medical School Hannover, Dept. of Plastic, Hand and Reconstructive Surgery. Spider MA dragline silk was collected from female spiders by a harmless fixation technique on a foam platform as previously described [27]. Stainless steel weaving frames were bent into square frames with a side length of 0.5–0.8 cm and spider MA dragline silk was woven onto the frames at a fixed rate (i.e. 30 revolutions per minute) for 7.5 minutes on each side thus creating a cross weaved mesh. Frames were autoclaved at 120°C for 1 hour and placed separately in 24-wells plates in sterile PBS at 37°C, 5% CO2, for several days prior to use to remove the 10–20 nm outermost lipid coat of the fibers [39]. For coating experiments, spider silk frames were incubated in 1 ml fibronectin (Sigma; 1:100 in PBS (10 μg/ml)) or 1 ml Poly-L-Lysine (ScienCell; 1:667 in PBS) at 37°C, 5% CO2 for 2 hours and washed twice with PBS prior to use.
Indirect determination of the mechanical forces of the spider silk fibers
Deformation by forces occurring while the silk is reeled on the weaving frames was determined by measuring the distances of the opposing wires of the frame before and after silking with n = 12. Forces necessary to restore the original size were measured with a spring balance, indicating indirectly the mechanical forces of the spider silk fibers.
Cell culture
HUCs were cultured as described by the cell provider (ScienCell) in serum-free Urothelial Cell Medium (UCM) (ScienCell). For culturing HUCs on spider silk, passage 5–6 HUCS (1x104/frame) were incubated for 1.5 hours at 37°C, 5% CO2 in 20 μl UCM to promote cell attachment. Subsequently, 1 ml of UCM was added to each well and media were changed every three days. T24 cells were cultured in IMDM medium (Gibco) supplemented with 1% penstrep (Gibco) and 10% FBS (PAA Cell culture Company) [40]. RT112 cells were cultured in fully supplemented RPMI+ Glutamax medium (Gibco) [41].
Cytocompatibility assay
To determine cytocompatibility, HUCs were cultured in unconditioned UCM, 1 mM H2O2 (VWR International), or conditioned medium generated by immersing a spider silk frame or a piece of SIS (Biodesign Cook Medical) in UCM for 2 days at 37°C, 5% CO2. Cytotoxicity was measured with the Vibrant® MTT cell proliferation assay (Molecular Probes) using a Microplate Reader (model 3550, Bio-Rad).
Histology
Frames were fixed in 4% formaldehyde (FA) (Merck) for 24 hours at RT and spider silk meshes were embedded in paraffin. The 4 μm thick paraffin sections were stained with Haematoxylin (Klinipath) and Eosin (Merck) and imaged with a Nikon DXM1200 digital camera.
(Immuno)fluorescence staining
For visualization of filamentous actin and nuclei, samples were fixed in 4% FA at RT, permeabilised with 5% Saponin and 1% BSA and stained with Phalloidin and DAPI (all from Sigma) and imaged using confocal microscopy (LSM700, Zeiss). To determine cell expansion, five fixed fields of vision (FOV, enlargement 10x) images were made with an EVOS® cell imaging system (Life Technologies) per sample and the amount of DAPI positive nuclei per FOV were counted using ImageJ software. To determine viability, a live/dead cell imaging assay (Molecular Probes) was performed. To determine HUC growth in time we measured the metabolic activity of HUCs by resorufin fluorescence using Presto Blue (PB, Molecular Probes) and a SpectraMax (Molecular Devices).
Scanning electron microscopy
For visualization of adhesion of HUCs on spider silk frames, constructs were fixed in 4% FA for 24 hours at RT and dehydrated as described earlier [42]. Ethanol was replaced with anhydrous acetone (acetone and 1% acidified 2.2-dimethoxypropane (DMP), 1:100) [43]. The samples were dried using a CPD-030 Leica critical point drying apparatus (Leica Microsystems, Vienna, Austria) and coated with 6 nm Pt/Pd using a sputter coater 208HR (Cressington Scientific Instruments Ltd., Chalk Hill Watford, England, UK). Specimens were viewed in an XL30 scanning electron microscopy (SEM) equipped with a field emission gun (FEI Europe, Eindhoven, The Netherlands).
RNA isolation and qRT-PCR
After six days of culturing, total RNA from HUCs, T24, and RT112 was isolated using the RNeasy mini kit (Qiagen, Hilden, Germany) and cDNA was synthesized using the iScriptTM cDNA Synthesis kit (Bio-Rad) in a T3000 Thermocycler (Biometra). QPCR was performed using SYBR green Supermix (Bio-Rad) using the indicated primers (Table 1, Sigma) and the MyiQ Single Color RT PCR detection system (BioRad). Relative gene expression was calculated using the 2-ΔΔCt method normalized to β2M levels for each individual sample.
Table 1. Primer sequences.
| Gene | Forward primer | Reverse primer |
|---|---|---|
| Vimentin | ACCAACGACAAAGCCCGCGT | CAGAGACGCATTGTCAACATCCTGT |
| E-cadherin | CACCACGTACAAGGGTCAGGTGC | CAGCCTCCCACGCTGGGGTAT |
| N-Cadherin | AGTCACCGTGGTCAAACCAATCGA | TGCAGTTGACTGAGGCGGGTG |
| Collagen 1 | CCCCAGCCACAAAGAGTCTAC | TGATTGGTGGGATGTCTTCGT |
| Cytokeratin 18 | CATGCAAAGCCTGAACGACC | ATTTGCGAAGATCTGAGCCCT |
| Cytokeratin 19 | TGAGGAGGAAATCAGTACGCTG | TTGGCTTCGCATGTCACTCA |
| Cytokeratin 17 | GGAGCAGCAGAACCAGGAATA | CTTGTACTGAGTCAGGTGGGC |
| Cytokeratin 7 | TCTTTGAGGCCCAGATTGCTG | GCAGCATCCACATCCTTCTTC |
| Integrin α2 | TTAGCGCTCAGTCAAGGCAT | CGGTTCTCAGGAAAGCCACT |
| Integrin β1 | CCGCGCGGAAAAGATGAATTT | CCACAATTTGGCCCTGCTTG |
| Integrin α6 | CAAATGCAGGCACTCAGGTTC | AGCCTTGTGATATGTGGCATCA |
| Integrin α5 | AAGACTTTCTTGCAGCGGGA | GCCACCTGACGCTCTTTTTG |
| CD44 | AGCAACTGAGACAGCAACCA | AGACGTACCAGCCATTTGTGT |
Flow cytometry
After six days of culturing, cells were collected and stained using anti-CD90, anti-CD49f (both BD Pharmingen), anti-CD44 (Immunotools) antibodies and SytoxBlue (Invitrogen) to exclude dead cells. Cells were measured on a FACSCanto II flow cytometer (BD Biosciences). Data analysis was performed using FlowJo version 7.6 (FlowJo LLC).
Statistical analysis
The mean and standard deviation (SD) were calculated with Microsoft Excel 2007 (Microsoft Corporation, Schiphol, The Netherlands) and Graphpad Prism 5® (GraphPad Software Inc., LaJolla, California, USA). Statistical analysis was performed using the unpaired student’s t-test and differences with p≤0.05 were considered statistically significant.
Results
Mechanics of spider silk weaving frames
To determine roughly the strain forces of the spider silk fibers on the weaving frame caused by the reeling process, deformation forces of the whole weaving frames were measured and calculated for single fibers. The length of one silk fiber used for one side of the frame was approximately 3 meters and the mean distance between single meshes (n = 297) was 32.42 ± 22.27 μm and the 95% confidence interval was 29.88–34.95 μm. Deformation of the steel frames by pulling forces occurred with a mean of 6.69% +/- 2.08% (SD) of the original side length but could be considered as tolerable, as they did not exceed 10%. Force needed to reverse deformation (Fmeasured) were also ruled out by measuring the force necessary to reverse deformation and deformation strength (Scalculated) was calculated by dividing Fmeasured by thickness d of straight wire with equation (1): S calculated = F measured d −1 [Pa]. As mean, Fmeasured was measured to 1.55 N +/- 0.66 N (SD) and Scalculated with d = 0.7 mm was calculated to 2.21 MPa.
Spider silk supports HUC adhesion and survival
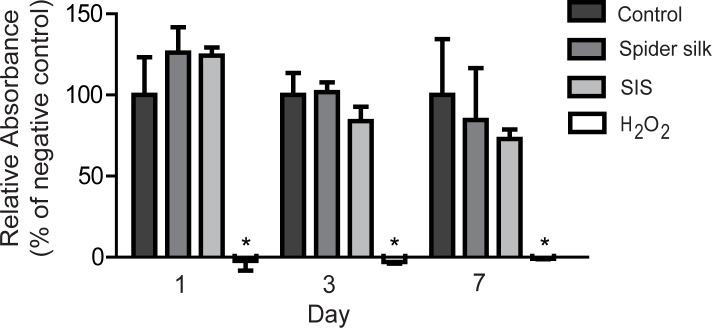
To test whether spider silk can be used as a potential biomaterial for bladder reconstruction, we exposed HUCs to an extract of spider silk, or SIS, which is the current gold standard in bladder reconstruction, and measured cytotoxicity by MTT assay. Conditioned medium of spider silk or SIS did not significantly affect HUC expansion (Fig 1), indicating that spider silk extract has no cytotoxic effects on HUCs.
Fig 1. Spider silk extract is not cytotoxic for HUCs.
MTT assays showing HUC viability and expansion when exposed to spider silk and SIS conditioned medium, UCM (negative control) and H2O2 at indicated days of culturing. Relative MTT absorbance as compared to negative control values per time point are shown for each sample. Data are means ±SD (n = 3). * p≤0.05
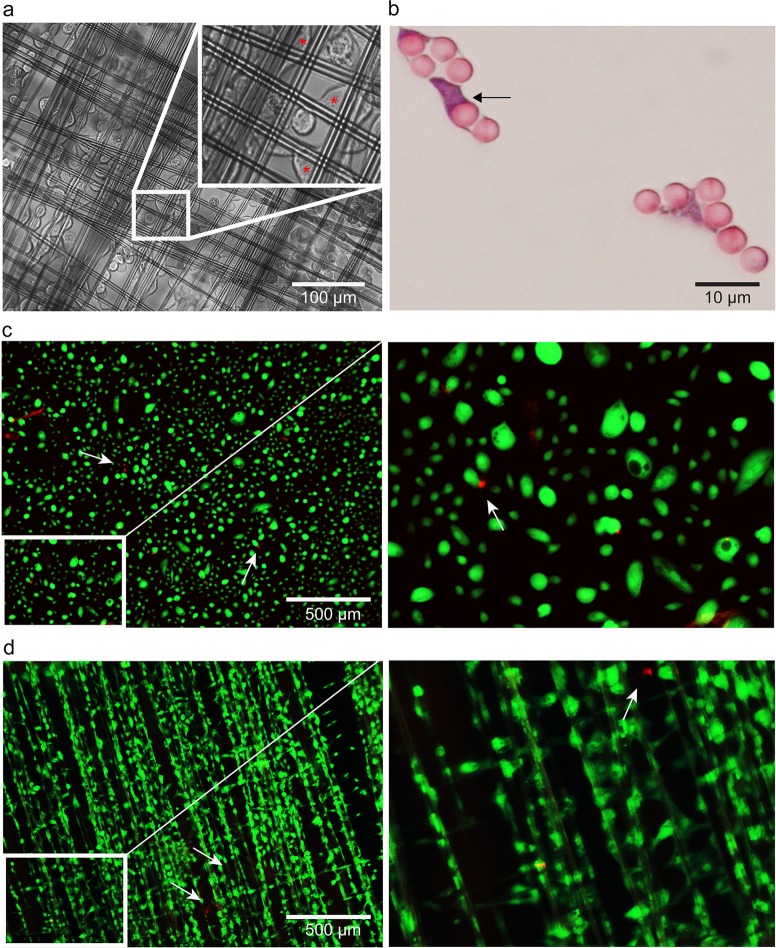
To determine whether HUCs are able to adhere to spider silk, we used light microscopy. Within 90 minutes after seeding, HUCs adhered to the spider silk matrices and cell bodies stretched longitudinally alongside the spider silk fibers and bridged the gaps between the spider silk fibers (Fig 2A). H&E staining of paraffin cross-sections confirmed this ‘bridging’ capacity (Fig 2C and 2D, black arrows). Live/dead assay performed on HUCs seeded on either spider silk or glass at 6 days post seeding revealed that dead (red) cells were only detected occasionally (white arrows, Fig 2E and 2F). Together, these results demonstrate that spider silk matrices allow adherence and survival of HUCs.
Fig 2. HUC adhesion and survival on spider silk.
(a) Representative phase contrast microscopy images of HUC-seeded spider silk matrices at 90 minutes post seeding. Inserts show non-adhered cells with a rounded appearance, red asterisks indicate HUCs adhered to spider silk. (b) Representative H&E staining of paraffin cross-section of HUC-seeded spider silk matrices at 7 days post seeding. HUCs appear purple and spider silk fibers pink. Arrows indicate the HUCs’ ‘bridging’ capacity. (c-d) Representative microscopy images of live/dead staining of HUCs cultured on glass coverslips (c) or spider silk (d) in which live and dead cells stain with green cytoplasmic fluorescence and red nucleic fluorescence (white arrows), respectively. Inserts show magnification of the indicated (white box) area.
HUC adhesion induces actin-rearrangements and filopodia development
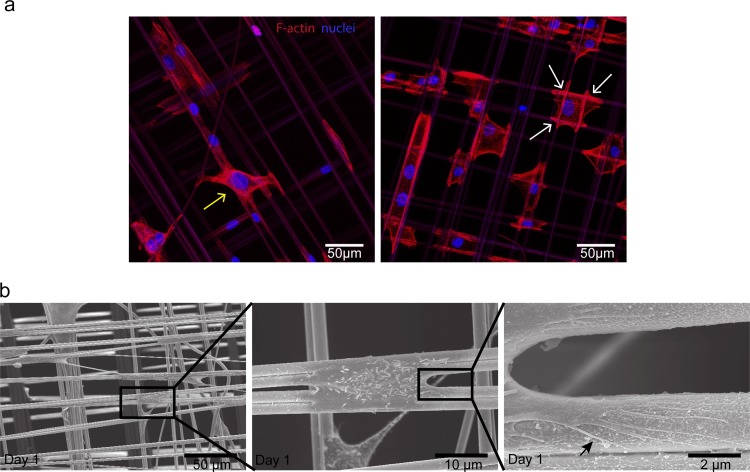
To obtain insight in the processes involved in HUC adherence, we stained HUCs seeded on spider silk matrices with DAPI and phalloidin to detect the nucleus and filamentous actin, respectively. Confocal imaging confirmed that HUCs stretched in various directions (Fig 3A). Furthermore, actin filaments specifically accumulated in proximity to the spider silk fibers as indicated by the bright intracellular phalloidin staining in these areas (Fig 3A, white arrows), suggesting an important role for actin-rearrangements in adhesion of HUCs to spider silk. Subsequent SEM revealed that HUCs wrap numerous lengthy filopodia around the spider silk fibers (Fig 3B), which were determined to be approximately 2 μm thick. Together, these results demonstrate that HUCs rearrange their cytoskeleton and form filopodia while adhering to the spider silk fibers.
Fig 3. HUC adhesion is associated with actin-rearrangements and filopodia development.
(a) Representative confocal images of DAPI (blue) and phalloidin (red) stained HUC-seeded spider silk matrices at day 1 post seeding. White arrows indicate some of the attachment sites of HUCs concentrated around the fiber mesh that stain brightly with fluorescing phalloidin, the yellow arrow indicate cells capable of bridging the gap between two spider silk fibers. (b) Representative electron micrographs of spider silk matrices at day 1 post HUC seeding. Consecutive magnifications are shown in sequence. The black arrow indicates one of the many filopodia present on the fiber surface protruding from a single HUC.
Spider silk allows HUC expansion
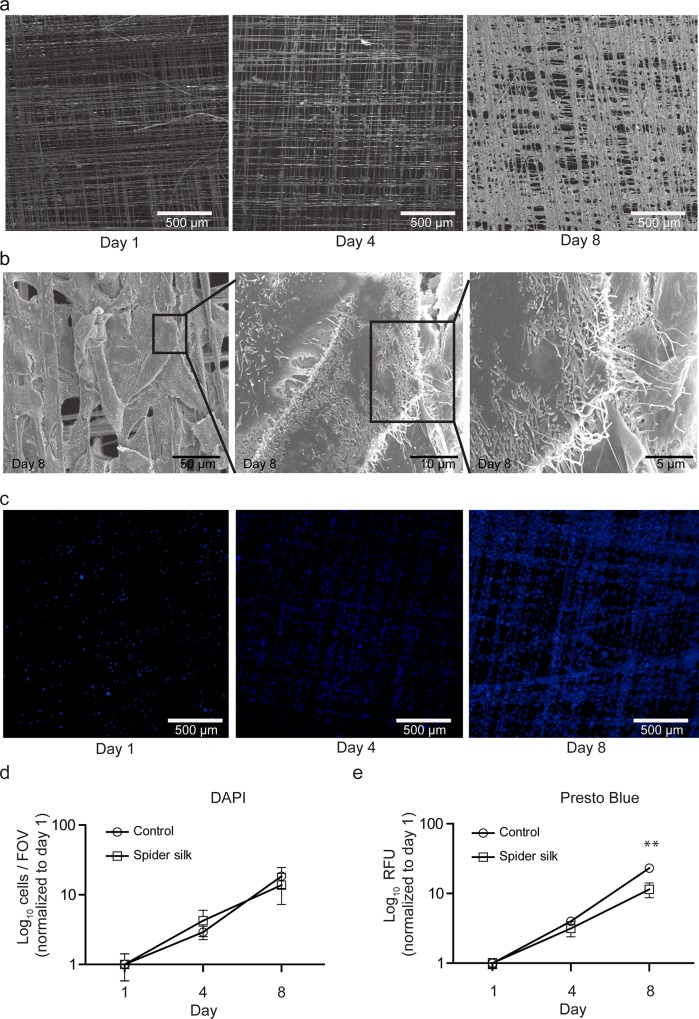
To investigate HUC growth over time we performed SEM analysis at different time points after seeding (Fig 4A). Within 8 days HUCs progressively covered most of the spider silk fibers (Fig 4A). High magnification electron micrographs at 8 days post seeding showed multiple layers of cells covering the spider silk matrix (Fig 4B).
Fig 4. Spider silk supports expansion of HUCs.
Visualization of HUC growth on spider silk frames by SEM (a-b) or DAPI immunostaining (c-d). Representative images of HUC growth at the indicated days post seeding (a and c). Detailed SEM images at day 8 post seeding (b). Quantification of HUC expansion by counting DAPI positive cells (d) or by Presto Blue fluorescence in relative fluorescence units (RFU) (e). Data are means ±SD (n = 3). ** p≤0.01 between spider silk and control.
Quantification of cell nuclei per field of view (FOV) in immunofluorescence images of DAPI-stained HUCs (Fig 4C and 4D) revealed no difference in cell growth between HUCs grown on spider silk or plastic. To confirm this, we followed HUC growth by measuring the metabolic activity of HUCs by resorufin fluorescence. Initially (day 1–4) no significant differences were observed in HUC growth on either plastic or spider silk (Fig 4E). However, at day 8 HUC growth on spider silk was slightly decreased. Together, these results show that spider silk allows the expansion of HUCs into a multilayered HUC network within 8 days of culture.
Coating spider silk improves HUC adhesion but does not compromise growth
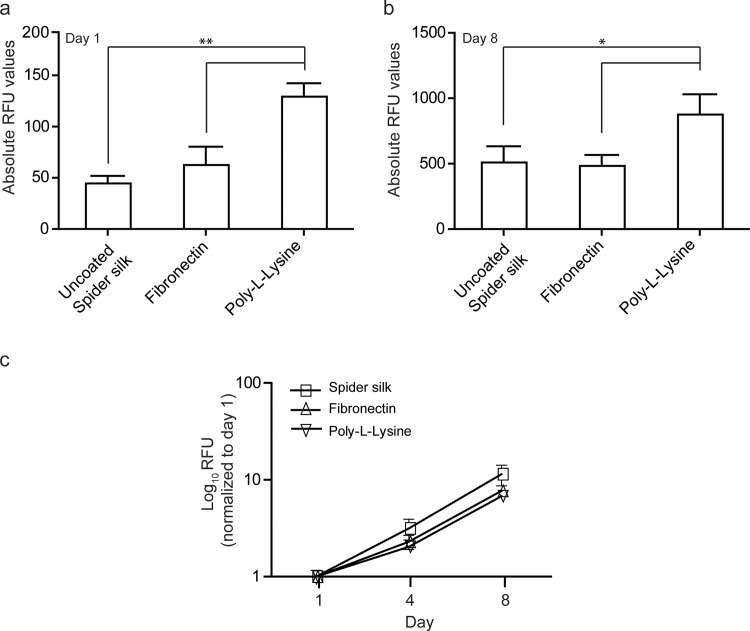
To determine whether HUC adherence and growth can be further improved we applied bioactive coatings on the spider silk. One day post-seeding, spider silk coated with poly-l-lysine showed a significant increase in the number of viable HUCs as compared to fibronectin and non-coated spider silk (+/- 3 fold), indicating that poly-L-lysine can improve the adherence of HUCs to spider silk (Fig 5A). Subsequent analysis over an 8 days period revealed that HUCs adhering to either coated or uncoated spider silk did not show any significant difference in expansion (Fig 5B). When normalized to day 1, relative cell growth was highest in HUCs cultured on non-coated spider silk compared to fibronectin and poly-l-lysine coated spider silk (Fig 5C).
Fig 5. Coated spider silk enhances cell adherence but diminishes growth.
Presto Blue-based quantification of the amount of viable HUCs on spider silk coated with the indicated substrates. (a) RFU at day 1 post seeding as percentage of HUCs cultured on uncoated spider silk. (b) Absolute RFU values at day 8. (c) RFU between day 1 to 8 normalized to day 1 demonstrating cell growth. Data are means ±SD (n = 3). * p≤0.05, ** p≤0.01.
Spider silk-exposed HUCs show minimal gene expression changes
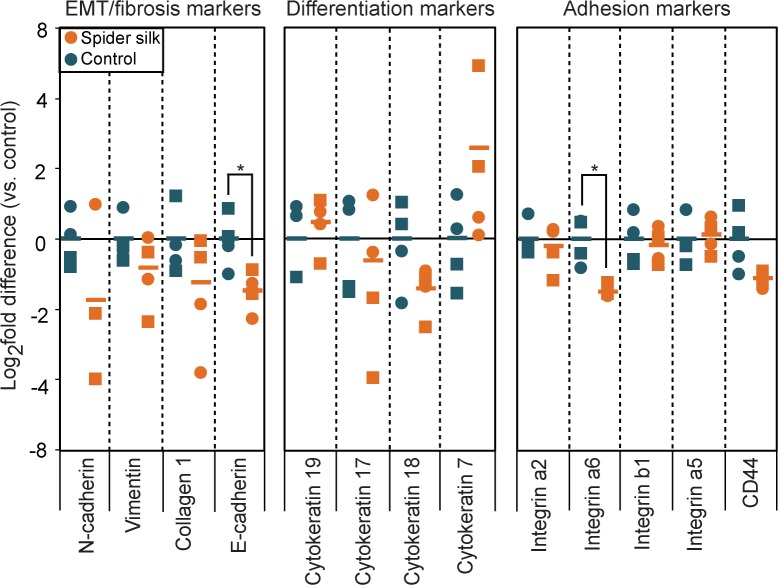
To determine whether exposure to spider silk leads to molecular alterations in HUCs, we analyzed the mRNA expression levels of various genes involved in epithelial-to-mesenchymal transition (EMT), fibrosis, differentiation and adhesion by qRT PCR. Spider silk-exposed HUCs only showed small decreases (1.5–1.7 fold) in the levels of E-cadherin and Integrin α6 (ITG6), which are involved in cell-cell or cell-matrix interactions, but showed no differences in any other genes involved in EMT, fibrosis or differentiation (Fig 6).
Fig 6. Spider-silk exposure minimally affects the gene expression profile of HUCs.
qRT PCR-based gene expression analysis of EMT/fibrosis markers, differentiation markers and adhesion markers in HUCs cultured on plastic or spider silk for six days. Data are normalized to β2M and expressed as log2 fold change relative to average level in HUCs cultured on plastic. Data are means ±SD (n = 4). * p≤0.05, ** p≤0.01.
Taken together, these results indicate that spider silk has marginal effects on the studied gene expression profile of HUCs.
Spider silk-exposure maintains the undifferentiated nature of HUCs
We used a recently reported flow cytometry-based approach to determine the effects of spider silk on HUC differentiation 39 [44]. This method uses surface markers to identify urothelial stem cells as CD90+CD44+CD49f+ cells, basal cells as CD90-CD44+CD49f+, intermediate cells as CD90-CD44-CD49f+ and umbrella cells as CD90-CD44-CD49f-. In line with their reported basal cell phenotype HUCs were positive for both CD44 and CD49f and negative for CD90 (Fig 7A) [45]. Furthermore, FBS-induced differentiation resulted in a decrease in CD44 and CD49f expression [46,47]. In accordance with Ho et al., who implicated basal subtype cancers with poor clinical outcomes and better outcomes in intermediate subtype cancers, the high-grade metastatic human T24 showed a higher expression level of CD44 than the non-metastatic well differentiated RT122, respectively [44].
Fig 7. Slight differentiation of a subpopulation of spider silk-exposed HUCs.
(a) Representative FACS plots of T24 cells, RT112 cells, HUCs and HUCs treated with fetal bovine serum (FBS) and overlays of HUCs cultured on plastic or spider silk (b) that were stained for CD90, CD44, CD49f and Sytox Blue. Dead cells and doublets were excluded.
Spider silk exposure induced a significant (p≤0.05, n = 3) increase in the CD90 expression HUCs. Furthermore, in line with the trend towards a decrease in CD44 mRNA expression (Fig 6B), HUCs cultured on spider silk contain a small subpopulation of CD44 negative cells (Fig 7), suggesting that spider silk induces the differentiation of a small fraction of silk-exposed cells. Together, these results demonstrate the usefulness of the flow cytometry-based approach to identify various bladder cell subsets and indicate that HUCs for most part maintain their basal phenotype upon exposure to spider silk.
Discussion
Previous studies have shown that various scaffold materials can be used to reconstruct the bladder. Although SIS or composite materials (such as collagen-PGA) showed promising results, long term side effects including reduced bladder capacity, fibrosis and graft shrinkage has shown that these biomaterials are not optimal for bladder augmentation [9–12]. Given the reported outstanding strength, elasticity, biocompatibility and biodegradability of spider MA silk, which exceed characteristics of B. Mori silk, the scope of this study was to test the cell—material interactions of HUCS with spider MA dragline silk from Nephila edulis and provide an in-depth assessment on cell behavior of HUCs when in contact with spider silk with respect to adherence, survival, cell growth and differentiation.
Mechanical consideration
To achieve a scaffold usable for tissue engineering purposes, dental straight wire was bent to square weaving frames. Straight wire is a medical product which can be sterilized and shows neither in vivo nor in vitro cytotoxicity. With a bending strength of 1.9 x 104 MPa, a Young’s modulus of 1.7 x 103 MPa and a bending stiffness of 670 kPa (according to manufacturers’ manual), straight wire holds certain stability to theoretically withstand greater bending forces. However, a distinct deformation could be observed, which occurred obviously due to the forces caused by the silking process. As this deformation was reversible, i.e. an elastic deformation for which Hooke’s Law holds, it can be stated that forces that occur during silking spiders, in general surpass bending stiffness of 670 kPa, but below Young’s modulus of 1.7 x 103 MPa. Interestingly, Scalculated was calculated 2.21 MPa, further substantiating this thesis. It has to be considered that these values are very roughly as with the experimental setting in this pioneering study measurement errors were relatively high. Nevertheless, even if tolerances of >100% were considered, Scalculated was still in the expected range. Interestingly, our results correspond very well with values of forces occurring during rearing process (i.e. spinning) described in literature [26,48]. While the most exact measurement probably was performed by Pérez-Rigeiro et al., the measured spinning force was constant in the mentioned studies around 5.5–7.5 mN [48,49]. It has to be considered that Fmeasured needed to restore original frame had to be divided by 240 fibers to achieve Fmeasured for one single fiber (Fsingle fiber), resulting in a Fsingle fiber of 6.46 mN +/- 2.75 mN (SD), matching exactly to the spinning forces determined by Pérez-Rigeiro et al. Additionally, relative deformation was measured and calculated to 6.68%, indicating that no greater deformation rates occurred even with high tolerance rates. This deformation also influenced neither mesh pattern nor cell growth, thus we could found no disadvantages by the minor deformations that occurred.
Cytocompatibility, adhesion and cell expansion
We report that spider silk extract is non-toxic for HUCs [22,23,27,29,30]. Furthermore, HUCs that adhere to spider silk showed good viability comparable to HUCs cultured on glass. In all our experiments the spider silk matrices were pre-incubated in PBS to remove the 10–20 nm outermost lipid coat of the fibers thereby revealing the glycoprotein layer of spider silk containing fine fibrils and promoting cell adherence [39]. In contrast to what was previously reported that fetal calf serum (FCS) is necessary for HUCs to adhere to SIS [50], HUCs can be cultured on spider silk without addition of FCS. H&E and phalloidin staining revealed that the adhering HUCs stretch along the spider silk where filamentous actin was specifically localized to areas where HUCs were in close contact to spider silk. The HUCs bridge the gaps between the individual fibers in the spider silk mesh, indicating an attractive morphology of the spider silk for HUCs to adhere to and good geometry of mesh fabrication (i.e. inter-fiber distance). Furthermore, SEM revealed that HUCs adhered to the spider silk by using filopodia. Recent studies demonstrate that integrin-extracellular matrix contacts occur in fibroblasts at the tips of filopodia which organize into focal adhesions and serve as anchor points for the cytoskeleton [51]. Together this could indicate that in HUCs a similar process occurs in which integrin and actin containing filopodia form the initial cell-cell and cell-extracellular matrix contacts, and could be responsible for cell attachment and growth on spider silk.
Our study revealed that HUCs grown on spider silk show a slightly decreased expansion as compared to HUCs grown on tissue culture polystyrene, which is in accordance with studies demonstrating lower growth rates of fibroblasts when cultured on spider silk matrices as compared to cover slips [27]. This decreased expansion of cells on spider silk might be related to the requirement of cells to bridge the gaps in between the spider silk fibers when grown on silk, whereas on plastic the surface area to attach and expand is much larger. This is also supported by SEM images demonstrating HUCs growing near confluent on spider silk after 8 days of cultivation. In contrast, previous studies testing the growth of HUCs on SIS demonstrate generally show much slower growth [52–54]. Furthermore, the reported expansion of HUCs on spider silk in this study is much faster than the reported growth of HUCs on uncoated B. Mori silk constructs [55]. Together, this indicates that spider silk is a biomaterial that properly supports the growth of HUCs, which might even be superior over other biomaterials.
Similar to what has been reported by Franck et al. for silkworm-derived silk, coating spider silk with fibronectin, which is expressed in and near the urothelial basement area, did not improve HUC adherence and slowed down the growth of HUC cells [55]. The only method that improved adherence of HUCs to the spider silk was coating the spider silk with Poly-L-Lysine, a synthetic compound which is highly positively charged and which is also often used for culturing HUCs on tissue culture plastic and is well known to improve adhesion of cells [56]. However, since spider silk without any additional bioactive coating spider silk already showed good biocompatibility, adherence and growth support for primary HUCs, we focused on analyzing HUC behavior on uncoated spider silk.
Differentiation
Although it is known that substrate properties such as surface chemistry and matrix stiffness can influence cellular behavior and differentiation [57], qRT PCR analysis revealed that many genes are expressed at similar levels in HUCs cultured on spider silk or on tissue culture plastic. The only genes that showed significant differential expression between HUCs cultured on either plastic or spider silk were the genes encoding E-cadherin and integrin α6, which are both involved in cell-cell and cell-extracellular matrix interactions. Downregulation of ITGa6 was only marginal and only observed at the mRNA level. In contrast, CD49f protein expression, which is the product of the ITGa6 gene and was analyzed by flow cytometry, remained unchanged upon exposure to spider silk. Although it has been described that E-cadherin loss can be an indication of EMT, we did not observe changes in the mesenchymal genes encoding Vimentin and N-cadherin. Overall, these results suggest that exposure of HUCs to spider silk only minimally affects the behavior of HUCs.
We used a recently described flow cytometry-based method that uses CD44 as well as the cell surface markers CD49f and CD90 to identify different cell types within the normal urothelium to further elucidate the effect of spider silk on HUC behavior and differentiation [58]. As expected, HUCs showed a basal cell phenotype (i.e. CD90-CD44+CD49f+) [45]. In line with the trend towards decreased CD44 mRNA expression in spider silk exposed HUCs, flow cytometric analysis revealed that exposure of HUCs to silk results in decreased levels of CD44 protein albeit in only a small subpopulation of HUCs, rather than in the total cell population. So rather than adjusting to a specific differentiated state, this indicates that most silk-exposed HUCs remain undifferentiated and keep their basal phenotype. This is beneficial for clinical application, since the in vivo microenvironment should allow the proper differentiation of HUCs to epithelial bladder cell layers that mimics the native bladder wall, similar to previous studies in which undifferentiated HUCs were seeded onto collagen-based scaffolds [10].
Conclusions
We have shown that native spider MA dragline silk from Nephila edulis supports the adhesion, survival and growth of HUCs, while maintaining their undifferentiated state. In combination with the reported outstanding strength, elasticity, biocompatibility and biodegradability of spider MA silk [13–17], these results establish spider MA dragline silk from Nephila edulis as a promising biomaterial for bladder reconstruction. In order to make a functional construct capable of miction and urine storage, it is of great importance to incorporate a layer of smooth muscle cells (SMCs) into the construct. Atala et al. demonstrate successful engraftment of an a-cellular scaffold seeded with both HUCs and SMCs in patients. In this study the focus was on the specific interaction between HUCs and spider silk. However, future research should determine the interaction between SMCs and spider silk as well as a combination of HUCs, SMCs and spider silk. A current drawback of spider silk for in vivo use is the difficulty of large-scale production. In addition, wetted spider silk fibers show supercontraction [59]. In this study, we used supporting weaving frames to overcome this problem. The demonstration that in an in vivo fascia replacement model supercontraction can be overcome by suturing the silk mesh into a defect before releasing it from the frame, encourages the in vivo use of spider silk without the presence of a supporting scaffold. [28]. Additionally, great efforts are made to overcome these issues with the intensive research on recombinant spider silk [34,60]. By tuning the structure to suite the physical and environmental demands of an application, mechanical properties could be adapted to the specific requirements. The next step would be to test this new biomaterial in pre-clinical models, in which, amongst others, effects of bladder environment on spider silk, such as degradation due to ureum and mechanical forces, cell and tissue formation and tensile forces affecting adherence and functionality, should be investigated. When such hurdles can successfully be taken, possibilities of spider silk as a reconstructive biomaterial for urinary tissues could be endless.
Acknowledgments
We thank the members of the Department of Plastic, Hand and Reconstructive Surgery (Medical School Hannover, Germany) for help with spider silk frame manufacturing, culturing and staining techniques. We thank I.M. Hoepelman (UMCU) and J.A. Schalken (UMC St. Radboud, Nijmegen) for kindly providing the cell lines T24 and RT112, respectively. We thank Veerle Fleskens for help with confocal microscopy and flow cytometry, Regina Korlaar and Liesbeth Verhagen for help with immunohistochemical staining, Dr. George Posthuma for help with specimen preparation for SEM, Winand Omta for help with statistical analyses, the UMC Utrecht Pathology Core and all members of the Cell Biology Department of UMC Utrecht for critical insights, discussions and suggestions.
Data Availability
All relevant data are within the paper.
Funding Statement
The authors have no support or funding to report.
References
- 1. Frimberger D, Cheng E, Kropp BP. The current management of the neurogenic bladder in children with spina bifida. Pediatr Clin North Am. 2012. August;59(4):757–67. 10.1016/j.pcl.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 2. Sakakibara R, Hattori T, Uchiyama T, Kamura K, Yamanishi T. Uroneurological assessment of spina bifida cystica and occulta. Neurourol Urodyn. 2003. January;22(4):328–34. [DOI] [PubMed] [Google Scholar]
- 3. Soergel TM, Cain MP, Misseri R, Gardner TA, Koch MO, Rink RC. Transitional cell carcinoma of the bladder following augmentation cystoplasty for the neuropathic bladder. J Urol. 2004. October;172(4 Pt 2):1649–51; discussion 1651–2. [DOI] [PubMed] [Google Scholar]
- 4. Gilbert SM, Hensle TW. Metabolic consequences and long-term complications of enterocystoplasty in children: a review. J Urol. 2005. April;173(4):1080–6. [DOI] [PubMed] [Google Scholar]
- 5. Pariente JL, Kim BS, Atala A. In vitro biocompatibility assessment of naturally derived and synthetic biomaterials using normal human urothelial cells. J Biomed Mater Res. 2001. April;55(1):33–9. [DOI] [PubMed] [Google Scholar]
- 6. Atala A. Tissue engineering of human bladder. Br Med Bull. 2011. January;97:81–104. 10.1093/bmb/ldr003 [DOI] [PubMed] [Google Scholar]
- 7. Rohrmann D, Albrecht D, Hannappel J, Gerlach R, Schwarzkopp G, Lutzeyer W. Alloplastic replacement of the urinary bladder. J Urol. 1996. December;156(6):2094–7. [PubMed] [Google Scholar]
- 8. Barrera DA, Zylstra E, Lansbury PT, Langer R. Synthesis and RGD peptide modification of a new biodegradable copolymer: poly(lactic acid-co-lysine). J Am Chem Soc. American Chemical Society; 1993. November;115(23):11010–1. [Google Scholar]
- 9. Oberpenning F, Meng J, Yoo JJ, Atala A. De novo reconstitution of a functional mammalian urinary bladder by tissue engineering. Nat Biotechnol. 1999. February;17(2):149–55. [DOI] [PubMed] [Google Scholar]
- 10. Atala A, Bauer SB, Soker S, Yoo JJ, Retik AB. Tissue-engineered autologous bladders for patients needing cystoplasty. Lancet. 2006. April 15;367(9518):1241–6. [DOI] [PubMed] [Google Scholar]
- 11. Caione P, Boldrini R, Salerno A, Nappo SG. Bladder augmentation using acellular collagen biomatrix: a pilot experience in exstrophic patients. Pediatr Surg Int. 2012. April;28(4):421–8. 10.1007/s00383-012-3063-0 [DOI] [PubMed] [Google Scholar]
- 12. Wang DS, Anderson DA, Fretz PC, Nguyen TT, Winfield HN. Laparoscopic augmentation cystoplasty: a comparison between native ileum and small intestinal submucosa in the porcine model. BJU Int. 2007. March;99(3):628–31. [DOI] [PubMed] [Google Scholar]
- 13. Wong Po Foo C, Kaplan DL. Genetic engineering of fibrous proteins: spider dragline silk and collagen. Adv Drug Deliv Rev. 2002. October 18;54(8):1131–43. [DOI] [PubMed] [Google Scholar]
- 14. Rising A, Nimmervoll H, Grip S, Fernandez-Arias A, Storckenfeldt E, Knight DP, et al. Spider silk proteins—mechanical property and gene sequence. Zoolog Sci. 2005. March;22(3):273–81. [DOI] [PubMed] [Google Scholar]
- 15. Altman GH, Diaz F, Jakuba C, Calabro T, Horan RL, Chen J, et al. Silk-based biomaterials. Biomaterials. 2003. Feb;24(3):401–16. [DOI] [PubMed] [Google Scholar]
- 16. Hakimi O, Knight DP, Vollrath F, Vadgama P. Spider and mulberry silkworm silks as compatible biomaterials. Compos Part B Eng. 2007. April;38(3):324–37. [Google Scholar]
- 17. Gosline JM, Guerette PA, Ortlepp CS, Savage KN. The mechanical design of spider silks: from fibroin sequence to mechanical function. J Exp Biol. 1999. December;202(Pt 23):3295–303. [DOI] [PubMed] [Google Scholar]
- 18. Seth A, Chung YG, Gil ES, Tu D, Franck D, Di Vizio D, et al. The performance of silk scaffolds in a rat model of augmentation cystoplasty. Biomaterials. 2013. July;34(20):4758–65. 10.1016/j.biomaterials.2013.03.038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Wen CM, Ye ST, Zhou LX, Yu Y. Silk-induced asthma in children: a report of 64 cases. Ann Allergy. 1990. November;65(5):375–8. [PubMed] [Google Scholar]
- 20. Meinel L, Hofmann S, Karageorgiou V, Kirker-Head C, McCool J, Gronowicz G, et al. The inflammatory responses to silk films in vitro and in vivo. Biomaterials. 2005. January;26(2):147–55. [DOI] [PubMed] [Google Scholar]
- 21. Zaoming W, Codina R, Fernández-Caldas E, Lockey RF. Partial characterization of the silk allergens in mulberry silk extract. J Investig Allergol Clin Immunol. 1996;6(4):237–41. [PubMed] [Google Scholar]
- 22. Allmeling C, Jokuszies A, Reimers K, Kall S, Choi CY, Brandes G, et al. Spider silk fibres in artificial nerve constructs promote peripheral nerve regeneration. Cell Prolif. 2008. June;41(3):408–20. 10.1111/j.1365-2184.2008.00534.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Radtke C, Allmeling C, Waldmann K-H, Reimers K, Thies K, Schenk HC, et al. Spider silk constructs enhance axonal regeneration and remyelination in long nerve defects in sheep. PLoS One. 2011. January;6(2):e16990 10.1371/journal.pone.0016990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Vollrath F, Barth P, Basedow A, Engström W, List H. Local tolerance to spider silks and protein polymers in vivo. In Vivo. 2002;16(4):229–34. [PubMed] [Google Scholar]
- 25. Cunniff PM, Fossey SA, Auerbach MA, Song JW, Kaplan DL, Adams WW, et al. Mechanical and thermal properties of dragline silk from the spider Nephila clavipes. Polym Adv Technol. 1994. August;5(8):401–10. [Google Scholar]
- 26. Vollrath F, Knight DP. Liquid crystalline spinning of spider silk. Nature. 2001. March 29;410(6828):541–8. [DOI] [PubMed] [Google Scholar]
- 27. Kuhbier JW, Allmeling C, Reimers K, Hillmer A, Kasper C, Menger B, et al. Interactions between spider silk and cells—NIH/3T3 fibroblasts seeded on miniature weaving frames. PLoS One. 2010. January;5(8):e12032 10.1371/journal.pone.0012032 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Schäfer-Nolte F, Hennecke K, Reimers K, Schnabel R, Allmeling C, Vogt PM, et al. Biomechanics and biocompatibility of woven spider silk meshes during remodeling in a rodent fascia replacement model. Ann Surg. 2014. April;259(4):781–92. 10.1097/SLA.0b013e3182917677 [DOI] [PubMed] [Google Scholar]
- 29. Gellynck K, Verdonk PCM, Van Nimmen E, Almqvist KF, Gheysens T, Schoukens G, et al. Silkworm and spider silk scaffolds for chondrocyte support. J Mater Sci Mater Med. 2008. November;19(11):3399–409. 10.1007/s10856-008-3474-6 [DOI] [PubMed] [Google Scholar]
- 30. Wendt H, Hillmer A, Reimers K, Kuhbier JW, Schäfer-Nolte F, Allmeling C, et al. Artificial skin—culturing of different skin cell lines for generating an artificial skin substitute on cross-weaved spider silk fibres. PLoS One. 2011. January;6(7):e21833 10.1371/journal.pone.0021833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gellynck K, Verdonk P, Forsyth R, Almqvist KF, Van Nimmen E, Gheysens T, et al. Biocompatibility and biodegradability of spider egg sac silk. J Mater Sci Mater Med. 2008. August;19(8):2963–70. 10.1007/s10856-007-3330-0 [DOI] [PubMed] [Google Scholar]
- 32. Gomes S, Gallego-Llamas J, Leonor IB, Mano JF, Reis RL, Kaplan DL. Biological responses to spider silk-antibiotic fusion protein. J Tissue Eng Regen Med. 2012. May;6(5):356–68. 10.1002/term.437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kearns V, MacIntosh A, Crawford A. Silk-based biomaterials for tissue engineering. Top Tissue Eng. 2008;4. [DOI] [PubMed] [Google Scholar]
- 34. Slotta U, Mougin N, Römer L, Leimer AH. Synthetic Spider Silk Proteins and Threads. Chem Eng Prog. 2012. [Google Scholar]
- 35. Apodaca G. The uroepithelium: not just a passive barrier. Traffic. 2004. March;5(3):117–28. [DOI] [PubMed] [Google Scholar]
- 36. Birder L. Role of the urothelium in bladder function. Scand J Urol Nephrol Suppl. 2004. January;(215):48–53. [DOI] [PubMed] [Google Scholar]
- 37. Romih R, Korosec P, de Mello W, Jezernik K. Differentiation of epithelial cells in the urinary tract. Cell Tissue Res. 2005. May;320(2):259–68. [DOI] [PubMed] [Google Scholar]
- 38. Lazzeri M. The physiological function of the urothelium—more than a simple barrier. Urol Int. 2006. January;76(4):289–95. [DOI] [PubMed] [Google Scholar]
- 39. Sponner A, Vater W, Monajembashi S, Unger E, Grosse F, Weisshart K. Composition and hierarchical organisation of a spider silk. Scheibel T, editor. PLoS One. Public Library of Science; 2007. January;2(10):e998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Bubenïk J, Perlmann P, Helmstein K, Moberger G. Cellular and humoral immune responses to human urinary bladder carcinomas. Int J Cancer. 1970. May 15;5(3):310–9. [DOI] [PubMed] [Google Scholar]
- 41. Marshall CJ, Franks LM, Carbonell AW. Markers of Neoplastic Transformation in Epithelial Cell Lines Derived From Human Carcinomas. J Natl Cancer Inst. 1977. June 1;58(6):1743–51. [DOI] [PubMed] [Google Scholar]
- 42. Denk W, Horstmann H. Serial block-face scanning electron microscopy to reconstruct three-dimensional tissue nanostructure. PLoS Biol. Public Library of Science; 2004. November;2(11):e329 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Muller LL, Jacks TJ. Rapid chemical dehydration of samples for electron microscopic examinations. J Histochem Cytochem. 1975. February;23(2):107–10. [DOI] [PubMed] [Google Scholar]
- 44. Ho PL, Kurtova A, Chan KS. Normal and neoplastic urothelial stem cells: getting to the root of the problem. Nat Rev Urol. 2012. October;9(10):583–94. 10.1038/nrurol.2012.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Kawakami S, Arai G, Hayashi T, Fujii Y, Xia G, Kageyama Y, et al. PPARgamma ligands suppress proliferation of human urothelial basal cells in vitro. J Cell Physiol. 2002. June;191(3):310–9. [DOI] [PubMed] [Google Scholar]
- 46. Tian H, Bharadwaj S, Liu Y, Ma PX, Atala A, Zhang Y. Differentiation of human bone marrow mesenchymal stem cells into bladder cells: potential for urological tissue engineering. Tissue Eng Part A. 2010. May;16(5):1769–79. 10.1089/ten.TEA.2009.0625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Zhu Y, Lei C, Luo Y, Liu N, He C, Chen W, et al. A modified method for isolation of bladder cancer stem cells from a MB49 murine cell line. BMC Urol. 2013. January;13(1):57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pérez-Rigueiro J, Elices M, Plaza G, Real JI, Guinea G V. The effect of spinning forces on spider silk properties. J Exp Biol. 2005. July;208(Pt 14):2633–9. [DOI] [PubMed] [Google Scholar]
- 49. Ortlepp CS, Gosline JM. Consequences of forced silking. Biomacromolecules. January;5(3):727–31. [DOI] [PubMed] [Google Scholar]
- 50. Ram-Liebig G, Meye A, Hakenberg OW, Haase M, Baretton G, Wirth MP. Induction of proliferation and differentiation of cultured urothelial cells on acellular biomaterials. BJU Int. 2004. October;94(6):922–7. [DOI] [PubMed] [Google Scholar]
- 51. Partridge MA, Marcantonio EE. Initiation of attachment and generation of mature focal adhesions by integrin-containing filopodia in cell spreading. Mol Biol Cell. 2006. October;17(10):4237–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Feil G, Christ-Adler M, Maurer S, Corvin S, Rennekampff H-O, Krug J, et al. Investigations of urothelial cells seeded on commercially available small intestine submucosa. Eur Urol. 2006. December;50(6):1330–7. [DOI] [PubMed] [Google Scholar]
- 53. Zhang Y, Kropp BP, Moore P, Cowan R, Furness PD, Kolligian ME, et al. Coculture of bladder urothelial and smooth muscle cells on small intestinal submucosa: potential applications for tissue engineering technology. J Urol. 2000. September;164(3 Pt 2):928–34; discussion 934–5. [DOI] [PubMed] [Google Scholar]
- 54. Campodonico F, Benelli R, Michelazzi A, Ognio E, Toncini C, Maffezzini M. Bladder cell culture on small intestinal submucosa as bioscaffold: experimental study on engineered urothelial grafts. Eur Urol. 2004. October;46(4):531–7. [DOI] [PubMed] [Google Scholar]
- 55. Franck D, Gil ES, Adam RM, Kaplan DL, Chung YG, Estrada CR, et al. Evaluation of silk biomaterials in combination with extracellular matrix coatings for bladder tissue engineering with primary and pluripotent cells. Egles C, editor. PLoS One. Public Library of Science; 2013. January;8(2):e56237 10.1371/journal.pone.0056237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Liberio MS, Sadowski MC, Soekmadji C, Davis RA, Nelson CC. Differential effects of tissue culture coating substrates on prostate cancer cell adherence, morphology and behavior. PLoS One. Public Library of Science; 2014. January 6;9(11):e112122 10.1371/journal.pone.0112122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Discher DE, Janmey P, Wang Y-L. Tissue cells feel and respond to the stiffness of their substrate. Science. 2005. November 18;310(5751):1139–43. [DOI] [PubMed] [Google Scholar]
- 58. Ho PL, Kurtova A, Chan KS. Normal and neoplastic urothelial stem cells: getting to the root of the problem. Nat Rev Urol. Nature Publishing Group, a division of Macmillan Publishers Limited. All Rights Reserved.; 2012. October;9(10):583–94. 10.1038/nrurol.2012.142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Blackledge TA, Boutry C, Wong S-C, Baji A, Dhinojwala A, Sahni V, et al. How super is supercontraction? Persistent versus cyclic responses to humidity in spider dragline silk. J Exp Biol. 2009. July;212(Pt 13):1981–9. 10.1242/jeb.028944 [DOI] [PubMed] [Google Scholar]
- 60. Brown CP, Whaite AD, MacLeod JM, Macdonald J, Rosei F. With great structure comes great functionality: Understanding and emulating spider silk. J Mater Res. Cambridge University Press; 2015. February 6;30(01):108–20. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.