Abstract
Concurrent use of cocaine and heroin (speedball) has been shown to exert synergistic effects on dopamine neurotransmission in the nucleus accumbens (NAc), as observed by significant increases in extracellular dopamine levels and compensatory elevations in the maximal reuptake rate (Vmax) of dopamine. The present studies were undertaken to determine whether chronic self-administration of cocaine, heroin or a combination of cocaine:heroin led to compensatory changes in the abundance and/or affinity of high- and low-affinity DAT binding sites. Saturation binding of the cocaine analog [125I] 3β-(4-iodophenyl)tropan-2β-carboxylic acid methyl ester ([125I]RTI-55) in rat NAc membranes resulted in binding curves that were best fit to two-site binding models, allowing calculation of dissociation constant (Kd) and binding density (Bmax) values corresponding to high- and low-affinity DAT binding sites. Scatchard analysis of the saturation binding curves clearly demonstrate the presence of high- and low- affinity binding sites in the NAc, with low-affinity sites comprising 85 to 94% of the binding sites. DAT binding analyses revealed that self-administration of cocaine and a cocaine:heroin combination increased the affinity of the low-affinity site for the cocaine congener RTI-55 compared to saline. These results indicate that the alterations observed following chronic speedball self-administration are likely due to the cocaine component alone; thus further studies are necessary to elaborate upon the synergistic effect of cocaine:heroin combinations on the dopamine system in the NAc.
Keywords: reinforcement, drug addiction, polydrug, drug abuse, operant conditioning, neuropharmacology, psychopharmacology, speedball
INTRODUCTION
Polydrug use is more prevalent among substance abusers in the US as compared to abuse of a single drug or drug class. Over the past few decades, clinical and experimental consideration has been given to a phenomenon in which individuals co-abuse cocaine and opioids (commonly heroin), either simultaneously or within a short period of time. This form of polysubstance abuse is referred to as “speedballing” and the cocaine:heroin combination as “speedball” (Hunt et al., 1984; Leri et al., 2003). It has been previously shown that, compared to heroin alone, patients with co-dependence on cocaine and opioids engage in more risky sexual behaviors (Hudgins et al., 1995), exhibit antisocial personality disorders (King et al., 2001), engage in more income-generating crimes (Cross et al., 2001), and most importantly, have worse treatment outcomes (DeMaria et al., 2000; Downey et al., 2000; Perez de los Cobos et al., 1997; Preston et al., 1998; Sofuoglu et al., 2003; Tzilos et al., 2009). In order to develop more efficacious treatment options for polydrug abusers, it is necessary to use animal models of self-administration in order to characterize the neurobiological correlates that underlie speedball abuse.
Following our development of the first preclinical model of cocaine:heroin addiction (Hemby et al., 1996), we demonstrated that self-administration of cocaine:heroin combinations elevated extracellular dopamine levels in the NAc in a synergistic manner (~1000% of baseline), compared to cocaine (~400% of baseline) and heroin (0–50% of baseline) alone (Hemby et al., 1999). In addition to significantly elevated extracellular dopamine concentrations, speedball self-administration induced a significant increase in the baseline maximal reuptake rate for the dopamine transporter (DAT), an effect not observed with self-administration of cocaine or heroin alone (Pattison et al., 2012). Elevated dopamine levels observed during speedball self-administration may reflect a more significant change in DAT binding than observed with cocaine alone, specifically, a greater decrease in DAT density or abundance as well as an increase in the DAT binding affinity.
Several studies have reported increased DAT binding in the NAc following cocaine administration in rodents (Bailey et al., 2008; Miguens et al., 2008), monkeys (Beveridge et al., 2006; Letchworth et al., 1997; Letchworth et al., 2001) and humans (Little et al., 1999; Mash et al., 2002; Staley et al., 1994a; Staley et al., 1994b) (but see (Hurd and Herkenham, 1993)). To date, no studies have reported the effect of speedball self-administration on DAT binding in the brain. Changes in DAT binding can be due to changes in the abundance or affinity of the transporter. Moreover, analysis of drug effects on DAT binding is complicated by the fact that cocaine analogs bind to DAT in a biphasic nature, with high- and low-affinity sites in striatal membrane preparations (Boja et al., 1992; Letchworth et al., 1999; Rothman et al., 1995), although the functional and structural significance of these two binding sites have not been determined. Determining how the affinity of cocaine-like ligands for DAT may be altered following self-administration of cocaine, heroin and speedball may provide insights into the molecular mechanisms of action that underlie the synergistic elevations observed during speedball self-administration and ultimately may aid in the development of novel treatments for polysubstance abuse. In the present study, membrane binding with a potent cocaine-like DAT radioligand was used to assess the affinities and densities of high- and low-affinity DAT binding sites.
MATERIALS AND METHODS
Subjects
Male Fisher F-344 rats (120–150 days; 270–320 g; Charles River, Wilmington, MA) were housed in a temperature-controlled vivarium on a 12-hour reversed light/dark cycle (lights on at 6:00 PM). Rats were group-housed before surgery and housed individually after catheterization. Water was available ad libitum while food access was restricted to maintain consistent body weight during the experiment. Experimental sessions were conducted during the dark phase of the light/dark cycle. All procedures were performed in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH Publication No. 80-23) revised in 1996.
Drugs
Cocaine hydrochloride and heroin hydrochloride were obtained from the Drug Supply Program of the National Institute on Drug Abuse (Bethesda, MD). Pentobarbital and sodium thiopental were purchased from the pharmacy at Wake Forest Baptist Hospital (Winston-Salem, NC). Sodium heparin was purchased from Elkin-Sinn (Cherry Hill, NJ), methyl atropine nitrate was purchased from Sigma-Aldrich (St Louis, MO) and penicillin G was purchased from Webster Vet Supply (Devens, MA). Iodinated 3β-(4-iodophenyl)tropan-2β-carboxylic acid methyl ester ([125I]RTI-55; 2200 Ci/mmol) was purchased from PerkinElmer (Waltham, MA). Unlabeled RTI-55 was supplied by the National Institute on Drug Abuse Drug Supply Program (Bethesda, MD). WF-23 [2β-propanoyl-3β-(2-naphthyl) tropane] was previously synthesized and dissolved in phosphate-buffered saline (Davies et al., 1994). TR tritium-sensitive phosphoimage screens were purchased from PerkinElmer (Waltham, MA).
Intravenous self-administration
Animals were pretreated with atropine nitrate (10 mg/kg; i.p.) and anesthesia was induced by pentobarbital (40 mg/kg; i.p.). Under anesthesia, rats were implanted with chronic indwelling venous catheters as described previously (Hemby et al., 1999; Hemby et al., 1997; Hemby et al., 1995; Hemby et al., 1996). Infusions of sodium thiopental (150 μl; 15 mg/kg; i.v.) were manually administered as needed to assess catheter patency. Rats were administered penicillin G procaine (75,000 units in 0.25 ml, i.m.) at the termination of catheter implantation. Health of the rats was monitored daily by the experimenters and weekly by institutional veterinarians according to the guidelines issued by the Wake Forest University Animal Care and Use Committee and the National Institute of Health.
Self-administration was performed as described previously (Pattison et al., 2012). Briefly, rats were assigned randomly into groups (n = 8–10) to self-administer cocaine (250 μg/inf), heroin (10 μg/inf) or speedball (250/10 μg/inf cocaine:heroin). Infusions were delivered in a volume of 200 μl. Responding was engendered under a fixed-ratio (FR) 1 schedule and the daily session terminated after 25 infusions or 4 hours. Once rats consistently obtained 25 infusions per session, the schedule was increased to FR2 and then FR5 for several training days, followed by 25 consecutive days of 25 infusions per day, where stable responding was maintained under an FR5 reinforcement schedule (as monitored by average inter-infusion intervals within 10%). Approximately 24 hours following the final self-administration session, animals were sacrificed by rapid decapitation and brains were promptly removed and hemisected, then each hemisphere was flash-frozen in isopentane at −35°C to −50°C and stored at −80°C until further use.
DAT radioligand binding in membranes
Brain hemispheres used in membrane binding assays were brought up to −20°C and the NAc was dissected under a 10X microscope. The entire NAc from one hemisphere of each animal was microdissected from approximately +3.00 mm to +0.45 mm relative to bregma and stored at −80°C. NAc tissue was thawed on ice and homogenized in 10 ml cold assay buffer (10 mM sodium phosphate buffer, pH 7.4, with 0.32 M sucrose). Membranes were prepared by high-speed centrifugation (10 min at 48,000 × g) and re-suspended in 50 ml cold buffer. Protein concentration was determined using colorimetric bicinchoninic acid (BCA) protein assay reagents (Thermo Scientific) by absorbance at 562 nm.
To determine dissociation constant (Kd) and binding density (Bmax) values of [125I]RTI-55 binding to DAT, saturation binding of [125I]RTI-55 (Boja et al., 1991; Rothman et al., 1994) was accomplished using a constant concentration of [125I]RTI-55 with a range of concentrations of unlabeled RTI-55 (0.001–30 nM) in rat NAc membranes. Each NAc membrane sample was assayed in triplicate (carried out in ice-cold assay buffer, total volume 2 ml) using 0.1 nM [125I]RTI-55. Assays were incubated at 25°C for 50 min and reactions were terminated by rapid filtration with 3 × 5 ml of cold 50 mM Tris-HCl, pH 7.4, through Whatman GF/B glass fiber filters pre-soaked in Tris buffer containing 0.1% bovine serum albumin. Nonspecific binding was determined with 1 μM WF-23 (Davies et al., 1994). Radioactivity was determined by liquid scintillation spectrometry in filters eluted for 8 hours in 5 ml of Ecolite scintillation fluid. Binding curves were fit to one-site and two-site models by iterative non-linear curve fitting, where the contribution of the high-affinity Bmax values have been subtracted from those of low-affinity sites.
Data Analysis
Differences in the number of infusions and inter-infusion intervals between the groups were analyzed using a two-way ANOVA with repeated measures with Drug as the main factor and Time as the repeated measure. For post hoc analysis, Bonferroni with comparisons between speedball and cocaine and speedball and heroin was used (P<0.05). Saturation binding data were analyzed using GraphPad Prism 5.0 for nonlinear regression curve fitting to determine the Kd and Bmax from data fit to one- and two-site binding hyperbolae. Statistical analyses using an F test were performed to determine whether those data could be best described by a single- or two-site model, and P < 0.05 determined the best-fit model. Group differences for membrane binding were determined using one-way analysis of variance (ANOVA) with Bonferroni test for selected group comparisons (P < 0.05).
RESULTS
Self-administration
Rats were allowed to self-administer i.v. cocaine (250 μg/inf), heroin (10 μg/inf) or speedball (250/10 μg/inf cocaine:heroin) under an FR5 schedule for 25 consecutive days for 4 hours per day or until a maximum of 25 infusions was acquired. Control subjects were given access to self-administer saline for approximately 30 days. The number of infusions was significantly greater for speedball, cocaine and heroin self-administration compared with saline self-administration. There was no statistically significant difference in the number of infusions between the speedball group and either the cocaine or heroin groups ([F(1,16)=4.24, P=0.0562] and [F(1,16)=0.66, P=0.4298], respectively) (Fig. 1A). There was no significant effect of self-administered drug on the inter-infusion intervals [F(2, 24)=1.67, P=0.209]. In addition, there was no significant difference between the self-administered drugs across the 30 sessions [F(58, 696)=1.21, P=0.143]. Inter-infusion intervals were not significantly different between speedball and cocaine [F(1,16)=1.12, P=0.3047] or speedball and heroin [F(1,16)=0.60, P=0.450]. Furthermore, there was no significant difference between speedball and cocaine [F(29, 464)=0.84, P=0.7129] or speedball and heroin [F(29,464)=0.66, P=0.9103] over the 30 self-administration sessions (Fig. 1B).
Figure 1. Self-administration for rats responding under an FR5 for i.v. cocaine (250 μg/inf), heroin (10 μg/inf) or speedball (250/10 μg/inf cocaine:heroin).
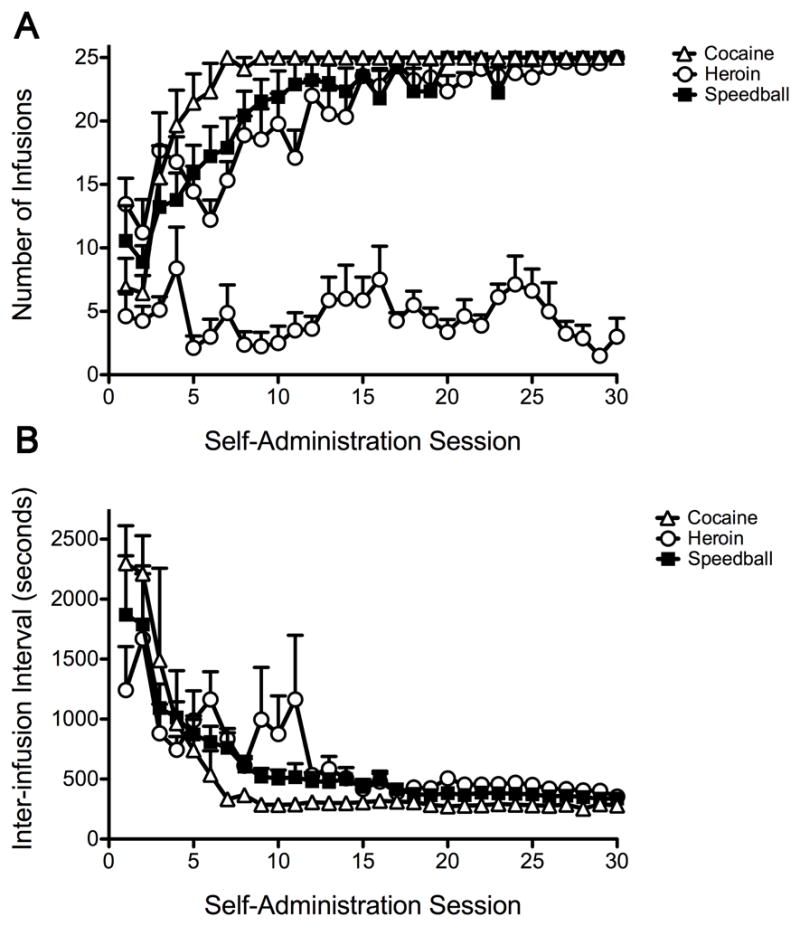
Responding was engendered under an FR1 (and remained FR1 for saline controls) then increased to FR5. (A) Number of infusions per day (mean ± SEM). Speedball, cocaine and heroin self-administration resulted in a significantly greater number of infusions compared with saline across the thirty days. There was no significant difference in the number of infusions between the speedball, cocaine and heroin groups. (B) The average (mean ± SEM) inter-infusion interval was not significantly different between the groups. In addition, there was no significant difference in the inter-infusion interval between the groups across the 30 self-administration sessions.
DAT binding
[125I]RTI-55 and unlabeled RTI-55 were used to create saturation binding plots in NAc membrane preparations in order to assess the affinity and Bmax of this cocaine analog at high- and low-affinity DAT binding sites. Figure 2 shows a representative Scatchard plot of saturation RTI-55 binding to NAc membranes, showing the clear biphasic nature of this binding. Both one- and two-site binding fits were applied to the specific binding data for each subject and F tests were used to determine which fit best described the data. In agreement with previously published results (Boja et al., 1992; Letchworth et al., 1999; Rothman et al., 1995), saturation curves were best represented by a two-site binding model (F value range, 4.47–802.9; P < 0.05). Table 1 depicts the binding parameters Kd and Bmax for both high- and low-affinity DAT binding sites. There was a significant decrease in the Kd at the low-affinity sites between the groups [F(3,19)= 5.748, P=0.008]. Post hoc analysis revealed Kd values following cocaine and speedball self-administration were significantly lower compared to saline. No statistically significant differences in the Kd values for the high-affinity sites were observed [F(3,19)= 2.8, P=0.076]. Due to differences in sample variance among the groups for Bmax values for the high-affinity site, the Kruskal-Wallis test was used to compare differences amongst groups. No statistically significant difference in the Bmax values for the high-affinity sites was observed [KW= 6.429, P=0.0925]. There was no significant difference between groups in Bmax for low-affinity sites [F(3,19)= 1.885, P=0.173].
Figure 2. Scatchard plots of saturation RTI-55 binding to NAc membrane preparations from representative subjects in saline (SAL), cocaine (COC), heroin (HER) and speedball (SBL) groups.
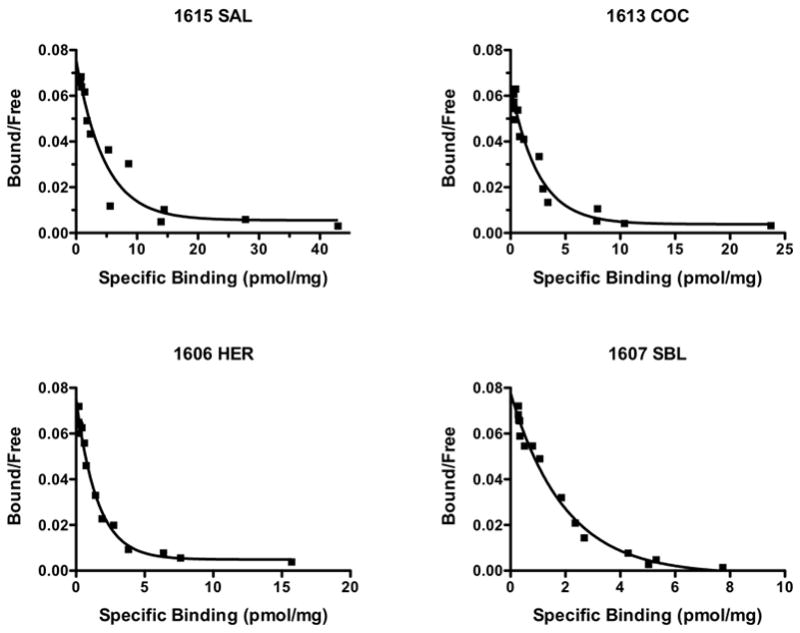
Membranes were incubated with 0.1 nM [125I]RTI-55 along with 15 concentrations of unlabeled RTI-55 (0.001–30 nM) in triplicate. Binding data were analyzed by Prism 5 software using a nonlinear curve fit and a two site-specific binding model. Plots display high- and low-affinity binding components.
Table 1.
Effects of chronic cocaine, heroin and speedball self-administration on parameters of high- and low-affinity DAT binding sites.
| SA Group | High Kd (pM) | Low Kd (pM) | High Bmax (pmol/mg) | Low Bmax (pmol/mg) |
|---|---|---|---|---|
|
| ||||
| Saline | 132.6 ± 46.1 | 16880 ± 2938 | 0.286 ± 0.140 | 4.561 ± 1.340 |
| Cocaine | 410.3 ± 98.2 | 7960 ± 2190* | 0.284 ± 0.095 | 3.035 ± 0.470 |
| Speedball | 255.6 ± 81.9 | 4993 ± 1256* | 0.994 ± 0.355 | 5.878 ± 0.815 |
| Heroin | 237.3 ± 22.7 | 11442 ± 1774 | 0.228 ± 0.083 | 3.011 ± 1.159 |
Binding parameters for high-affinity (high Kd and Bmax) and low-affinity (low Kd and Bmax) DAT binding sites (mean ± SEM) determined by nonlinear regression for two-site modeling of membrane binding assays with [125I]RTI-55 in rat NAc membranes.
p < 0.05 vs. saline.
DISCUSSION
The present study was undertaken to determine if chronic self-administration of cocaine, heroin and speedball leads to compensatory changes in the abundance and/or affinity of high and low affinity DAT binding sites. Scatchard analyses of the saturation binding curves clearly demonstrate the presence of high- and low-affinity binding sites in the NAc, with low-affinity sites comprising 85 to 94% of the binding sites. These results are in agreement with results from human (Staley et al., 1994a; Staley et al., 1994b) and non-human primate (Madras et al., 1989a; Madras et al., 1989b) striatal tissue. We found significant alterations in the binding parameters for DAT binding sites in rat NAc following chronic self-administration of cocaine and cocaine:heroin combinations. DAT binding analysis revealed that the self-administration of cocaine and a cocaine:heroin combination increased the affinity of the low-affinity site for the cocaine congener RTI-55. These results indicate that the alterations observed following chronic speedball self-administration are likely due to the cocaine component alone, thus further studies are necessary to elaborate upon the synergistic effect of cocaine:heroin combinations on the dopamine system in the NAc.
The current analyses of radioligand binding in membranes allowed modeling of both high- and low-affinity DAT binding sites, in agreement with previous studies (Gracz and Madras, 1995; Katz et al., 2000; Letchworth et al., 1999; Madras et al., 1989a; Madras et al., 1989b; Mash et al., 2002; Pristupa et al., 1994; Rothman et al., 1995; Staley et al., 1994a; Staley et al., 1994b). The physical nature of the high- and low-affinity sites remains debated, and, in fact, may depend upon precise assay conditions. Specifically, there are questions as to whether they represent binding sites for multiple substrates and whether the sites are physically distinct, identical or overlapping. Correlational analysis between physical binding locations of DAT substrates and observed high- and low-affinity binding characteristics of the ligands would provide valuable insight to the functional alterations in DAT following chronic drug abuse.
A goal of the present study was to assess the regulation of high- and low-affinity DAT sites by chronic cocaine self-administration. The affinity of psychostimulants for DAT is positively correlated with the behavioral potency of those compounds in in rodents and nonhuman primates (Bergman et al., 1989; Ritz et al., 1987; Wilcox et al., 1999). However, some drugs that bind to DAT with high-affinity are not efficacious as cocaine in terms of behavioral stimulation, are weak positive reinforcers compared to cocaine (Stafford et al., 2001; Tella et al., 1996; Wilcox et al., 2000; Woolverton et al., 2001) and do not share subjective effects with cocaine (Katz et al., 1999; Newman et al., 1995). The different effects suggest that cocaine and its analogs share a similar bindings site(s) that mediates uptake inhibition, but that a separate site may regulate the behavioral effects of cocaine related to its abuse liability (Izenwasser, 1998). Previous studies suggest that the high-affinity binding site exerts a low-capacity uptake process that maintains extracellular dopamine at relatively low concentrations where as the low-affinity site contributes to a high-capacity uptake process. Previous studies have reported increased DAT binding following chronic cocaine self-administration in rats (Miguens et al., 2008) and monkeys (Letchworth et al., 2001). Such changes in binding could be due to increased abundance of DAT or an increase in the affinity of the binding sites for cocaine. Results from the present study extend previous findings by demonstrating chronic cocaine self-administration increases affinity of the low-affinity DAT binding site. Similar to cocaine, speedball self-administration increased affinity of the low-affinity DAT binding site. Given the lack of effect of heroin self-administration on the low-affinity site Kd, we conclude that the changes observed with speedball are due to cocaine. Thus, the change in affinity of the low affinity DAT binding site alone does not account for the significant difference in extracellular dopamine concentrations in the NAc during speedball versus cocaine self-administration (Hemby et al., 1999; Smith et al., 2006). Studies are needed to assess the bindings characteristics of similar DAT radioligands to confirm the specificity of the binding site regulation by cocaine. The functional impact of changes in the low-affinity DAT binding remains to be determined and is dependent upon the development of pharmacological tools that have selectivity for the different DAT binding sites.
Computational modeling of DAT binding and substrate translocation mechanisms have recently utilized the crystal structure of a homologue to Na+/Cl−-dependent neurotransmitter transporters, bacterial leucine transporter from Aquifex aeolicus (LeuTAa) (Yamashita et al., 2005) and reported divergent sites of DAT protein interactions with different ligands, namely dopamine and cocaine. Several groups have reported a secondary pocket as the binding site for cocaine distinct from the primary substrate binding site for dopamine (Gedeon et al., 2010; Huang et al., 2009; Indarte et al., 2008; Merchant and Madura, 2012; Quick et al., 2009; Shan et al., 2011; Shi et al., 2008; Singh et al., 2007). Computational modeling employing minimum free energy profiles and atomic distances between DAT and its ligands suggest that these distinct binding sites may be located adjacently within a single binding pocket, where the primary binding site for dopamine is located more internally and secondary binding site located more toward the extracellular surface (Huang et al., 2009; Merchant and Madura, 2012; Shi et al., 2008). Insight into the physical characteristics of DAT binding sites and the proposed mechanisms of substrate translocation from the external secondary to the internal primary binding site (Huang et al., 2009; Shi et al., 2008) may lend support for the current results. The existence of allosteric and other atypical ligands for dopamine transporters (Schmitt et al., 2013) also supports these concepts. However, further studies are necessary to accurately compare these computational mechanisms to biochemical findings.
In DAT binding assays using [125I]RTI-55, there are potential analytical artifacts that could confound these results. First, sufficient quantities of endogenous dopamine could possibly be present to bind to DAT and reduce binding of the radioligand. Such a scenario is highly unlikely in this study since the membranes were washed thoroughly prior to the binding assay (which would have removed endogenous dopamine) and the preparations were suspended in vast excess of buffer (50 ml) (which would have diluted endogenous dopamine to undetectable levels). For the same reasons, as well as a 24 hour withdrawal period prior to sacrifice, it also unlikely that residual cocaine would remain in the membrane preparations to affect [125I]RTI-55 binding. Second, since RTI-55 binds to the serotonin transporter (SERT) as well as the DAT (Boja et al, 1992), sufficient abundance of SERT in the NAc membrane preparations would confound the interpretation of the results. However, SERT levels in the rat NAc are at least 20-fold lower abundance than DAT levels (D’Amato et al., 1987; Javitch et al., 1985), suggesting minimal contribution from SERT binding in the assay. Addition of 30 nM fluoxetine to the [125I]RTI-55 binding assays in rat NAc membranes did not alter the binding on high or low affinity [125I]RTI-55 binding of DAT (data not shown).
The parameters of the experimental design were chosen to be similar to those used in previous studies from our lab, including dose selection, drug access and exposure (Hemby et al., 1999; Hemby et al., 1996; Pattison et al., 2012; Smith et al., 2006). In the present study, the number of infusions was limited in order to negate the influence of differential intake on DAT binding between the cocaine and speedball groups and the heroin and speedball groups, respectively. Studies investigating the effects of extended drug access and escalated intake would likely yield different results from limited access procedures due to greater overall intake. A previous study demonstrated that limited access versus extended access to cocaine self-administration resulted in significant differences in the NAc dopaminergic response to cocaine (Ahmed et al., 2003). The effects of cocaine, heroin or cocaine:heroin combinations on DAT binding under these conditions remain to be determined. As noted above, doses were selected to parallel doses used in previous studies by the authors, in order to compare pharmacological, behavioral and neurochemical measures between cocaine, heroin and cocaine:heroin combinations for the purpose of developing a comprehensive comparative analysis. The investigators acknowledge that multiple historical, experiential and environmental factors influence the extent and manner in which drugs are self-administered (Barrett, 1977; Barrett and Witkin, 1986) and the neurobiological outcomes are determined. Further studies are warranted to explore the contribution of such factors.
A vast literature indicates that dopamine is a common substrate for the reinforcing effects of food and abuse drugs such as cocaine and heroin (Kelley and Berridge, 2002; Pelchat, 2002); thus, it is conceivable that food restriction may have influenced the result of the present study. Previous studies have shown that the behavioral effects of cocaine and other stimulants are increased upon food restriction (80% for 3+ weeks) (Cabeza de Vaca and Carr, 1998), an effect due in part to post-synaptic dopamine receptor sensitivity (Carr et al., 2001; Carr et al., 2003). Decreased basal levels of dopamine caused by increased uptake could lead to increased post-synaptic receptor sensitivity as a result of food restriction. However, analysis of presynaptic dopamine dynamics reveals acute food restriction (seven days) does not affect DAT binding (Bello et al., 2003) and induced a modest reduction in Vmax without altering Km (Patterson et al., 1998), whereas chronic food restriction did not alter Vmax or Km in the striatum (Galici et al., 1998) (but see (Zhen et al., 2006)). Given the lack of effect of chronic food restriction on dopamine uptake dynamics and DAT binding and the fact that all subjects were food restricted in the current study, we conclude that the present results were not influenced by food restriction.
Previously, we have shown that self-administration of cocaine:heroin combinations synergistically increased extracellular dopamine levels in the NAc. The present study was undertaken to determine whether the synergistic elevations in dopamine were associated with alterations in the binding profile of DAT. While self-administration of the cocaine:heroin combination increased the affinity of the low-affinity site for the cocaine congener RTI-55, similar changes were observed after cocaine self-administration, suggesting that changes in DAT binding do not account for the speedball-induced synergistic dopamine elevations. Further studies are warranted to determine the physiological and pharmacological mechanisms that enable the synergistic dopaminergic response observed during cocaine:heroin self-administration.
Acknowledgments
The study was supported in part by R01 DA012498 (SEH) and P50 DA006634 (SRC, SEH). The authors appreciate Amanda Grigg assisting with self-administration.
Footnotes
Conflict of Interest
The authors declare no conflicts of interest.
References
- Ahmed SH, Lin D, Koob GF, Parsons LH. Escalation of cocaine self-administration does not depend on altered cocaine-induced nucleus accumbens dopamine levels. Journal of Neurochemistry. 2003;86(1):102–113. doi: 10.1046/j.1471-4159.2003.01833.x. [DOI] [PubMed] [Google Scholar]
- Bailey A, Metaxas A, Yoo JH, McGee T, Kitchen I. Decrease of D2 receptor binding but increase in D2-stimulated G-protein activation, dopamine transporter binding and behavioural sensitization in brains of mice treated with a chronic escalating dose ‘binge’ cocaine administration paradigm. Eur J Neurosci. 2008;28(4):759–770. doi: 10.1111/j.1460-9568.2008.06369.x. [DOI] [PubMed] [Google Scholar]
- Barrett JE. Behavioral history as a determinant of the effects of d-amphetamine on punished behavior. Science. 1977;198(4312):67–69. doi: 10.1126/science.408925. [DOI] [PubMed] [Google Scholar]
- Barrett JE, Witkin JM. The role of behavioral and pharmacological history in determining the effects of abused drugs. In: Goldberg SR, Stolerman IP, editors. Behavioral Approaches to Drug Dependednce. New York: Academic Press; 1986. pp. 195–223. [Google Scholar]
- Bello NT, Sweigart KL, Lakoski JM, Norgren R, Hajnal A. Restricted feeding with scheduled sucrose access results in an upregulation of the rat dopamine transporter. Am J Physiol Regul Integr Comp Physiol. 2003;284(5):R1260–1268. doi: 10.1152/ajpregu.00716.2002. [DOI] [PubMed] [Google Scholar]
- Bergman J, Madras BK, Johnson SE, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. III. Self-administration by squirrel monkeys. J Pharmacol Exp Ther. 1989;251(1):150–155. [PubMed] [Google Scholar]
- Beveridge TJ, Smith HR, Daunais JB, Nader MA, Porrino LJ. Chronic cocaine self-administration is associated with altered functional activity in the temporal lobes of non human primates. The European journal of neuroscience. 2006;23(11):3109–3118. doi: 10.1111/j.1460-9568.2006.04788.x. [DOI] [PubMed] [Google Scholar]
- Boja JW, Mitchell WM, Patel A, Kopajtic TA, Carroll FI, Lewin AH, Abraham P, Kuhar MJ. High-affinity binding of [125I]RTI-55 to dopamine and serotonin transporters in rat brain. Synapse. 1992;12(1):27–36. doi: 10.1002/syn.890120104. [DOI] [PubMed] [Google Scholar]
- Boja JW, Patel A, Carroll FI, Rahman MA, Philip A, Lewin AH, Kopajtic TA, Kuhar MJ. [125I]RTI-55: a potent ligand for dopamine transporters. European Journal of Pharmacology. 1991;194:133–134. doi: 10.1016/0014-2999(91)90137-f. [DOI] [PubMed] [Google Scholar]
- Cabeza de Vaca S, Carr KD. Food restriction enhances the central rewarding effect of abused drugs. J Neurosci. 1998;18(18):7502–7510. doi: 10.1523/JNEUROSCI.18-18-07502.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr KD, Kim GY, Cabeza de Vaca S. Rewarding and locomotor-activating effects of direct dopamine receptor agonists are augmented by chronic food restriction in rats. Psychopharmacology. 2001;154(4):420–428. doi: 10.1007/s002130000674. [DOI] [PubMed] [Google Scholar]
- Carr KD, Tsimberg Y, Berman Y, Yamamoto N. Evidence of increased dopamine receptor signaling in food-restricted rats. Neuroscience. 2003;119(4):1157–1167. doi: 10.1016/s0306-4522(03)00227-6. [DOI] [PubMed] [Google Scholar]
- Cross JC, Johnson BD, Davis WR, Liberty HJ. Supporting the habit: income generation activities of frequent crack users compared with frequent users of other hard drugs. Drug Alcohol Depend. 2001;64(2):191–201. doi: 10.1016/s0376-8716(01)00121-1. [DOI] [PubMed] [Google Scholar]
- D’Amato RJ, Largent BL, Snowman AM, Snyder SH. Selective labeling of serotonin uptake sites in rat brain by [3H]citalopram contrasted to labeling of multiple sites by [3H]imipramine. Journal of Pharmacology and Experimental Therapeutics. 1987;242:364–371. [PubMed] [Google Scholar]
- Davies HM, Saikali E, Huby NJ, Gilliatt VJ, Matasi JJ, Sexton T, Childers SR. Synthesis of 2 beta-acyl-3 beta-aryl-8-azabicyclo[3.2.1]octanes and their binding affinities at dopamine and serotonin transport sites in rat striatum and frontal cortex. J Med Chem. 1994;37(9):1262–1268. doi: 10.1021/jm00035a005. [DOI] [PubMed] [Google Scholar]
- DeMaria PA, Jr, Sterling R, Weinstein SP. The effect of stimulant and sedative use on treatment outcome of patients admitted to methadone maintenance treatment. Am J Addict. 2000;9(2):145–153. doi: 10.1080/10550490050173217. [DOI] [PubMed] [Google Scholar]
- Downey KK, Helmus TC, Schuster CR. Treatment of heroin-dependent poly-drug abusers with contingency management and buprenorphine maintenance. Exp Clin Psychopharmacol. 2000;8(2):176–184. doi: 10.1037//1064-1297.8.2.176. [DOI] [PubMed] [Google Scholar]
- Galici R, Pinna G, Stephens DN, Schneider HH, Turski L. Tolerance to and Dependence on Alprazolam are Due to Changes in GABAa Receptor Function and Are Independent of Exposure to Experimental Set-up. Restor Neurol Neurosci. 1998;12(4):233–237. [PubMed] [Google Scholar]
- Gedeon PC, Indarte M, Surratt CK, Madura JD. Molecular dynamics of leucine and dopamine transporter proteins in a model cell membrane lipid bilayer. Proteins. 2010;78(4):797–811. doi: 10.1002/prot.22601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gracz LM, Madras BK. [3H]WIN 35,428 ([3H]CFT) binds to multiple charge-states of the solubilized dopamine transporter in primate striatum. J Pharmacol Exp Ther. 1995;273(3):1224–1234. [PubMed] [Google Scholar]
- Hemby SE, Co C, Dworkin SI, Smith JE. Synergistic elevations in nucleus accumbens extracellular dopamine concentrations during self-administration of cocaine:heroin combinations (Speedball) in rats. J Pharmacol Exp Ther. 1999;288(1):274–280. [PubMed] [Google Scholar]
- Hemby SE, Co C, Koves TR, Smith JE, Dworkin SI. Differences in extracellular dopamine concentrations in the nucleus accumbens during response-dependent and response-independent cocaine administration in the rat. Psychopharmacology (Berl) 1997;133(1):7–16. doi: 10.1007/s002130050365. [DOI] [PubMed] [Google Scholar]
- Hemby SE, Martin TJ, Co C, Dworkin SI, Smith JE. The effects of intravenous heroin administration on extracellular nucleus accumbens dopamine concentrations as determined by in vivo microdialysis. J Pharmacol Exp Ther. 1995;273(2):591–598. [PubMed] [Google Scholar]
- Hemby SE, Smith JE, Dworkin SI. The effects of eticlopride and naltrexone on responding maintained by food, cocaine, heroin and cocaine:heroin combinations in rats. The Journal of Pharmacology and Experimental Therapeutics. 1996;277(3):1247–1258. [PubMed] [Google Scholar]
- Huang X, Gu HH, Zhan CG. Mechanism for cocaine blocking the transport of dopamine: insights from molecular modeling and dynamics simulations. J Phys Chem B. 2009;113(45):15057–15066. doi: 10.1021/jp900963n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudgins R, McCusker J, Stoddard A. Cocaine use and risky injection and sexual behaviors. Drug Alcohol Depend. 1995;37(1):7–14. doi: 10.1016/0376-8716(94)01060-x. [DOI] [PubMed] [Google Scholar]
- Hunt DE, Lipton DS, Goldsmith D, Strug D. Street pharmacology: uses of cocaine and heroin in the treatment of addiction. Drug Alcohol Depend. 1984;13(4):375–387. doi: 10.1016/0376-8716(84)90005-x. [DOI] [PubMed] [Google Scholar]
- Hurd YL, Herkenham M. Molecular alterations in the neostriatum of human cocaine addicts. Synapse. 1993;13(4):357–369. doi: 10.1002/syn.890130408. [DOI] [PubMed] [Google Scholar]
- Indarte M, Madura JD, Surratt CK. Dopamine transporter comparative molecular modeling and binding site prediction using the LeuT(Aa) leucine transporter as a template. Proteins. 2008;70(3):1033–1046. doi: 10.1002/prot.21598. [DOI] [PubMed] [Google Scholar]
- Izenwasser S. Basic pharmacological mechanisms of cocaine. In: Higgins S, Katz JL, editors. Cocaine abuse: Behavior, Pharmacology and Clinical Applications. San Diego: Academic Press: 1998. pp. 1–20. [Google Scholar]
- Javitch JA, Strittmatter SM, Snyder SH. Differential visualization of dopamine and norepinephrine uptake sites in rat brain using [3H]mazindol autoradiography. Journal of Neuroscience. 1985;5:1513–1521. doi: 10.1523/JNEUROSCI.05-06-01513.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz JL, Izenwasser S, Kline RH, Allen AC, Newman AH. Novel 3alpha-diphenylmethoxytropane analogs: selective dopamine uptake inhibitors with behavioral effects distinct from those of cocaine. The Journal of Pharmacology and Experimental Therapeutics. 1999;288(1):302–315. [PubMed] [Google Scholar]
- Katz JL, Izenwasser S, Terry P. Relationships among dopamine transporter affinities and cocaine-like discriminative-stimulus effects. Psychopharmacology (Berl) 2000;148(1):90–98. doi: 10.1007/s002130050029. [DOI] [PubMed] [Google Scholar]
- Kelley AE, Berridge KC. The neuroscience of natural rewards: relevance to addictive drugs. J Neurosci. 2002;22(9):3306–3311. doi: 10.1523/JNEUROSCI.22-09-03306.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King VL, Kidorf MS, Stoller KB, Carter JA, Brooner RK. Influence of antisocial personality subtypes on drug abuse treatment response. J Nerv Ment Dis. 2001;189(9):593–601. doi: 10.1097/00005053-200109000-00004. [DOI] [PubMed] [Google Scholar]
- Leri F, Bruneau J, Stewart J. Understanding polydrug use: review of heroin and cocaine co-use. Addiction. 2003;98(1):7–22. doi: 10.1046/j.1360-0443.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Daunais JB, Hedgecock AA, Porrino LJ. Effects of chronic cocaine administration on dopamine transporter mRNA and protein in the rat. Brain Res. 1997;750(1–2):214–222. doi: 10.1016/s0006-8993(96)01384-4. [DOI] [PubMed] [Google Scholar]
- Letchworth SR, Nader MA, Smith HR, Friedman DP, Porrino LJ. Progression of changes in dopamine transporter binding site density as a result of cocaine self-administration in rhesus monkeys. J Neurosci. 2001;21(8):2799–2807. doi: 10.1523/JNEUROSCI.21-08-02799.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letchworth SR, Sexton T, Childers SR, Vrana KE, Vaughan RA, Davies HM, Porrino LJ. Regulation of rat dopamine transporter mRNA and protein by chronic cocaine administration. J Neurochem. 1999;73(5):1982–1989. [PubMed] [Google Scholar]
- Little KY, Zhang L, Desmond T, Frey KA, Dalack GW, Cassin BJ. Striatal dopaminergic abnormalities in human cocaine users. Am J Psychiatry. 1999;156(2):238–245. doi: 10.1176/ajp.156.2.238. [DOI] [PubMed] [Google Scholar]
- Madras BK, Fahey MA, Bergman J, Canfield DR, Spealman RD. Effects of cocaine and related drugs in nonhuman primates. I. [3H]cocaine binding sites in caudate-putamen. The Journal of Pharmacology and Experimental Therapeutics. 1989a;251(1):131–141. [PubMed] [Google Scholar]
- Madras BK, Spealman RD, Fahey MA, Neumeyer JL, Saha JK, Milius RA. Cocaine receptors labeled by [3H]2 beta-carbomethoxy-3 beta-(4-fluorophenyl)tropane. Molecular Pharmacology. 1989b;36(4):518–524. [PubMed] [Google Scholar]
- Mash DC, Pablo J, Ouyang Q, Hearn WL, Izenwasser S. Dopamine transport function is elevated in cocaine users. Journal of Neurochemistry. 2002;81(2):292–300. doi: 10.1046/j.1471-4159.2002.00820.x. [DOI] [PubMed] [Google Scholar]
- Merchant BA, Madura JD. Insights from molecular dynamics: The binding site of cocaine in the dopamine transporter and permeation pathways of substrates in the leucine and dopamine transporters. Journal of Molecular Graphics and Modelling. 2012;38(0):1–12. doi: 10.1016/j.jmgm.2012.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miguens M, Crespo JA, Del Olmo N, Higuera-Matas A, Montoya GL, Garcia-Lecumberri C, Ambrosio E. Differential cocaine-induced modulation of glutamate and dopamine transporters after contingent and non-contingent administration. Neuropharmacology. 2008;55(5):771–779. doi: 10.1016/j.neuropharm.2008.06.042. [DOI] [PubMed] [Google Scholar]
- Newman AH, Kline RH, Allen AC, Izenwasser S, George C, Katz JL. Novel 4′-substituted and 4′,4″-disubstituted 3 alpha-(diphenylmethoxy)tropane analogs as potent and selective dopamine uptake inhibitors. Journal of Medicinal Chemistry. 1995;38(20):3933–3940. doi: 10.1021/jm00020a006. [DOI] [PubMed] [Google Scholar]
- Patterson TA, Brot MD, Zavosh A, Schenk JO, Szot P, Figlewicz DP. Food deprivation decreases mRNA and activity of the rat dopamine transporter. Neuroendocrinology. 1998;68(1):11–20. doi: 10.1159/000054345. [DOI] [PubMed] [Google Scholar]
- Pattison LP, McIntosh S, Budygin EA, Hemby SE. Differential regulation of accumbal dopamine transmission in rats following cocaine, heroin and speedball self-administration. Journal of Neurochemistry. 2012;122(1):138–146. doi: 10.1111/j.1471-4159.2012.07738.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pelchat ML. Of human bondage: food craving, obsession, compulsion, and addiction. Physiology & Behavior. 2002;76(3):347–352. doi: 10.1016/s0031-9384(02)00757-6. [DOI] [PubMed] [Google Scholar]
- Perez de los Cobos J, Trujols J, Ribalta E, Casas M. Cocaine use immediately prior to entry in an inpatient heroin detoxification unit as a predictor of discharges against medical advice. Am J Drug Alcohol Abuse. 1997;23(2):267–279. doi: 10.3109/00952999709040946. [DOI] [PubMed] [Google Scholar]
- Preston KL, Silverman K, Higgins ST, Brooner RK, Montoya I, Schuster CR, Cone EJ. Cocaine use early in treatment predicts outcome in a behavioral treatment program. J Consult Clin Psychol. 1998;66(4):691–696. doi: 10.1037//0022-006x.66.4.691. [DOI] [PubMed] [Google Scholar]
- Pristupa ZB, Wilson JM, Hoffman BJ, Kish SJ, Niznik HB. Pharmacological heterogeneity of the cloned and native human dopamine transporter: disassociation of [3H]WIN 35,428 and [3H]GBR 12,935 binding. Mol Pharmacol. 1994;45(1):125–135. [PubMed] [Google Scholar]
- Quick M, Winther AM, Shi L, Nissen P, Weinstein H, Javitch JA. Binding of an octylglucoside detergent molecule in the second substrate (S2) site of LeuT establishes an inhibitor-bound conformation. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(14):5563–5568. doi: 10.1073/pnas.0811322106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ritz MC, Lamb RJ, Goldberg SR, Kuhar MJ. Cocaine receptors on dopamine transporters are related to self-administration of cocaine. Science. 1987;237(4819):1219–1223. doi: 10.1126/science.2820058. [DOI] [PubMed] [Google Scholar]
- Rothman RB, Cader JL, Akunne HC, Silverthorn ML, Bauman MH, Carroll FI, Rice KC, de Costa BR, Partilla JS, Wang J-B, Uhl G, Glowa JR, Dersch CM. Studies of the biogenic amine transporters. IV. Demonstration of a multiplicity of binding sites in rat caudate membranes for the cocaine analog [125I]RTI-55. Journal of Pharmacology and Experimental Therapeutics. 1994;270:296–309. [PubMed] [Google Scholar]
- Rothman RB, Silverthorn ML, Baumann MH, Goodman CB, Cadet JL, Matecka D, Rice KC, Carroll FI, Wang JB, Uhl GR, et al. Studies of the biogenic amine transporters. VI. Characterization of a novel cocaine binding site, identified with [125I]RTI-55, in membranes prepared from whole rat brain minus caudate. J Pharmacol Exp Ther. 1995;274(1):385–395. [PubMed] [Google Scholar]
- Schmitt KC, Rothman RB, Reith ME. Nonclassical pharmacology of the dopamine transporter: atypical inhibitors, allosteric modulators, and partial substrates. J Pharmacol Exp Ther. 2013;346:2–10. doi: 10.1124/jpet.111.191056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan J, Javitch JA, Shi L, Weinstein H. The substrate-driven transition to an inward-facing conformation in the functional mechanism of the dopamine transporter. PLoS One. 2011;6(1):e16350. doi: 10.1371/journal.pone.0016350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Quick M, Zhao Y, Weinstein H, Javitch JA. The mechanism of a neurotransmitter:sodium symporter--inward release of Na+ and substrate is triggered by substrate in a second binding site. Mol Cell. 2008;30(6):667–677. doi: 10.1016/j.molcel.2008.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh SK, Yamashita A, Gouaux E. Antidepressant binding site in a bacterial homologue of neurotransmitter transporters. Nature. 2007;448(7156):952–956. doi: 10.1038/nature06038. [DOI] [PubMed] [Google Scholar]
- Smith JE, Co C, Coller MD, Hemby SE, Martin TJ. Self-administered heroin and cocaine combinations in the rat: additive reinforcing effects-supra-additive effects on nucleus accumbens extracellular dopamine. Neuropsychopharmacology. 2006;31(1):139–150. doi: 10.1038/sj.npp.1300786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sofuoglu M, Gonzalez G, Poling J, Kosten TR. Prediction of treatment outcome by baseline urine cocaine results and self-reported cocaine use for cocaine and opioid dependence. Am J Drug Alcohol Abuse. 2003;29(4):713–727. doi: 10.1081/ada-120026256. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Rice KC, Glowa JR. A comparison of cocaine, GBR 12909, and phentermine self-administration by rhesus monkeys on a progressive-ratio schedule. Drug and Alcohol Dependence. 2001;62(1):41–47. doi: 10.1016/s0376-8716(00)00158-7. [DOI] [PubMed] [Google Scholar]
- Staley JK, Basile M, Flynn DD, Mash DC. Visualizing dopamine and serotonin transporters in the human brain with the potent cocaine analogue [125I]RTI-55: in vitro binding and autoradiographic characterization. Journal of Neurochemistry. 1994a;62(2):549–556. doi: 10.1046/j.1471-4159.1994.62020549.x. [DOI] [PubMed] [Google Scholar]
- Staley JK, Hearn WL, Ruttenber AJ, Wetli CV, Mash DC. High affinity cocaine recognition sites on the dopamine transporter are elevated in fatal cocaine overdose victims. The Journal of Pharmacology and Experimental Therapeutics. 1994b;271(3):1678–1685. [PubMed] [Google Scholar]
- Tella SR, Ladenheim B, Andrews AM, Goldberg SR, Cadet JL. Differential reinforcing effects of cocaine and GBR-12909: biochemical evidence for divergent neuroadaptive changes in the mesolimbic dopaminergic system. J Neurosci. 1996;16(23):7416–7427. doi: 10.1523/JNEUROSCI.16-23-07416.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzilos GK, Rhodes GL, Ledgerwood DM, Greenwald MK. Predicting cocaine group treatment outcome in cocaine-abusing methadone patients. Exp Clin Psychopharmacol. 2009;17(5):320–325. doi: 10.1037/a0016835. 310.1037/a0016835. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Paul IA, Woolverton WL. Comparison between dopamine transporter affinity and self-administration potency of local anesthetics in rhesus monkeys. European Journal of Pharmacology. 1999;367(2–3):175–181. doi: 10.1016/s0014-2999(98)00967-4. [DOI] [PubMed] [Google Scholar]
- Wilcox KM, Rowlett JK, Paul IA, Ordway GA, Woolverton WL. On the relationship between the dopamine transporter and the reinforcing effects of local anesthetics in rhesus monkeys: practical and theoretical concerns. Psychopharmacology. 2000;153(1):139–147. doi: 10.1007/s002130000457. [DOI] [PubMed] [Google Scholar]
- Woolverton WL, Hecht GS, Agoston GE, Katz JL, Newman AH. Further studies of the reinforcing effects of benztropine analogs in rhesus monkeys. Psychopharmacology. 2001;154(4):375–382. doi: 10.1007/s002130000616. [DOI] [PubMed] [Google Scholar]
- Yamashita A, Singh SK, Kawate T, Jin Y, Gouaux E. Crystal structure of a bacterial homologue of Na+/Cl--dependent neurotransmitter transporters. Nature. 2005;437(7056):215–223. doi: 10.1038/nature03978. Epub 2005 Jul 2024. [DOI] [PubMed] [Google Scholar]
- Zhen J, Reith ME, Carr KD. Chronic food restriction and dopamine transporter function in rat striatum. Brain Research. 2006;1082(1):98–101. doi: 10.1016/j.brainres.2006.01.094. [DOI] [PubMed] [Google Scholar]


