Abstract
Thyroid-associated ophthalmopathy (TAO) remains the vexing and undertreated ocular component of Graves’ disease where orbital tissues undergo extensive remodeling. We have recently introduced the concept that CD34+ fibrocytes, bone marrow derived monocyte lineage precursor cells express the thyrotropin receptor (TSHR) and several other proteins traditionally thought to be expressed uniquely in the thyroid. TSHR-engaged fibrocytes generate extremely high levels of several inflammatory cytokines. Acting in concert with TSHR, the insulin-like growth factor 1 receptor (IGF-1R) expressed by fibrocytes appears to be necessary for TSHR-dependent cytokine production since anti-IGF-1R blocking antibodies attenuate these actions of TSH. Further, circulating fibrocytes become more abundant and appear to infiltrate orbital connective tissues in TAO where they may transition to CD34+ fibroblasts. We currently postulate that the infiltration of fibrocytes into the orbit and their unique biosynthetic repertoire and proinflammatory/profibrotic phenotype account for the characteristic properties exhibited by orbital connective tissues that render them susceptible to TAO. Further, it may be possible to utilize these very recent insights to therapeutically target pathogenic orbital fibrocytes selectively utilizing recently developed biologic agents which interfere with TSHR and IGF-1R signaling.
Introduction
Thyroid-associated ophthalmopathy (TAO) represents an autoimmune process that affects the orbit and adjacent tissues of the upper face in the syndrome known as Graves’ disease (GD)1,2. Unlike most other forms of autoimmunity, TAO is associated with a distinctive, frequently predictable, and self-limited pattern of disease activity3. Initially, it is manifested by an often-intense active phase where inflammation, tissue expansion, and orbital congestion predominate. This eventually culminates in a chronic, stable period which is typified by the absence of changing symptoms or ocular measurements. This phase may be dominated by mechanical restrictions resulting from frank fibrosis4. Uncertainty persists as to why the orbital contents are singled out for involvement in GD. Most investigators have focused on the orbital connective tissue as the primary target of immune reactivity in TAO2,5 while a minority believes that the extraocular muscles rather than orbital connective tissue are primarily involved6. Their contention is based largely on the detection of serum antibodies in individuals with TAO directed against several muscle proteins. Intrinsic differences appear to distinguish orbital fat and connective tissues from those inhabiting other regions of the body. We postulate that the unique properties of orbital fibroblasts may render the orbit susceptible to the characteristic inflammation, volume expansion, and remodeling in TAO4,7. A characteristic pattern of inflammatory gene expression can be detected in affected orbital connective tissues including several immediate early genes8,9. Many believe that expression by orbital tissue of the thyrotropin receptor (TSHR), the central pathogenic autoantigen in GD, underlies TAO. In this review, I attempt to introduce the concept that CD34+ fibrocytes are potential participants in the pathogenesis of TAO. These monocyte lineage-derived cells express several “thyroid specific” proteins, including TSHR, and have been identified in the diseased orbit10. Because of their striking immunological and biosynthetic properties, we believe that a strong case can be made for fibrocytes as prime candidates for therapeutic targeting in TAO.
Early studies associating TSHR with TAO
Cloning of TSHR by Parmentier and colleagues11 represented a pivotal breakthrough in gaining insight into normal thyroid glandular function and the pathogenesis of GD. This notable accomplishment opened the flood gates through which molecular interrogation of the genetics, protein structure/function, patterns of TSHR expression and signaling could be accomplished. Detailed characterization of TSHR structure has allowed a better understanding of the molecular rationale for its involvement in thyroid autoimmunity12. It has yielded detailed mapping of immunogenic determinants of the receptor protein and has disclosed details of how TSHR signals within the thyroid epithelial cell13–19. These insights have proven invaluable to better defining the molecular basis for hyperthyroidism in GD. In that process, activating antibodies, known as thyroid-stimulating immunoglobulins (TSI) underlie excessive production of thyroid hormones by mimicking the actions of TSH20. But unlike the well-regulated production of TSH in the anterior pituitary, TSI production and activity are not subjected to negative feedback. Thus the trophic actions of TSI can result in the hyper-metabolism characteristic of GD1. Of potential importance, TSHR signaling in thyroid epithelial cells differs when TSH or TSIs activate the receptor19.
Substantial but largely circumstantial evidence supports involvement of TSI and TSHR in TAO. Recent detection of TSHR outside the anatomic boundaries of the thyroid and insinuation of its functions in extra-thyroidal tissues has generated interest in critically examining broader physiological and pathological roles for the protein. Finding TSHR in orbital fat and extra-ocular muscles21 has prompted inquiry into whether it might be involved in TAO. Theoretically, the receptor could participate in at least two pathogenically distinct ways: first as an autoantigen shared by the thyroid and orbit and second, as a molecular conduit for conveying disease-related molecular signaling. With regard to the former function, this concept is born out of its central involvement in the hyperthyroidism associated with GD1. Several studies have demonstrated detectable TSI in the vast majority of individuals with TAO22,23. However, evidence that TSI levels or activity correlate longitudinally within individuals with regard to disease duration, severity, or activity has not been conclusive. Further, TAO appearing in individuals where TSI is undetectable, in those with euthyroid GD, or in Hashimoto’s thyroiditis complicates any model insinuating TSHR in orbital disease pathogenesis. Direct involvement of TSHR in TAO is implied by the promiscuous expression of TSHR in orbital connective tissues. Feliciello et al21 reported detecting TSHR mRNA in orbital connective tissue shortly after the molecular cloning of TSHR. That report contained evidence for orbital expression of TSHR mRNA regardless of the health status of the donor. Derivative orbital fibroblasts also express the receptor at extremely low levels in vitro compared to those found on thyroid epithelium24. This expression can be fractionally enhanced by subjecting the fibroblast cultures to conditions favoring their differentiation into adipocytes. But TSHR expression has also been identified in several other tissues and cell-types and thus appears to not be anatomically restricted to tissues ordinarily associated with the manifestations of GD25–29. Reports have appeared suggesting direct effects of TSH and TSI on cultured orbital fibroblasts,30–33. Both provoke the synthesis of cytokines and hyaluronan (HA), the principal glycosaminoglycan accumulating in TAO4,30,33,34. Whether signaling downstream from TSHR in extra-thyroidal tissues diverges when TSH or TSI activate the receptor, as appears to be the case in thyroid19, has not yet been determined. Potentially important differences in post-TSHR signaling in thyroid epithelium versus non-thyroid cells have recently been identified. TSH induces gene expression in some cells that do not express adenylate cyclase and where TSHR signaling is thus completely independent of cAMP generation30. Definition of how the receptor might play a role in TAO is currently the topic of substantial debate that centers not only on examining TSHR within the orbit but also in other extra-thyroidal compartments, such as circulating cells originating in the bone marrow. An expanded appreciation for the potential roles assumed by TSHR in extra-thyroidal tissues should better define the potential for targeting this receptor as a therapeutic strategy in TAO.
Chemoattractants and their potential role in T cells trafficking to the orbit
Another aspect of localized tissue activation in TAO relates to the underlying mechanisms through which professional immune cells such as T and B cells, monocytes, and mast cells might be trafficked to the orbit. An array of chemoattractants expressed by orbital fibroblasts, endothelial cells, and vascular smooth muscle could help explain the infiltration of CD4+ and CD8+T cells that have been identified in affected tissues35–38. These include molecules that are classified as chemokines by virtue of their harboring signature protein sequences and others that lack these motifs but function similarly. Sciaky et al39 reported that orbital fibroblasts activated by IL-1β express IL-16, a non-chemokine chemoattractant that specifically targets CD4+ T cells, and regulated on activation, normal T cell expressed and secreted (RANTES or CCL5), a member of the IL-8 super-family that targets T cells and monocytes. Moreover, these activated fibroblasts exhibit substantially enhanced T cell migration in vitro. Activation of orbital fibroblasts through the CD40/CD40 ligand bridge results in the elaboration of IL-6, IL-8, and macrophage chemoattractant protein-1 (MCP-1)40. On the other hand, interferon γ in combination with TNF-α induces both CCL2 and CXCL10 production in these cells41. Thus under the influence of several stimuli, orbital fibroblasts can express and release multiple chemokines and other chemoattractants that may underlie the orbital tissue infiltration with a variety of immune mononuclear cells in TAO. What remains uncertain is the means by which balance among multiple signals is achieved in these infiltrating target cells.
Emergence of insulin-like growth factor 1 receptor (IGF-1R) as a putative autoantigen in TAO
A long-standing and as yet unanswered question concerns whether the pathogenic TSI might initiate or in some manner promote mononuclear cell infiltration of the orbit. Another concerns the possibility that additional autoantigens besides TSHR could share relevance to GD and TAO. Among these, Weightman et al42 demonstrated that IgGs from patients with TAO could displace radiolabeled IGF-1 from the surfaces of cultured orbital fibroblasts. This finding implied that a specific IGF-1 binding site might be recognized by these antibodies. While their studies failed to identify or characterize the nature of this fibroblast site, they introduced the then novel concept that the IGF-1 pathway might be involved in TAO. We now know that multiple proteins bind IGF-1 and IGF-2 with high affinity and high specificity, including the IGF-1 receptor (IGF-1R) and the family of IGF-binding proteins43. Subsequent studies by Pritchard et al44 revealed that IgGs from patients with GD but not those from healthy individuals could induce IL-16 and RANTES in TAO orbital fibroblasts by activating the Akt/FRAP/mTor/p70s6k pathway. The responses elicited by IgGs were inhibited by rapamycin and glucocorticoids. In later studies, these activating IgGs were found to target IGF-1R45. Moreover, this receptor was found to be over-expressed by TAO fibroblasts compared to those from healthy controls. Induction of neither chemoattractant could be detected in fibroblasts from healthy donors.
Finding that antibodies harbored by individuals with GD and TAO can target IGF-1R is congruent with the initial studies42. Subsequent reports have either confirmed that anti-IGF-1R antibodies can be detected differentially in at least a subset of patients with GD46 or have failed to distinguish the presence of anti-IGF-1R antibodies in healthy individuals compared to those with the disease47. A number of technical issues may have confounded attempts to replicate the original findings of Weightman et al42 and Pritchard et al44,45. It would appear that further refinement and standardization of experimental conditions and designing assays that are best suited for detecting these antibodies will be necessary before any firm conclusions can be drawn about whether IGF-1R represents a second antigen in GD and TAO48. Of particular importance would be a means of distinguishing anti-IGF-1R antibodies that can activate the receptor from those that merely bind but fail to initiate signaling. In any event, identification of the potential involvement of IGF-1R and its signaling pathway in TAO and in mediating actions initiated by TSH/TSI on extra-thyroidal tissues has led to the conduct of the largest therapeutic trial for TAO ever conducted in North America [http://clinicaltrials.gov/show/NCT01868997]. This ongoing, multicenter placebo controlled study examines the safety and efficacy of the human monoclonal anti-IGF-1R blocking antibody, teprotumamab, in severe, active TAO.
Besides the evidence that IGF-1R and antibodies directed against it are involved in the immune pathogenesis of GD, the IGF-1 pathway appears to share an intimate relationship with the physiological regulation by TSH of thyroid function. Initial evidence for this involvement was provided by Tramontano et a49 who demonstrated either synergy or antagonism between TSH action and IGF-1 on thyroid epithelial cells in culture. They demonstrated that IGF-1 could enhance the effects of TSH on FRTL-5 cell proliferation. In contrast, IGF-1 dampens the induction by TSH of NIS, an action mediated by PI3 kinase50. IGF-1 and TSH synergistically enhance 1,2-diacylglycerol production in rat thyroid cells51. Very recently, Sastre-Perona and Santisteban52 reported that TSH and IGF-1 increase the nuclear accumulation of β-catenin and in so doing enhance thyroid epithelial cell proliferation in a Wnt-independent manner. IGF-1R appears to play an indispensable role in specific aspects of TSHR signaling. In mice where tissue-specific IGF-1R expression was knocked down in the thyroid, TSH levels were increased while thyroxine levels were decreased53. On the other hand, over-expressing IGF-1R in thyroid reduces the TSH concentration requirement for regulating the synthesis of thyroid hormones54. Tsui et al55 demonstrated that IGF-1R and TSHR form a physical complex in TAO orbital fibroblasts and thyroid epithelial cells. Interrupting IGF-1R-dependent signaling with an IGF-1R blocking antibody could attenuate the actions of TSH and TSI on post-TSHR signaling. Further, the report contained evidence for TSHR/IGF-1R co-localization as assessed by confocal microscopy and through co-precipitation pull-down studies. Dependence on IGF-1R for TSHR-initiated signaling in orbital fibroblasts has been confirmed more recently by another laboratory group34. The potential involvement of IGF-1R and activating antibodies against it in TAO has provoked lively discussions. While strong opinions have been voiced56, essentially nothing is currently known about the relative contributions of either receptor to the pathophysiological events occurring in vivo. Thus determination of the merits of either side of the debate will necessarily await further study.
Reconciling the detection of low-level thyroid antigens in the orbit
Among the first reports suggesting that proteins thought to be restricted to thyroid could also be detected in the orbit was that of Kriss57. He proposed that thyroid antigens such as thyroglobulin (Tg) might translocate from the thyroid to the orbit in TAO. He employed thyroidolymphography to examine lymphatic drainage in hyperthyroid patients with GD.99mTc Sulfur colloid injected directly into the hyperplastic thyroids of these patients exhibited rapid movement into their lymphatics. This same group successfully immunized mice with human extra-ocular muscle58. This resulted in the generation of anti-Tg antibodies. Subsequently, Marino and colleagues59 have characterized Tg in orbital contents. They detected intact Tg in tissues from individuals with TAO and could identify T4 in the material. This led them to conclude that the Tg most likely originated in the thyroid and somehow translocated to the orbit. The protein was undetectable in tissues from healthy donors. This same group found that Tg in orbital tissues formed a complex with glycosaminoglycans60. Further, they found a potential relationship between orbital Tg content and previous treatment with radioactive iodine61.
Orbital fibroblasts exhibit exaggerated responses to inflammatory mediators
Among the properties that appear to distinguish orbital fibroblasts from those inhabiting other anatomic regions are the repertoire of proteins they express62 and their particularly robust responses to inflammatory cytokines. The magnitude of these responses appears to set these cells apart from other fibroblasts. Evidence suggests inherent differences between orbital fibroblasts from donors with TAO and those from healthy individuals63. In addition, external molecular cues derived from infiltrating mononuclear cells may play important roles in fibroblast behavior in situ64,65. Among these are responses to members of the IL-1 cytokine family. In particular IL-1β frequently elicits dramatic inductions of genes such as those encoding other inflammation-related proteins, including IL-6 and IL-866,67 chemokines and chemoatractants (IL-16, RANTES)39, and enzymes involved in the synthesis of immune mediators of inflammation68,69. Notable enzymes which are expressed and differentially induced in TAO orbital fibroblasts include 15-lipoxygenase68 and prostaglandin endoperoxide H synthase-269 (PGHS-2, AKA COX-2, the inflammatory cyclooxygenase) and their production of 15-HETE and prostaglandin E2, respectively. A peculiar profile of IL-1 receptor antagonist (IL-1RA) isoforms appears to be among the factors underlying these exaggerated responses to cytokines. These patterns of expression diverge from those identified in fibroblasts from other connective tissue depots70. Of particular note is the relatively low level of secreted IL-1RA (sIL-1RA) generated by orbital fibroblasts in response to multiple stimuli. sIL-1RA acts as a dominant modulator of IL-1 actions71 . Therefore, its low levels renders orbital fibroblasts particularly susceptible to the actions of IL-1β. In contrast, intracellular IL-1RA (icIL-1RA) is highly expressed by these cells. While this isoform attenuates responses downstream from IL-1 receptor, it does not block the actions of exogenous IL-1β72. IL-1RA expression is provoked by several factors in these cells, including IL-1β and TSH70,73. A very recent report has disclosed the modulatory influence of phosphatase and tensin homologue (PTEN) on IL-1RA expression by fibroblasts as well as that of IL-1α and IL-1β in response to TSH74. Thus the array of inflammatory genes as well as their modulators expressed in orbital connective tissues may determine the magnitude and duration of immune reactivity in TAO.
Orbital fibroblasts produce hyaluronan and undergo adipogenesis in TAO
The propensity of soft tissues to expand in TAO is a consequence, at least in part, of an accumulation of HA. This is associated with increased de novo adipogenesis, resulting in increased fat volume. Orbital fibroblasts express the molecular machinery necessary for HA synthesis, including UDP-glucose dehydrogenase75,76 and the terminal hyaluronan synthases, of which three have been identified77,78. When activated by cytokines such as IL-1β, CD40 ligand, leukoregulin, prostaglandin D2, or growth factors such as IGF-1 and bTSH, fibroblasts express levels of these enzymes that result in accelerated HA production33,79–82. It is currently uncertain whether the apparent accumulation of HA in the TAO orbit results from altered rates of synthesis or delayed macromolecular degradation. Because HA chain length is an important determinant of its biological impact83, a systematic analysis of HA size and determination of whether this becomes skewed in TAO remain important voids in our current understanding. Further, the relative abundance of other glycosaminoglycans, such as chondroitin sulfate, heparan sulfate, and dermatan sulfate, has yet to be quantified.
Characterization of orbital connective tissue reveals multiple cell types: emerging evidence for orbital fibroblast heterogeneity
Identifying substantial differences in the behavior in vitro of orbital fibroblasts from those found in other tissues has prompted a thorough examination of their distinguishing phenotypic attributes, including their embryonic lineages. Determination of the derivation of orbital connective tissue can be traced to the work of Noden84 who called attention to the origins of its components, fate, and interactions with epithelial elements. Among its peculiar attributes, orbital fat comprises a heterogeneous population of fibroblast-like cells, especially in TAO. Fibroblasts derived from this fat can be divided into discrete populations based upon display of cell surface molecules85. Despite sharing similar morphologies86 and ultrastructure87 in culture, these cells exhibit distinct properties85,88–92. Fibroblasts from healthy orbital connective tissues as well as those from individuals with TAO can be bisected into two subsets based on the display of CD90 (akaThy-1), a membrane spanning glycoprotein. Thy-1+ and Thy-1− subsets exhibited divergent patterns of terminal differentiation. The former expresses smooth muscle-specific actin and develops into myofibroblasts when treated with TGF-β. In contrast, Thy-1− fibroblasts express high levels of peroxisome proliferator-activated receptor γ (PPARγ) and respond to thiazolidinediones by accumulating cytoplasmic lipid droplets and expressing lipogenic enzymes. Differences in these cell subsets were characterized recently by Li et al using a 3-dimensional model92. Responses to cytokines such as IL-1β appear to differ in the two subsets. Subsequent studies by Lehmann et al93 have demonstrated recently a molecular interplay between Thy-1+ and Thy-1− orbital fibroblasts. They found that Thy-1+ orbital fibroblasts secrete a soluble factor which seems to prevent cells of that phenotype from differentiating into adipocytes. The actions of this inhibitory factor may be mediated by altering the binding of PPAR-γ to its DNA binding site. Thus it is possible that the balance between Thy-1+ and Thy-1− fibroblasts might determine whether the orbital tissues undergo adipogenic differentiation or fibrosis. Further investigation into the relationship between these orbital fibroblast subsets, including better definition of their phenotypic attributes and biosynthetic repertoires, should yield fundamental insights into the biology of tissue changes associated with TAO.
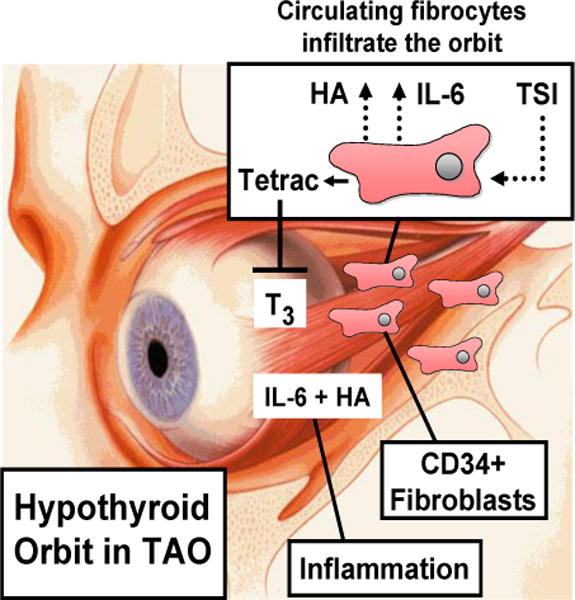
Identifying infiltrating CD34+ fibrocyte in the orbit allows reconciliation of several unexplained tissue characteristics associated with TAO
Fibrocytes are monocyte linage progenitors from the bone marrow that have been implicated in many aspects of wound repair, tissue remodeling, and immune function94,95. They have been associated with several diseases and repair processes including thermal burns96, glomerular disease97, hepatic fibrosis98, asthma99, and rheumatoid arthritis100. They are identified by a specific pattern of protein expression and display of cell surface markers101. These include CD45, CXCR4, CD34, and the production of collagen I. Fibrocytes can terminally differentiate into either myofibroblasts or adipocytes depending on the molecular environment to which they are subjected102. Specifically, when exposed to PPARγ, they accumulate triglycerides and differentiate into mature adipocytes. In contrast, when activated through the Smad pathway following treatment in vitro with TGF-β, they differentiate into myofibroblasts and express high levels of α-smooth muscle specific actin. Fibrocytes play roles in immune function, including their ability to present antigens through MHC II to T cells103. These cells are extremely rare in the circulation of healthy individuals, where they account for less than one percent of mononuclear cells. Their abundance increases dramatically in certain pathological states, such as those associated with extensive acute or chronic tissue injury104,105. This appears to be the case in thermal injury where the cells not only circulate in greater abundance but infiltrate affected tissue106. This homing occurs through chemokine activities. Animal-based studies have implicated the CXCR4/CXCL12 chemokine network as an important localization determinant involved in the pathogenesis of lung fibrosis107.
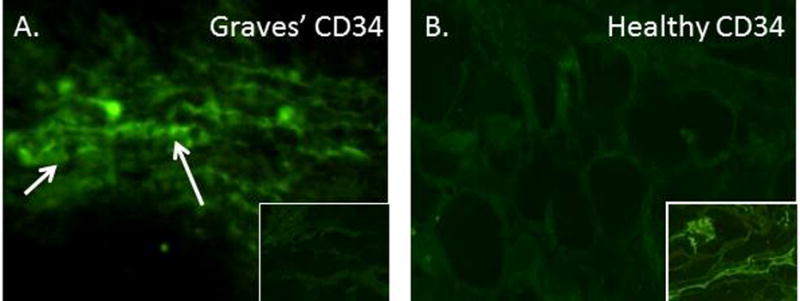
Douglas et al10 have reported that the frequency of circulating CD34+ fibrocytes is substantially increased in GD (Fig. 1). These cells were detected in situ in TAO orbital fat but appear to be absent in tissues from healthy individuals (Fig. 2). The authors identified an abundant subset of TAO orbital fibroblasts displaying a CD34+CD31−CXCR4+ phenotype, suggesting that they represent fibrocytes that had infiltrated orbital tissues and transitioned to CD34+ fibroblasts. The cells no longer express CD45 but produce collagen I. They reside in a cellular context which includes CD34− fibroblasts in the TAO orbit. CD34− fibroblasts are believed to be the identical fibroblasts that are uniformly identified in healthy tissues but that have been partially replaced in TAO. Since both fat expansion and fibrosis represent important components of tissue remodeling associated with TAO108, fibrocytes are now considered prime candidates for therapeutic targeting.
Figure 1.
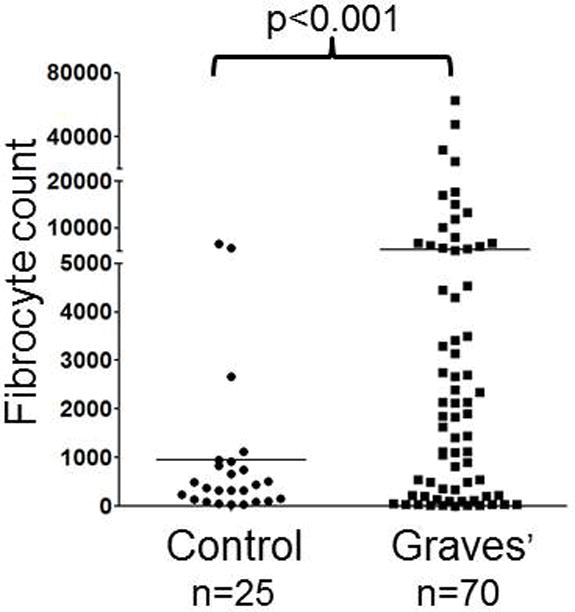
Increased frequency of CD34+ fibrocyte generation from the PBMCs of 70 patients with GD compared with 25 healthy control donors. PBMCs were inoculated at a density of 5 × 106 cells/well. Cultures were incubated for 14d. Adherent cells (<5% of starting cells) were collected and counted. (Reprinted with permission; Douglas et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy, Reference 6, Copyright 2010. The Endocrine Society.)
Figure 2.
CD34+ LSP-1+ TSHR+ fibrocytes can be identified in the orbital tissue of patients with TAO but are absent in tissues from healthy donors. A, CD34 expression (arrows, green fluorescein isothiocyanate) in TAO- derived tissue (inset, negative control staining). B, Absent, CD34 expression in healthy tissue (inset, positive control). C, LSP-1 expression in TAO-derived tissue [red, arrows, nuclei counter-stained with 4′,6′ –diamino-2-phenylindole (DAPI)(blue)(inset, negative, control). D, Absence of LSP-1 expression in healthy tissue (inset, negative controls). E, CD31 expression in disease-derived tissue is limited to vascular endothelium (red, arrows). F, Hematoxylin and eosin-stained consecutive thin sections of the same orbital tissue (×40). G, Fibrocytes present in orbital tissue from patients with TAO co-express CD34 and TSHR. Thin-sectioned tissue from a donor with TAO was stained with anti-CD34 (green) and anti-TSHR (red) antibodies. Nuclei were counterstained with DAPI (blue). Thin sections were then subjected to confocal microscopy. Inset contains a negative staining control. (Reprinted with permission; Douglas et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy, Reference 6, Copyright 2010. The Endocrine Society.)
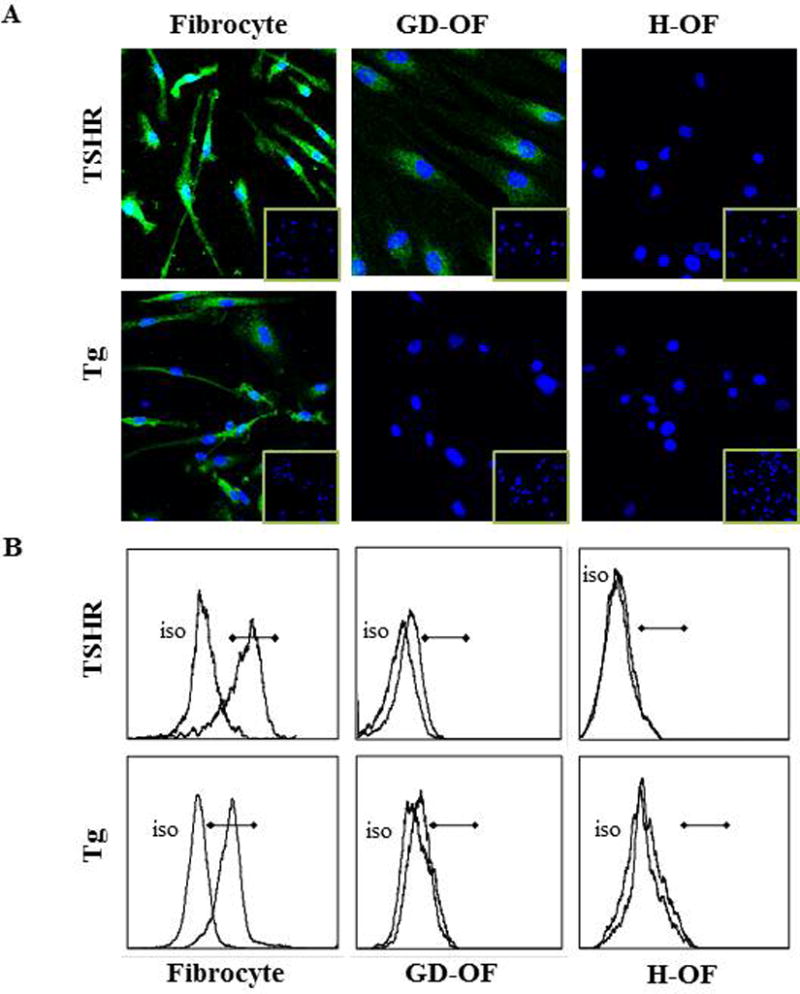
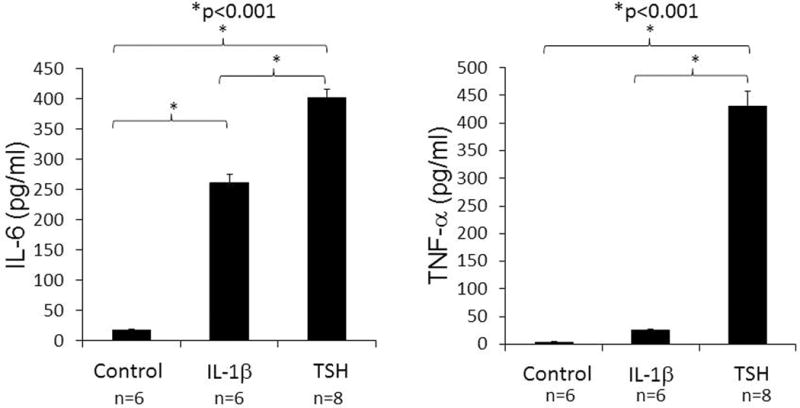
Among the attributes recently ascribed to fibrocytes is a high level of TSHR mRNA expression that is unprecedented in the extra-thyroidal compartment10. Steady-state abundance of the transcript is considerably lower than that found in thyroid tissue but levels of the protein appear to be equivalent on fibrocytes and thyroid epithelial cells and substantially higher than those on orbital fibroblasts (Fig. 3)55. Its presence on fibrocytes suggests that the receptor might mediate actions of TSH and TSI locally in the connective tissue compartment7. Several reports have appeared documenting responses of fibrocytes to TSH and M22, a monoclonal TSI. These have demonstrated increases in cytokine production, including IL-1β, IL-1RA, TNF-α, IL-6, IL-8, and MCP8,30,73,109 (Fig. 4). These responses appear to be qualitatively similar in cells from patients with TAO and healthy individuals but the magnitude of response appears greater in the former106. In contrast to fibrocytes, TAO orbital fibroblasts exhibit substantially less robust responses to TSH. This divergence in the magnitude of response might be attributed to higher TSHR levels in fibrocytes and qualitative differences in the relevant signaling pathways.
Figure 3.
Analysis of TSHR and Tg expression by human fibrocytes and fibroblasts by (A) immunofluorescence staining and (B) flow cytometry. Cultivated cells were (A) fixed, blocked, and stained with either anti-TSHR, anti-Tg, or isotype control mouse IgG followed by Alexa Fluor 488-conjugated goat anti-mouse Abs. Nuclei were stained with DAPI, and thin sections were subjected to confocal fluorescence microscopy. (Insets) Isotype IgG staining. (B) Fibrocytes and fibroblasts were incubated with phycoerythrin-conjugated anti-TSHR, anti-TG, or isotype mAbs and Alexa Fluor 488 goat anti-mouse IgG. They were subjected to flow cytometry. Horizontal lines denote fluorescence intensity compared with isotype controls. (Reprinted from; Fernando et al. Human fibrocytes co-express thyroglobulin and thyrotropin receptor, Proceedings of the National Academy of Sciences, Reference 103, Copyright 2012.)
Figure 4.
TSHR displayed on fibrocytes generated from PBMCs can initiate cytokine production. Cultures cells from a patient with GD were treated with bTSH (5 mU/ml) or IL-1β (10 ng/ml) for 48h. Medium was subjected to ELISAs specific for IL-6 (left panel) or TNF- α (right panel). Data are expressed as the mean ± SEM of three replicate culture wells. *, P < 0.001. (Reprinted with permission; Douglas et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy, Reference 6, Copyright 2010. The Endocrine Society.)
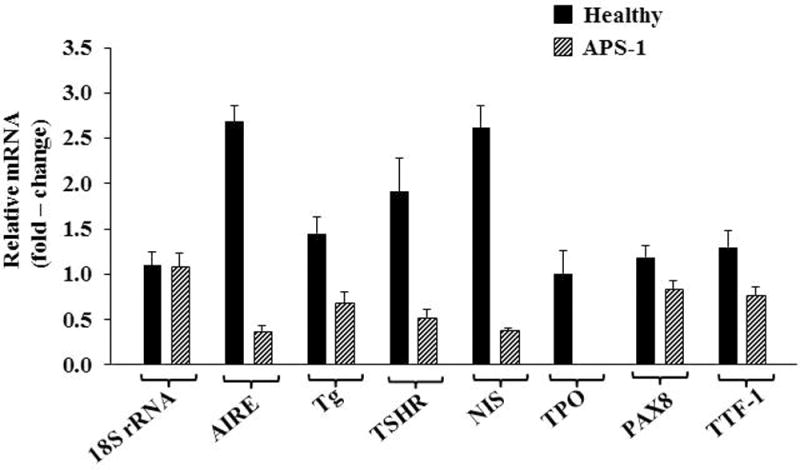
Besides TSHR, other “thyroid specific” proteins, such as Tg110, sodium iodide symporter (NIS), and thyroid peroxidase (TPO) are also expressed by cultured fibrocytes111. Levels of Tg as well as these other proteins are substantially lower than those found in thyroid but are still readily detectable using standard techniques. Fibrocyte-expressed Tg can incorporate iodine, a process that appears to be TSH-independent. As with TSHR, Tg, NIS, and TPO mRNAs are considerably less abundant in TAO orbital fibroblasts than in cultured fibrocytes and the respective proteins are expressed at the limits of detection. It is thus possible that the expression of TSHR and Tg by infiltrating fibrocytes could account for the low levels of both proteins detected in TAO orbital tissues21.57–61. In addition to those proteins associated with thyroid function and disease, fibrocytes express autoantigens associated with type 1 diabetes mellitus112. Of potential importance is the identification of autoimmune regulator protein (AIRE), a non-traditional transcription factor113, as an essential participant in the expression by fibrocytes of these 4 proteins111. Knocking down AIRE expression substantially reduces the levels of all 4 thyroid proteins (Fig. 5). Further, interrogation of TSHR, Tg, NIS, and TPO expression in a patient with loss of function AIRE mutation reveals a similar reduction (Fig. 6).
Figure 5.
Interfering with AIRE expression knocked-down levels of AIRE, Tg, TSHR, NIS, TPO, PAX8, and TTF-1 but not PGHS-2 or 18S RNA. A, Fibrocytes from four different donors were transfected, either with scrambled (control) siRNA or siRNA directed against AIRE. Cultures were then incubated for 48 hours, and RNA was harvested and then subjected to real-time PCR. Data are expressed as mean ± SD of three independent determinants. B, Fibrocyte cultures were transfected with either siRNA or siRNA targeting AIRE for 96 hours. Tg protein was quantified by labeling with [35S]methionine (40μCi/mL) and then immunoprecipitated. TSHR and NIS were quantified by flow cytometry. Levels of TPO were assessed by Western blotting. (Reprinted with permission; Fernando et al. Expression of thyrotropin receptor, thyroglobulin, sodium iodide symporter, and thyroperoxidase by fibrocytes depends on AIRE, Reference 104, Copyright 2014. The Endocrine Society.)
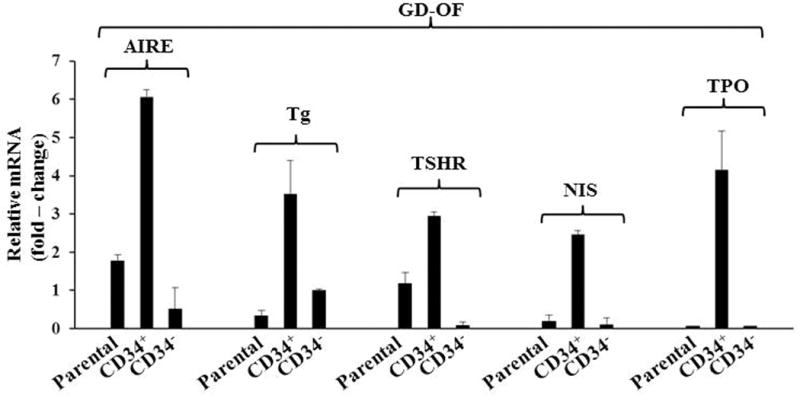
Recent studies have disclosed interactions between native (CD34−) orbital fibroblasts and CD34+ orbital fibroblasts which are putatively derived from CD34+ fibrocytes. It appears that signals from CD34− fibroblasts may repress the expression of thyroid proteins, thereby accounting for their lower levels as fibrocytes transition to CD34+ orbital fibroblasts110,111. Supporting this possibility are studies revealing that parental orbital fibroblast strains, comprising a mixture of CD34+ and CD34− cells and subjected to cytometric cell sorting into pure subsets on the basis of CD34 display yield dramatically higher thyroid protein expression in the CD34+ subset (Fig. 7). These findings carry substantial implications for the potential mechanisms through which aspects of CD34+ fibroblast phenotype might be modulated by CD34− fibroblasts. The expression repertoire of antigens by fibrocytes infiltrating the orbit in TAO therefore might be governed by the capacity of residential CD34− fibroblasts to modulate their magnitude and diversity. In so doing, they might help determine the pathogenic immune responses. Could insinuation of fibrocytes into TAO reveal attractive therapeutic targets?
In our view, identifying relatively high-level expression of functional TSHR in fibrocytes and the apparent infiltration of these cells into the TAO orbit might be exploited therapeutically. Strategies for interrupting TSHR signaling are being developed and the properties of these agents appear promising as potential drugs. Highly selective small molecule antagonists of TSHR have been reported by Neumann et al114 and among the latest is an agent designated ANTAG3 which demonstrates activity in vitro as well as in mice. Alternatively, stimulatory and blocking antibodies targeting TSHR have been developed115,116 and their divergent interactions with the receptor have been scrutinized in great detail117. Either approach might prove effective in reducing the impact of the inflammatory phenotype exhibited by TSH-engaged fibrocytes. Alternatively, targeting IGF-1R signaling with a blocking antibody such as teprotumamab has been shown to substantially reduce TSHR-mediated cytokine expression. That blocking antibody substantially reduced the induction by TSH of IL-6 in fibrocytes118, strongly suggesting that the IGF-1R pathway in these cells might be interrupted as another therapeutic approach. As yet untested but worthy of investigation are strategies that interfere with fibrocyte infiltration, such as those interrupting relevant chemokine pathways. It would appear that emerging insights into the biology of the orbit will yield more effective treatments of TAO.
Conclusion
Bone derived fibrocytes have recently been identified as becoming more numerous in GD and TAO. They appear to infiltrate the orbit in TAO where they putatively transition into a subset of orbital fibroblasts. Fibrocytes express relatively high levels of functional TSHR through which TSH and TSIs provoke expression of an array of inflammatory genes including those encoding cytokines. It would appear that TSHR signaling in fibrocytes and orbital fibroblasts is dependent, at least partially on functional IGF-1R. Taken together, these recent findings insinuate fibrocytes in the pathogenesis of TAO. When considering this evidence, it is imperative that the reader consider that virtually all of these findings come from a single research laboratory. This work must therefore be independently corroborated by others and expanded to in vivo models before any firm conclusions can be drawn.
Acknowledgments
The author gratefully acknowledges the great editorial assistance of Ms. Linda Polonsky and the help of Ms. Justyna Piernicka in the preparation of this manuscript. This work was supported in part by National Institutes of Health grant EY08976, Center for Vision grant EY007003 from the National Eye Institute, and unrestricted grants from Research to Prevent Blindness, and the Bell Charitable Foundation.
Footnotes
The author affirms no competing interests.
References
- 1.Brent GA. Graves’ Disease. N Engl J Med. 2008;358:2594–2605. doi: 10.1056/NEJMcp0801880. [DOI] [PubMed] [Google Scholar]
- 2.Bahn RS. Mechanisms of Disease Graves’ Ophthalmopathy. N Engl J Med. 2010;362:726–738. doi: 10.1056/NEJMra0905750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rundle FF, Wilson CW. Development and course of exophthalmos and ophthalmoplegia in Graves’ disease with special reference to the effect of thyroidectomy. Clin Sci. 1945;5:177–194. [PubMed] [Google Scholar]
- 4.Smith TJ, Bahn RS, Gorman CA. Connective tissue, glycosaminoglycans, and diseases of the thyroid. Endocr Rev. 1989;10:366–391. doi: 10.1210/edrv-10-3-366. [DOI] [PubMed] [Google Scholar]
- 5.Smith TJ, Tsai CC, Shih JJ, Tsui S, et al. Unique attributes of orbital fibroblasts and global alterations in IGF-1 receptor signaling could explain thyroid-associated ophthalmopathy. Thryoid. 2008;18:983–988. doi: 10.1089/thy.2007.0404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tani J, Gopinath B, Nguyen B, Wall JR. Extraocular muscle autoimmunity and orbital flat inflammation in thyroid-associated ophthalmopathy. Expert Rev Clin Immunol. 2007;3:299–311. doi: 10.1586/1744666X.3.3.299. [DOI] [PubMed] [Google Scholar]
- 7.Wang Y, Smith TJ. Current concepts in the molecular pathogenesis of thyroid-associated ophthalmopathy. Invest Ophthalmol. 2014;55:1735–1748. doi: 10.1167/iovs.14-14002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lantz M, Vondrichova T, Parikh H, Frenander C, et al. Overexpression of immediate early genes in active Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2005;90:4784–4791. doi: 10.1210/jc.2004-2275. [DOI] [PubMed] [Google Scholar]
- 9.Vondrichova T, de Capretz A, Parikh H, Frenander C, et al. COX-2 and SCD, markers of inflammation and adipogenesis, are related to disease activity in Graves’ ophthalmopathy. Thyroid. 2007;17:511–517. doi: 10.1089/thy.2007.0028. [DOI] [PubMed] [Google Scholar]
- 10.Douglas RS, Afifiyan NF, Hwang CJ, Chong K, et al. Increased generation of fibrocytes in thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2010;95:430–438. doi: 10.1210/jc.2009-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parmentier M, Libert F, Maenhaut C, Lefort A, et al. Molecular cloning of the thyrotropin receptor. Science. 1989;246:1620–1622. doi: 10.1126/science.2556796. [DOI] [PubMed] [Google Scholar]
- 12.Davies TF, Ando T, Lin RY, Latif R. Thyrotropin receptor-associated diseases: from adenomata to Graves disease. J Clin Invest. 2005;115:1972–1983. doi: 10.1172/JCI26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Allgeier A, Offermanns S, Van Sande J, Spicher K, et al. The human thyrotropin receptor activates G-proteins Gs and Gq/11. J Biol Chem. 1994;269:13733–13735. [PubMed] [Google Scholar]
- 14.Fuse M, Tanaka T, Shibata T, Yoshida T, et al. Regulation of geranylgeranyl pyrophosphate synthase in the proliferation of rat FRTL-5 cells: involvement of both cAMP-PKA and PI3-AKT pathways. Biochem Biophys Res Commun. 2004;315:1147–1153. doi: 10.1016/j.bbrc.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Hara T, Namba H, Takamura N, Yang TT, et al. Thyrotropin regulates c-Jun N-terminal kinase (JNK) activity through two distinct signal pathways in human thyroid cells. Endocrinology. 1999;140:1724–1730. doi: 10.1210/endo.140.4.6619. [DOI] [PubMed] [Google Scholar]
- 16.Zaballos MA, Garcia B, Santisteban P. Gβγ dimers released in response to thyrotropin activate phosphoinositide 3-kinase and regulate gene expression in thyroid cells. Mol Endocrinol. 2008;22:1183–1199. doi: 10.1210/me.2007-0093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saunier B, Tournier C, Jacquemin C, Pierre M. Stimulation of mitogen-activated protein kinase by thyrotropin in primary cultured human thyroid follicles. J Biol Chem. 1995;270:3693–3697. doi: 10.1074/jbc.270.8.3693. [DOI] [PubMed] [Google Scholar]
- 18.Suh JM, Song JH, Kim DW, Chung HK, et al. Regulation of the phosphatidylinositol 3-kinase, Akt/protein kinase B, FRAP/mammalian target of rapamycin, and ribosomal S6 kinase 1 signaling pathways by thyroid-stimulating hormone (TSH) and stimulating type TSH receptor antibodies in the thyroid gland. J Biol Chem. 2003;278:21960–21971. doi: 10.1074/jbc.M300805200. [DOI] [PubMed] [Google Scholar]
- 19.Morshed SA, Latif R, Davies TF. Characterization of thyrotropin receptor antibody-induced signaling cascades. Endocrinology. 2009;150:519–529. doi: 10.1210/en.2008-0878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smith BR, Sanders J, Furmaniak J. TSH receptor antibodies. Thyroid. 2007;17:923–938. doi: 10.1089/thy.2007.0239. [DOI] [PubMed] [Google Scholar]
- 21.Feliciello A, Porcellini A, Ciullo I, Bonavolontà G, et al. Expression of thyrotropin-receptor mRNA in healthy and Graves’ disease retro-orbital tissue. Lancet. 1993;342:337–338. doi: 10.1016/0140-6736(93)91475-2. [DOI] [PubMed] [Google Scholar]
- 22.Gerding MN, van der Meer JW, Broenink M, Bakker O, et al. Association of thyrotrophin receptor antibodies with the clinical features of Graves’ ophthalmopathy. Clin Endocrinol(Oxf) 2000;52:267–271. doi: 10.1046/j.1365-2265.2000.00959.x. [DOI] [PubMed] [Google Scholar]
- 23.Lytton SD, Ponto KA, Kanitz M, Matheis N, et al. A novel thyroid stimulating immunoglobulin bioassay is a functional indicator of activity and severity of Graves’ orbitopathy. J Clin Endocrinol Metab. 2010;95:2123–2131. doi: 10.1210/jc.2009-2470. [DOI] [PubMed] [Google Scholar]
- 24.Heufelder AE, Bahn RS. Evidence for the presence of a functional TSH-Receptor in a retroocular fibroblasts from patients with Graves’ ophthalmopathy. Exp Clin Endocrinol. 1992;100:62–67. doi: 10.1055/s-0029-1211178. [DOI] [PubMed] [Google Scholar]
- 25.Slominski A, Wortsman J, Kohn L, Ain KB, et al. Expression of hypothalamic-pituitary-thyroid axis related genes in the human skin. J Invest Dermatol. 2002;119:1149–1455. doi: 10.1046/j.1523-1747.2002.19617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimura H, Miyazaki A, Haraguchi K, Endo T, et al. Analysis of differentiation-induced expression mechanisms of thyrotropin receptor gene in adipocytes. Mol Endocrinol. 1998;12:1473–1486. doi: 10.1210/mend.12.10.0175. [DOI] [PubMed] [Google Scholar]
- 27.Bell A, Gagnon A, Grunder L, Parikh SJ, et al. Functional TSH receptor in human abdominal preadipocytes and orbital fibroblasts. Am J Physiol Cell Physiol. 2000;279:C335–C340. doi: 10.1152/ajpcell.2000.279.2.C335. [DOI] [PubMed] [Google Scholar]
- 28.Cianfarani F, Baldini E, Cavalli A, Marchioni E, et al. TSH receptor and thyroid-specific gene expression in human skin. J Invest Dermatol. 2010;130:93–101. doi: 10.1038/jid.2009.180. [DOI] [PubMed] [Google Scholar]
- 29.Endo T, Ohta K, Haraguchi K, Onaya T. Cloning and functional expression of a thyrotropin receptor cDNA from rat fat cells. J Biol Chem. 1995;270:10833–10837. doi: 10.1074/jbc.270.18.10833. [DOI] [PubMed] [Google Scholar]
- 30.Raychaudhuri N, Fernando R, Smith TJ. Thyrotropin regulates IL-6 expression in CD34+ fibrocytes: Clear delineation of its cAMP-independent actions. PLoS One. 2013;8:E75100. doi: 10.1371/journal.pone.0075100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rotella CM, Zonefrati R, Toccafondi R, Valente WA, et al. Ability of monoclonal antibodies to the thyrotropin receptor to increase collagen synthesis in human fibroblasts: an assay which appears to measure exophthalmogenic immunoglobulins in Graves’ sera. J Clin Endocrinol Metab. 1986;62:357–367. doi: 10.1210/jcem-62-2-357. [DOI] [PubMed] [Google Scholar]
- 32.Kumar S, Schiefer R, Coenen MJ, Bahn RS. A stimulatory thyrotropin receptor antibody (M22) and thyrotropin increase interleukin-6 expression and secretion in Graves’ orbital preadipocyte fibroblasts. Thyroid. 2010;20:59–65. doi: 10.1089/thy.2009.0278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhang L, Bowen T, Grennan-Jones F, Paddon C, et al. Thyrotropin receptor activation increases hyaluronan production in preadipocyte fibroblasts: contributory role in hyaluronan accumulation in thyroid dysfunction. J Biol Chem. 2009;284:26447–26455. doi: 10.1074/jbc.M109.003616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kumar S, Iyer S, Bauer H, Coenen M, et al. A stimulatory thyrotropin receptor antibody enhances hyaluronic acid synthesis in graves’ orbital fibroblasts: inhibition by an IGF-I receptor blocking antibody. J Clin Endocrinol Metab. 2012;97:1681–1687. doi: 10.1210/jc.2011-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pappa A, Calder V, Ajjan R, Fels P, et al. Analysis of extraocular muscle-infiltrating T cells in thyroid-associated ophthalmopathy (TAO) Clin Exp Immunol. 1997;109:362–369. doi: 10.1046/j.1365-2249.1997.4491347.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Grubeck-Loebenstein B, Trieb K, Sztankay A, Holter W, et al. Retrobulbar T cells from patients with Graves’ ophthalmopathy are CD8+ and specifically recognize autologous fibroblasts. J Clin Invest. 1994;93:2738–2743. doi: 10.1172/JCI117289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ecksteink AK, Quadbeck B, Tews S, Mann K, et al. Thyroid associated ophthalmopathy: evidence for CD4(+) gammadelta T cells; de novo differentiation of RFD7(+) macrophages, but not of RFD1(+) dendritic cells; and loss of gammadelta and alphabeta T cell receptor expression. Br J Ophthalmol. 2004;88:803–808. doi: 10.1136/bjo.2003.035915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.de Carli M, D’Elios MM, Mariotti S, Marcocci C, et al. Cytolytic T cells with Th1-like cytokine profile predominate in retroorbital lymphocytic infiltrates of Graves’ ophthalmopathy. J Clin Endocrinol Metab. 1993;77:1120–1124. doi: 10.1210/jcem.77.5.8077301. [DOI] [PubMed] [Google Scholar]
- 39.Sciaky D, Brazer W, Center DM, Cruikshank WW, et al. Cultured human fibroblasts express constitutive IL-16 mRNA: Cytokine induction of active IL-16 protein synthesis through a caspase-3-dependent mechanism. J Immunol. 2000;164:3806–3814. doi: 10.4049/jimmunol.164.7.3806. [DOI] [PubMed] [Google Scholar]
- 40.Hwang CJ, Afifiyan N, Sand D, Naik V, et al. Orbital fibroblasts from patients with thyroid-associated ophthalmopathy overexpress CD40: CD154 hyperinduces IL-6, IL-8, and MCP-1. Invest Ophthalmol Vis Sci. 2009;50:2262–2268. doi: 10.1167/iovs.08-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Antonelli A, Ferrari SM, Frascerra S, Ruffilli I, et al. β (CCL2) and α (CXCL10) chemokine modulations by cytokines and peroxisome proliferator-activated receptor-α agonists in Graves’ ophthalmopathy. J Endocrinol. 2012;213:183–191. doi: 10.1530/JOE-11-0488. [DOI] [PubMed] [Google Scholar]
- 42.Weightman DR, Perros P, Sherif IH, Kendall-Taylor P. Autoantibodies to IGF-1 binding sites in thyroid associated ophthalmopathy. Autoimmunity. 1993;16:251–257. doi: 10.3109/08916939309014643. [DOI] [PubMed] [Google Scholar]
- 43.Smith TJ. Insulin-like growth factor-I regulation of immune function: a potential therapeutic target in autoimmune diseases? Pharmacol Rev. 2010;62:199–236. doi: 10.1124/pr.109.002469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pritchard J, Horst N, Cruikshank W, Smith TJ. Igs from patients with Graves’ disease induce the expression of T cell chemoattractants in their fibroblasts. J Immunol. 2002;168:942–950. doi: 10.4049/jimmunol.168.2.942. [DOI] [PubMed] [Google Scholar]
- 45.Pritchard J, Han R, Horst N, Cruikshank WW, et al. Immunoglobulin activation of T cell chemoattractant expression in fibroblasts from patients with Graves’ disease is mediated through the IGF-1 receptor pathway. J Immunol. 2003;170:6348–6354. doi: 10.4049/jimmunol.170.12.6348. [DOI] [PubMed] [Google Scholar]
- 46.Varewijck AJ, Boelen A, Lamberts SW, Fliers E, et al. Circulating IgGs may modulate IGF-I receptor stimulating activity in a subset of patients with Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2013;98:769–776. doi: 10.1210/jc.2012-2270. [DOI] [PubMed] [Google Scholar]
- 47.Minich WB, Dehina N, Welsink T, Schwiebert C, et al. Autoantibodies to the IGF1 receptor in Graves’ orbitopathy. J Clin Endocrinol Metab. 2013;98:752–760. doi: 10.1210/jc.2012-1771. [DOI] [PubMed] [Google Scholar]
- 48.Smith TJ. Is IGF-I receptor a target for autoantibody generation in Graves’ disease? J Clin Endocrinol Metab. 2013;98:515–518. doi: 10.1210/jc.2013-1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tramontano D, Cushing GW, Moses AC, Ingbar SH. Insulin-like growth factor-I stimulates the growth of rat thyroid cells in culture and synergizes the stimulation of DNA synthesis induced by TSH and Graves’-IgG. Endocrinology. 1986;119:940–942. doi: 10.1210/endo-119-2-940. [DOI] [PubMed] [Google Scholar]
- 50.Garcia B, Santisteban P. PI3K is involved in the IGF-I inhibition of TSH-induced sodium/iodide symporter gene expression. Mol Endocrinol. 2002;16:342–352. doi: 10.1210/mend.16.2.0774. [DOI] [PubMed] [Google Scholar]
- 51.Brenner-Gati L, Berg KA, Gershengorn MC. Insulin-like growth factor-I potentiates thyrotropin stimulation of adenylyl cyclase in FRTL-5 cells. Endocrinology. 1989;125:1315–1320. doi: 10.1210/endo-125-3-1315. [DOI] [PubMed] [Google Scholar]
- 52.Sastre-Perona A, Santisteban P. Wnt-independent role of β-catenin in thyroid cell proliferation and differentiation. Mol Endocrinol. 2014;28:681–695. doi: 10.1210/me.2013-1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ock S, Ahn J, LeeSastre-Perona A, Santisteban P. Wnt-independent role of β-catenin in thyroid cell proliferation and differentiation, S.H. Kang, H. et al. IGF-1 receptor deficiency in thyrocytes impairs thyroid hormone secretion and completely inhibits TSH-stimulated goiter. FASEB J. 2013;27:4899–4908. doi: 10.1096/fj.13-231381. [DOI] [PubMed] [Google Scholar]
- 54.Clement S, Refetoff S, Robaye B, Dumont JE, et al. Low TSH requirement and goiter in transgenic mice overexpressing IGF-I and IGF-Ir receptor in the thyroid gland. Endocrinology. 2001;142:5131–5139. doi: 10.1210/endo.142.12.8534. [DOI] [PubMed] [Google Scholar]
- 55.Tsui S, Naik V, Hoa N, Hwang CJ, et al. Evidence for an association between thyroid stimulating hormone and insulin-like growth factor 1 receptors: A tale of two antigens implicated in Graves’ disease. J Immunol. 2008;181:4397–4405. doi: 10.4049/jimmunol.181.6.4397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wiersinga WM. Autoimmunity in Graves; ophthalmopathy: the result of an unfortunate marriage between TSH receptors and IGF-1 receptors? J Clin Endocrinol Metab. 2011;96:2386–2394. doi: 10.1210/jc.2011-0307. [DOI] [PubMed] [Google Scholar]
- 57.Kriss JP. Radioisotopic thyroidolymphography in patients with Graves’ disease. J Clin Endocrinol Metab. 1970;31:315–323. doi: 10.1210/jcem-31-3-315. [DOI] [PubMed] [Google Scholar]
- 58.Tao TW, Cheng PJ, Pham H, Leu SL, et al. Monoclonal antithyroglobulin antibodies derived from immunizations of mice with human eye muscle and thyroid membranes. J Clin Endocrinol Metab. 1986;63:577–582. doi: 10.1210/jcem-63-3-577. [DOI] [PubMed] [Google Scholar]
- 59.Marinò M, Lisi S, Pinchera A, Mazzi B, et al. Identification of thyroglobulin in orbital tissues of patients with thyroid-associated ophthalmopathy. Thyroid. 2001;11:177–185. doi: 10.1089/105072501300042929. [DOI] [PubMed] [Google Scholar]
- 60.Marinò M, Lisi S, Pinchera A, Marcocci C, et al. Glycosaminoglycans provide a binding site for thyroglobulin in orbital tissues of patients with thyroid-associated ophthalmopathy. Thyroid. 2003;13:851–859. doi: 10.1089/105072503322401041. [DOI] [PubMed] [Google Scholar]
- 61.Lisi S, Marinò M, Pinchera A, Mazzi B, et al. Thyroglobulin in orbital tissues from patients with thyroid-associated ophthalmopathy: predominant localization in fibroadipose tissue. Thyroid. 2002;12:351–360. doi: 10.1089/105072502760043413. [DOI] [PubMed] [Google Scholar]
- 62.Young DA, Evans CH, Smith TJ. Leukoregulin induction of protein expression in human orbital fibroblasts: evidence for anatomical site-restricted cytokine-target cell interactions. Proc Natl Acad Sci USA. 1998;95:8904–8909. doi: 10.1073/pnas.95.15.8904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Meyer zu Hörste M, Ströher E, Berchner-Pfannschmidt U, Schmitz-Spanke S, et al. A novel mechanism involved in the pathogenesis of Graves ophthalmopathy (GO): clathrin is a possible targeting molecule for inhibiting local immune response in the orbit. J Clin Endocrinol Metab. 2011;96:E1727–E1736. doi: 10.1210/jc.2011-1156. [DOI] [PubMed] [Google Scholar]
- 64.van Steensel L, Paridaens D, van Meurs M, van Hagen PM, et al. Orbit-infiltrating mast cells, monocytes, and macrophages produce PDGF isoforms that orchestrate orbital fibroblast activation in Graves’ ophthalmopathy. J Clin Endocrinol Metab. 2012;97:E400–E408. doi: 10.1210/jc.2011-2697. [DOI] [PubMed] [Google Scholar]
- 65.van Steensel L, Paridaens D, Dingian GM, van Daele PL, et al. Platelet-derived growth factor-BB: a stimulus for cytokine production by orbital fibroblasts in Graves’ ophthalmopathy. Invest Ophthalmol Vis Sci. 2010;51:1002–1007. doi: 10.1167/iovs.09-4338. [DOI] [PubMed] [Google Scholar]
- 66.Hwang CJ, Afifiyan N, Sand D, Naik V, et al. Orbital fibroblasts from patients with thyroid-associated ophthalmopathy overexpress CD4: CD154 hyperinduces IL-6, IL-8, and MCP-1. Invest Ophthalmol Vis Sci. 2009;50:2262–2268. doi: 10.1167/iovs.08-2328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Raychaudhuri N, Douglas RS, Smith TJ. PGE2 induces IL-6 in orbital fibroblasts through EP2 receptors and increased gene promoter activity: implications to thyroid-associated ophthalmopathy. PLoS One. 2010;5:E15296. doi: 10.1371/journal.pone.0015296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Chen B, Tsui S, Boeglin WE, Douglas RS, et al. IL-4 induces 15‐lipoxygenase-1 expression in human orbital fibroblasts from patients with Graves’ disease: Evidence for anatomic site-selective action of TH2 cytokines. J Biol Chem. 2006;281:18296–18306. doi: 10.1074/jbc.M603484200. [DOI] [PubMed] [Google Scholar]
- 69.Han R, Tsui S, Smith TJ. Up-regulation of prostaglandin E2 synthesis by interleukin-1β in human orbital fibroblasts involves coordinate induction of prostaglandin-endoperoxide H synthase-2 and glutathione-dependent PGE2 synthase expression. J Biol Chem. 2002;277:16355–16364. doi: 10.1074/jbc.M111246200. [DOI] [PubMed] [Google Scholar]
- 70.Li B, Smith TJ. Divergent expression of IL-1 receptor antagonists in CD34+ fibrocytes and orbital fibroblasts in thyroid-associated ophthalmopathy: Contribution of fibrocytes to orbital inflammation. J Clin Endocrinol Metab. 2013;98:2783–2790. doi: 10.1210/jc.2013-1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gabay C, Lamacchia C, Palmer G. IL-1 pathways in inflammation and human diseases. Nat Rev Rheumatol. 2010;6:232–241. doi: 10.1038/nrrheum.2010.4. [DOI] [PubMed] [Google Scholar]
- 72.Watson JM, Lofguist AK, Rinehart CA, Olsen JC, et al. The intracellular IL-1 receptor antagonist alters IL-1-inducible gene expression without blocking exogenous signaling by IL-1 beta. J Immunol. 1995;155:4467–4475. [PubMed] [Google Scholar]
- 73.Li B, Smith TJ. Regulation of IL-1 Receptor Antagonist by TSH in Fibrocytes and Orbital Fibroblasts. J Clin Endocrinol Metab. 2014;99:E625–E633. doi: 10.1210/jc.2013-3977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Li B, Smith TJ. PI3K/AKT pathway mediates induction of IL-1RA by TSH in fibrocytes: Modulation by PTEN. J Clin Endocrinol Metab. 2014;99:3363–3372. doi: 10.1210/jc.2014-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Spicer AP, Kaback LA, Smith TJ, Seldin MF. Molecular cloning and characterization of the human and mouse UDP-glucose dehydrogenase genes. J Biol Chem. 1998;273:25117–25124. doi: 10.1074/jbc.273.39.25117. [DOI] [PubMed] [Google Scholar]
- 76.Tsui S, Fernando R, Chen B, Smith TJ. Divergent Sp1 levels may underlie differential expression of UDP glucose dehydrogenase by fibroblasts: Role in susceptibility to orbital Graves’ disease. J Biol Chem. 2011;286:24487–24499. doi: 10.1074/jbc.M111.241166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kaback LA, Smith TJ. Expression of hyaluronan synthase messenger ribonucleic acids and their induction by interleukin-1ß in human orbital fibroblasts: Potential insight into the molecular pathogenesis of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 1999;84:4079–4084. doi: 10.1210/jcem.84.11.6111. [DOI] [PubMed] [Google Scholar]
- 78.Guo N, Woeller CF, Feldon SE, Phipps RP. Peroxisome proliferator-activated receptor gamma ligands inhibit transforming growth factor-beta-induced, hyaluronan-dependent, T cell adhesion to orbital fibroblasts. J Biol Chem. 2011;286:18856–18867. doi: 10.1074/jbc.M110.179317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smith TJ, Wang HS, Evans CH. Leukoregulin is a potent inducer of hyaluronan synthesis in cultured human orbital fibroblasts. Am J Physiol. 1995;268:C382–C388. doi: 10.1152/ajpcell.1995.268.2.C382. [DOI] [PubMed] [Google Scholar]
- 80.Guo N, Baglole CJ, O’Loughlin CW, Feldon SE, et al. Mast cell-derived prostaglandin D2 controls hyaluronan synthesis in human orbital fibroblasts via DP1 activation: implications for thyroid eye disease. J Biol Chem. 2010;285:15794–15804. doi: 10.1074/jbc.M109.074534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cao HJ, Wang HS, Zhang Y, Lin HY, et al. Activation of human orbital fibroblasts through CD40 engagement results in a dramatic induction of hyaluronan synthesis and prostaglandin endoperoxide H synthase-2 expression: Insights into potential pathogenic mechanisms of thyroid associated ophthalmopathy. J Biol Chem. 1998;273:29615–29625. doi: 10.1074/jbc.273.45.29615. [DOI] [PubMed] [Google Scholar]
- 82.Smith TJ, Hoa N. Immunoglobulins from patients with Graves’ disease induce hyaluronan synthesis in their orbital fibroblasts through the self-antigen, IGF-1 receptor. J Clin Endocrinol Metab. 2004;89:5076–5080. doi: 10.1210/jc.2004-0716. [DOI] [PubMed] [Google Scholar]
- 83.Jackson DG. Immunological functions of hyaluronan and its receptors in the lymphatics. Immunol Rev. 2009;230:216–231. doi: 10.1111/j.1600-065X.2009.00803.x. [DOI] [PubMed] [Google Scholar]
- 84.Noden DM. The role of the neural crest in patterning of avian cranial skeletal, connective, and muscle tissues. Dev Biol. 1983;96:144–165. doi: 10.1016/0012-1606(83)90318-4. [DOI] [PubMed] [Google Scholar]
- 85.Smith TJ, Sempowski GD, Wang HS, DelVecchio PJ, et al. Evidence for cellular heterogeneity in primary cultures of human orbital fibroblasts. J Clin Endocrinol Metab. 1995;80:2620–2625. doi: 10.1210/jcem.80.9.7673404. [DOI] [PubMed] [Google Scholar]
- 86.Smith TJ, Wang HS, Hogg MG, Henrikson RC, et al. Prostaglandin E2 elicits a morphological change in cultured orbital fibroblasts from patients with Graves ophthalmopathy. Proc Natl Acad Sci USA. 1994;91:5094–5098. doi: 10.1073/pnas.91.11.5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Henrikson RC, Smith TJ. Ultrastructure of cultured human orbital fibroblasts. Cell Tissue Res. 1994;278:629–631. doi: 10.1007/BF00331384. [DOI] [PubMed] [Google Scholar]
- 88.Koumas L, Smith TJ, Phipps RP. Fibroblast subsets in the human orbit: Thy-1+ and Thy-1− subpopulations exhibit distinct phenotypes. Eur J Immunol. 2002;32:477–485. doi: 10.1002/1521-4141(200202)32:2<477::AID-IMMU477>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- 89.Sorisky A, Pardasani D, Gagnon A, Smith TJ. Evidence of adipocyte differentiation in human orbital fibroblasts in primary culture. J Clin Endocrinol Metab. 1996;81:3428–3431. doi: 10.1210/jcem.81.9.8784110. [DOI] [PubMed] [Google Scholar]
- 90.Koumas L, Smith TJ, Feldon S, Blumberg N, et al. Thy-1 expression in human fibroblast subsets defines myofibroblastic or lipofibroblastic phenotypes. American J Pathology. 2003;163:1291–1300. doi: 10.1016/S0002-9440(10)63488-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Smith TJ, Koumas L, Gagnon A, Bell A, et al. Orbital fibroblast heterogeneity may determine the clinical presentation of thyroid-associated ophthalmopathy. J Clin Endocrinol Metab. 2002;87:385–392. doi: 10.1210/jcem.87.1.8164. [DOI] [PubMed] [Google Scholar]
- 92.Li H, Fitchett C, Kozdon K, Jayaram H, et al. Independent adipogenic and contractile properties of fibroblasts in Graves’ orbitopathy: an in vitro model for the evaluation of treatments. PLos One. 2014;9:e95586. doi: 10.1371/journal.pone.0095586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lehmann GM, Woeller CF, Pollock SJ, O’Loughlin CW, et al. Novel anti-adipogenic activity produced by human fibroblasts. Am J Physiol Cell Physiol. 2010;299:C672–C681. doi: 10.1152/ajpcell.00451.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Bucala R, Spiegel LA, Chesney J, Hogan M, et al. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 95.Chesney J, Metz C, Stavitsky AB, Bacher M, et al. Regulated production of type I collagen and inflammatory cytokines by peripheral blood fibrocytes. J Immunol. 1998;160:419–425. [PubMed] [Google Scholar]
- 96.Yang L, Scott PG, Giuffre J, Shankowsky HA, et al. Peripheral blood fibrocytes from burn patients: identification and quantification of fibrocytes in adherent cells cultured from peripheral blood mononuclear cells. Lab Inves. 2002;82:1183–1192. doi: 10.1097/01.lab.0000027841.50269.61. [DOI] [PubMed] [Google Scholar]
- 97.Bohle A, Wehrmann M, Mackensen-Haen S, Gise H, et al. Pathogenesis of chronic renal failure in primary glomerulopathies. Nephrol Dial Transplant Suppl. 1994;3:4–12. [PubMed] [Google Scholar]
- 98.Scholten D, Reichart D, Paik YH, Lindert J, et al. Migration of fibrocytes in fibrogenic liver injury. Am J Pathol. 2011;179:189–198. doi: 10.1016/j.ajpath.2011.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Wang CH, Huang CD, Lin HC, Lee KY, et al. Increased circulating fibrocytes in asthma with chronic airflow obstruction. Am J Respir Crit Care Med. 2008;178:583–591. doi: 10.1164/rccm.200710-1557OC. [DOI] [PubMed] [Google Scholar]
- 100.Galligan CL, Siminovitch KA, Keystone EC, Bykerk V, et al. Fibrocyte activation in rheumatoid arthritis. Rheumatology (Oxford) 2010;49:640–651. doi: 10.1093/rheumatology/kep265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Pilling D, Fan T, Huang D, Kaul B, et al. Identification of markers that distinguish monocyte-derived fibrocytes from monocytes, macrophages, and fibroblasts. PLoS One. 2009;4:e7475. doi: 10.1371/journal.pone.0007475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Hong KM, Belperio JA, Keane MP, Burdick MD, et al. Differentiation of human circulating fibrocytes as mediated by transforming growth factor-beta and peroxisome proliferator-activated receptor gamma. J Biol Chem. 2007;282:22910–22920. doi: 10.1074/jbc.M703597200. [DOI] [PubMed] [Google Scholar]
- 103.Chesney J, Bacher M, Bender A, Bucala R. The peripheral blood fibrocyte is a potent antigen-presenting cell capable of priming naive T cells in situ. Proc Natl Acad Sci USA. 1997;94:9307–6312. doi: 10.1073/pnas.94.12.6307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Abe R, Donnelly SC, Peng T, Bucala R, et al. Peripheral blood fibrocytes: differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 105.Moeller A, Gilpin SE, Ask K, Cox G, et al. Circulating fibrocytes are an indicator of poor prognosis in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med. 2009;179:588–594. doi: 10.1164/rccm.200810-1534OC. [DOI] [PubMed] [Google Scholar]
- 106.Wang JF, Jiao H, Stewart TL, Shankowsky HA, et al. Fibrocytes from burn patients regulate the activities of fibroblasts. Wound Repair Regen. 2007;15:113–121. doi: 10.1111/j.1524-475X.2006.00192.x. [DOI] [PubMed] [Google Scholar]
- 107.Phillips RJ, Burdick MD, Hong K, Lutz MA, et al. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest. 2004;114:438–446. doi: 10.1172/JCI20997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kazim M, Goldberg RA, Smith TJ. Insights into the pathogenesis of thyroid-associated orbitopathy: evolving rationale for therapy. Arch Ophthalmol. 2002;120:380–386. doi: 10.1001/archopht.120.3.380. [DOI] [PubMed] [Google Scholar]
- 109.Gillespie EF, Papageorgiou KI, Fernando R, Raychaudhuri N, et al. Increased expression of TSH receptor by fibrocytes in thyroid-associated ophthalmopathy leads to chemokine production. J Clin Enodcrinol Metab. 2012;97:E740–E746. doi: 10.1210/jc.2011-2514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Fernando R, Atkins S, Raychaudhuri N, Lu Y, et al. Human fibrocytes coexpress thyroglobulin and thyrotropin receptor. Proc Natl Acad Sci USA. 2012;109:7427–7432. doi: 10.1073/pnas.1202064109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Fernando R, Lu Y, Atkins SJ, Mester T, et al. Expression of thyrotropin receptor, thyroglobulin, sodium-iodide symporter, and thyroperoxidase by fibrocytes depends on AIRE. J Clin Endocrinol Metab. 2014;99:E1236–E1244. doi: 10.1210/jc.2013-4271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nagamine K, Peterson P, Scott HS, Kudoh J, et al. Positional cloning of the APECED gene. Nature. 1997;17:393–398. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 113.Fernando R, Vonberg A, Atkins SJ, Pietropaolo S, et al. Human fibrocytes express multiple antigens associated with autoimmune endocrine diseases. J Clin Endocrinol Metab. 2014;99:E796–E803. doi: 10.1210/jc.2013-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Neumann S, Nir EA, Eliseeva E, Huang W, et al. A selective TSH receptor antagonist inhibits stimulation of thyroid function in female mice. Endocrinology. 2014;155:310–314. doi: 10.1210/en.2013-1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sanders P, Young S, Sanders J, Kabelis K, et al. Crystal structure of the TSH receptor (TSHR) bound to a blocking-type TSHR autoantibody. J Mol Endocrinol. 2011;46:81–99. doi: 10.1530/JME-10-0127. [DOI] [PubMed] [Google Scholar]
- 116.Sanders P, Young S, Sanders J, Kabelis K, et al. Characteristics of a human monoclonal autoantibody to the thyrotropin receptor: sequence structure and function. Thyroid. 2004;14:560–570. doi: 10.1089/1050725041692918. [DOI] [PubMed] [Google Scholar]
- 117.Núñez Miguel R, Sanders J, Sanders P, Young S, et al. Similarities and differences in interactions of thyroid stimulating and blocking autoantibodies with the TSH receptor. J Mol Endocrinol. 2012;49:137–151. doi: 10.1530/JME-12-0040. [DOI] [PubMed] [Google Scholar]
- 118.Chen H, Mester T, Raychaudhuri N, Kauh CY, et al. Teprotumumab, an IGF-1R Blocking Monoclonal Antibody Inhibits TSH and IGF-1 Action in Fibrocytes. J Clin Endocrinol Metab. 2014 doi: 10.1210/jc.2014-1580. E-Pub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]


