Abstract
The gel mobility shift assay is a powerful technique for detecting and quantifying protein–RNA interactions. While other techniques such as filter binding and isothermal titration calorimetry (ITC) are available for quantifying protein–RNA interactions, gel shift analysis provides the added advantage that you can visualize the protein–RNA complexes. In the gel shift assay, protein–RNA complexes are typically separated from the unbound RNA using native polyacrylamide gels in Tris/borate/EDTA buffer, although an alternative Tris-glycine buffering system is superior in many situations. Here, we describe both gel shift methods, along with strategies to improve separation of protein–RNA complexes from free RNA, which can be a particular challenge for small RNA binding proteins.
Keywords: Protein–RNA interaction, Gel mobility shift assay, Electrophoretic mobility shift assay, EMSA, RNA binding protein, CsrA, TRAP
1. Introduction
Gel mobility shift (gel shift) assays, also referred to as electrophoretic mobility shift assays (EMSA), allow rapid detection and quantification of protein–RNA interactions (1–5). In vitro generated RNA is typically end-labeled at the 5′ end with [γ-32P]ATP, while the protein is unlabeled. Following an incubation step to allow protein–RNA complex formation, complexes are separated from unbound (free) RNA by native (nondenaturing) polyacrylamide gel electrophoresis (PAGE). Visualization of bound RNA in the complex and free RNA is accomplished using a phosphorimager. The intensity of the bound and free RNA species are then quantified using commercially available software (e.g., ImageQuant from Molecular Dynamics). The fraction of bound RNA is plotted as a function of protein concentration to derive the apparent equilibrium binding constant (Kd), which is defined as the concentration of protein in which 50% of the RNA is bound. Thus, the Kd value is a measure of the affinity that the protein has for its RNA binding target (3).
The use of a Tris/borate/EDTA (TBE) buffering system (1, 2, 6) has been widely used for gel shift analysis. While this buffering system works well for a variety of applications, we found that an alternative Tris-glycine buffering system, commonly used for separating proteins under denaturing conditions (7), offers superior results in many situations (3–5, 8–10). Thus, we recommend that both buffering systems be tested to determine which system works best for the particular application.
Another important consideration is the length of the RNA to be used in the analysis, to ensure that PAGE adequately resolves the bound and free RNA species. In general, the separation of bound and free RNA species improves as the length of RNA is decreased. This consideration is critical for small RNA binding proteins, such as CsrA of Escherichia coli (MW = 13.7 kDa), and less so for larger proteins such as TRAP of Bacillus subtilis (MW = 91.6 kDa). Gel shift analyses with these two proteins will be used as examples throughout this chapter.
2. Materials
It is critical that RNase-free conditions be maintained throughout the procedure. All glassware, metal spatulas, and stir bars used for preparing solutions should be baked in a 250°C oven for 4 h to destroy RNases. As diethylpyrocarbonate (DEPC) destroys RNases, all solutions for protein–RNA binding reactions should be prepared with DEPC-treated and autoclaved water (see Note 1). Prepare gel casting and running buffer solutions with distilled water.
2.1. RNA Binding by CsrA
DEPC-treated water: Add 1 mL of DEPC to 0.5 L of distilled water in a 1-L bottle. Mix, incubate overnight at room temperature, and autoclave for 30 min the next day. Store at room temperature.
2 M Tris–HCl, pH 7.5: Weigh 24.2 g Tris base and transfer to a 125-mL glass beaker containing a stir bar. Add DEPC-treated water to a volume of ~75 mL. Mix on a magnetic stirrer and adjust the pH to 7.5 with HCl. Add DEPC-treated water to a final volume of 100 mL. Store at room temperature.
2 M MgCl2: Weigh 20.3 g magnesium chloride hexahydrate and transfer to a 50-mL plastic conical tube. Add DEPC-treated water to a final volume of 50 mL. Store at room temperature.
4 M KCl: Weigh 29.8 g potassium chloride and transfer to 125-mL glass beaker. Add DEPC-treated water to a final volume of 100 mL. Store at room temperature.
0.5 M EDTA, pH 8.0: Weigh 18.6 g EDTA, disodium salt, and transfer to a 125-mL glass beaker containing a stir bar. Add DEPC-treated water to a volume of ~75 mL. Mix on a magnetic stirrer and adjust the pH to 8.0 with 10 M sodium hydroxide. Add DEPC-treated water to a final volume of 100 mL. Store at room temperature.
TE buffer: 10 mM Tris–HCl, pH 7.5, 1 mM EDTA. Mix 0.5 mL of 2 M Tris–HCl, pH 7.5, 0.2 mL of 0.5 M EDTA, and 99.3 mL of DEPC-treated water.
1 M Dithiothreitol (DTT): Dissolve 154 mg DTT in a 1.5-mL microcentrifuge tube with 1 mL of DEPC-treated water. Store at −20°C.
50% Glycerol (v/v): Weigh 31.5 g glycerol (25 mL) and transfer to a 50-mL plastic conical tube. Add 25 mL DEPC-treated water and mix. Store at room temperature.
5% Xylene cyanol: Dissolve 50 mg xylene cyanol in a 1.5-mL microcentrifuge tube with 1 mL DEPC-treated water. Store at −20°C.
10 mg/mL yeast RNA: Dissolve 10 mg yeast RNA in a 1.5-mL microcentrifuge tube with 1 mL of DEPC-treated water. Store at −20°C (see Note 2).
10× CsrA binding buffer: Mix 50 μL of 2 M Tris–HCl, pH 7.5 (100 mM final concentration), 50 μL of 2 M MgCl2 (100 mM final concentration), 250 μL of 4 M KCl (1 M final concentration), and 650 μL of DEPC-treated water. Store at room temperature (see Note 3).
CsrA dilution buffer: Mix 5 μL of 2 M Tris–HCl, pH 7.5 (10 mM final concentration), 2 μL of 1 M DTT (2 mM final concentration), 200 μL 50% glycerol (10% final concentration), and 793 μL DEPC-treated water. Store at −20°C.
RNasin (Promega)
5′ End-labeled RNA: (see Note 4).
2.2. RNA Binding by TRAP
DEPC-treated water: See Subheading 2.1, step 1.
1 M Tris-acetate, pH 8.0: Weigh 12.1 g Tris base and transfer to a 125-mL glass beaker containing a stir bar. Add DEPC-treated water to a volume of ~75 mL. Mix on a magnetic stirrer and adjust the pH with acetic acid. Add DEPC-treated water to a final volume of 100 mL. Store at room temperature.
2 M magnesium acetate: Weigh 21.4 g magnesium acetate tertrahydrate and transfer to a 50-mL plastic conical tube. Add DEPC-treated water to a final volume of 50 mL. Store at room temperature.
0.5 M EDTA, pH 8.0: See Subheading 2.1, step 5.
TE buffer: See Subheading 2.1, step 6.
1 M DTT: See Subheading 2.1, step 7.
50% Glycerol (v/v): See Subheading 2.1, step 8.
5% Xylene cyanol: See Subheading 2.1, step 9.
10 mg/mL E. coli tRNA: Dissolve 10 mg tRNA (E. coli MRE600) (Roche) in a 1.5-mL microcentrifuge tube with 1 mL of DEPC-treated water. Store at −20°C (see Note 2).
20 mM l-tryptophan: Dissolve 41 mg l-tryptophan in a 15-mL plastic conical tube with 10 mL of DEPC-treated water. Store at −20°C.
6× TRAP binding buffer: Mix 300 μL of 1 M Tris-acetate, pH 8.0 (300 mM final concentration), 12 μL of 2 M magnesium acetate (24 mM final concentration), 30 μL of 1 M DTT (30 mM final concentration), 120 μL of 10 mg/mL tRNA (1.2 mg/mL final concentration), 12 μL of 5% xylene cyanol (0.6 mg/mL final concentration), and 526 μL of DEPC-treated water. Store at −20°C (see Notes 3 and 5).
TRAP dilution buffer: Mix 10 μL of 1 M Tris-acetate, pH 8.0 (10 mM final concentration), 2 μL of 1 M DTT (2 mM final concentration), 200 μL 50% glycerol (10% final concentration), and 788 μL DEPC-treated water. Store at −20°C.
5′ End-labeled RNA: (see Note 4).
2.3. Tris-Glycine Polyacrylamide Gel Components
Gel buffer: 1.5 M Tris–HCl, pH 8.8. Weigh 36.3 g Tris base and transfer to a 250-mL glass beaker containing a stir bar. Add water to a volume of ~150 mL. Mix on a magnetic stirrer and adjust pH with HCl. Add water to a final volume of 200 mL. Store at 4°C.
5× Tris-glycine gel running buffer: 135 mM Tris, 960 mM glycine, 5 mM EDTA, pH 8.3. Weigh 16.3 g Tris base, 72 g glycine, and 1.8 g EDTA, disodium salt. Transfer to a 1-L glass beaker containing a stir bar. Add ~600 mL water. Mix on a magnetic stirrer. Add water to a final volume of 1 L. The pH does not require adjustment. Store at room temperature.
1× Tris-glycine gel running buffer: Mix 200 mL of 5× Tris-glycine gel running buffer with 800 mL of water. Store at room temperature.
40% Acrylamide:bisacrylamide, 37.5:1 solution. Store at 4°C.
Ammonium persulfate, 10% solution in water. Store at −20°C.
N, N, N′, N′-tetramethylethylenediamine (TEMED). Store at 4°C.
Fixing solution, 30% methanol and 10% acetic acid: Mix 0.3 L of methanol, 0.1 L of glacial acetic acid, and 0.6 L of water. Store at room temperature.
2.4. TBE Polyacrylamide Gel Components
5× TBE buffer: 465 mM Tris, 445 mM boric acid, 10 mM EDTA, pH 8.3. Weigh 56.3 g of Tris base, 27.6 g of boric acid, and 3.7 g of EDTA, disodium salt. Transfer to a 1-L glass beaker containing a stir bar. Add water to a volume of ~600 mL. Mix on a magnetic stirrer. Add water to a final volume of 1 L. pH does not require any adjustment. Store at room temperature.
0.5× TBE buffer: 47 mM Tris, 45 mM boric acid, 1 mM EDTA, pH 8.3. Mix 100 mL of 5× TBE buffer with 900 mL of water. Store at room temperature.
40% Acrylamide:bisacrylamide solution: See Subheading 2.3, step 4.
Ammonium persulfate (10%): See Subheading 2.3, step 5.
TEMED: See Subheading 2.3, step 6.
Fixing solution: See Subheading 2.3, step 7.
3. Methods
3.1. Gel Casting for Tris-Glycine System
6% Polyacrylamide gel: Mix 1.8 mL of 40% acrylamide: bisacrylamide, 1.2 mL of 50% glycerol, 2.4 mL of 1.5 M Tris–HCl (pH 8.8), 6.6 mL of water, 12 μL of TEMED, and 120 μL of 10% ammonium persulfate. This mixture is sufficient for two gels. Cast gels using 8 × 10-cm gel plates with 0.5-mm spacers. Insert a 10-well comb in each gel. Allow the gels to polymerize for 30 min. Transfer gels into a vertical gel running apparatus and fill the buffer reservoirs with 1× Tris-glycine gel running buffer (see Notes 6 and 7).
10% Polyacrylamide gel: Mix 3.0 mL of 40% acrylamide: bisacrylamide, 1.2 mL of 50% glycerol, 2.4 mL of 1.5 M Tris–HCl (pH 8.8), 5.4 mL of water, 12 μL of TEMED, and 120 μL of 10% ammonium persulfate. This mixture is sufficient for two gels. Cast gels using 8 × 10-cm gel plates with 0.5-mm spacers. Insert a 10-well comb in each gel. Allow the gels to polymerize for 30 min. Transfer gels into a vertical gel running apparatus and fill the buffer reservoirs with 1× Tris-glycine gel running buffer.
3.2. Gel Casting for TBE System
6% Polyacrylamide gel: Mix 1.8 mL of 40% acrylamide: bisacrylamide, 0.6 mL of 50% glycerol, 1.2 mL of 5× TBE buffer (0.5× final concentration), 8.4 mL of water, 12 μL of TEMED, and 120 μL of 10% ammonium persulfate. This mixture is sufficient for two gels. Cast gels using 8 × 10-cm gel plates with 0.5-mm spacers. Insert a 10-well comb in each gel. Allow the gels to polymerize for 30 min. Transfer gels into a vertical gel running apparatus and fill the buffer reservoirs with 0.5× TBE buffer (see Notes 6 and 7).
10% Polyacrylamide gel: Mix 3 mL of 40% acrylamide: bisacrylamide, 0.6 mL of 50% glycerol, 1.2 mL of 5× TBE buffer (0.5× final concentration), 7.2 mL of water, 12 μL of TEMED, and 120 μL of 10% ammonium persulfate. This mixture is sufficient for two gels. Cast gels using 8 × 10-cm gel plates with 0.5-mm spacers. Insert a 10-well comb in each gel. Allow the gels to polymerize for 30 min. Transfer gels into a vertical gel running apparatus and fill the buffer reservoirs with 0.5× TBE buffer.
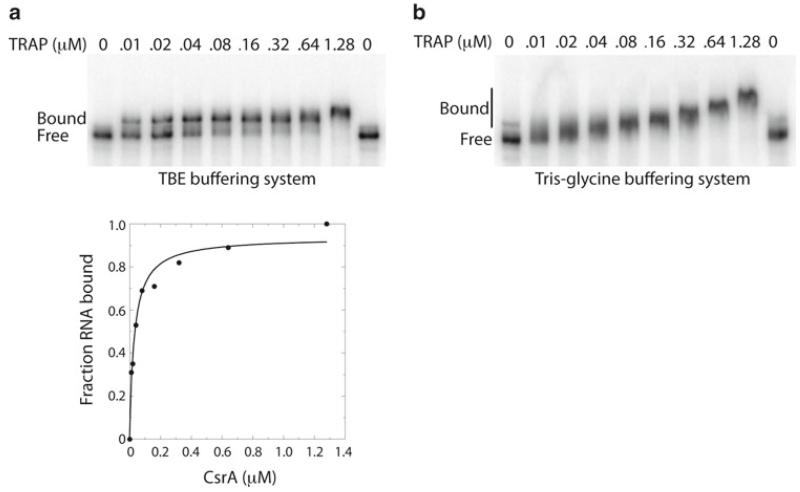
3.3. CsrA-RNA Gel Shift Analysis (Fig. 1)
Fig. 1.
Gel mobility shift analysis of CsrA-csrA RNA interaction. 5′ end-labeled csrA RNA (0.1 nM) was incubated with the concentration of CsrA shown at the top of each lane. Samples were loaded onto 10% polyacrylamide gels with a 37.5:1 acrylamide:bisacrylamide ratio. Positions of bound and free RNA are shown. (a) TBE buffering system. (b) Tris-glycine buffering system. The TBE buffering system gave superior results in this particular case. The simple binding curve for these data is shown below the gel. The Kd for this reaction was calculated to be 30 nM CsrA.
Make up to 8 twofold serial protein dilutions in CsrA dilution buffer. The concentration of RNA should be at least tenfold lower than the lowest protein concentration used in the binding reaction.
Label ten Eppendorf tubes (0.65 mL) 1 through 10. Add 2 μL of protein dilution buffer to tubes 1 and 10. Add 2 μL of each protein dilution into tubes 2 through 9. As the bound and free RNA species run close to one another with the small CsrA protein, it is helpful to have unbound controls (no CsrA) in the first and last lanes of the gel.
Dilute 5′ end-labeled RNA (gel purified) to 1 nM in TE and heat to 85°C for 3 min. Slow cool for 10 min at room temperature (see Note 8).
Prepare binding mixture (80 μL). Mix 10 μL of 10× CsrA binding buffer, 10 μL of 1 nM RNA (0.1 nM final concentration), 15 μL of 50% glycerol, 2 μL 1 M DTT, 1 μL of RNasin, 2 μL of 10 μg/μL yeast tRNA, 10 μL 5% xylene cyanol, and 30 μL of DEPC-treated water. Mix well. Add 8 μL to each tube containing protein dilution buffer or protein (see Notes 5 and 9).
Incubate tubes at 37°C for 30 min in a heat block and then immediately load 2–5 μL aliquots onto a polyacrylamide gel (see Note 10).
Gel running for TBE system: Pre-run the gel using 0.5× TBE for 10–15 min at 200 V (constant). Wash the wells with gel running buffer using a syringe and needle. Load the gel and run for 1–2 h at 200 V (constant).
Gel running for Tris-glycine system: Do not pre-run the gel. Wash the wells with 1× Tris-glycine gel running buffer using a syringe and needle. Load the gel and run at 12 mA (constant) and 150 V (maximum) for 2–3 h (see Note 11).
Transfer the gel to Whatman paper, cover with plastic wrap and dry in a gel dryer. Place the dried gel overnight in a Phosphorimager cassette. Scan and quantify the bound and free RNA species using ImageQuant software (Molecular Dynamics) (see Notes 12 and 13).
Fit the binding data to simple and cooperative binding equations (see Note 14).
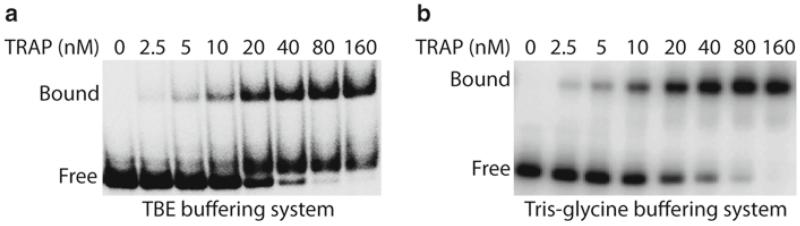
3.4. TRAP-RNA Gel Shift Analysis (Fig. 2)
Fig. 2.
Gel mobility shift analysis of TRAP-trp leader RNA interaction. 5′ end-labeled trp leader RNA (0.1 nM) was incubated with the concentration of TRAP shown at the top of each lane. Samples were loaded onto 6% polyacrylamide gels with a 37.5:1 acrylamide:bisacyrylamide ratio. Positions of bound and free RNA are shown. (a) TBE buffering system. (b) Tris-glycine buffering system. The Tris-glycine buffering system gave superior results in this particular case. The Kd for this reaction was calculated to be 17 nM TRAP.
Make up to 9 twofold serial dilutions of protein in TRAP dilution buffer. The concentration of RNA should be at least ten-fold lower than the lowest protein concentration used in the binding reaction.
Label ten Eppendorf tubes (0.65 mL) 1 through 10. Add 2 μL of protein dilution buffer to tube 1. Add 2 μL of each protein dilution to tubes 2 through 10.
Dilute 5′ end-labeled B. subtilis trp leader RNA (gel purified) to 1 nM in TE and heat to 85°C for 3 min. Cool slowly for 10 min at room temperature (see Note 15).
Prepare binding mixture (80 μL). Mix 16.7 μL of 6× TRAP binding buffer, 10 μL of 1 nM RNA (0.1 nM final concentration), 5 μL of 20 mM l-tryptophan, and 48.3 μL of DEPC-treated water. Mix well. Add 8 μL to each tube containing protein dilution buffer or protein (see Note 16).
Incubation: Same as for CsrA-RNA interaction (see Note 10). See Subheading 3.3, step 5.
Gel running for TBE system: Same as for CsrA-RNA interaction. See Subheading 3.3, step 6.
Gel running for Tris-glycine system: Same as for CsrA-RNA interaction (see Note 11). See Subheading 3.3, step 7.
Gel handling and quantification (see Notes 12 and 13). See Subheading 3.3, step 8.
Fit the binding data to simple and cooperative binding equations (see Note 14).
Acknowledgement
This work was supported by NIH grant GM059969.
Footnotes
Establishing and maintaining RNase-free conditions is a critical consideration. Gloves should be worn at all times to prevent the introduction of RNases from your fingers. Do not use pipeters that have been used for procedures containing RNase A (e.g., plasmid miniprep procedures). While DEPC inhibits RNases, it is critical to ensure that DEPC is destroyed by autoclaving. It is recommended that chemicals and solutions used for RNA work be used exclusively for that purpose.
A nonspecific RNA competitor must be included in the binding reaction in large excess over labeled RNA to prevent nonspecific protein–RNA interactions. Although tRNA is routinely used as a competitor, a pilot experiment must be performed to determine whether tRNA is suitable for any given protein. For example, tRNA interferes with CsrA binding to its targets. Instead, total yeast RNA was found to be an effective nonspecific competitor for CsrA-RNA binding studies.
Binding conditions, such as pH and the concentration of monovalent and divalent salts, must be determined empirically.
PCR products containing a T7 RNA polymerase promoter are used as DNA templates. RNA is synthesized in vitro with a commercially available transcription kit by following the manufacturer’s protocol. Gel-purified RNA is dephosphorylated with calf intestinal alkaline phosphatase and subsequently 5′-end labeled with (γ-32P)-ATP and T4 polynucleotide kinase (4). Labeled RNAs are gel purified and suspended in TE buffer.
Inclusion of glycerol and xylene cyanol in the binding reaction simplifies gel loading. Glycerol also protects diluted proteins from denaturation. Both CsrA and TRAP remain active in their binding buffers at low concentrations. However, inclusion of acetylated BSA at 100 μg/mL (final concentration) to binding reactions may be required for less stable proteins.
The concentration of the polyacrylamide gel is an important consideration for gel shift analysis. The size of the protein and the RNA length must be taken into consideration to obtain adequate resolution of bound and free RNA species. In general, separation of these species is not a problem for a large protein such as TRAP (MW = 91.6 kDa). In this case, we typically use 100–200 nucleotide transcripts and obtain good separation in 6% gels. However, adequate separation with small RNA binding proteins such as CsrA (MW = 13.7 kDa) is more challenging. In general, the separation with CsrA improves as the transcript size is decreased (e.g., 100–16 nucleotide). However, care must be taken to ensure that unbound (free) short RNAs do not run too close to the gel front. Thus, for small RNA binding proteins we suggest trying a variety of RNA lengths and/or acrylamide concentrations (e.g., 8, 10 and 15%) in gels. Resolution may also be improved using different acrylamide:bisacrylamide ratios (e.g., 37.5:1 vs. 19:1).
We use the Hoefer SE 250 Mighty Small II 10 × 8 cm electrophoresis units. Other similar vertical slab gel systems can be substituted.
As CsrA and TRAP do not contain cysteine residues, reducing agents such as DTT are not required. However, DTT is required to maintain activity of RNase inhibitor (RNasin). Therefore, DTT should be included in the binding reaction if RNasin is used. RNasin may be omitted if proper RNase-free conditions are maintained.
The length of time required to establish an equilibrium in which association and dissociation of the protein–RNA complex are balanced must be determined empirically. A time course of binding (e.g., 10, 30, 60 min) should be performed using the same protein dilution. Equilibrium is obtained when the fraction bound no longer increases with time.
Each protein–RNA pair requires appropriate pH and salt concentrations for interaction. Loading of large reaction volumes (more than 10 μL) containing salt concentrations above 150 mM may disturb gel running and/or the shape of the bound and free RNA bands. In general, gels run in TBE buffer are more sensitive to salt perturbation than gels run in Tris-glycine buffer. Loading smaller volumes of binding reactions containing lower salt concentrations provides better separation and sharper bands.
High percentage polyacrylamide gels do not stick well to Whatman paper. Gels ≤10% will stick to Whatman paper. Be careful so as not to tear or distort the shape of the gel during this process.
Fractionation of the binding reaction in native gels may result in dissociation of protein–RNA complexes during gel running. The newly released RNA will run between the free and bound species, sometimes running as a smear. Because this newly released RNA was part of a protein–RNA complex at the time of gel loading, it should be considered as bound RNA for calculation of the apparent Kd value.
TRAP binds to 11 (G/U)AG repeats in the B. subtilis trp leader (3).
l-tryptophan activates TRAP such that it can bind to the B. subtilis trp leader (3).
References
- 1.Fried M, Crothers DM. Equilibria and kinetics of lac repressor-operator interactions by polyacrylamide gel electrophoresis. Nucleic Acids Res. 1981;9:6505–6525. doi: 10.1093/nar/9.23.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Garner MM, Revzin A. A gel electrophoresis method for quantifying the binding of proteins to specific DNA regions: application to components of the Escherichia coli lactose operon regulatory system. Nucleic Acids Res. 1981;9:3047–3060. doi: 10.1093/nar/9.13.3047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yakhnin AV, Trimble JJ, Chiaro CR, Babitzke P. Effects of mutations in the l-tryptophan binding pocket of the Trp RNA-binding attenuation protein of Bacillus subtilis. J Biol Chem. 2000;275:4519–4524. doi: 10.1074/jbc.275.6.4519. [DOI] [PubMed] [Google Scholar]
- 4.Dubey AK, Baker CS, Romeo T, Babitzke P. RNA sequence and secondary structure participate in high-affinity CsrA-RNA interaction. RNA. 2005;11:1579–1587. doi: 10.1261/rna.2990205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yakhnin H, Pandit P, Petty TJ, Baker CS, Romeo T, Babitzke P. CsrA of Bacillus subtilis regulates translation initiation of the gene encoding the flagellin protein (hag) by blocking ribosome binding. Mol Microbiol. 2007;64:1605–1620. doi: 10.1111/j.1365-2958.2007.05765.x. [DOI] [PubMed] [Google Scholar]
- 6.Yakhnin H, Yakhnin AV, Baker CS, Sineva E, Berezin I, Romeo T, Babitzke P. Complex regulation of the global regulatory gene csrA : CsrA-mediated translational repression, transcription from five promoters by Eσ70 and EσS, and indirect transcriptional activation by CsrA. Mol Microbiol. 2011;81:689–70. doi: 10.1111/j.1365-2958.2011.07723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 8.Baker CS, Morozov I, Suzuki K, Romeo T, Babitzke P. CsrA regulates glycogen biosynthesis by preventing translation of glgC in Escherichia coli. Mol Microbiol. 2002;44:1599–1610. doi: 10.1046/j.1365-2958.2002.02982.x. [DOI] [PubMed] [Google Scholar]
- 9.Yakhnin H, Yakhnin AV, Babitzke P. The trp RNA-binding attenuation protein (TRAP) of Bacillus subtilis regulates translation initiation of ycbK, a gene encoding a putative efflux protein, by blocking ribosome binding. Mol Microbiol. 2006;61:1252–1266. doi: 10.1111/j.1365-2958.2006.05278.x. [DOI] [PubMed] [Google Scholar]
- 10.Baker CS, Eöry LA, Yakhnin H, Mercante J, Romeo T, Babitzke P. CsrA inhibits translation initiation of Escherichia coli hfq by binding to a single site overlapping the Shine-Dalgarno sequence. J Bacteriol. 2007;189:5472–5481. doi: 10.1128/JB.00529-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Edwards AN, Patterson-Fortin LM, Vakulskas CA, Mercante JW, Potrykus K, Vinella D, Camacho MI, Fields JA, Thompson SA, Georgellis D, Cashel M, Babitzke P, Romeo T. Circuitry linking the Csr and stringent response global regulatory systems. Mol Microbiol. 2011;80:1561–1580. doi: 10.1111/j.1365-2958.2011.07663.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yang M, Chen X, Militello K, Hoffman R, Fernandez B, Baumann C, Gollnick P. Alanine-scanning mutagenesis of Bacillus subtilis trp RNA-binding attenuation protein (TRAP) reveals residues involved in tryptophan binding and RNA binding. J Mol Biol. 1997;270:696–710. doi: 10.1006/jmbi.1997.1149. [DOI] [PubMed] [Google Scholar]
- 13.Schaak JE, Yakhnin H, Bevilacqua PC, Babitzke P. A Mg2+-dependent RNA tertiary structure forms in the Bacillus subtilis trp operon leader transcript and appears to interfere with trpE translation control by inhibiting TRAP binding. J Mol Biol. 2003;332:555–574. doi: 10.1016/s0022-2836(03)00969-0. [DOI] [PubMed] [Google Scholar]