Abstract
Protein degradation plays key roles in diverse pathways in cell division, growth and differentiation. Aberrant stabilization of crucial proteins participating in oncogenic pathways is often observed in cancer. The importance of proper protein turnover is exemplified by the SCFFbxw7 ubiquitin ligase, which is frequently mutated in human cancer, including T cell acute lymphoblastic leukemia. Recent studies have revealed novel substrates of Fbxw7 and shed light on its role on differentiation of stem cells and expansion of stem-cell-like cells driving tumorigenesis. Detailed understanding of the contribution of the Fbxw7-regulated network of proteins in initiation and progression of cancer will facilitate the identification of candidate intervention targets in human cancer.
Introduction
The ubiquitin-proteasome system (UPS) controls spatially and temporarily a broad range of cellular processes. Ubiquitin is covalently attached to substrates by the consecutive activities of three enzymes and specificity in the system is provided by the E3 ubiquitin ligases. Defective function of the UPS has been linked to an array of diseases, including cancer [1•]. The importance of the UPS as a drug target is underlined by the successful use of proteasome inhibitors against multiple myeloma. Drugs targeting other components of the UPS have also shown promising results in preclinical or early clinical trials [2]. Importantly, various cancers carry mutations affecting the UPS. A prominent mutational target is Fbxw7, a substrate targeting subunit of the SCF (Skp1-Cul1-F box) ubiquitin ligase complex. Leukemias represent a special example of Fbxw7-associated cancer, with T cell acute lymphoblastic leukemia (T-ALL) patients carrying mutations in up to 30% of cases. Fbxw7 targets several key regulators of proliferation, cell growth and apoptosis for proteasomal degradation. Fbxw7 has also a central role in the maintenance of somatic stem cells. Additionally, Fbxw7 controls pluripotency of embryonic stem cells (ESCs) by regulating the stability of key proteins and affects the reprogramming efficiency of induced pluripotent stem cells (iPSCs). This review will focus on the recent advances that have deepened our understanding on the role of Fbxw7 in supporting tumorigenesis and in the maintenance of normal stem cells.
Roles of Fbxw7 in pluripotent stem cells
ESCs are derived from the inner cell mass of the developing embryo. ESCs self-renew extensively in vitro, however, in differentiation they must readily alter their transcriptional program in order to allow proper cell specification. c-Myc is highly abundant in ESCs and orchestrates their proliferation program resulting in rapid progression through cell cycle, whereas its levels are lower in response to differentiation [3]. Conversely, Fbxw7 is maintained at low levels in self-renewing ESCs but is upregulated in differentiation, suggesting direct regulation of c-Myc by Fbxw7. Indeed, Fbxw7 appears dispensable for self-renewal since its loss does not affect markers of pluripotency [3]. However, knockdown of Fbxw7 during conditions of differentiation results in maintenance of self-renewal markers and inhibition of differentiation [4]. Furthermore, ubiquitylation levels of c-Myc correlate with high Fbxw7 expression, and its knockdown results in higher protein levels of c-Myc in differentiating cells.
Regulation of ESC differentiation by Fbxw7 led to the hypothesis that c-Myc ubiquitylation may also regulate iPSCs function. c-Myc is one of the four Yamanaka factors (together with Oct4, Sox2 and Klf4) which induce somatic cell reprogramming [5]. Consistent with that, Fbxw7 knockdown in reprogrammable fibroblasts [6] resulted in increased efficiency of iPSC formation [4]. Furthermore, downregulation of Fbxw7 in reprogrammable fibroblasts expressing only 3 reprogramming factors (Oct4, Sox2, Klf4) resulted in enhanced reprogramming efficiency, suggesting that Fbxw7 regulates endogenous c-Myc protein abundance in iPSC generation. However, an open question remains whether additional Fbxw7 substrates might also contribute in reprogramming [7]. These data suggest that the Fbxw7 – c-Myc axis regulates cellular identity and pluripotent stem cell function.
Roles of Fbxw7 in adult stem and progenitor cells
Hematopoietic stem cells
In contrast to pluripotent stem cells, which are characterized by rapid proliferation, adult stem cells are often quiescent. Recent studies demonstrated that Fbxw7 plays pivotal roles in the regulation of different stem cell types. Hematopoietic stem cells (HSCs) are among the most well-characterized somatic stem cells and serve as an example of how maintenance of quiescence is crucial for the balance between homeostasis and malignant transformation. Fbxw7 deletion in HSCs was found to promote active cell cycle and defective maintenance of quiescence [8,9]. Furthermore, Fbxw7 functions in the hematopoietic compartment were attributed to c-Myc stabilization. Both in bone marrow HSCs and in fetal liver, c-Myc was specifically stabilized by Fbxw7 deletion [3,9]. On the other hand, overexpression of Fbxw7 results in diminished c-Myc levels in HSCs, which correlates with quiescent state [10]. In sharp contrast with stem cells being able to sustain their lineage throughout the life of the organism, ~30% of Fbxw7-null mice develop rapid exhaustion of HSCs and those animals succumb to pancytopenia [8]. Notably, enhanced c-Myc activation leads to elevated p53-dependent induction of apoptosis. On the other hand, mice that do not upregulate the Fbxw7-induced p53 response develop T-ALL [8]. Furthermore, cyclin E has been also implicated in the maintenance of HSC genomic stability in a p53 and Fbxw7-dependent manner [11]. These results indicate that p53-induced apoptosis serves as a balance between maintenance of HSCs and malignant transformation.
Intestinal stem cells
In sharp contrast to quiescent HSCs, intestinal stem cells (ISCs) are characterized by continuous cycling [12]. The intestinal epithelium is organized in crypt-villus structures in which the crypt-based ISCs rapidly proliferate and differentiate into cells of the enterocyte and secretory lineages that form the villi. Ubiquitylation and proteasomal degradation in the intestinal system is critical to regulate abundance and function of transcription factors. Fbxw7 is an important player that orchestrates cell specification and its impairment leads to transformation. Goblet cells, which are representative of the secretory lineage, are responsive to Notch signaling, since intestinal cells can convert to Goblet cells after Notch inhibition [13]. Fbxw7 heterozygous-null mice exhibit defective Goblet cell differentiation [14], as well as heterozygous mutations of FBXW7 have been observed in human colorectal carcinoma [15]. Furthermore, homozygous-null Fbxw7 mice manifest intestinal adenomas [16], which exhibit elevated levels of c-Myc, Notch1 and c-Jun, all known substrates of Fbxw7. In the context of adenomatous polyposis coli (APC) loss, Fbxw7 deficiency accelerated intestinal tumorigenesis, which promoted stability of DEK, an oncoprotein that plays critical roles in tumor progression [17]. Fbxw7 directly promotes phospho-DEK ubiquitylation and degradation in a GSK3β-dependent manner. As a consequence, Fbxw7 loss promotes deregulation of cell growth that together with APC deficiency leads to β-catenin stabilization, resulting to adenocarcinoma progression [16]. In a similar approach, p53 deletion can also accelerate phenotypes of Fbxw7 loss, leading to metastatic intestinal adenocarcinomas [18]. These results highlight the importance of Fbxw7 in intestinal stem and progenitor cells, emerging as a key player balancing differentiation and malignant transformation.
Neural stem cells
Neural stem and progenitor cells are able to differentiate to downstream neurons and glia that collectively form the nervous system. Mechanisms that regulate specification to downstream cell types such as neurons, astrocytes and oligodendrocytes govern nervous system viability and development as well as organism behavior. The Notch1 signaling pathway has been proposed to play important roles in the balance of neuronal stem cell (NSC) self-renewal and differentiation [19]. Ubiquitylation by Fbxw7 was recently described to regulate these processes. Deletion of Fbxw7 in the brain results in accumulation of Notch1 both in vivo and in vitro [20,21]. Aberrant Notch signaling triggers impaired NSC differentiation and neurogenesis in the brain. In the embryonic brain, Fbxw7 deletion associates with several morphological abnormalities [21]. Neuronal differentiation is halted whereas astroglial differentiation is enhanced and Fbxw7-null mice succumb soon after birth. Furthermore, differentiation is blocked at the expense of self-renewal in response to Notch accumulation. Fbxw7 deletion enhances neurosphere formation in vitro, a phenotype attributed to Notch, since inhibition of Notch signaling attenuates abnormalities in NSC differentiation [21].
Furthermore, Fbxw7 deletion resulted in decreased survival of neural progenitor cells and c-Jun was found to accumulate following Fbxw7 deletion [20]. c-Jun is an important regulator of neuronal viability [22] and it was previously proposed to be a substrate for ubiquitylation and degradation by the proteasome by Fbxw7 [23]. Hence, Fbxw7 was proposed to possess a dual role in NSCs, by regulating both Notch and c-Jun levels thus controlling neural cell differentiation and neural cell viability, respectively.
Reprogramming and transdifferentiation in the pancreas
Besides regulating stem cell fates in different somatic systems, Fbxw7 has been also implicated in shaping cellular plasticity of differentiated cell types. The pancreas is devoid of adult stem cells rendering differentiation of pancreatic cells restricted to embryonic development [24]. Efforts have focused on strategies to expand pancreatic regenerative capacity or transdifferentiation of adult pancreatic cell types. Post-translational modification by Fbxw7 was found to play critical roles both in embryonic and adult pancreatic cell fate decisions. Inactivation of Fbxw7 in pancreatic ductal cells leads to their reprograming into α, β and δ cells [25•]. These induced β cells resemble their islet β cell counterparts both morphologically and immunologically. Dissection of the molecular pathways that lead to β cell neogenesis revealed that deletion of Fbxw7 results in increased Ngn3 protein levels, a major endocrine cell factor whose suppression leads to ductal cell maintenance [25•]. Furthermore, Fbxw7 binds to and ubiquitylates Ngn3, a process mediated my GSK3β phosphorylation, leading to Ngn3 degradation by the proteasome. Although it is unclear if additional Fbxw7 substrates promote ductal to β cell reprogramming, these studies suggest that ubiquitylation by Fbxw7 can promote adult cell reprogramming and transdifferentiation besides stem cell proliferation or differentiation. It would be interesting to investigate further therapeutic implications in response to injury or disease.
A central role of FBXW7 in blood cancers
FBXW7 is frequently mutated in T-ALL [26]. The majority of mutations result in amino-acid substitutions at three key arginine residues (Arg465Cys/His, Arg479Leu and Arg505Cys) within the WD40 domain that forms a β-propeller structure important for the binding of phosphorylated substrates [27,28]. These point mutations are frequently found in a heterozygous state and because Fbxw7 acts as a dimer, they differentially affect the stability of their substrates depending on the Fbxw7-substrate affinity [29].
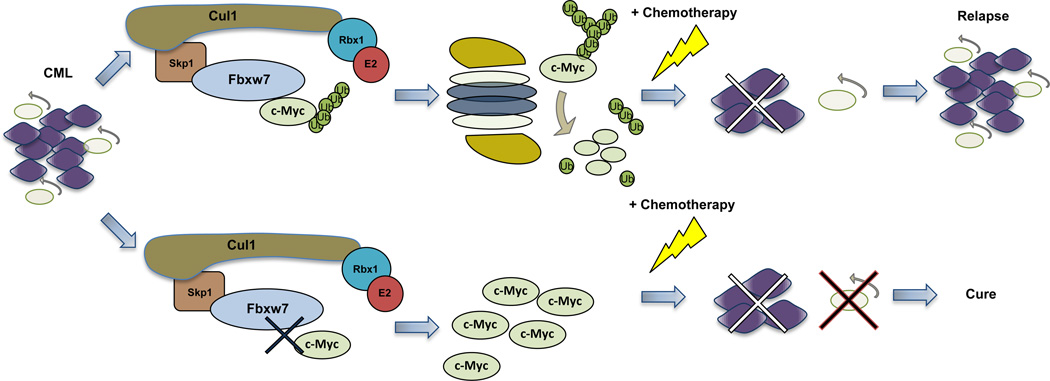
Leukemia-initiating cells (LICs) consist of a rare subpopulation of cells vital for the propagation of leukemia [30]. LICs resemble HSCs in several aspects including self-renewal, pluripotency and quiescence [31]. The quiescent state of LICs has been proposed to contribute to resistance of leukemia to conventional chemotherapy. In addition, quiescence contributes to relapse of chronic myeloid leukemia (CML) patients in complete remission, after discontinuation of treatment with the tyrosine kinase inhibitor imatinib. Therefore, understanding the mechanism by which LICs maintain quiescence is important. Fbxw7 has a central role in LICs quiescence maintenance by degrading c-Myc [32]. Fbxw7 is critical for the initiation and maintenance of CML and regulates LIC homeostasis. In addition, Fbxw7 expression is necessary for maintaining a threshold of c-Myc advantageous for the CML LIC cells [33••]. From a therapeutic perspective, Fbxw7 loss led to increased sensitivity of LICs to imatinib while therapy combining Fbxw7 ablation and imatinib administration resulted in LICs eradication in a mouse model of CML [32••] (Figure 1). Interestingly, combination of FBXW7 depletion and chemotherapy induced apoptosis in human CML LICs while leaving HSCs relatively unaffected.
Figure 1.
Fbxw7 ablation and subsequent c-Myc accumulation depletes LICs in CML. In wild type for Fbxw7 tumors, Fbxw7 targets c-Myc for proteasomal degradation and maintains LICs. Although chemotherapy eliminates the bulk of tumor, LICs survive leading to relapse upon drug discontinuation (top part). In Fbxw7 deficient tumors, c-Myc accumulates resulting in p53-dependent LIC apoptosis. The remaining cycling cancer cells are vulnerable to chemotherapy (bottom part).
Fbxw7 levels can cause subtle quantitative changes in the abundance of normal proteins that consequently may become oncogenic. The function of a common mutant, Fbxw7R465C, has been investigated in T-ALL. Fbxw7 mutations act synergistically with Notch pathway deregulation to accelerate leukemia. Mice transplanted with bone marrow cells that express Notch1ΔE, a truncated form of Notch1 that is constitutively active in a ligand-independent manner, and mutant Fbxw7 developed disease faster than the mice transplanted with cells that expressed Notch1ΔE and were wild-type for Fbxw7 or Fbxw7+/− [34••]. This indicates that, missense mutations that disrupt the arginine residues which bind the phosphorylated substrates, are not simply loss-of-function alleles but instead serve as dominant-negative alleles. This explains the strong evolutionary selection in cancer for missense mutations in these stringent positions [1]. Strikingly, the intermediate levels of Myc induced by the heterozygous Fbxw7 missense mutation did not compromise the HSCs function. The LIC population was significantly higher in Fbxw7-mutant T-ALL than in Fbxw7 wild type T-ALL. Mutation of Fbxw7 leads to stabilization of Myc protein, a key T-ALL oncogene. By tracking Myc protein levels during leukemia progression in vivo, the authors showed that mice transplanted with high-Myc LICs had significantly increased numbers of leukemic cells in the peripheral blood and succumbed to T-ALL while the low-Myc recipients exhibited complete leukemia-free survival. Notch1 and Myc, a direct transcriptional target of Notch1, colocalize in most sites in T-ALL cells. This results in Myc-mediated amplification of the Notch1-orchestrated oncogenic pathway. Targeting Myc transcription using BET-Brd4 inhibitors JQ1 reduced the expression of genes that were upregulated due to mutant Fbxw7 activity [34••,35]. Therefore, small molecule targeting of Myc in T-ALL and subsequent depletion of the LIC population might be an effective therapeutic strategy.
The complexity of the molecular networks regulated by Fbxw7 is exemplified by the good prognosis and early glucocorticoid treatment response in T-ALL patients carrying FBXW7 mutations. FBXW7 ubiquitylates the glucocorticoid receptor α (GRα) and targets it for proteasomal degradation. FBXW7 deficiency sensitizes leukemic cells to apoptosis in response to glucocorticoids through stabilization of GRα and upregulation of expression of its target genes including pro-apoptotic genes [36].
In addition to mutations and allelic loss, other mechanisms also disrupt FBXW7 function in blood cancer. The oncogenic transcription factor TAL1 is overexpressed in 60% of human T-ALL. TAL1 regulates the expression of the microRNA miR-223 that targets FBXW7 [37]. miR-223 overexpression represses FBXW7 leading to stabilization of critical for the T-ALL pathogenesis oncoproteins.
TCGA studies have provided a wealth of data on the mutational status of FBXW7 in various types of cancer. Interestingly, FBXW7 mutations are not detected in all blood cancers. Although FBXW7 acts primarily as a tumor suppressor, in multiple myeloma it serves as a pro-survival factor through the degradation of the p100 protein, the main inhibitor of the non-canonical NF-κB pathway [38]. Interfering with Fbxw7-mediated p100 ubiquitylation in these tumors results in decreased cell survival and proliferation.
Emerging substrates
Accumulating data on new Fbxw7 substrates have strengthened our understanding of the diverse oncogenic and non-oncogenic pathways regulated by Fbxw7. Heat-Shock Factor 1 (HSF1) is a multifaceted modifier of carcinogenesis [39,40]. HSF1 is a mediator of non-oncogene addiction and orchestrates a transcriptional program that provides critical relief to the proteotoxic stress encountered by cancer cells. FBXW7 interacts with HSF1 through a conserved HSF1 phosphodegron motif phosphorylated by GSK3β and ERK1. FBXW7 deficiency leads to stabilization of nuclear HSF1 specifically and defective heat-shock response attenuation during recovery from exogenous stress [41••]. In melanoma, FBXW7 is frequently mutated or silenced [26,42]. FBXW7 deficiency results in nuclear HSF1 accumulation and subsequent activation of a metastasis-promoting transcriptional program in melanoma [41••]. FBXW7’s substrates comprise transcription factors that regulate complex transcriptional programs such as MYC, NOTCH and HSF1. The finding that FBXW7 regulates the amount of CDK8 module bound to Mediator through targeting MED13/13L for degradation indicates that the FBXW7 role in global transcriptional control might be even more broad [43••]. FBXW7 suppresses the inflammatory gene expression through degradation of the CCAAT/enhancer binding protein delta (C/EBPδ) transcription factor [44]. Given that inflammatory pathways modulate tumor progression, it remains to be investigated whether part of the FBXW7 tumor suppressor activity is mediated through attenuation of pro-inflammatory gene expression.
Outlook
GSK3β phosphorylates the phosphodegron motif of many substrates, instigating the interaction with FBXW7. Also, ERK1 phosphorylation plays an important role in the FBXW7-HSF1 interaction [41••]. The rewiring of signaling pathways that mediate degron phosphorylation in cancer and the subsequent stabilization or degradation of proteins with key roles in in cell proliferation begs further investigation. In addition, FBXW7 regulates the stability of a network of proteins with key roles in diverse cellular pathways. Understanding the crosstalk of deregulated molecular pathways driven by aberrantly stabilized proteins in the presence of FBXW7 mutations will enable the simultaneous targeting of multiple oncogenic pathways.
Inhibition of Fbxw7 appears as a promising therapeutic approach for the treatment of CML. However, Fbxw7 has a pivotal role in normal hematopoiesis and its deletion results in progressive adult stem exhaustion [8,9]. Interestingly, the kinetics of response of normal and tumor stem cells to deletion of Fbxw7 are distinct. Therefore, therapeutic windows for targeting of Fbxw7 specifically in malignant stem and progenitor cells while sparing normal HSCs should be defined. Also, the stabilization of proto-oncoproteins and anti-apoptotic proteins should also be considered during the design of therapeutic approaches based on Fbxw7 inhibition. Fbxw7 mutations have been associated with resistance to chemotherapy [45,46]. Identification of the Fbxw7 cancerspecific substrates that mediate drug resistance will allow the design of novel synthetic lethality therapeutic approaches.
Our understanding on the impact of the Fbxw7 post-translational modifications on its function is still limited. It was recently shown that the deubiquitinase Usp28 deubiquitinates and stabilizes Fbxw7 [47]. Loss of Usp28 promotes autocatalytic degradation of Fbxw7, stabilization of its substrates and malignant transformation in the presence of oncogenic insults. Elucidation of the pathways that alter the function of the Fbxw7 modifying enzymes in cancer will lead to better understanding of the mechanisms by which oncogenic and tumor-supporting Fbxw7 substrates are stabilized in the absence of missense mutations or allelic loss of Fbxw7.
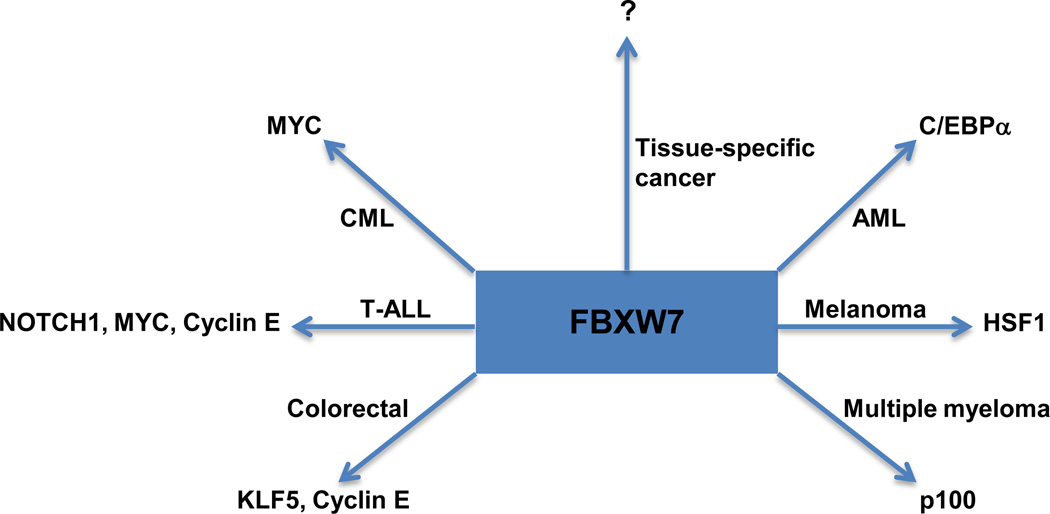
The systems degrading or stabilizing key players of carcinogenesis may be cell and tissue specific. Identification of the specific FBXW7 substrates that are vital for the initiation and maintenance of each cancer type will facilitate the discovery of candidate therapeutic targets (Figure 2).
Figure 2.
FBXW7 controls the turnover of diverse critical substrates depending on the tissue type. Identification of the cancer-specific network of FBXW7-stabilized proteins will elucidate the rewiring of signaling pathways in cancer due to abnormal proteostasis.
Acknowledgments
N.K. is supported by a European Molecular Biology Organization (EMBO) Long Term Fellowship and a Human Frontiers Science Program (HFSP) Long Term Fellowship. A.S. is supported by the NYSTEM institutional NYU Stem Cell Training Grant (C026880). I.A. is supported by the NIH (RO1CA133379, RO1CA105129, RO1CA149655, 5RO1CA173636) and the NYSTEM program of the New York State Health Department (NYSTEM-N11G-255). I.A. is also supported by the William Lawrence and Blanche Hughes Foundation, The Leukemia and Lymphoma Society, The Chemotherapy Foundation and the St. Baldrick’s Foundation. I.A is a Howard Hughes Medical Institute Early Career Scientist.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors declare no competing financial interests.
References
- 1. Davis RJ, Welcker M, Clurman BE. Tumor suppression by the Fbw7 ubiquitin ligase: mechanisms and opportunities. Cancer Cell. 2014;26:455–464. doi: 10.1016/j.ccell.2014.09.013. • This review provides an excellent overview of the mechanisms and consequences of Fbxw7 deregulation in cancer.
- 2.Skaar JR, Pagan JK, Pagano M. SCF ubiquitin ligase-targeted therapies. Nat Rev Drug Discov. 2014;13:889–903. doi: 10.1038/nrd4432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reavie L, Della Gatta G, Crusio K, Aranda-Orgilles B, Buckley SM, Thompson B, Lee E, Gao J, Bredemeyer AL, Helmink BA, et al. Regulation of hematopoietic stem cell differentiation by a single ubiquitin ligase-substrate complex. Nat Immunol. 2010;11:207–215. doi: 10.1038/ni.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley SM, Aranda-Orgilles B, Strikoudis A, Apostolou E, Loizou E, Moran-Crusio K, Farnsworth CL, Koller AA, Dasgupta R, Silva JC, et al. Regulation of pluripotency and cellular reprogramming by the ubiquitin-proteasome system. Cell Stem Cell. 2012;11:783–798. doi: 10.1016/j.stem.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 6.Stadtfeld M, Maherali N, Borkent M, Hochedlinger K. A reprogrammable mouse strain from gene-targeted embryonic stem cells. Nat Methods. 2010;7:53–55. doi: 10.1038/nmeth.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Okita Y, Matsumoto A, Yumimoto K, Isoshita R, Nakayama KI. Increased efficiency in the generation of induced pluripotent stem cells by Fbxw7 ablation. Genes Cells. 2012;17:768–777. doi: 10.1111/j.1365-2443.2012.01626.x. [DOI] [PubMed] [Google Scholar]
- 8.Matsuoka S, Oike Y, Onoyama I, Iwama A, Arai F, Takubo K, Mashimo Y, Oguro H, Nitta E, Ito K, et al. Fbxw7 acts as a critical fail-safe against premature loss of hematopoietic stem cells and development of T-ALL. Genes Dev. 2008;22:986–991. doi: 10.1101/gad.1621808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thompson BJ, Jankovic V, Gao J, Buonamici S, Vest A, Lee JM, Zavadil J, Nimer SD, Aifantis I. Control of hematopoietic stem cell quiescence by the E3 ubiquitin ligase Fbw7. J Exp Med. 2008;205:1395–1408. doi: 10.1084/jem.20080277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Iriuchishima H, Takubo K, Matsuoka S, Onoyama I, Nakayama KI, Nojima Y, Suda T. Ex vivo maintenance of hematopoietic stem cells by quiescence induction through Fbxw7α overexpression. Blood. 2011;117:2373–2377. doi: 10.1182/blood-2010-07-294801. [DOI] [PubMed] [Google Scholar]
- 11.Siu KT, Xu Y, Swartz KL, Bhattacharyya M, Gurbuxani S, Hua Y, Minella AC. Chromosome instability underlies hematopoietic stem cell dysfunction and lymphoid neoplasia associated with impaired Fbw7-mediated cyclin E regulation. Mol Cell Biol. 2014;34:3244–3258. doi: 10.1128/MCB.01528-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clevers H. The intestinal crypt, a prototype stem cell compartment. Cell. 2013;154:274–284. doi: 10.1016/j.cell.2013.07.004. [DOI] [PubMed] [Google Scholar]
- 13.van Es JH, de Geest N, van de Born M, Clevers H, Hassan BA. Intestinal stem cells lacking the Math1 tumour suppressor are refractory to Notch inhibitors. Nat Commun. 2010;1:18. doi: 10.1038/ncomms1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sancho R, Blake SM, Tendeng C, Clurman BE, Lewis J, Behrens A. Fbw7 repression by hes5 creates a feedback loop that modulates Notch-mediated intestinal and neural stem cell fate decisions. PLoS Biol. 2013;11:e1001586. doi: 10.1371/journal.pbio.1001586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sancho R, Jandke A, Davis H, Diefenbacher ME, Tomlinson I, Behrens A. F-box and WD repeat domain-containing 7 regulates intestinal cell lineage commitment and is a haploinsufficient tumor suppressor. Gastroenterology. 2010;139:929–941. doi: 10.1053/j.gastro.2010.05.078. [DOI] [PubMed] [Google Scholar]
- 16.Babaei-Jadidi R, Li N, Saadeddin A, Spencer-Dene B, Jandke A, Muhammad B, Ibrahim EE, Muraleedharan R, Abuzinadah M, Davis H, et al. FBXW7 influences murine intestinal homeostasis and cancer, targeting Notch, Jun, and DEK for degradation. J Exp Med. 2011;208:295–312. doi: 10.1084/jem.20100830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wise-Draper TM, Mintz-Cole RA, Morris TA, Simpson DS, Wikenheiser-Brokamp KA, Currier MA, Cripe TP, Grosveld GC, Wells SI. Overexpression of the cellular DEK protein promotes epithelial transformation in vitro and in vivo. Cancer Res. 2009;69:1792–1799. doi: 10.1158/0008-5472.CAN-08-2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grim JE, Knoblaugh SE, Guthrie KA, Hagar A, Swanger J, Hespelt J, Delrow JJ, Small T, Grady WM, Nakayama KI, et al. Fbw7 and p53 cooperatively suppress advanced and chromosomally unstable intestinal cancer. Mol Cell Biol. 2012;32:2160–2167. doi: 10.1128/MCB.00305-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Faigle R, Song H. Signaling mechanisms regulating adult neural stem cells and neurogenesis. Biochim Biophys Acta. 2013;1830:2435–2448. doi: 10.1016/j.bbagen.2012.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoeck JD, Jandke A, Blake SM, Nye E, Spencer-Dene B, Brandner S, Behrens A. Fbw7 controls neural stem cell differentiation and progenitor apoptosis via Notch and c-Jun. Nat Neurosci. 2010;13:1365–1372. doi: 10.1038/nn.2644. [DOI] [PubMed] [Google Scholar]
- 21.Matsumoto A, Onoyama I, Sunabori T, Kageyama R, Okano H, Nakayama KI. Fbxw7-dependent degradation of Notch is required for control of "stemness " and neuronal-glial differentiation in neural stem cells. J Biol Chem. 2011;286:13754–13764. doi: 10.1074/jbc.M110.194936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Raivich G, Bohatschek M, Da Costa C, Iwata O, Galiano M, Hristova M, Nateri AS, Makwana M, Riera-Sans L, Wolfer DP, et al. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron. 2004;43:57–67. doi: 10.1016/j.neuron.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Nateri AS, Riera-Sans L, Da Costa C, Behrens A. The ubiquitin ligase SCFFbw7 antagonizes apoptotic JNK signaling. Science. 2004;303:1374–1378. doi: 10.1126/science.1092880. [DOI] [PubMed] [Google Scholar]
- 24.Dor Y, Brown J, Martinez OI, Melton DA. Adult pancreatic beta-cells are formed by self-duplication rather than stem-cell differentiation. Nature. 2004;429:41–46. doi: 10.1038/nature02520. [DOI] [PubMed] [Google Scholar]
- 25. Sancho R, Gruber R, Gu G, Behrens A. Loss of Fbw7 reprograms adult pancreatic ductal cells into alpha, delta, and beta cells. Cell Stem Cell. 2014;15:139–153. doi: 10.1016/j.stem.2014.06.019. • This study illustrates that Fbxw7 regulates transdifferentiation in the pancreas by the regulation of its substrate Ngn3 and promotes β cell neogenesis in vivo.
- 26.Akhoondi S, Sun D, von der Lehr N, Apostolidou S, Klotz K, Maljukova A, Cepeda D, Fiegl H, Dafou D, Marth C, et al. FBXW7/hCDC4 is a general tumor suppressor in human cancer. Cancer Res. 2007;67:9006–9012. doi: 10.1158/0008-5472.CAN-07-1320. [DOI] [PubMed] [Google Scholar]
- 27.Hao B, Oehlmann S, Sowa ME, Harper JW, Pavletich NP. Structure of a Fbw7-Skp1-cyclin E complex: multisite-phosphorylated substrate recognition by SCF ubiquitin ligases. Mol Cell. 2007;26:131–143. doi: 10.1016/j.molcel.2007.02.022. [DOI] [PubMed] [Google Scholar]
- 28.Orlicky S, Tang X, Willems A, Tyers M, Sicheri F. Structural basis for phosphodependent substrate selection and orientation by the SCFCdc4 ubiquitin ligase. Cell. 2003;112:243–256. doi: 10.1016/s0092-8674(03)00034-5. [DOI] [PubMed] [Google Scholar]
- 29.Welcker M, Larimore EA, Swanger J, Bengoechea-Alonso MT, Grim JE, Ericsson J, Zheng N, Clurman BE. Fbw7 dimerization determines the specificity and robustness of substrate degradation. Genes Dev. 2013;27:2531–2536. doi: 10.1101/gad.229195.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lapidot T, Sirard C, Vormoor J, Murdoch B, Hoang T, Caceres-Cortes J, Minden M, Paterson B, Caligiuri MA, Dick JE. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 31.Clevers H. The cancer stem cell: premises, promises and challenges. Nat Med. 2011;17:313–319. doi: 10.1038/nm.2304. [DOI] [PubMed] [Google Scholar]
- 32. Takeishi S, Matsumoto A, Onoyama I, Naka K, Hirao A, Nakayama KI. Ablation of Fbxw7 eliminates leukemia-initiating cells by preventing quiescence. Cancer Cell. 2013;23:347–361. doi: 10.1016/j.ccr.2013.01.026. •• This study provides evidence that Fbxw7 function is essential for the initiation and progression of CML.
- 33. Reavie L, Buckley SM, Loizou E, Takeishi S, Aranda-Orgilles B, Ndiaye-Lobry D, Abdel-Wahab O, Ibrahim S, Nakayama KI, Aifantis I. Regulation of c-Myc ubiquitination controls chronic myelogenous leukemia initiation and progression. Cancer Cell. 2013;23:362–375. doi: 10.1016/j.ccr.2013.01.025. •• This study provides evidence that Fbxw7 function is essential for the initiation and progression of CML.
- 34. King B, Trimarchi T, Reavie L, Xu L, Mullenders J, Ntziachristos P, Aranda-Orgilles B, Perez-Garcia A, Shi J, Vakoc C, et al. The ubiquitin ligase FBXW7 modulates leukemia-initiating cell activity by regulating MYC stability. Cell. 2013;153:1552–1566. doi: 10.1016/j.cell.2013.05.041. •• This important study provides solid evidence that Fbxw7 deficiency stabilizes c-Myc protein that defines LICs in T-ALL.
- 35.Roderick JE, Tesell J, Shultz LD, Brehm MA, Greiner DL, Harris MH, Silverman LB, Sallan SE, Gutierrez A, Look AT, et al. c-Myc inhibition prevents leukemia initiation in mice and impairs the growth of relapsed and induction failure pediatric T-ALL cells. Blood. 2014;123:1040–1050. doi: 10.1182/blood-2013-08-522698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Malyukova A, Brown S, Papa R, O'Brien R, Giles J, Trahair TN, Dalla Pozza L, Sutton R, Liu T, Haber M, et al. FBXW7 regulates glucocorticoid response in T-cell acute lymphoblastic leukaemia by targeting the glucocorticoid receptor for degradation. Leukemia. 2013;27:1053–1062. doi: 10.1038/leu.2012.361. [DOI] [PubMed] [Google Scholar]
- 37.Mansour MR, Sanda T, Lawton LN, Li X, Kreslavsky T, Novina CD, Brand M, Gutierrez A, Kelliher MA, Jamieson CH, et al. The TAL1 complex targets the FBXW7 tumor suppressor by activating miR-223 in human T cell acute lymphoblastic leukemia. J Exp Med. 2013;210:1545–1557. doi: 10.1084/jem.20122516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Busino L, Millman SE, Scotto L, Kyratsous CA, Basrur V, O'Connor O, Hoffmann A, Elenitoba-Johnson KS, Pagano M. Fbxw7alpha- and GSK3-mediated degradation of p100 is a pro-survival mechanism in multiple myeloma. Nat Cell Biol. 2012;14:375–385. doi: 10.1038/ncb2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dai C, Whitesell L, Rogers AB, Lindquist S. Heat shock factor 1 is a powerful multifaceted modifier of carcinogenesis. Cell. 2007;130:1005–1018. doi: 10.1016/j.cell.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mendillo ML, Santagata S, Koeva M, Bell GW, Hu R, Tamimi RM, Fraenkel E, Ince TA, Whitesell L, Lindquist S. HSF1 drives a transcriptional program distinct from heat shock to support highly malignant human cancers. Cell. 2012;150:549–562. doi: 10.1016/j.cell.2012.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Kourtis N, Moubarak RS, Aranda-Orgilles B, Lui K, Aydin IT, Trimarchi T, Darvishian F, Salvaggio C, Zhong J, Bhatt K, et al. FBXW7 modulates cellular stress response and metastatic potential through HSF1 post-translational modification. Nat Cell Biol. 2015;17:322–332. doi: 10.1038/ncb3121. •• In this report, the investigators identify the transcription factor HSF1 as a novel substrate of FBXW7. Moreover, they demonstrate that FBXW7 deficiency leads to stabilization of nuclear HSF1 and defective heat-shock response attenuation. In the context of melanoma, nuclear HSF1 accumulation, due to FBXW7 deficiency, activates an invasion-supportive transcriptional program.
- 42.Cheng Y, Chen G, Martinka M, Ho V, Li G. Prognostic significance of Fbw7 in human melanoma and its role in cell migration. J Invest Dermatol. 2013;133:1794–1802. doi: 10.1038/jid.2013.58. [DOI] [PubMed] [Google Scholar]
- 43. Davis MA, Larimore EA, Fissel BM, Swanger J, Taatjes DJ, Clurman BE. The SCF-Fbw7 ubiquitin ligase degrades MED13 and MED13L and regulates CDK8 module association with Mediator. Genes Dev. 2013;27:151–156. doi: 10.1101/gad.207720.112. •• This report illustrates that FBXW7 controls the stability of MED13 and MED13L, suggesting an extensive role of FBXW7 in transcriptional control.
- 44.Balamurugan K, Sharan S, Klarmann KD, Zhang Y, Coppola V, Summers GH, Roger T, Morrison DK, Keller JR, Sterneck E. FBXW7alpha attenuates inflammatory signalling by downregulating C/EBPdelta and its target gene Tlr4. Nat Commun. 2013;4:1662. doi: 10.1038/ncomms2677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inuzuka H, Shaik S, Onoyama I, Gao D, Tseng A, Maser RS, Zhai B, Wan L, Gutierrez A, Lau AW, et al. SCF(FBW7) regulates cellular apoptosis by targeting MCL1 for ubiquitylation and destruction. Nature. 2011;471:104–109. doi: 10.1038/nature09732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wertz IE, Kusam S, Lam C, Okamoto T, Sandoval W, Anderson DJ, Helgason E, Ernst JA, Eby M, Liu J, et al. Sensitivity to antitubulin chemotherapeutics is regulated by MCL1 and FBW7. Nature. 2011;471:110–114. doi: 10.1038/nature09779. [DOI] [PubMed] [Google Scholar]
- 47.Schulein-Volk C, Wolf E, Zhu J, Xu W, Taranets L, Hellmann A, Janicke LA, Diefenbacher ME, Behrens A, Eilers M, et al. Dual regulation of Fbw7 function and oncogenic transformation by Usp28. Cell Rep. 2014;9:1099–1109. doi: 10.1016/j.celrep.2014.09.057. [DOI] [PubMed] [Google Scholar]