Abstract
The APOE4 allele confers greater risk of Alzheimer’s Disease (AD) for women than men, in conjunction with greater clinical deficits per unit of AD neuropathology (plaques, tangles). Cerebral microbleeds, which contribute to cognitive dysfunctions during AD, also show APOE4 excess, but sex-APOE allele interactions are not described. We report that elderly men diagnosed for mild cognitive impairment (MCI) and AD showed a higher risk of cerebral cortex microbleeds with APOE4 allele dose effect in two clinical cohorts (ADNI and KIDS). Sex-APOE interactions were further analyzed in EFAD mice carrying human APOE alleles and familial AD genes. At 7 months, E4FAD mice had cerebral cortex microbleeds with female excess, in contrast to humans. Cerebral amyloid angiopathy (CAA), plaques, and soluble Aβ also showed female excess. Both the cerebral microbleeds and CAA increased in proportion to individual Aβ load. In humans, the opposite sex bias of APOE4 allele for microbleeds vs the plaques and tangles is the first example of organ-specific, sex-linked APOE allele effects, and further shows AD as a uniquely human condition.
Keywords: ADNI, Alzheimer’s Disease Neuroimaging Initiative; Alzheimer’s Disease, AD; Aβ, amyloid β-peptide; APOE, apolipoprotein E; CAA, cerebral amyloid angiopathy; EFAD mice; KIDS, Karolinska Institute Dementia Study; microbleeds, cerebral cortex microbleeds or microhemorrhages; sex bias
1. Introduction
The APOE4 allele is the strongest heritable risk factor for sporadic AD, with sex-bias of >50% for women (Altmann et al., 2014; Farreret al., 1997; Payami et al., 1996). The neuropathological load showed parallel sex and APOE differences in benchmark postmortem studies of AD brains, with female excess of senile plaques and of neurofibrillary tangles (NFT) (Barnes et al., 2005; Corder et al., 2004). Moreover, women incurred 5-fold greater cognitive deficits per unit of brain β-amyloid (Aβ) than men (Barnes et al., 2005). Other studies also show female excess vulnerability in brain aging, with 1–1.5% faster brain atrophy in MRI studies of probable AD (Hua et al., 2010), and higher tau in cerebrospinal fluid of prodromal AD (MCI) (Altmann et al., 2014), but also in cognitively healthy elderly (Damoiseaux et al., 2012). Correspondingly, women APOE4 carriers had excess of Aβ plaque and NFT (Barnes et al., 2005; Corder et al., 2004) and more hippocampal atrophy in prodromal AD (Fleisher et al., 2005). Mouse transgenic models of AD with familial AD mutations (FAD) have shown consistent female excess of brain Aβ and cognitive deficits (Carroll et al., 2010; Dubal et al., 2012; Sturchler-Pierrat and Staufenbiel, 2000; Vest and Pike, 2013).
In contrast to brain Aβ and NFT, cerebral cortex microbleeds (microhemorrhages) typically show male 50% excess, e.g. in the Karolinska Imaging Dementia Study (KIDS) for AD (Shams et al., 2015) and the Amsterdam Dementia Cohort (Benedictus et al., 2015). Microbleeds, together with CAA (Shams et al., 2015), are of emerging importance as a contributing factor to pre-clinical cognitive decline and as an additional clinical burden in AD (Meier et al., 2014; Reijmer et al., 2015). In cognitively normal elderly, the presence of cerebral cortex microbleeds was associated with lower resting-state cerebral blood flow and subtle cognitive deficits (Gregg et al., 2015). APOE4 is also associated with cerebral microbleeds (Rannikmae et al., 2013; Yates et al., 2014) and CAA in leptomeningeal and cerebral cortex vessels (Rannikmae et al., 2013; Schmechel et al., 1993). Because microbleeds are associated with CAA (Premkumar et al., 1996; Yates et al., 2014), it is cogent to evaluate their association with sex. Some postmortem studies show a slight female excess of CAA (Lee and Stemmermann, 1978; Masuda et al., 1988) that was not seen in other samples (Gilbert and Vinters, 1983; Love et al., 2003; Allen et al., 2014). FAD mice develop CAA (Fryer et al., 2005) but sex differences and interactions of FAD with human APOE transgenes are not reported.
AD may have uniquely evolved in humans, because no FAD rodent or aging primate has clinical grade AD-like cognitive impairment with major neuron loss (Finch and Austad, 2015). While aging great apes develop CAA (Gearing et al., 1994) and incur sporadic strokes (Rosen et al., 2008; Jean et al., 2012), no aging wildtype rodent has shown CAA or stroke (Sullivan et al., 2008). Wildtype rodents are famously resistant to diet-induced atherosclerosis, unless made hypercholesterolemic by lipoprotein gene knockout, e.g. APOE-KO mice on high cholesterol diets developed aortic atheromas and intimal-media thickening (Smith et al., 2010). However, spontaneous cerebrovascular ischemic lesions are unknown in lab rodents even with induced hypercholesterolemia. Thus, four major pathological changes associated with human brain aging and AD have not been reported for aging wildtype rodents: brain Aβ-amyloid deposits, major neurodegeneration or neuron loss, CAA, and cerebrovascular ischemic lesions. One clue may be in APOE differences by species.
Humans have a unique multi-allele APOE system, while the primates and rodents examined so far have a single APOE isoform which resembles APOE4 in some amino acid positions (Fullerton et al., 2000; McIntosh et al., 2012). Notably during aging, APOE-TR mice transgenic for human APOE by targeted replacement (knock-in’) developed gross lobar hemorrhages by 18 months (41% of APOE4-TR; 8% of APOE3-TR)(Sullivan et al., 2008). Moreover, these hemorrhages were associated with classic CAA. The vascular thioflavin-positive fibrillar amyloid on wildtype background was unexpected because the endogenous murine Aβ has lower aggregatability than human Aβ (Boyd-Kimball et al., 2004). The greater prevalence of CAA in APOE4- vs E3-TR is consistent with findings from mice transgenic for the combination of FAD genes together human APOE, in which APOE4 increased CAA and plaques above APOE3 (Fryer et al., 2005). These studies did not examine sex differences.
To extend the correlations of Aβ with CAA for sex-APOE allele interactions in FAD mice and in human AD (see above), we used the EFAD mouse (Youmans et al., 2012) transgenic for human APOE3 or -E4 alleles (APOE-TR) (Sullivan et al., 1997) in combination with five familial AD genes, ‘5xFAD’ (Oakley et al., 2006). The early deposition of Aβ in EFAD mice gives an attractive model to study quantitative relationships between CAA and the Aβ load at young ages before changes in sex steroids. Cerebral cortex was analyzed at age 7 months for sex-APOE interactions in microbleeds and CAA, and for correlations with plaque Aβ. We also assayed oligomeric Aβ, a driver of neurodegeneration in AD (Klein et al., 2001; Mucke and Selkoe, 2012), which has not been reported for associations with sex or APOE in mouse models. Novel sex-APOE allele interactions were found in microvascular pathologies of brain aging that differ from AD, with species differences that may arise during sexual differentiation.
2. Material and Methods
2.1. Subjects
Informed written consent was obtained from all participants at each site.
2.1.1. Alzheimer’s Disease Neuroimaging Initiative (ADNI) (U.S.A. and Canada)
The ADNI database (www.adni-info.org) represents a longitudinal study of MRI and PET, combined with biological markers and clinical assessment of mild cognitive impairment (MCI) and early AD. More than 1500 community dwelling subjects, aged 48 to 91 years, were recruited at 50 sites across the U.S. and Canada. Microbleeds were assessed by MRI (http://adni.loni.usc.edu/methods/documents/mri-protocols) as hypointense lesions within the brain parenchyma <10 mm dia. on the MRI sequence: GRE/T2*; MRI field strength: 1.5T/3.0T (Greenberg et al., 2009; Wardlaw et al., 2013). Excluding APOE2, the sample was 658 subjects (166 cognitively normal, 402 with mild cognitive impairment, and 90 with early AD.
2.1.2. Karolinska Institute Dementia Study (KIDS) cohort (Sweden)
The KIDS database represents a cross-sectional cohort of consecutive patients undergoing memory investigation with an accompanying MRI scan for hemosiderin at Karolinska University Hospital, Stockholm, Swe. (n=1572, 2006–2012, age 36 to 88 years). Informed consent: if the patient was too confused, consent was obtained from a legal guardian. Ethics approval was obtained from the regional ethics board, Stockholm. After excluding scans of insufficient quality and APOE2, the analysis cohort consisted of 448 subjects: 152 AD, 152 MCI, and 144 controls which were a group of patients with subjective cognitive impairment (not clinical grade) (Caracciolo et al., 2012). Diagnoses were set according to ICD-10 by senior geriatricians at the memory clinic in multidisciplinary meetings that considered all diagnostic analyses. MRI scans were made on 1.5–3.0T scanners with full protocols with axial hemosiderin sequences T2* and/or SWI. Microbleeds were characterized by the microbleed anatomical rating scale and identified as hypointense lesions <10 mm dia. (Gregoire et al., 2009).
2.2. Animals
Procedures were approved by the USC Institutional Animal Care and Use Committee. EFAD mice were generously given by Mary Jo LaDu (Univ. Illinois at Chicago). E3FAD and E4FAD mice were generated by crossing 5XFAD to homozygous APOE3-, and APOE4-TR. APOE-TR mice were generated by Dr. Patrick Sullivan (Sullivan et. al., 1997). 5xFAD mice are transgenic for 5 distinct FAD mutations (APP K670N/M671L+ I716V+ V717I and PS1 M146L+L286V) controlled by the neuron-specific mouse Thy-1 promoter (Oakley, et al., 2006). Mice were examined at age 6–7 mo, when the female bias in behavioral deficits emerges in NSE-APOE mouse (Raber et al., 1998). C57BL/6NJ mice were used as the background mice strain. Two different cohorts of mice were used: one (4–5 mice/group) for microbleed (Fig. 2) and one for CAA (6–7 mice/group) (Fig. 3) analysis. Both were analyzed for Aβ load (Fig. 4, 5).
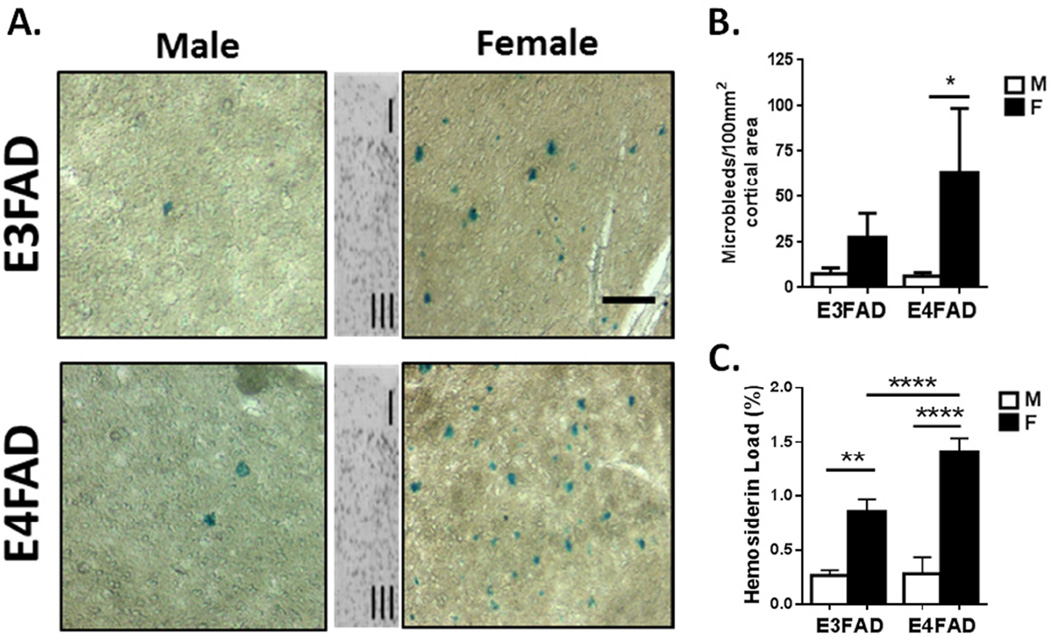
Figure 2. Microbleeds in cerebral cortex of EFAD mice: female excess is additive with APOE4.
A. Microbleeds visualized as extravasated hemosiderin by Prussian blue histochemistry in cerebral cortex sagital sections (0.5–2 mm lateral from midline; counterstained by nuclear fast red; scale bar 50 µm). The inset shows cortical layers I and III. B. Number of microbleeds per 100 mm2 of cortical area for EFAD mice. Female excess was observed in both EFAD mice. C. Hemosiderin load (total Prussian blue area, %) showed female excess and additivity with APOE4 in EFAD. * p<0.05; ** p <0.01; **** p<0.001. Male microbleeds did not differ by APOE allele.
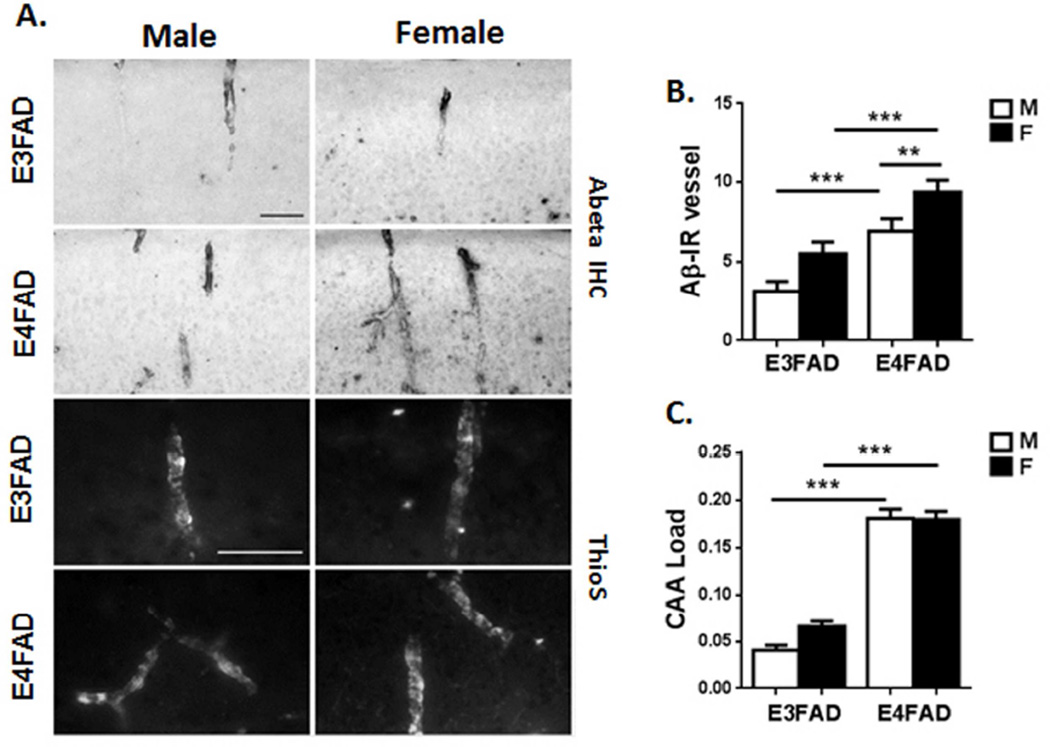
Figure 3. Cerebrovascular amyloid in EFAD mice shows female excess.
A. Cortical vessels immunopositive for Aβ (upper panels) and for thioflavin-S (lower panels) in EFAD mice. Scale bars: 50um. B. The number of Aβ-immunopositive (Aβ-IR) vessels was higher in females, with additive effects of APOE4. C. CAA load of Aβ within vessels was higher in E4FAD mice but did not differ by sex. Mean ±SEM, N=6–8/group ** p <0.01 ; *** p<0.005.
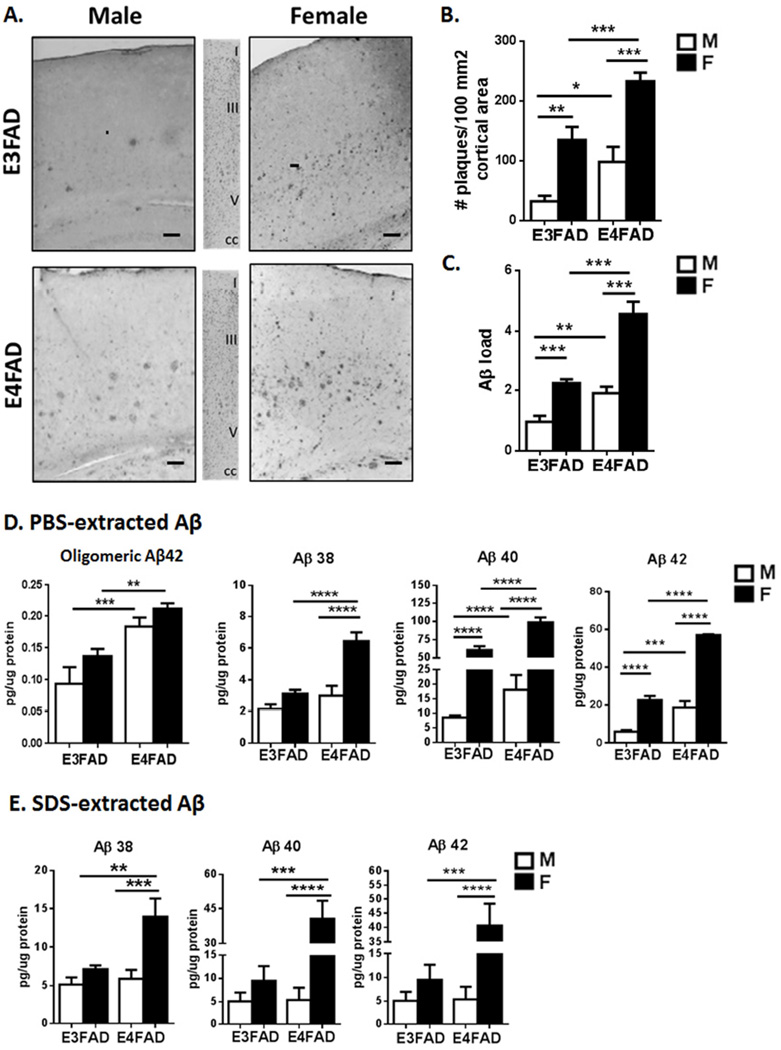
Figure 4. Levels of plaque and soluble Aβ show female excess in EFAD mice.
A. Cerebral cortex sagital sections (0.5–2 mm lateral from midline) were immunostained for Aβ (4G8 antibody); middle panels, Cresyl violet histochemistry showing cortical layers; scale bar, 100 µm. B. Number of plaques per per 100 mm2 of cortical area C. Aβ load, total plaque Aβ immunostaining. The subiculum had similar female and APOE4 excess Aβ plaque (not shown). The sex-apoE interaction was significant for Aβ load (p= 0.02). D–E. Soluble Aβ peptides in cerebral cortex. Oligomeric Aβ42 in PBS extract (oligo-specific ELISA) differed by APOE allele, but not by sex (MOAB-1 ELISA). Total Aβ peptides (multiplex ELISA) showed female excess, with additive effects for APOE4 for Aβ38, Aβ40, and Aβ42. PBS extracts had consistently 2-fold more Aβ40 than Aβ42 (Aβ40:42 ratio male: E3, 1.5±0.1, E4, 1.7±0.5; female: E3, 2.6±0.4, E4, 1.6±0.1), whereas the ratio was closer to 1 in SDS extracts (male: E3, 1.5±0.1, E4, 1.7±0.5; female: E3, 2.6±0.4, E4, 1.6±0.1). Sex-apoE allele interactions were significant for total soluble Aβ levels in both the PBS (Aβ 38, p<0.01; Aβ 40, p<0.01; Aβ 42, p= 0.001) and SDS extractions (Aβ 40, p<0.005; Aβ 42 p<0.01). Mean ± SEM, N=6–8/group. CC: corpus callosum; * p<0.05; ** p <0.01, *** p<0.005.
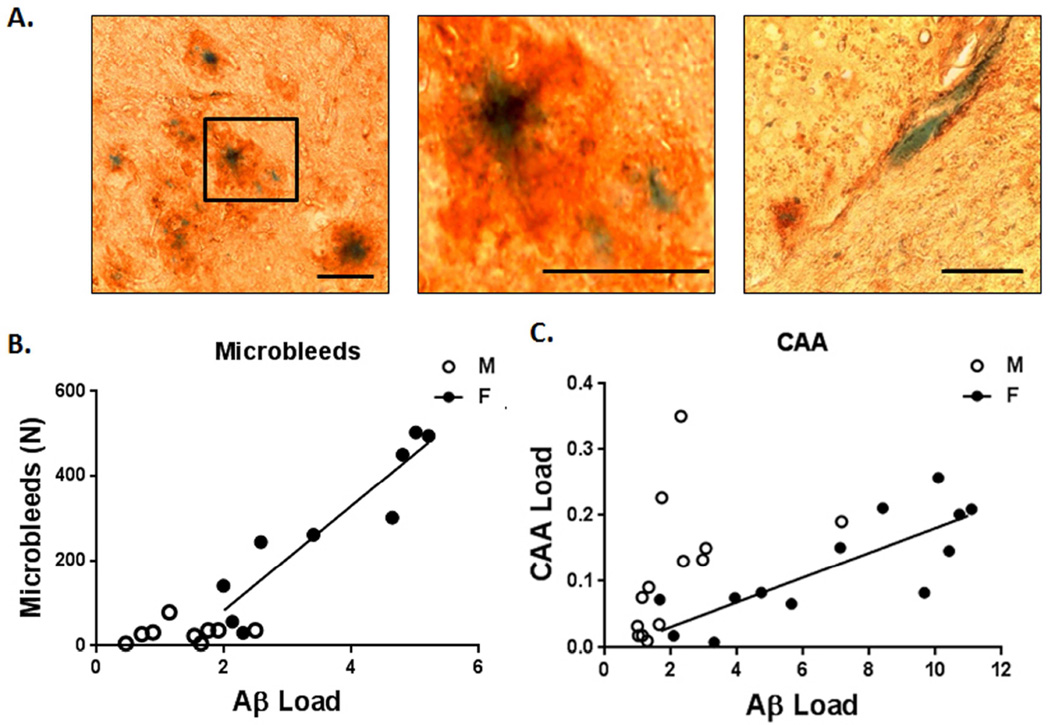
Figure 5. Aβ total load correlated with microbleeds and CAA only in EFAD females.
A. Cerebral cortex histochemistry co-staining for Aβ (4G8 antibody, brown) and microbleeds (Prussian Blue, hemosiderin); these images show the diverse morphology of hemosiderin cores within Aβ deposits, scale bars, 50 um. Middle panel shows particular from left panel (black box). B. The number of microbleeds correlated most strongly with total Aβ plaque load for female EFAD mice, but not males, in rank order of total plaque Aβ load (M, r2=0.09, p=0.3; F, r2=0.89, p<0.001) >PBS-extracted Aβ42 (M, r2=0.002, p=0.9; F, r2 =0.64, p<0.01) and PBS extracted Aβ40 (M, r2=0.002, p=0.9; F, r2=0.47, p<0.05), > oligomeric Aβ42 (M, r2=0.003, p=0.9; F, r2=0.27, p=0.15). The threshold level of total Aβ for linear correlation with microbleed numbers was determined iteratively: Aβ ≥1.70, t = 8.6294, df = 10, p = 6.03e-06; Aβ <1.70, t = 0.4536, df = 5, p = 0.6692. C. CAA load correlated with total Aβ load in females, but not males (M, r2=0.22, p=0.1; F, r2=0.75, p<0.001). CAA load represents 1.8% of the total Aβ load in females and 5.3% in males. Removal of one male outlier in the Aβ plaque load yields a significant correlation (p=0.05); nonetheless, the resulting r2 value for males (0.34) would still be below the females (0.75).
2.3. Tissues
After euthanization by isoflurane anesthesia, mice were perfused transcardially with PBS. Brains were hemi-sected and fixed in 4% paraformaldehyde/24 h, immersed in sequential sucrose (10–20–30%, 24 h each) and Optimal Cutting Temperature compound (OCT, Sakura, Torrance, CA), and frozen on dry ice. The other hemisphere was dissected and stored at −80 °C. All assays used observer-blinded protocols.
2.4. Immunohistochemistry
After sagittal sectioning 0.5–2 mm from midline (25 um), sections were stored at −80 °C. Aβ amyloid was immunostained with 4G8 (residues 17–24 at N-terminal of APP; Covance, Princeton, NJ) (Rasool et al., 2013). Sections were immersed in 70% formic acid/5 min. Endogenous peroxidases were blocked by 3% H2O2 and 10% methanol in Tris-buffered saline (TBS), 30 min/22 °C. Sections were permeabilized in 0.1% Triton X-100/15 min, blocked by 30 min incubation in TBS with 2% BSA and 0.1% Triton, followed by primary and then biotinylated anti-mouse secondary antibodies (1:250), and stained by ABC peroxidase and DAB substrate (Vector, Burlingame, CA). For plaque quantification bright field microscopic images were converted to 8-bit grayscale, thresholded to highlight plaques and to diminish background signal with visual inspection to confirm each object as a plaque. The entire cortex section was evaluated for total plaque number and percentage of area covered (Aβ load) by the “analyze particles” function of NIH ImageJ software.
Fibrillar Aβ was stained by 0.1% thioflavin-S (Youmans et al., 2012). CAA was assessed using a primary antibody directed against Ab (Invitrogen, Grand Island, NY) with a standard avidin: biotinylated enzyme complex immunoperoxidase method and ABC Elite and diaminobenzidine kits (Vector Laboratories, CA), see above. Non-overlapping images of cerebral cortex (6–9 per immunostained section) were collected from 7–8 horizontal sections (40 µm) per brain with an average of 65 images. Images were captured with an Olympus DP73 digital camera paired with cellSens software and a 10× objective to include the entire medial-lateral span of the cortex in one image. NIH ImageJ 1.48 software was used to define the region of interest (ROI) within each image, which included all cortical layers. The white matter at the edge of the cortex was excluded and served as the medial boundary. Gray scale images were thresholded for discrimination of immunopositive and negative Aβ zones. Total Aβ load represents the percentage of pixels within the cerebrocortical ROI that showed positive Aβ immunoreactivity. To determine CAA load, Aβ-immunoreactive vessels were identified morphologically. Identified vessels were analyzed only if they were visually distinct from cells with diameter >10 µm and length of ≥ 20 µm. The entirety of each immunoreactive vessel within a section was outlined to generate a CAA ROI that was contained within the larger cerebro-cortical ROI. The CAA load was calculated as the number of immunoreactive pixels within the CAA ROI and expressed as % the total pixels in the cerebrocortical ROI. Numbers of Aβ immunoreactive vessels within each section were determined. CAA load from each section was averaged per brain.
Microbleeds were assayed by Prussian blue staining for hemosiderin (Sullivan et al., 2008), with counterstaining by nuclear fast red (Sigma-Aldrich). Hemosiderin deposits (puncta) were individually analyzed for number and area.
2.5. ELISA
Aβ peptides were assayed in brain supernates (Jayaraman et al., 2012). Cerebral half-cortexes were homogenized in DEA buffer (0.2% diethylamine, 50 mm NaCl; 1 ml/200 mg tissue) with complete protease inhibitor cocktail (Sigma). After centrifugation (20,800g × 30 min), supernatants were neutralized with Tris-HCl, pH 6.2 (‘Tris extract’). Pellets were resuspended in 1% SDS-PBS and centrifuged (supernatants, ‘SDS extract’). Oligomeric Aβ was assayed in Tris extracts by oligo-specific MOAB-2 ELISA (Biosensis, Thebarton, Australia). Aβ 38, −40, −42 fragments were assayed by Peptide Panel 1 (4G8) Kit V-PLEX™ (Meso Scale Discovery, Rockville, MD).
2.6. Statistical Analyses
Mouse: Two-way ANOVA and post-doc t-test by GraphPad Prism version 5; Human: STATA 13 (StataCorp, College Station, TX) and SPSS 22.0. APOE allele association with number of microbleeds was analyzed by Kruskal-Wallis test. All analyses were observer-blinded.
3. Results
3.1. Microbleeds show opposite sex bias in human and mice
3.1.1. Humans
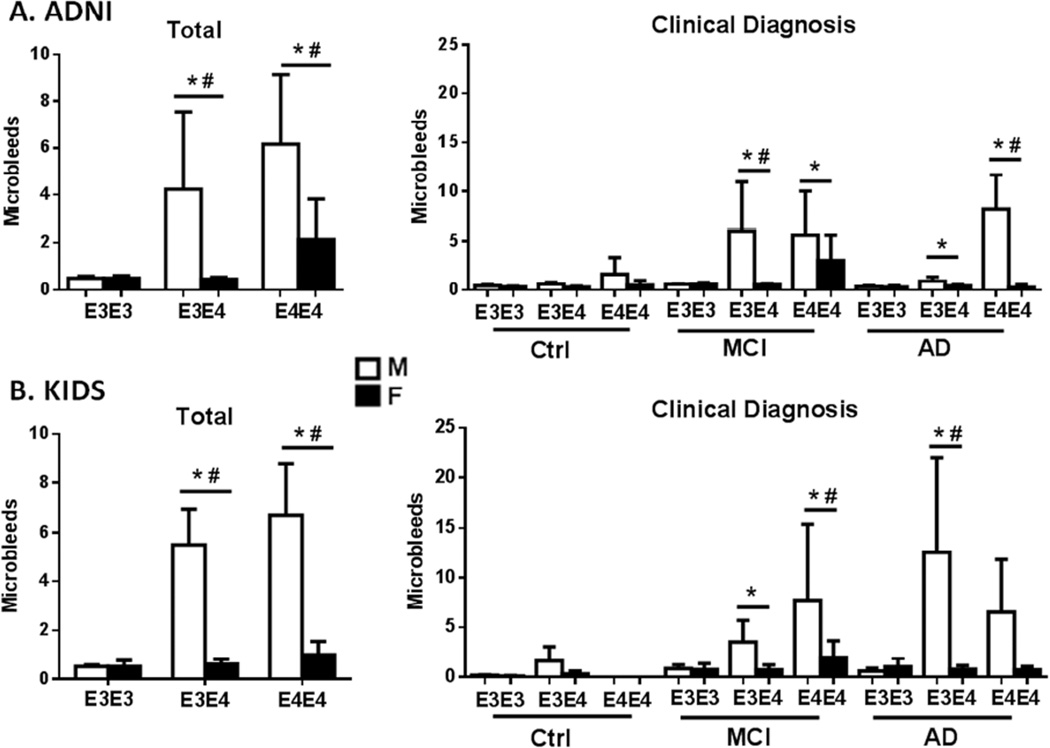
Because sex-APOE interactions for human microbleeds have not been reported, we interrogated two clinical MRI studies of well-characterized elderly for microbleeds: the ADNI Study (Alzheimer’s Disease Neuroimaging Initiative), a longitudinal MRI study of a large North American cohort, and the Karolinska Imaging Dementia Study (KIDS), a cross-sectional cohort study (Methods). In both ADNI and KIDS, APOE3 homozygotes did not differ by sex in microbleeds. Both ADNI and KIDS male subjects carrying APOE4 had more microbleeds; for both sexes, APOE4 homozygotes had more microbleeds (Fig.1A, B, left panels). Only men showed an APOE4 allele interaction with the microbleed burden (Table 1C). After stratification by clinical diagnosis, only the MCI and AD subjects had significant male-APOE4 excess of microbleeds (Fig.1A, B, right panels).
Figure 1. Human male APOE4 carriers have more cerebral microbleeds.
MRI analysis of ADNI and KIDS clinical cohorts showed male excess of microbleeds, increasing with APOE4 allele dose. APOE3 did not differ by sex in microbleed numbers. In both studies, a minority of subjects had microbleeds: ADNI, 35%; KIDS, 19%.Data are shown as total sample (left panel) and by diagnostic group (right) for mean number of microbleeds ± 95% CI. Table 1 shows statistical findings and subject numbers by diagnostic group and APOE allele. Both studies used a negative binomial regression model adjusted for age and a diagnostic group specified for each study. Ctrl: control group; MCI: mild cognitive impairment; AD: Alzheimer Disease. A. ADNI, the Alzheimer Disease Neuroimaging Initiative, representing the U.S.A and Canada. Total subjects (N= 658); clinical diagnosis: Ctrl, cognitively normal; MCI, mild cognitive impairment; and clinical AD. MRI sequence: GRE-T2*; MRI field strength, 3T. B. KIDS, Karolinska Imaging Dementia Study. Total subjects (N=448); clinical diagnosis: Ctrl, subjective cognitive impairment (not clinical grade); MCI, and clinical AD; same MRI as ADNI. *, APOE allele dose effect; #, APOE allele- male sex interaction (see Table1 for details).
Table 1.
Statistical analysis of human cohorts
| A. Demography | ||
|---|---|---|
| ADNI (%) | KIDS (%) | |
| Patients | 658 | 448 |
| Age (years) | ||
| Total | 48–91, median 73 (IQR: 67–78) | 36–88, median 62 (IQR 57–66) |
| Control | 56–90, median 74 (IQR: 70–79) | 36–79, median 57 (IQR 53–63) |
| MCI | 48–89, median 72 (IQR: 66–77) | 40–81, median 63 (IQR: 57–67) |
| AD | 56–91, median 76 (IQR: 70–80) | 50–88, median 64 (IQR: 60–72) |
| Male | 363 (53) | 183 (41) |
| Patients with microbleeds | 228 (35) | 88 (19) |
| APOE 3/3h | 335 (51) | 206 (46) |
| APOE 3/4 | 249 (38) | 174 (39) |
| APOE 4/4 | 74 (11) | 68 (15) |
| Control | 166 (25) | 144 (32) |
| MCI | 402 (61) | 152 (34) |
| AD | 90 (14) | 152 (34) |
| B. Clinical Diagnosis | ||||||
|---|---|---|---|---|---|---|
| ADNI | KIDS | |||||
| APOE allele |
E3/E3 | E3/E4 | E4/E4 | E3/E3 | E3/E4 | E4/E4 |
| Ctrl (M/F) | 58/51 | 22/30 | 3/2 | 26/47 | 21/36 | 2/4 |
| MCI (M/F) | 106/95 | 91/63 | 29/18 | 27/23 | 22/41 | 10/29 |
| AD (M/F) | 10/15 | 24/19 | 15/7 | 38/38 | 29/25 | 8/15 |
| C. Sex differences | ||||
|---|---|---|---|---|
| ADNI | KIDS | |||
| Male excess | ||||
| coeff | p-value | coeff | p-value | |
| Total | 0.61 | 0.003 | 1.7 | <0.001 |
| Control | 1.73 | <0.001 | ||
| MCI | 1.12 | <0.001 | ||
| AD | 0.9 | 0.07 | 2.02 | <0.001 |
| D. APOE allele interactions | ||||||||
|---|---|---|---|---|---|---|---|---|
| ADNI | KIDS | |||||||
| APOE allele |
E3/E4 | E4/E4 | E3/E4 | E4/E4 | ||||
| coeff | p-value | coeff | p-value | coeff | p-value | coeff | p-value | |
| APOE allele dose * | ||||||||
| Total | 1.4 | 0.003 | 2.4 | <0.001 | 0.6 | <0.001 | 0.8 | <0.001 |
| Control | 2.26 | <0.001 | ||||||
| MCI | 1.7 | <0.001 | 2.2 | <0.001 | 1.2 | <0.001 | ||
| AD | 1.2 | 0.04 | 3.1 | <0.001 | 0.69 | 0.004 | ||
| APOE allele-MALE sex interaction # | ||||||||
| Total | 1.6 | <0.001 | 1.6 | =0.007 | 0.6 | <0.001 | 0.8 | 0.02 |
| Control | 1.8 | 0.06 | ||||||
| MCI | 1.9 | 0.001 | 1.2 | 0.04 | ||||
| AD | 3.0 | =0.02 | 1.6 | <0.001 | 0.7 | <0.08 | ||
Coeff: coefficient of regression analysis. Non-significant values are not shown.
3.1.2 Mice
In EFAD mice, cerebral cortex microbleeds had the opposite sex bias from humans. By Prussian blue histochemistry, females had 2-fold more microbleeds, with 5-fold greater total hemosiderin (Fig. 2A–C). Only female EFAD mice showed APOE4 allele effects for total hemosiderin (Fig. 2C).
We also analyzed for sex effects that may be independent of FAD genes. The APOE-TR siblings without FAD transgenes in these groups also had a 2-fold female excess of microbleeds. However, their 20-fold lower levels did not show significant APOE allele effects (Fig.1S). 7 month old C57BL/6 wildtype mice from an independent sample also had cerebral microbleeds in both sexes at very low levels and of small size; these low levels did not allow resolution of possible sex differences (not shown).
3.2. EFAD associations of Aβ levels with microbleeds and CAA
Because microbleeds are associated with CAA in humans (Premkumar et al., 1996; Yates et al., 2014) and in mice (Zipfel et al., 2009), and because Aβ peptide levels drove leptomeningeal CAA in mice above a threshold Aβ level (Han et al., 2008; Zipfel et al., 2009), we evaluated effects of APOE alleles and sex on these relationships in EFAD mice.
3.2.1. CAA
In cerebral cortex, Aβ immunoreactivity exhibited a patchy pattern within morphologically identified vessels (Fig. 3A). Thioflavin S staining for fibrillar amyloid showed a similar pattern characteristic of CAA (Fig. 3A). Female EFAD mice had more Aβ positive vessels, with additive effects of APOE4 (Fig. 3B). The Aβ load per vessel was higher in E4FAD, with no significant sex effect (Fig. 3C).
3.2.2. Plaque Aβ
In cerebral cortex, female EFAD mice had 2-fold more Aβ as plaque deposits than males for both APOE alleles (Fig.4A–C). Female sex and the APOE4 allele had additive effects for plaque number (Fig. 4B) and for Aβ load (total immunoreactive area) (Fig. 4C). Plaque size differed 5-fold by sex, with no additive APOE allele effect (modal frequency size in µm2: 127, male E3FAD; 146, male E4; 1020, female E3; 1320, female E4).
3.2.3. Soluble Aβ peptides
Oligomeric Aβ42 was higher in Tris-extracts of cerebral cortex (no detergents) for E4FAD mice (Fig. 4D), with effects of APOE4 but not sex. For total soluble Aβ in Tris-extracts, females of both APOE alleles had higher levels of Aβ38, −40, and −42, with the greatest female excess for Aβ40 by >7-fold (Fig. 4D). Sequential extraction of the initial Tris-extracted pellet with SDS-PBS also showed the highest Aβ levels in female E4FAD (Fig. 4F). The Aβ42:40 ratios did not differ by sex or APOE allele (Fig. 4 legend).
3.2.4. Correlations of cerebrovascular pathology with Aβ
Most hemosiderin puncta (67%) resided within Aβ immunopositive deposits (Fig. 5A), which represent a minority of all Aβ plaques (15%). Some hemosiderin deposits (33%) were not embedded within immunodetectable Aβ, which we designate as ‘naked microbleeds’.
Only female EFAD mice showed a significant correlation of microbleed numbers with total Aβ load in immunohistochemically defined plaques (Fig. 5B). The number of microbleeds was chosen for this correlation analysis in parallel with the human MRI studies (Fig.1). Since the effect of sex was stronger than APOE alleles, data were combined for both APOE alleles. Besides immunohistochemically detected Aβ, other Aβ fractions also showed correlation with microbleeds, again restricted to females but with lower statistical strength, in rank order: Aβ plaque number >PBS extracted Aβ42 > PBS extracted Aβ40 >oligomeric Aβ42 (Fig. 5 legend). A threshold level of Aβ plaque load for a linear correlation with microbleeds was determined iteratively to be significant at ≥1.70 Aβ units (Fig. 5 legend). The CAA load also strongly correlated with Aβ plaque load for both APOE alleles, again only in females (Fig. 5C). Because different cohorts of mice were analyzed for microbleeds or CAA with Aβ, it was not possible to examine correlations of CAA and microbleeds in this study.
4. Discussion
We report novel sex-APOE allele interactions for cerebral cortex microbleeds and for brain Aβ amyloid levels with species differences. Microbleeds were analyzed from two clinical MRI imaging studies from different national populations (ADNI, U.S and Canada; KIDS, Sweden). In both ADNI and KIDS, elderly men with clinical grade symptoms of MCI or AD had 2-fold more microbleeds than women, with APOE4 allele effects. The control groups had much fewer microbleeds in both ADNI and KIDS and did not show sex or APOE allele associations. The male bias for microbleeds confirms prior findings (Shams et al., 2015; Benedictus et al., 2015). This sex-bias is opposite to the >50% higher risk of AD in women carrying APOE4 (Altmann et al., 2014; Corder et al., 2004).
In contrast to these human findings, EFAD mice showed female APOE4 excess of microbleeds with correspondingly higher CAA and Aβ levels. The strong correlation of CAA and microbleeds with the levels of Aβ in EFAD female mice does not appear to generalize to human AD. Although APOE4 carriers have excess Aβ (Altmann et al., 2014; Corder et al., 2004; Damoiseaux et al., 2012; Reznick et al., 2015), women have fewer microbleeds than men (Shams et al., 2015; Benedictus et al., 2015; this study). Thus, APOE4 has sex-specificity for different causes of cognitive decline, with greater cerebrovascular contributions in men than in women. The neurobiological basis for these sex-APOE allele interactions and the species differences is unknown.
The excess of microbleeds in APOE4 men suggests its role in the human male excess of stroke (Go et al., 2013) and of vascular disease generally (Beltran-Sanchez et al. 2015). Population-based studies (Ding et al., 2015’ Jeerakathil et al., 2004; Romero et al., 2012; Sveinbjornsdottir et al., 2008) and memory clinic-based cohorts (Shams et al., 2015) also showed male bias of microbleeds, but did not assess sex-APOE4 interactions. The differing sex bias between mice and humans for microbleeds could reflect the influence of hypertension interacting with CAA. Hypertension is more prevalent in men (Piper et al., 2014), whereas hypertension during aging is not reported for most mouse genotypes. Future studies could consider ethnic differences of sex-APOE allele interactions in cerebrovascular pathology, e.g. Asians generally have a lower prevalence of CAA than Caucasians (Chen et al., 2010).
Cerebral atrophy also shows sex differences, but with fewer sex-APOE interactions. In ADNI, older women incurred 1% faster cerebral cortex atrophy than men, with greatest effect in MCI and in proportion to APOE4 allele dose (Hua et al., 2010), but with no sex-APOE4 interaction (Xue Hua, pers. comm.). Similarly, a study of healthy elderly did not find sex-APOE effects on grey matter volume, despite strong sex-APOE interactions in functional brain connectivity (Damoiseaux et al., 2012). These sex differences also imply mechanisms of neuronal atrophy that are independent of focal vascular pathology.
The mouse models showed new cerebrovascular features. Two transgenic APOE models, EFAD and APOE-TR developed cortical microbleeds with female bias in 100% of the mice by 7 months. In contrast, only a minority of elderly human patients showed microbleeds with male bias (35% ADNI; 18.5% KIDS; Fig.1 legend). Female EFAD mice showed strong correlations of microbleeds with the total Aβ load above an Aβ threshold level (Fig. 5). These Aβ-microbleed correlations extend those for single FAD gene transgenic mice with endogenous murine APOE, in which vascular Aβ levels in leptomeningeal arteries correlated with impaired vasodilation (sex unspecified) (Han et al., 2008; Zipfel et al., 2009). These authors hypothesized that increasing microvascular Aβ levels above a threshold can drive a pathological progression, from impaired dilation to microaneurysms and white matter lesions (Dumas et al., 2012; Peca et al., 2013). Future human in vivo imaging studies could evaluate possible Aβ thresholds for microbleeds.
Intriguingly, wildtype C57BL/6 mice, the background ‘wildtype’ strain, also developed microbleeds within the lower range of APOE-TR, confirming Liu et al., (2014). With human transgenic Aβ at high levels, the EFAD mice showed a 20-fold increase of microbleeds. Because a subgroup of 18 month and older APOE-TR mice developed gross cerebral hemorrhages in association with thioflavin-positive CAA (Sullivan et al., 2008), we conclude that the endogenous murine Aβ is permissive for cerebrovascular pathology in the presence of human APOE. In APOE-TR mice age 7 months, we did not detect amyloid by 4G8 immunostaining or thioflavin histochemistry (not shown). Analysis of a full range of ages in APOE-TR mice may reveal transitional stages from microbleeds to gross hemorrhages and the onset age of sex-APOE allele interactions. The rapid development of Aβ deposits and CAA in EFAD mice by 3–7 months gives a model for Aβ-driven changes, distinct from neuroinflammatory changes that arise during middle-age in healthy mice.
Most microbleeds in EFAD mice were co-localized within Aβ deposits, also seen in another FAD model by dye-visualization of blood leaks (Tanifum et al., 2014). The correlations of microbleed numbers and of the CAA load with human Aβ suggest that increased Aβ levels drive cerebrovascular degeneration with blood-brain barrier (BBB) leakage. Moreover, extravasated blood can induce Aβ deposition. We observed that one-third of microbleeds did not co-localize with immunohistochemically defined Aβ deposits, which we designate as ‘naked microbleeds’. Because trace bleeds from a fine needle can induce Aβ deposits in FAD mice (Chuang et al., 2012), we hypothesize that naked microbleeds may initiate local Aβ plaque genesis. Thus, the progressive leakiness of the BBB in normal human aging (Montagne et al., 2015) which is increased by APOE4 (Bell et al., 2012) could contribute to pre-clinical Aβ.
CAA is incompletely documented for sex-APOE interactions. This gap may reflect different operational definitions for sporadic CAA, as a pathological entity, a clinical syndrome (e.g. symptomatic intracerebral hemorrhage, cognitive impairment), or as a neuroimaging phenotype of small vessel disease markers. APOE4 associations with CAA as reported (Nelson et al., 2013; Premkumar et al., 1996; Walker et al., 2000; Yip, et al., 2005; Olichney et al., 2000; Berg et al., 1998), however were not replicated by others (Love et al., 2003; Xu et al., 2003). In EFAD mice, CAA showed independent effects of sex and APOE4. The stronger correlation of total cortical Aβ and CAA loads in females more than males of both APOE genotypes was paralleled by correlations of microbleeds with total Aβ (Fig. 5). The sex-specific correlation of both microbleeds and CAA with total Aβ load implies causal links to the female excess Aβ in human AD and in FAD mice. We note several caveats. By identifying vessels based upon morphological appearance, we may have missed smaller Aβ-containing vessels, thus underestimating total CAA. We also note a potential issue in interpreting findings from ADNI and KIDS groups of post-menopausal women with much lower sex steroid levels than EFAD mice at age 7 months before major decreases of sex steroids. However, young E3FAD and E4FAD female mice showed divergent Aβ responses to estrogen (Kunzler et al., 2014).
Could developmental variations of sex steroids alter the penetrance of APOE4 in AD risk? In FAD mice, blood levels of sex steroids influence neurodegenerative changes (Carroll et al., 2010; Rosario et al., 2010). Besides these ‘activating’ effects, sex steroids have ‘organizational’ effects on brain development that extend to Aβ deposits. As shown by the Pike lab, the ‘masculinization of neonatal female FAD mice by testosterone decreased adult brain Aβ; conversely, demasculinization of male neonates with flutamide increased adult Aβ (Rosario et al., 2010). Early sex differences include the greater vulnerability of neonatal male mouse neurons to hypoxia in primary culture (Fairbanks et al., 2013). In human congenital adrenal hyperplasia (CAH) syndrome, fetal exposure to excess androgens alters sex-linked behaviors (Hines et al., 2015) and thus might alter sex differences in CAA and AD. Cerebral arteries may also be sensitive to organizational effects of sex steroids, e.g. aneurysms, which are male biased in humans and mice, can be induced in female mice by neonatal androgenization (Zhang et al., 2012). Sex differences in cerebral vascular biology are unexplored.
Sex-APOE4 interactions may be relevant to AD as a human-specific disease (Finch and Austad, 2015), because only humans have APOE isoforms with differing lipid binding characteristics (Fullerton et al., 2000; McIntosh et al., 2012). The APOE4 allele was present in earlier Homo at least 600,000 years ago, while APOE3 emerged about 225,000 years ago (Fullerton et al., 2000). The persistence of APOE4 in modern populations, despite its later costs to lifespan and brain aging, may be due to benefits to young ages. APOE4 women have higher luteal phase blood progesterone (Jasienska et al., 2015), which may enhance fertility. Moreover, APOE4 may be adaptive in immunity by higher cytokine responses (Finch and Morgan, 2007) and in pathogen resistance (Azevedo et al. 2014; Finch and Martin, 2015; Rougeron et al., 2013).
Species differences in CAA and microbleeds may involve both APP and APOE. Mice and humans differ in Aβ sequence at three sites, which decrease aggregation and cytotoxicity (Boyd-Kimball et al., 2004; De Strooper et al., 1995). Because brain amyloid fibrils are unknown in aging wildtype mice, their presence in APOE-TR mice by 18 months (Sullivan et al., 2008) implies unknown interactions of human APOE with endogenous murine APP, possibly through allele-specific affinity binding to APP that modulates Aβ production (Theendakara et al., 2013).
5. Conclusion
For both humans and mice, APOE4 interacted with sex differences of microbleeds and Aβ levels. Comparison of human brain aging with mouse models reveals major gaps in our understanding of sex differences across species: aging men incur several-fold more microbleeds than women, opposite to male EFAD mice which had several-fold fewer. For CAA, the EFAD mice showed modest female excess in prevalence of Aβ-positive vessels, agreeing with some postmortem human studies. Because the mouse sex differences in brain Aβ and in aortic aneurysms are sensitive to organizational effects of steroids, we suggest that outcomes of cerebrovascular aging may also be shaped by sex steroids during development. This could be evaluated with postmortem brain studies of women exposed to congenital adrenal hyperplasia who show partial masculinization of behavior. Meanwhile, we need further analysis of sex-APOE interactions for CAA. The NIH directive of May 2014 to include both sexes in preclinical studies should soon expand the small data base on sex differences in brain aging. The greater risk of male APOE4 carriers for microbleeds may be a target for genotype informed therapy to reduce this contribution to cognitive decline.
Supplementary Material
Number of microbleeds per unit of cortical area (100 mm2) for APOE-TR (APOE3, APOE4) and EFAD mice. Female excess was observed in APOE4 mice and both EFAD mice. Mean ± SEM, N=6–8/group; * p<0.05
Highlights.
-
*
Elderly humans with MCI and AD had male sex-APOE4 bias for microbleeds, opposite to female Aβ bias
-
*
E4FAD mice had female bias of microbleeds and Aβ
-
*
In EFAD mice, CAA and microbleeds increase proportionately with Aβ
Acknowledgements
Experimental studies on mice were supported by the NIA: R21-AG040683 (CEF); P01-AG026572 (R.D.Brinton): Project 2 (CEF), Project 3 (CJP). EFAD mice were generously given by Mary Jo LaDu (Univ. Illinois at Chicago). APOE-TR mice were generated by Dr. Patrick Sullivan (Duke). Imaging studies were supported by the NIA: P01-AG05142 (H. Chui). Alzheimer's Disease Neuroimaging Initiative (ADNI) (NIA U01 AG-024904; DOD W81XWH-12-2-0012). ADNI is also funded by the National Institute of Biomedical Imaging and Bioengineering, and through generous contributions from: Alzheimer’s Association; Alzheimer’s Drug Discovery Foundation; Araclon Biotech; BioClinica, Inc.; Biogen Idec Inc.; Bristol-Myers Squibb Company; Eisai Inc.; Elan Pharmaceuticals, Inc.; Eli Lilly; EuroImmun; F. Hoffmann-La Roche Ltd and Genentech; Fujirebio; GE Healthcare; ; IXICO Ltd.; Janssen Alzheimer Immunotherapy Research & Development, LLC.; Johnson & Johnson Pharmaceutical Research & Development; Medpace, Inc.; Merck & Co., Inc.; Meso Scale Diagnostics; NeuroRx Research; Neurotrack Technologies; Novartis Pharmaceuticals; Pfizer Inc.; Piramal Imaging; Servier; Synarc; and Takeda Pharmaceutical Company. The Canadian Institutes of Health Research funds ADNI sites in Canada. Private contributions are facilitated by the Foundation for the NIH (www.fnih.org). The grantee organization is the Northern California Institute for Research and Education. The study is coordinated by the Alzheimer's Disease Cooperative Study at UC San Diego. ADNI data are disseminated by the Laboratory for NeuroImaging at USC. Data used in preparation of this article were obtained from the ADNI database (adni.loni.usc.edu). As such, the investigators within the ADNI contributed to the design and implementation of ADNI and/or provided data but did not participate in analysis or writing of this report. For ADNI investigators see: http://adni.loni.usc.edu/wp-ontent/uploads/how_to_apply/ADNI_Acknowledgement_List.pdf
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors have no conflicts of interest to disclose.
References
- Allen N, Robinson AC, Snowden J, Davidson YS, Mann DM. Patterns of cerebral amyloid angiopathy define histopathological phenotypes in Alzheimer's disease. Neuropathol. Appl. Neurobiol. 2014;40:136–148. doi: 10.1111/nan.12070. [DOI] [PubMed] [Google Scholar]
- Altmann A, Tian L, Henderson VW, Greicius MD Alzheimer's Disease Neuroimaging Initiative, I. Sex modifies the APOE-related risk of developing Alzheimer disease. Ann. Neurol. 2014;75:563–573. doi: 10.1002/ana.24135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attems J, Jellinger KA, Lintner F. Alzheimer's disease pathology influences severity and topographical distribution of cerebral amyloid angiopathy. Acta Neuropathol. 2005;110:222–231. doi: 10.1007/s00401-005-1064-y. [DOI] [PubMed] [Google Scholar]
- Azevedo OG, Bolick DT, Roche JK, Pinkerton RF, Lima, A.A, Vitek MP, Warren CA, Oriá RB, Guerrant RL. Apolipoprotein E plays a key role against cryptosporidial infection in transgenic undernourished mice. PLoS One. 2014;9(2):e89562. doi: 10.1371/journal.pone.0089562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes LL, Wilson RS, Bienias JL, Schneider JA, Evans DA, Bennett DA. Sex differences in the clinical manifestations of Alzheimer disease pathology. Arch. Gen. Psych. 2005;62:685–691. doi: 10.1001/archpsyc.62.6.685. [DOI] [PubMed] [Google Scholar]
- Bell RD, Winkler EA, Singh I, Sagare AP, Deane R, Wu Z, Holtzman DM, et al. Apolipoprotein E controls cerebrovascular integrity via cyclophilin A. Nature. 2012;485:512–516. doi: 10.1038/nature11087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beltrán-Sanchez H, Finchm CE, Crimmins EM. The 20th Century surge of excess adult male mortality. Proc. Nat’l. Acad. Sci. 2015;112:8993–8998. doi: 10.1073/pnas.1421942112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benedictus MR, Goos JD, Binnewijzend MA, Muller M, Barkhof F, Scheltens P, Prins ND, van der Flier WM. Specific risk factors for microbleeds and white matter hyperintensities in Alzheimer's disease. Neurobiol. Aging. 2013;34:2488–2494. doi: 10.1016/j.neurobiolaging.2013.04.023. [DOI] [PubMed] [Google Scholar]
- Berg L, McKeel DW, Jr, Miller JP, Storandt M, Rubin EH, Morris JC, et al. Clinicopathologic studies in cognitively healthy aging and Alzheimer's disease: relation of histologic markers to dementia severity, age, sex, and apolipoprotein E genotype. Arch. Neurol. 1998;55:326–335. doi: 10.1001/archneur.55.3.326. [DOI] [PubMed] [Google Scholar]
- Boyd-Kimball D, Sultana R, Mohmmad-Abdul H, Butterfield DA. Rodent Abeta(1–42) exhibits oxidative stress properties similar to those of human Abeta(1–42): Implications for proposed mechanisms of toxicity. J. Alz. Dis. 2004:515–525. doi: 10.3233/jad-2004-6509. [DOI] [PubMed] [Google Scholar]
- Caracciolo B, Gatz M, Xu W, Pedersen NL, Fratiglioni L. Differential distribution of subjective and objective cognitive impairment in the population: a nation-wide twin-study. J. Alz. Dis. 2012;29:393–403. doi: 10.3233/JAD-2011-111904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JC, Rosario ER, Kreimer S, Villamagna A, Gentzschein E, Stanczyk FZ, Pike CJ. Sex differences in beta-amyloid accumulation in 3xTg-AD mice: role of neonatal sex steroid hormone exposure. Brain. Res. 2010;1366:233–245. doi: 10.1016/j.brainres.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Lee MJ, Smith EE. Cerebral amyloid angiopathy in East and West. Int. J. Stroke. 2010;5:403–411. doi: 10.1111/j.1747-4949.2010.00466.x. [DOI] [PubMed] [Google Scholar]
- Chuang JY, Lee CW, Shih YH, Yang T, Yu L, Kuo YM. Interactions between amyloid-beta and hemoglobin: implications for amyloid plaque formation in Alzheimer's disease. PloS one. 2012;7:e33120. doi: 10.1371/journal.pone.0033120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corder EH, Ghebremedhin E, Taylor MG, Thal DR, Ohm TG, Braak H. The biphasic relationship between regional brain senile plaque and neurofibrillary tangle distributions: modification by age, sex, and APOE polymorphism. Ann. N. Y. Acad. Sci. 2004;1019:24–28. doi: 10.1196/annals.1297.005. [DOI] [PubMed] [Google Scholar]
- Damoiseaux JS, Seeley WW, Zhou J, Shirer WR, Coppola G, Karydas A, et al. Alzheimer's Disease Neuroimaging, I. Gender modulates the APOE epsilon4 effect in healthy older adults: convergent evidence from functional brain connectivity and spinal fluid tau levels. J. Neurosci. 2012;32:8254–8262. doi: 10.1523/JNEUROSCI.0305-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Strooper B, Simons M, Multhaup G, Van Leuven F, Beyreuther K, Dotti CG. Production of intracellular amyloid-containing fragments in hippocampal neurons expressing human amyloid precursor protein and protection against amyloidogenesis by subtle amino acid substitutions in the rodent sequence. EMBO J. 1995;14:4932–4938. doi: 10.1002/j.1460-2075.1995.tb00176.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J, Sigurdsson S, Garcia M, Phillips CL, Eiriksdottir G, Gudnason V, et al. Risk Factors Associated With Incident Cerebral Microbleeds According to Location in Older People: The Age, Gene/Environment Susceptibility (AGES)-Reykjavik Study. JAMA Neurol. 2015;72:682–688. doi: 10.1001/jamaneurol.2015.0174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donham RS, Stetson MH. Neonatal androgen abolishes clock-timed gonadotrophin release in prepubertal and adult female hamsters. J. Repr. Fert. 1985;73:215–221. doi: 10.1530/jrf.0.0730215. [DOI] [PubMed] [Google Scholar]
- Dubal DB, Broestl L, Worden K. Sex and gonadal hormones in mouse models of Alzheimer's disease: what is relevant to the human condition? Biol. Sex. Diff. 2012;3:24. doi: 10.1186/2042-6410-3-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumas A, Dierksen GA, Gurol ME, Halpin A, Martinez-Ramirez S, Schwab K, et al. Functional magnetic resonance imaging detection of vascular reactivity in cerebral amyloid angiopathy. Ann. Neurol. 2012;72:76–81. doi: 10.1002/ana.23566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fairbanks SL, Vest R, Verma S, Traystman RJ, Herson PS. Sex stratified neuronal cultures to study ischemic cell death pathways. J. Vis. Exp. 2013;82:e50758. doi: 10.3791/50758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer LA, Cupples LA, Haines JL, Hyman B, Kukull WA, Mayeux R, et al. Effects of age, sex, and ethnicity on the association between apolipoprotein E genotype and Alzheimer disease. A meta-analysis. APOE and Alzheimer Disease Meta Analysis Consortium. JAMA. 1997;278:1349–1356. [PubMed] [Google Scholar]
- Finch CE. Evolution of the human lifespan and diseases of aging: roles of infection, inflammation, and nutrition. Proc. Natl. Acad. Sci. USA. 2010;107(Suppl 1):1718–1724. doi: 10.1073/pnas.0909606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Austad SN. Commentary: is Alzheimer's disease uniquely human? Neurobiol. Aging. 2015;36:553–555. doi: 10.1016/j.neurobiolaging.2014.10.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finch CE, Morgan TE. Systemic inflammation, infection, ApoE alleles, and Alzheimer disease: a position paper. Curr. Alz. Res. 2007;42:185–189. doi: 10.2174/156720507780362254. [DOI] [PubMed] [Google Scholar]
- Finch CE, Martin GM. Dementias of the Alzheimer type: views through the lens of evolutionary biology suggest amyloid-driven brain aging is a trade-off with host defence. In: Alvergne A, editor. Evolutionary thinking in medicine: from research to policy and practice. 2015. [Google Scholar]
- Fleisher A, Grundman M, Jack CR, Jr, Petersen RC, Taylor C, Kim HT, Schiller, et al. Sex, apolipoprotein E epsilon 4 status, and hippocampal volume in mild cognitive impairment. Arch. Neurol. 2005;62:953–957. doi: 10.1001/archneur.62.6.953. [DOI] [PubMed] [Google Scholar]
- Fryer JD, Simmons K, Parsadanian M, Bales KR, Paul SM, Sullivan PM, Holtzman DM. Human apolipoprotein E4 alters the amyloid-beta 40:42 ratio and promotes the formation of cerebral amyloid angiopathy in an amyloid precursor protein transgenic model. J. Neurosci. 2005;25:2803–2810. doi: 10.1523/JNEUROSCI.5170-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fullerton SM, Clark AG, Weiss KM, Nickerson DA, Taylor SL, et al. Apolipoprotein E variation at the sequence haplotype level: implications for the origin and maintenance of a major human polymorphism. Am. J. Hum. Gen. 2000;67:881–900. doi: 10.1086/303070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gearing M, Rebeck GW, Hyman BT, Tigges J, Mirra SS. Neuropathology and apolipoprotein E profile of aged chimpanzees: implications for Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1994;91:9382–9386. doi: 10.1073/pnas.91.20.9382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert JJ, Vinters HV. Cerebral amyloid angiopathy: incidence and complications in the aging brain. I. Cerebral hemorrhage. Stroke. 1983;14:915–923. doi: 10.1161/01.str.14.6.915. [DOI] [PubMed] [Google Scholar]
- Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Borden WB, Bravata, et al. Heart disease and stroke statistics-2013 update: a report from the American Heart Association. Circulation. 2003;127:e6–e245. doi: 10.1161/CIR.0b013e31828124ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorski RA. The neuroendocrinology of reproduction: an overview. Biol. Reprod. 1979;20:111–127. doi: 10.1093/biolreprod/20.1.111. [DOI] [PubMed] [Google Scholar]
- Greenberg SM, Vernooij MW, Cordonnier C, Viswanathan A, Al-Shahi Salman R, et al. Cerebral microbleeds: a guide to detection and interpretation. Lancet. Neurol. 2009;8:165–174. doi: 10.1016/S1474-4422(09)70013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregg NM, Kim AE, Gurol ME, Lopez OL, Aizenstein HJ, Price JC, Mathis CA, et al. Incidental Cerebral Microbleeds and Cerebral Blood Flow in Elderly Individuals. JAMA Neurol. 2015;72:1021–1028. doi: 10.1001/jamaneurol.2015.1359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregoire SM, Chaudhary UJ, Brown MM, Yousry TA, Kallis C, Jager HR, Werring DJ. The Microbleed Anatomical Rating Scale (MARS): reliability of a tool to map brain microbleeds. Neurology. 2009;73:1759–1766. doi: 10.1212/WNL.0b013e3181c34a7d. [DOI] [PubMed] [Google Scholar]
- Han BH, Zhou ML, Abousaleh F, Brendza RP, Dietrich HH, Koenigsknecht-Talboo J, et al. Cerebrovascular dysfunction in amyloid precursor protein transgenic mice: contribution of soluble and insoluble amyloid-beta peptide, partial restoration via gamma-secretase inhibition. J. Neurosci. 2008;28:13542–13550. doi: 10.1523/JNEUROSCI.4686-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hines M, Constantinescu M, Spencer D. Early androgen exposure and human gender development. Biol. Sex. Diff. 2015;6:3. doi: 10.1186/s13293-015-0022-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua X, Hibar DP, Lee S, Toga AW, Jack CR, Jr, Weiner MW, et al. Sex and age differences in atrophic rates: an ADNI study with n=1368 MRI scans. Neurobiol. Aging. 2010;31:1463–1480. doi: 10.1016/j.neurobiolaging.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jasienska G, Ellison PT, Galbarczyk A, Jasienski M, Kalemba-Drozdz M, Kapiszewska M, et al. Apolipoprotein E (ApoE) polymorphism is related to differences in potential fertility in women: a case of antagonistic pleiotropy? Proc. Biol. Sci. Royal. Soc. 2015;282:2014–2395. doi: 10.1098/rspb.2014.2395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman A, Carroll JC, Morgan TE, Lin S, Zhao L, Arimoto JM, et al. 17beta-estradiol and progesterone regulate expression of beta-amyloid clearance factors in primary neuron cultures and female rat brain. Endocrinology. 2012;153:5467–5479. doi: 10.1210/en.2012-1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jean SM, Preuss TM, Sharma P, Anderson DC, Provenzale JM, Strobert E, et al. Cerebrovascular accident (stroke) in captive, group-housed, female chimpanzees. Compar. Med. 2012;62:322–329. [PMC free article] [PubMed] [Google Scholar]
- Jeerakathil T, Wolf PA, Beiser A, Hald JK, Au R, Kase CS, et al. Cerebral microbleeds: prevalence and associations with cardiovascular risk factors in the Framingham Study. Stroke. 2014;35:1831–1835. doi: 10.1161/01.STR.0000131809.35202.1b. [DOI] [PubMed] [Google Scholar]
- Klein WL, Krafft GA, Finch CE. Targeting small Abeta oligomers: the solution to an Alzheimer's disease conundrum? Trends. Neurosci. 2014;24:219–224. doi: 10.1016/s0166-2236(00)01749-5. [DOI] [PubMed] [Google Scholar]
- Kunzler J, Youmans KL, Yu C, Ladu MJ, Tai LM. APOE modulates the effect of estrogen therapy on Aβ accumulation EFAD-Tg mice. Neurosci. Lett. 2014;560:131– 136. doi: 10.1016/j.neulet.2013.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SS, Stemmermann GN. Congophilic angiopathy and cerebral hemorrhage. Arch. Pathn. Lab. Med. 1978;102:317–321. [PubMed] [Google Scholar]
- Love S, Nicoll JA, Hughes A, Wilcock GK. APOE and cerebral amyloid angiopathy in the elderly. Neuroreport. 2003;14:1535–1536. doi: 10.1097/00001756-200308060-00027. [DOI] [PubMed] [Google Scholar]
- Liu S, Grigoryan MM, Vasilevko V, Sumbria RK, Paganini-Hill A, Cribbs DH, Fisher MJ. Comparative analysis of H&E and Prussian blue staining in a mouse model of cerebral microbleeds. J. Histochem. Cytochem. 2014;62:767–773. doi: 10.1369/0022155414546692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masuda J, Tanaka K, Ueda K, Omae T. Autopsy study of incidence and distribution of cerebral amyloid angiopathy in Hisayama, Japan. Stroke. 1988;19:205–210. doi: 10.1161/01.str.19.2.205. [DOI] [PubMed] [Google Scholar]
- McIntosh AM, Bennett C, Dickson D, Anestis SF, Watts DP, Webster TH, et al. The apolipoprotein E (APOE) gene appears functionally monomorphic in chimpanzees (Pan troglodytes) PloS one. 2012;7:e47760. doi: 10.1371/journal.pone.0047760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier IB, Gu Y, Guzaman VA, Wiegman AF, Schupf N, Manly JJ, et al. Lobar microbleeds are associated with a decline in executive functioning in older adults. Cerebrovasc. Dis. 2014;38:377–383. doi: 10.1159/000368998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, et al. Blood-brain barrier breakdown in the aging human hippocampus. Neuron. 2015;85:296–302. doi: 10.1016/j.neuron.2014.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mucke L, Selkoe DJ. Neurotoxicity of amyloid beta-protein: synaptic and network dysfunction. Cold. Spring. Harb. Perspect. Med. 2012;2:a006338. doi: 10.1101/cshperspect.a006338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson PT, Pious NM, Jicha GA, Wilcock DM, Fardo DW, et al. APOE-epsilon2 and APOE-epsilon4 correlate with increased amyloid accumulation in cerebral vasculature. J. Neuropathol. Exp. Neurol. 2013;72:708–715. doi: 10.1097/NEN.0b013e31829a25b9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oakley H, Cole SL, Logan S, Maus E, Shao P, Craft J, et al. Intraneuronal beta-amyloid aggregates, neurodegeneration, and neuron loss in transgenic mice with five familial Alzheimer's disease mutations: potential factors in amyloid plaque formation. J. Neurosci. 2006;26:10129–10140. doi: 10.1523/JNEUROSCI.1202-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olichney JM, Hansen LA, Lee JH, Hofstetter CR, Katzman R, Thal LJ. Relationship between severe amyloid angiopathy, apolipoprotein E genotype, and vascular lesions in Alzheimer's disease. Ann. N. Y. Acad. Sci. 2000;903:138–143. doi: 10.1111/j.1749-6632.2000.tb06360.x. [DOI] [PubMed] [Google Scholar]
- Payami H, Zareparsi S, Montee KR, Sexton GJ, Kaye JA, Bird TD, et al. Gender difference in apolipoprotein E-associated risk for familial Alzheimer disease: a possible clue to the higher incidence of Alzheimer disease in women. Am. J. Hum. Gen. 1996;58:803–811. [PMC free article] [PubMed] [Google Scholar]
- Peca S, McCreary CR, Donaldson E, Kumarpillai G, Shobha N, Sanchez K, et al. Neurovascular decoupling is associated with severity of cerebral amyloid angiopathy. Neurology. 2013;81:1659–1665. doi: 10.1212/01.wnl.0000435291.49598.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piper MA, Evans CV, Burda BU, Margolis KL, O'Connor E, Smith N, et al. Screening for High Blood Pressure in Adults: A Systematic Evidence Review for the US Preventive Services Task Force, Rockville (MD) 2014 [PubMed] [Google Scholar]
- Premkumar DR, Cohen DL, Hedera P, Friedland RP, Kalaria RN. Apolipoprotein E-epsilon4 alleles in cerebral amyloid angiopathy and cerebrovascular pathology associated with Alzheimer's disease. Am. J. Pathol. 1996;148:2083–2095. [PMC free article] [PubMed] [Google Scholar]
- Raber J, Wong D, Buttini M, Orth M, Bellosta S, Pitas RE, et al. Isoform-specific effects of human apolipoprotein E on brain function revealed in ApoE knockout mice: increased susceptibility of females. Proc. Natl. Acad. Sci. USA. 1998;95:10914–10919. doi: 10.1073/pnas.95.18.10914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raffai RL, Dong LM, Farese RV, Jr, Weisgraber KH. Introduction of human apolipoprotein E4 "domain interaction" into mouse apolipoprotein E. Proc. Natl. Acad. Sci. USA. 2001;98:11587–11591. doi: 10.1073/pnas.201279298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rannikmae K, Samarasekera N, Martinez-Gonzalez NA, Al-Shahi Salman R, Sudlow CL. Genetics of cerebral amyloid angiopathy: systematic review and meta-analysis. J. Neurol. Neurosurg. Psychi. 2013;84:901–908. doi: 10.1136/jnnp-2012-303898. [DOI] [PubMed] [Google Scholar]
- Rasool S, Martinez-Coria H, Wu JW, LaFerla F, Glabe CG. Systemic vaccination with anti-oligomeric monoclonal antibodies improves cognitive function by reducing Abeta deposition and tau pathology in 3xTg-AD mice. J. Neurochem. 2013;126:473–482. doi: 10.1111/jnc.12305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reijmer YD, Fotiadis P, Martinez-Ramirez S, Salat DH, Schultz A, Shoamanesh A, et al. Structural network alterations and neurological dysfunction in cerebral amyloid angiopathy. Brain. 2015;138:179–188. doi: 10.1093/brain/awu316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnick SM, Bilgel M, Moghekar A, An Y, Cai Q, Wang MC, et al. Changes in Aβ biomarkers and associations with APOE genotype in 2 longitudinal cohorts. Neurobiol. Aging. 2015;36:2333–2339. doi: 10.1016/j.neurobiolaging.2015.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero JR, Preis SR, Beiser AS, DeCarli C, Lee DY, Viswanathan A, et al. Lipoprotein phospholipase A2 and cerebral microbleeds in the Framingham Heart Study. Stroke. 2012;43:3091–3094. doi: 10.1161/STROKEAHA.112.656744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosario ER, Carroll J, Pike CJ. Testosterone regulation of Alzheimer-like neuropathology in male 3xTg-AD mice involves both estrogen and androgen pathways. Brain. Res. 2012;1359:281–290. doi: 10.1016/j.brainres.2010.08.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen RF, Farberg AS, Gearing M, Dooyema J, Long PM, Anderson DC, et al. Tauopathy with paired helical filaments in an aged chimpanzee. J. Compar. Neurol. 2008;509:259–270. doi: 10.1002/cne.21744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rougeron V, Woods CM, Tiedje KE, Bodeau-Livinec F, Migot-Nabias F, Deloron P, et al. Epistatic Interactions between apolipoprotein E and hemoglobin S Genes in regulation of malaria parasitemia. PloS one. 2013;8:e76924. doi: 10.1371/journal.pone.0076924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmechel DE, Saunders AM, Strittmatter WJ, Crain BJ, Hulette CM, Joo SH, et al. Increased amyloid beta-peptide deposition in cerebral cortex as a consequence of apolipoprotein E genotype in late-onset Alzheimer disease. Proc. Natl. Acad. Sci. USA. 1993;90:9649–9653. doi: 10.1073/pnas.90.20.9649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shams S, Martola J, Granberg T, Li X, Shams M, Fereshtehnejad SM, Cavallin L, et al. Cerebral microbleeds: different prevalence, topography, and risk factors depending on dementia diagnosis-the Karolinska Imaging Dementia Study. Am. J. Neuroradiol. 2015;36:661–666. doi: 10.3174/ajnr.A4176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith DD, Tan X, Tawfik O, Milne G, Stechschulte DJ, Dileepan KN. Increased aortic atherosclerotic plaque development in female apolipoprotein E-null mice is associated with elevated thromboxane A2 and decreased prostacyclin production. J. Physiol. Pharmac. 2010;61:309–316. [PMC free article] [PubMed] [Google Scholar]
- Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, et al. Vascular contributions to cognitive impairment and dementia including Alzheimer's disease. Alzheimers. Dement. 2015;11:710–717. doi: 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sturchler-Pierrat C, Staufenbiel M. Pathogenic mechanisms of Alzheimer's disease analyzed in the APP23 transgenic mouse model. Ann. N. Y. Acad. Sci. 2000;920:134–139. doi: 10.1111/j.1749-6632.2000.tb06915.x. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mace BE, Estrada JC, Schmechel DE, Alberts MJ. Human apolipoprotein E4 targeted replacement mice show increased prevalence of intracerebral hemorrhage associated with vascular amyloid deposition. J. Stroke. Cerebrovasc. Dis. 2008;17:303–311. doi: 10.1016/j.jstrokecerebrovasdis.2008.03.011. [DOI] [PubMed] [Google Scholar]
- Sullivan PM, Mezdour H, Aratani Y, Knouff C, Najib J, Reddick RL, et al. Targeted replacement of the mouse apolipoprotein E gene with the common human APOE3 allele enhances diet-induced hypercholesterolemia and atherosclerosis. J. Biol. Chem. 1997;272:17972–17980. doi: 10.1074/jbc.272.29.17972. [DOI] [PubMed] [Google Scholar]
- Sveinbjornsdottir S, Sigurdsson S, Aspelund T, Kjartansson O, Eiriksdottir G, Valtysdottir B, et al. Cerebral microbleeds in the population based AGES-Reykjavik study: prevalence and location. J. Neurol. Neurosurg. Psych. 2008;79:1002–1006. doi: 10.1136/jnnp.2007.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanifum EA, Starosolski ZA, Fowler SW, Jankowsky JL, Annapragada AV. Cerebral vascular leak in a mouse model of amyloid neuropathology. J. Cereb. Blood. Flow. Metab. 2014;34:1646–1654. doi: 10.1038/jcbfm.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theendakara V, Patent A, Peters Libeu CA, Philpot B, Flores S, Descamps O, et al. Neuroprotective Sirtuin ratio reversed by ApoE4. Proc. Natl. Acad. Sci. USA. 2013;110:18303–18308. doi: 10.1073/pnas.1314145110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vamathevan JJ, Hasan S, Emes RD, Amrine-Madsen H, Rajagopalan D, Topp SD, et al. The role of positive selection in determining the molecular cause of species differences in disease. BMC. Evol. Biol. 2008;8:273. doi: 10.1186/1471-2148-8-273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vest RS, Pike CJ. Gender, sex steroid hormones, and Alzheimer's disease. Horm. Behav. 2013;63:301–307. doi: 10.1016/j.yhbeh.2012.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Pahnke J, Madauss M, Vogelgesang S, Pahnke A, Herbst EW, et al. Apolipoprotein E4 promotes the early deposition of Abeta42 and then Abeta40 in the elderly. Acta. Neuropathol. 2000;100:36–42. doi: 10.1007/s004010051190. [DOI] [PubMed] [Google Scholar]
- Wardlaw JM, Smith EE, Biessels GJ, Cordonnier C, Fazekas F, Frayne R, et al. Neuroimaging standards for research into small vessel disease and its contribution to ageing and neurodegeneration. Lancet. Neurol. 2013;12:822–838. doi: 10.1016/S1474-4422(13)70124-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu D, Yang C, Wang L. Cerebral amyloid angiopathy in aged Chinese: a clinico-neuropathological study. Acta. Neuropathol. 2003;106:89–91. doi: 10.1007/s00401-003-0706-1. [DOI] [PubMed] [Google Scholar]
- Yates PA, Desmond PM, Phal PM, Steward C, Szoeke C, Salvado O, et al. Incidence of cerebral microbleeds in preclinical Alzheimer disease. Neurology. 2014;82:1266–1273. doi: 10.1212/WNL.0000000000000285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yip AG, McKee AC, Green RC, Wells J, Young H, Cupples LA, Farrer LA. APOE, vascular pathology, and the AD brain. Neurology. 2005;65:259–265. doi: 10.1212/01.wnl.0000168863.49053.4d. [DOI] [PubMed] [Google Scholar]
- Youmans KL, Tai LM, Nwabuisi-Heath E, Jungbauer L, Kanekiyo T, Gan M, et al. APOE4-specific changes in Abeta accumulation in a new transgenic mouse model of Alzheimer disease. J. Biol. Chem. 2012;287:41774–41786. doi: 10.1074/jbc.M112.407957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Thatcher SE, Rateri DL, Bruemmer D, Charnigo R, Daugherty A, Cassis LA. Transient exposure of neonatal female mice to testosterone abrogates the sexual dimorphism of abdominal aortic aneurysms. Circulation. Res. 2012;110:e73–e85. doi: 10.1161/CIRCRESAHA.111.253880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipfel GJ, Han H, Ford AL, Lee JM. Cerebral amyloid angiopathy: progressive disruption of the neurovascular unit. Stroke. 2009;40:S16–S19. doi: 10.1161/STROKEAHA.108.533174. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Number of microbleeds per unit of cortical area (100 mm2) for APOE-TR (APOE3, APOE4) and EFAD mice. Female excess was observed in APOE4 mice and both EFAD mice. Mean ± SEM, N=6–8/group; * p<0.05