Abstract
Background
Human clonorchiasis is a prevailing food-borne disease caused by Clonorchis sinensis infection. Functional characterizations of key molecules from C. sinensis could facilitate the intervention of C. sinensis associated diseases.
Methods
In this study, immunolocalization of C. sinensis cathepsin B proteases (CsCBs) in C. sinensis worms was investigated. Four CsCBs were expressed in Pichia pastoris yeast cells. Purified yCsCBs were measured for enzymatic and hydrolase activities in the presence of various host proteins. Cell proliferation, wound-healing and transwell assays were performed to show the effect of CsCBs on human cells.
Results
CsCBs were localized in the excretory vesicle, oral sucker and intestinal tract of C. sinensis. Recombinant yCsCBs from yeast showed active enzymatic activity at pH 5.0–5.5 and at 37–42 °C. yCsCBs can degrade various host proteins including human serum albumin, human fibronectin, human hemoglobin and human IgG. CsCBs were detected in liver tissues of mice and cancer patients afflicted with clonorchiasis. Various bioassays collectively demonstrated that CsCBs could promote cell proliferation, migration and invasion of human cancer cells.
Conclusion
Our results demonstrated that CsCBs can degrade various human proteins and we proved that the secreted CsCBs are involved in the pathogenesis of clonorchiasis.
Electronic supplementary material
The online version of this article (doi:10.1186/s13071-015-1248-9) contains supplementary material, which is available to authorized users.
Keywords: Clonorchis sinensis, Clonorchiasis, Cathepsin B, Pathogenesis
Background
Clonorchiasis is a food-borne parasitic disease caused by infection with Clonorchis sinensis (C. sinensis). Mammals are often infected with C. sinensis by consuming raw or uncooked fish or shrimp containing infectious metacercaria. Adult worms reside in the bile ducts of hosts and secreted products from C. sinensis eventually lead to clonorchiasis resulting in: cholangectasis, cholecystitis, cholelithiasis, hepatic fibrosis, and even liver cancer and bile duct cancer [1–3]. It is estimated that about 35 million people are afflicted with clonorchiasis, with most cases in Asian countries such as Korea, China and Vietnam [4, 5]. Food security problems caused by liver flukes have attracted high attention of public health, increasing the urgency of finding new approaches to prevent the spread of clonorchiasis. Clonorchiasis is listed among food-borne parasitic diseases requiring urgent control in China.
With the recent progress of the C. sinensis genome and transcriptome [6, 7], scientific researchers have expended much effort to elucidate the underlying mechanism of carcinogenic liver fluke associated hepatobiliary diseases [8, 9]. Molecular characterizations of key pathogenic molecules could speed up the interventions of C. sinensis infection. Cysteine proteases of helminthes have been widely characterized for their biological functions, including digestion, encystation, excystation, immune evasion and tissue invasion [10, 11]. Although cysteine proteases are abundant in C. sinensis transcriptome, limited information is available to illustrate the biological roles for C. sinensis in the host. Biological roles of C. sinensis cathepsin B proteases (CsCBs) have not been sufficiently investigated, although extensive studies demonstrate the importance of cathepsins in other organisms.
In our previous work [12], we performed preliminary functional characterizations of four C. sinensis cathepsin B cysteine proteases (CsCB1, CsCB2, CsCB3 and CsCB4). CsCBs were cloned into a prokaryotic expression vector (pET-28a) and expressed in the form of inclusion bodies in E. coli. Purified proteins from E. coli (eCsCBs) were identified as C. sinensis excretory/secretory products and could trigger immune responses. However, we failed to perform further functional characterizations of these cysteine proteases due to loss of enzyme activity during the renaturation procedure. In this study, the eukaryotic expressing system in yeast was constructed using homologous recombination to express four yCsCBs (CsCB1, CsCB2, CsCB3 and CsCB4) in Pichia pastoris X33 yeast cells. Recombinant yCsCBs showed enzymatic activities and hydrolase activities in degrading various host proteins. CsCB was detected in the liver tissues of mice and cancer patients afflicted with clonorchiasis. Recombinant CsCB could promote cell proliferation, migration and invasion of human cancer cells. Our results provide evidence to support the role of CsCBs in the pathogenesis of clonorchiasis.
Methods
Parasites, animals and patient samples
C. sinensis worms (larva, juvenile and adult) were freshly isolated from artificially infected freshwater fishes or Sprague–Dawley rats as we previously described [13]. Ethical Approval: Male Sprague–Dawley rats were purchased from the animal center at Sun Yat-sen University and raised in accordance with the National Institutes of Health animal care and ethical guidelines. BALB/c mice (8-weeks-old) were intragastrically infected with metacercariae to establish the C. sinensis infected mice model. Mice in the control group were treated with phosphate-buffered saline (PBS). The mice were sacrificed at 8 weeks after the infection and liver tissues were isolated for immunohistochemistry. Clonorchiasis-induced liver cancer specimens acquired from People’s Hospital of HengXian, Guangxi Zhuang Autonomous Region were pathologically diagnosed. Normal liver specimens were acquired from the first affiliated hospital of Sun Yat-Sen University. Ethical approval to use patients’ samples in this study was obtained from local hospitals and animal procedures were approved by the animal care and use committee of Sun Yat-sen University (Permit Numbers: SCXK (Guangdong) 2009–0011).
Immunolocalization of CsCBs in adult worm, cercaria and metacercaria
C. sinensis worms (larva, juvenile and adult) were used for the immunolocalization assay. Sectioned worms in paraffin wax were deparaffinized and incubated with previously prepared anti-CsCBs sera (1: 400 in dilution). Pre-immune rat serum was applied as a negative control. Subsequently, the sections were incubated with Cy3 conjugated goat anti-rat IgG secondary antibody (1: 400 in dilution, Alexa Fluor 594, Molecular Probe) at RT for 1 h and imaged using an Axio Imager Z1fluorescent microscope (ZEISS).
Homologous recombination of CsCBs in yeast
As we previously reported, the complete coding sequences of CsCBs range from 1014 to 1044 bp, with an N-terminal hydrophobic signal peptide ranging from 18 to 22 aa. To obtain recombinant CsCBs from the eukaryotic expressing system for functional characterizations, we performed a homologous recombination of CsCBs in the Pichia pastoris X33 yeast strain. The gene fragments of CsCBs were amplified by PCR using primers (Table 1) according to CsCBs-ORF (signal peptide excluded) and restriction sites of shuttle vector pPICZαB. Recombinant colonies were screened by Zeocin followed by validation using PCR and sequencing. Confirmed plasmids were extracted from DH5α and Pichia pastoris X33 was transformed with a Sac I linearized recombinant pPICZαB vector. The transformants were selected for Zeocin resistance on YPD plates [14]. Genomic DNAs were extracted from positive transformants for PCR to further confirm homologous recombination.
Table 1.
Primers used in this study
| Gene | Primers | Gene length |
|---|---|---|
| CsCB1 | F: 5′GCCGAATTCACGAGTATATTCCATCTTTC3′ | 960 bp |
| R: 5′CGCTCTAGAAGCAGTTTTGGATGACCAG3′ | ||
| CsCB2 | F: 5′GTCGAATTCACGAAAATCTGGGGAGCGT3′ | 966 bp |
| R: 5′GCCTCTAGAACAAAATGCGGAATGGTGG3′ | ||
| CsCB3 | F: 5′CGACTGCAGGAACAGAATCGATTGGACT3′ | 960 bp |
| R: 5′GGCTCTAGAATCTTAAGTGGGATGCTGG3′ | ||
| CsCB4 | F: 5′GTACTGCAGGAAAACCAAAGCACGAAGC3′ | 987 bp |
| R: 5′GCGGTCTAGAGGCGAAAAGGATTCATGATT3′ |
Expression and purification of yCsCBs
Selected transformants were cultured in a BMGY medium for 16–18 h until OD600 of 2–6, cells were harvested by centrifugation and re-suspended in a BMMY medium at an OD600 of 1.0. The expression of yCsCBs was induced by the daily addition of 0.5 % (v/v) methanol at 24, 48, 72 and 96 h, respectively. The culture filtrate of recombinant X33 cells was concentrated using ammonium sulfate. Concentrated supernatant was used for SDS-PAGE and Western blotting experiments to examine the extracellular expression of yCsCBs in yeast. After that, recombinant protein was induced for 96 h and purified by His-bind resin chromatography (Novagen) followed by dialysis in PBS (pH7.2). Protein concentration was determined using the BCA method and stored at −80 °C for enzymatic assays.
SDS-PAGE and Western blotting
Concentrated supernatant was subjected to 12 % SDS-PAGE stained with Coomassie brilliant blue. To further confirm extracellular expression of CsCBs in X33 cells, concentrated supernatant was also subjected to Western Blotting. Protein samples were transferred onto a PVDF membrane (Millipore) followed by incubation with different antibodies: mouse anti-His antibody (1: 500 in dilution, Life Technologies), mouse anti-c-Myc monoclonal antibody (1: 500 in dilution, Life Technologies) and rat anti-CsCB1 antibody (1: 800 in dilution), which was produced in our previous study. HRP-conjugated goat anti-mouse IgG or goat anti-rat IgG (1: 2,000 in dilution) was further incubated with each membrane, followed by enhanced chemiluminescence (ECL).
Enzyme activity assays
The enzyme activity of yCsCBs was assayed fluorometrically according to the previous report [15]. Enzyme reactions were performed under different enzyme concentrations, pH values and temperatures, respectively. Typically, the measurements were performed at 37 °C for 1 h in a 100 μl mixture containing yCsCBs (0–20 μM), fluorescent Z-Phe-Arg-AMC/Z-Arg-Arg-AMC (20 μM, Bachem), 10 mM DTT, 0.05 % Brij-35 (AMRESCO), EDTANa2 (1 mM), and C2H3NaO2/Na3PO4/Tris–HCl (100 mM). The enzyme reaction was terminated by adding stop buffer (70 mM C2H4O2, 30 mM C2H3NaO2, 100 mM C2H2ClO2Na, pH 4.3). Fluorescent intensity was measured by plate reader at 348 nm.
Degradation of host proteins
We first investigated hydrolase activity of yCsCBs. Purified CsCBs from E. coli or from Pichia pastoris were loaded into a 12 % SDS-PAGE containing 0.1 % gelatin. The gel was washed with washing buffer (2.5 % Tritonx-100, 50 mM Tris–HCl, 5 mM CaCl2, pH 7.5), followed by incubation with Na3PO4 (100 mM, pH 7.5) at 37 °C for 24 h. The hydrolase activity of yCsCBs was visualized by Coomassie brilliant blue staining.
Second, we tested whether yCsCBs could degrade host proteins, given that CsCBs were proven components of secreted products of C. sinensis [12]. Purified yCsCBs were incubated with host proteins at 37 °C for 2 h. Human serum albumin (MB-CHEM), human hemoglobin (MB-CHEM), human IgG (MB-CHEM), human fibronectin (Sigma) and bovine serum albumin (MB-CHEM) were used as the substrates. The assays were performed in a 200 μl mixture containing Na3PO4 (100 mM, pH 5.5), EDTANa2 (1 mM), DTT (10 mM), yCsCBs (20 μM) and various host proteins (1 mg). The reactions were terminated by adding a reducing sample buffer and analyzed by SDS-PAGE.
Inhibition effect on enzyme activity of yCsCBs
To confirm the specificity of enzyme activity from the above-mentioned assays, we performed enzymatic inhibition experiments by using different enzyme inhibitors purchased from Sigma. Briefly, yCsCBs (20 μM) were pre-incubated with or without protease inhibitors, including E-64 (20 μM), iodoacetic acid (10 μM), PMSF (2 mM), EDTA (2 mM), AEBSF (200 μM), TPCK (200 μM) and CA-074 methyl ester (1 μM). Z-Phe-Arg-AMC (20 μM) was added to the reactions after 30 min and incubated for another 1 h. Each assay was performed in triplicate and enzyme activity was measured by plate reader at 348 nm.
Immunohistochemistry of CsCB in infected mouse and patient
Next, we sought to investigate whether CsCBs are involved in the pathogenesis of clonorchiasis using the yCsCB4 protein. First, we performed an immunohistochemistry using an anti-CsCB4 antibody to see the localization of CsCB4 in liver tissues of clonorchiasis afflicting mice and patients. Generally, tissue samples were fixed in 10 % formalin and sectioned to 4 μm in thickness. The sections were routinely treated with ethanol and slides were immersed in a 0.3 % hydrogen peroxide solution for 20 min to block the endogenous peroxidase activity. The sections were then incubated overnight at 4 °C with rat anti-CsCB4 antibody (1: 100 in dilution). Sections were subsequently incubated with horseradish peroxidase (HRP) conjugated rat-specific secondary antibodies (1: 200 in dilution). Immunohistochemistry results were developed using diaminobenzidine (DAB) and counterstained with hematoxylin. The images were taken under a light microscope (Leica DMI3000B) and subsequently analyzed using ImagePro Plus software (Media Cybernetics, Roper, USA). The brown staining was indicated as Integrated Optical Density (IOD), and IOD/Area was indicated as a relative expression level of CsCB4 in liver tissues.
Cell proliferation analysis
The cell proliferation level induced by yCsCB4 was measured in two human cancer cell lines, human hepatocellular carcinoma cell line (MHCC-97H, ATCC) and human cholangiocarcinoma cell line (RBE, ATCC). MHCC-97H and RBE cells were grown in DMEM (Hyclone, USA) and RPMI-1640 (Gibco, USA), respectively and supplemented with 10 % fetal bovine serum (Gibco, USA) and 1 % penicillin/streptomycin (Gibco, USA). Cells were incubated at 37 °C in a humidified chamber under 5 % CO2. Cells at the logarithmic phase were plated into 96-well plates in triplicate and treated with yCsCB4 protein (1 μg/ml). Cell viability at 24 h was measured using Cell Counting Kit-8 (CCK-8) as previously described [16]. For cell cycle analysis using flow cytometry, cells were incubated with yCsCB4 (1 μg/ml) for 24 h. Then the cells were trypsinized and fixed in 100 % ethanol at −20 °C overnight. Cell cycle distribution was determined by fluorescence activated cell sorting (FACS). Data was analyzed using the FlowJo software.
Cell migration and invasion assay
To further confirm the role of yCsCB4 in human cancer cell growth, wound-healing assays were performed to evaluate the effect of yCsCB4 on cell migration according to the previous method [17]. MHCC-97H and RBE cells seeded in 6-well plates were grown to 80 % confluence and incubated with yCsCB4 (1 μg/ml) or PBS for 24 h. Cells were wounded by scratching with pipette tips. Wounds at 24 h were observed and photographed under a light microscope (Leica DMI3000B).
To evaluate the effect of yCsCB4 on cell invasion, we performed transwell assays according to the method described elsewhere [18]. MHCC-97H and RBE cells were suspended in serum-free media and placed in 8 μm pores. These inserts were placed in wells with serum-containing media. Cells were incubated with yCsCB4 (1 μg/ml) or PBS for 24 h. Invasion assays were performed using matrigel-coated membranes (BD, USA). The migration assay was similar to the invasion assay, except that the upper side of the membranes was not coated with the matrigel. Cells attached to the lower surface of the membranes at 24 h were counted under a light microscope.
Statistical analysis
Experimental data were obtained from three independent experiments with a similar pattern; data are expressed as means ± standard deviation. All the data were analyzed by SPSS 13.0. Student’s t-test and ANOVA were used to analyze the data. P value <0.05 was considered statistically significant.
Results
Immunolocalization of CsCBs in C. sinensis worms
In our previous work, we demonstrated that CsCBs are components of C. sinensis secreted products by Western Blotting assay [12]. In this study, we first investigated the immunolocalization of four CsCBs in C. sinensis worms. As shown in Fig. 1, in metacercaria (Fig. 1, panel A1-A4) and cercaria (Fig. 1, panel C1-C4), four CsCBs could be detected in the excretory vesicle and oral sucker. In adult worm, four CsCBs could be specifically observed in the intestinal tract (Fig. 1, panel E1-E4). No fluorescent signal could be detected in negative controls treated with pre-immune serum (Fig. 1, panel A5, C5 and E5).
Fig. 1.
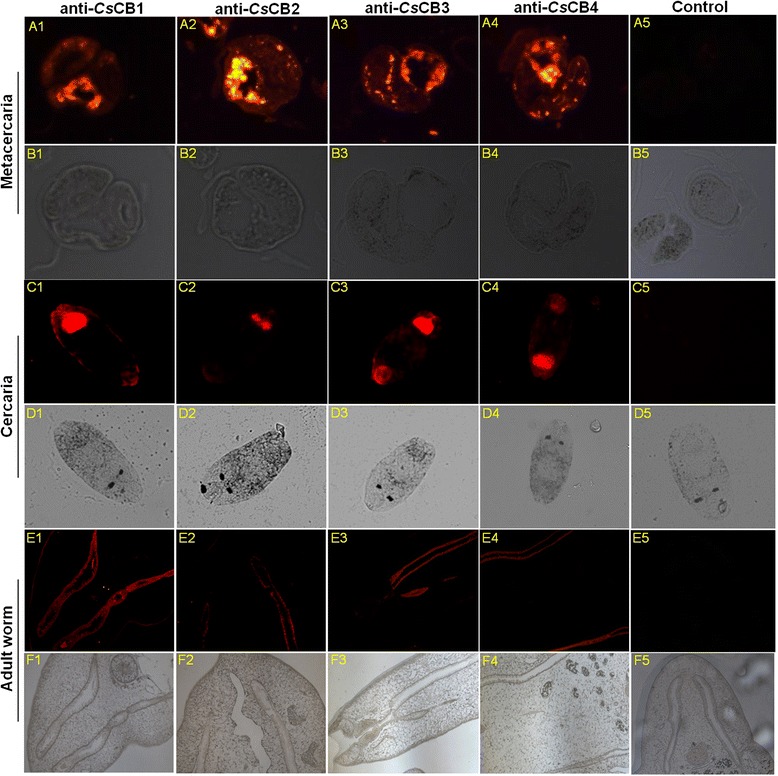
Immunolocalization of CsCBs in C. sinensis worms. Sectioned worms were deparaffinized and incubated with anti-CsCBs sera (1: 400). Pre-immune rat serum was applied as a negative control. The sections were incubated with Cy3 conjugated goat anti-rat IgG secondary antibody (1: 400) in dark and imaged using an Axio Imager Z1 fluorescent microscope. 1–5 indicated anti-CsCB1 serum, anti-CsCB2 serum, anti-CsCB3 serum, anti-CsCB4 serum and pre-immune serum, respectively. a, b Metacercaria. c, d Cercaria. e, f Adult worm. a, c, e Images under fluorescent objective. b, d, f Images in bright field
Homologous recombination of CsCBs in yeast
As shown in Additional file 1: Figure S1, the ORFs of four CsCBs were successfully inserted into the shuttle vector pPICZαB. Selected transformants with CsCBs were used for protein expression induced by methanol. Cells were collected at different time points to monitor the expression level of yCsCBs. As shown in Additional file 1: Figure S2, a band of interest around 45 kDa appeared in the yeast culture medium at 24 h with an increased expressed level afterward, indicating the successful expression of yCsCBs in yeast (Additional file 1: Figure S2A-D). To further confirm whether the interesting band (45 kDa) was yCsCBs, a concentrated culture medium was used for Western blotting assays probed with different antibodies (Fig. 2 and Additional file 1: Figure S3). As expected, the interesting bands seen in SDS-PAGE could be recognized by His-tag (Fig. 2a). Expression of yCsCBs was also demonstrated by reactions with anti-c-Myc antibody and anti-CsCB1 antibody when yCsCB1 was used as the example (Fig. 2b and c). Eventually, four yCsCBs were purified by His-bind resin chromatography and analyzed by 12 % SDS-PAGE, resulting in a highly pure yCsCBs (Additional file 1: Figure S4, A-D).
Fig. 2.
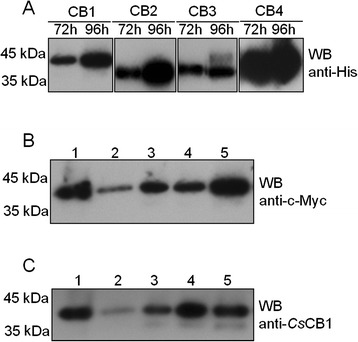
Identification of extracellular expression of yCsCBs by Western Blotting. a Cell culture medium of yCsCBs (yCsCB1, yCsCB2, yCsCB3 and yCsCB4) was probed with mouse anti-His antibody (1: 500). b Cell culture medium of yCsCB1 was probed with mouse anti-c-Myc antibody (1: 500). c Cell culture medium of yCsCB1 was probed with rat anti-CsCB1 antibody (1: 800). Lanes 1–5 indicated ammonium sulfate precipitate, 72-h culture medium, 96-h culture medium, 120-h culture medium and 144-h culture medium, respectively
Enzyme activity of yCsCB
We tested the enzyme activity of yCsCBs by using two fluorescent substrates (Z-Phe-Arg-AMC and Z-Arg-Arg-AMC). As shown in Fig. 3a, four yCsCBs were demonstrated to be active enzymes when the enzyme concentration ranged from 0 to 20 μM. Compared to Z-Arg-Arg-AMC, yCsCBs showed higher enzymatic activity when Z-Phe-Arg-AMC was used as the substrate. In addition, optimal enzyme reaction pH values and temperatures were investigated. yCsCBs showed the highest enzymatic activity when enzyme assays were performed at pH 5.0–5.5 (Fig. 3b) and at 37–42 °C (Fig. 3c). The results suggested that yCsCBs were stable enzymes under acidic conditions when temperatures ranged from 37 to 42 °C.
Fig. 3.
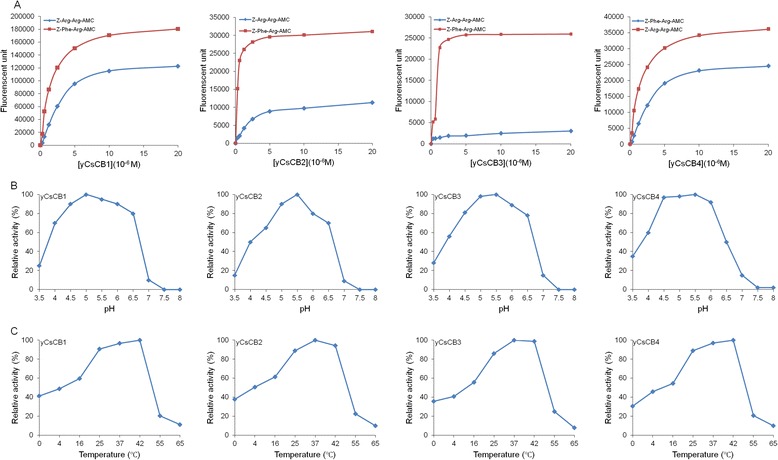
Enzyme activity of yCsCB. a Enzyme activity of yCsCBs was assayed by using two fluorescent substrates (Z-Phe-Arg-AMC and Z-Arg-Arg-AMC) under different enzyme concentrations (0, 0.3125, 0.625, 1.25, 2.5, 5, 10 and 20 μM). b Enzyme activity of yCsCBs was assayed by using fluorescent Z-Phe-Arg-AMC as a substrate under different pH values (3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, 7.5 and 8.0). c Enzyme activity of yCsCBs was assayed by using fluorescent Z-Phe-Arg-AMC as substrate under different temperatures (0, 4, 16, 28, 37, 42 and 55 °C). Fluorescent intensity was measured at 348 nm to calculate relative enzyme activity
Host proteins degradation by yCsCBs
Since CsCBs have been demonstrated to be the components of secreted products of C. sinensis, we tested hydrolase activity of yCsCBs within the context of host proteins under acidic conditions (pH 5.5). In gelatin hydrolysis assay (Fig. 4a), yCsCBs could obviously hydrolyze gelatin, while eCsCBs could not. When bovine serum albumin was used as the substrate, four yCsCBs also showed a degradation effect with different activities (Fig. 4b). We then employed different host proteins as the substrate; four yCsCBs could degrade host proteins including human serum albumin (Fig. 4c), human fibronectin (Fig. 4d), human hemoglobin (Fig. 4e) and human IgG (Fig. 4f).
Fig. 4.
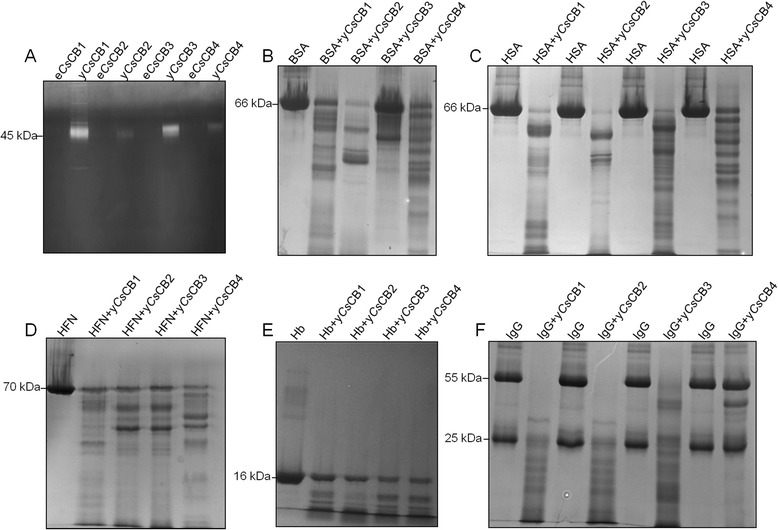
Host proteins degradation by yCsCBs. The assays were performed in a 200 μl mixture containing Na3PO4 (100 mM, pH 5.5), EDTANa2 (1 mM), DTT (10 mM), yCsCBs (20 μM) and various host proteins (1 mg). The reactions were terminated by adding a reducing sample buffer and analyzed by SDS-PAGE. a Gelatin hydrolysis assay using yCsCBs versus eCsCBs. b Degradation assay using bovine serum albumin (BSA) as the substrate. c Degradation assay using human serum albumin (HSA) as the substrate. d Degradation assay using human fibronectin (HFN) as the substrate. e Degradation assay using human hemoglobin (Hb) as the substrate. f Degradation assay using human IgG as the substrate. The molecular mass of host proteins was indicated
Inhibition effect on enzyme activity of yCsCBs
As we showed above, four yCsCBs were demonstrated as active enzymes. We carried out inhibiting assays using different enzyme inhibitors to confirm that observed enzyme activity was specific to cathepsin B proteases (Fig. 5). Compared to controls without enzyme inhibitors, enzymatic activities of yCsCBs could be completely inhibited by cathepsin B specific inhibitors or cysteine protease specific inhibitors (CA-074 methyl ester, E-64 and iodoacetic acid). However, serine protease specific inhibitors (PMSF and AEBSF) and trypsin specific inhibitor TPCK could only partially inhibit enzyme activity. EDTA had no inhibition on the activity, indicating that CsCBs belongs to the typical cathepsin B cysteine protease family.
Fig. 5.
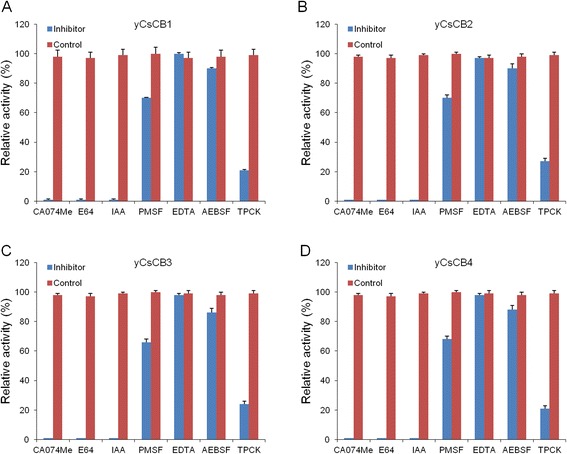
Inhibition effect on enzyme activity of yCsCBs. a-d yCsCB1, yCsCB2, yCsCB3 and yCsCB4, respectively. yCsCBs (20 μM) were pre-incubated with or without protease inhibitors including E-64 (20 μM), iodoacetic acid (10 μM), PMSF (2 mM), EDTA (2 mM), AEBSF (200 μM), TPCK (200 μM) and CA-074 methyl ester (1 μM). Z-Phe-Arg-AMC (20 μM) was added to the reactions after 30 min and incubated for another 1 h. Fluorescent intensity was measured at 348 nm to calculate relative enzyme activity. Errors represent data from triplicate samples
Immunohistochemistry of CsCB in infected mice and liver cancer patients
The liver tissues from mice model and patient samples were analyzed by immunohistochemistry using rat anti-CsCB4 antibody. Positive staining was indicated with brown. Compared to normal mice, strong staining was detected in the liver tissue of infected mice (Fig. 6a). The IOD of infected mice livers was significantly higher than the IOD of normal mice livers (Fig. 6b, P < 0.001). Strong staining dispersed throughout the liver tissues from clonorchiasis patients, while little brown staining was evident in liver tissues from healthy people (Fig. 6c). The IOD of liver cancer specimens was higher than the IOD of normal liver specimens (Fig. 6d, P < 0.01).
Fig. 6.
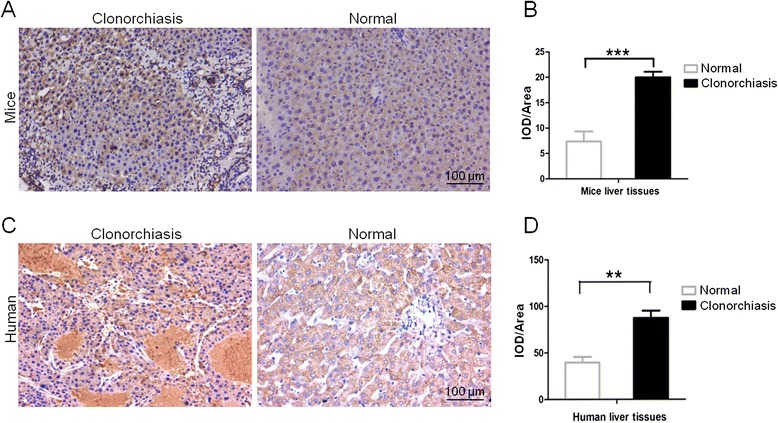
Immunohistochemistry assays of CsCB4 in liver tissues. a Representative localization image of CsCB4 in mice liver. c Representative localization image of CsCB4 in human liver. b, d Quantification of Integrated Optical Density (IOD). The images were magnified at 200X and scale bar is 100 μm. The brown staining was indicated as IOD; IOD/Area was indicated as a relative expression level of CsCB4 in liver tissues. Five random fields from each sample were analyzed using ImagePro Plus software. The sections were developed by DAB and counterstained with hematoxylin. **P < 0.01, ***P < 0.001, compared to normal tissue
Cell proliferation promoted by CsCB4
The proliferation level of MHCC-97H and RBE cells treated with yCsCB4 was measured by CCK-8 assays. As shown in Fig. 7a, both MHCC-97H and RBE cells treated with yCsCB4 showed significantly a higher proliferative level when compared to control cells (P < 0.05). To further confirm the effect of yCsCB4 on cell proliferation, we evaluated the distribution of the cell cycle by flow cytometry (Additional file 1: Figure S5). As shown in Fig. 7b, the G2/S percentage of MHCC-97H and RBE cells treated with yCsCB4 was statistically higher than those of cells treated with PBS control, respectively (P < 0.05).
Fig. 7.
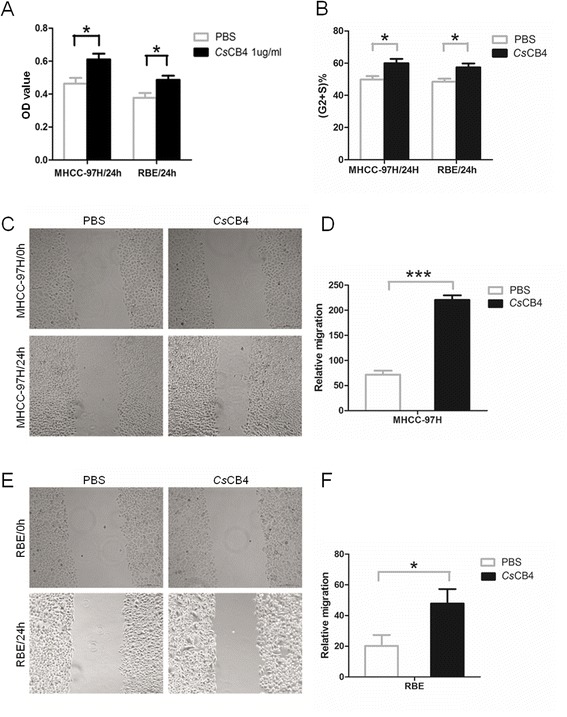
The effect of yCsCB4 on human MHCC-97H and RBE cells. a Cell proliferation level measured by CCK-8 assay. b Cell cycle analysis by flow cytometry, the percentage of cells in the G2/S period was analyzed and quantified using FlowJo software. c, d Cell migration of MHCC-97H cells shown by wound-healing assay. Cells were observed using light microscope under 10X objective. e, f Cell migration of RBE cells shown by wound-healing assays; cells were observed using a light microscope under 10X objective. Generally, MHCC-97H cells or RBE cells were incubated with 1 μg/ml of yCsCB4 or PBS for 24 h and assays were performed. Assays were performed in triplicate. Relative cell migration level was calculated by normalizing to cell migration level at 0 h. *P < 0.05, ***P < 0.001, compared to PBS control
Cell migration and invasion triggered by CsCB4
We wondered if yCsCB4 could play any role in cancer cell migration. To determine this, we carried out wound-healing assays. For both MHCC-97H (Fig. 7c) and RBE cells (Fig. 7e), at the concentration of 1 μg/ml, yCsCB4 could induce significant cell migration, which is 3-fold (Fig. 7d, P < 0.001) and 2-fold (Fig. 7f, P < 0.05) when compared to the PBS control, respectively. Similarly, in transwell assays, yCsCB4 (1 μg/ml) promoted a higher cell migration level in MHCC-97H (Fig. 8a) and RBE cells (Fig. 8b). In addition, the cell invasion level could also be reflected in transwell assays, indicating that at the concentration of 1 μg/ml yCsCB4 could induce a 3-fold (Fig. 8a, P < 0.001) and 5-fold (Fig. 8b, P < 0.001) invasion level compared to the PBS control, respectively.
Fig. 8.
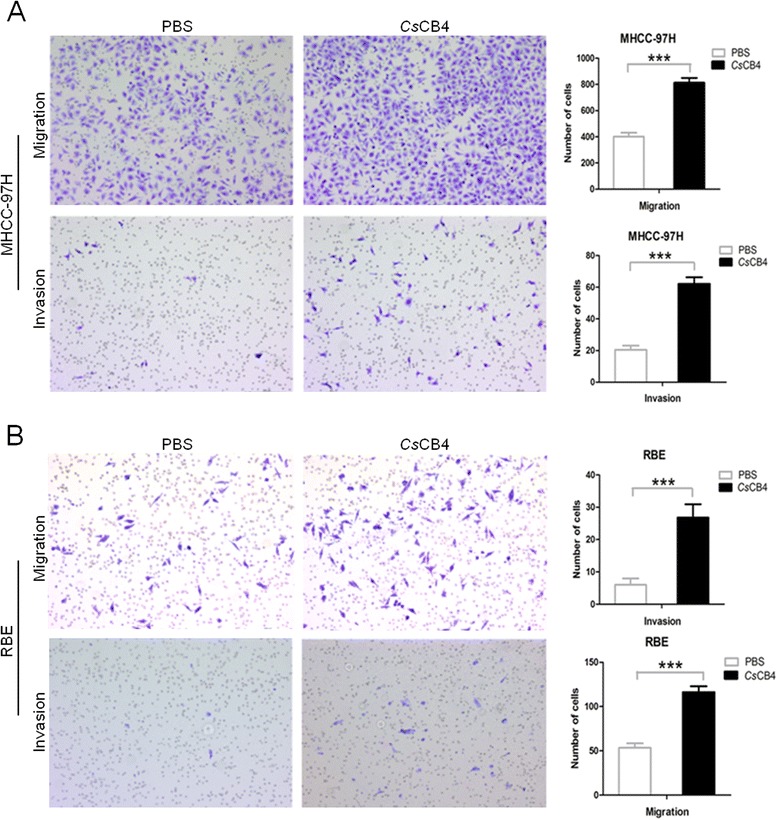
Cell migration and invasion triggered by CsCB4 in transwell assay. MHCC-97H cells (a) or RBE cells (b) were suspended in serum-free media. Cells were incubated with 1 μg/ml of yCsCB4 or PBS for 24 h. Invasion assays were performed using matrigel-coated membranes. Migration assay was similar to the invasion assay, except that the upper side of the membranes was not coated with the matrigel. Cells attached to the lower surface of the membranes at 24 h were counted under a light microscope. Ten random visual fields were selected to quantify the migration and invasion. ***P < 0.001, compared to PBS control
Discussion
Proteases are ubiquitous in nature and most organisms. In addition to their housekeeping functions, proteases are involved in the digestion of host proteins such as fibronectin, collagen and albumin, to facilitate migration and feeding in the host [19]. Cathepsins are of particular interest to parasitologists because there is considerable evidence that cathepsins are involved in parasitism. All of the trematodes have been shown to contain genes encoding cathepsin B-like proteins. For example, in Fasciola hepatica, cathepsin B was identified as an important factor associated with invasion of the mammalian host [20] and cathepsin B was suggested as a potential digestive factor in newly excysted juvenile parasites [21]. Cathepsin B was also identified as a stage and tissue-specific expression protease in Fasciola gigantica [22]. In Schistosoma mansoni, secreted cathepsin B was proposed to interact with host molecules and thus be a vital factor in parasitism [23]. In Angiostrongylus cantonensis, cathepsin B plays a potential role in the invasion of the central nervous system during parasite-host interactions [24]. Thus, cathepsin B proteases clearly play an important role in the biology of trematode parasites. Cysteine proteases were abundant genes in C. sinensis genome and transcriptome. As the main components of C. sinensis excretory/secretory products, CsCBs were proved to be potential vaccine candidates and diagnostic markers [11, 12]. In this study, we constructed a eukaryotic expressing system by homologous recombination to express four CsCBs in yeast. Active yCsCBs were purified for biochemical and functional characterizations. The cellular effect of CsCBs on human cancer cells was observed using various cellular assays. Our results provide evidence to support the role of CsCBs in the pathogenesis of clonorchiasis.
At this time, CsCBs were expressed in soluble form with enzyme activity because of the advantages of the methylotropic yeast, Pichia pastoris, and the shuttle vector pPICZαB [25, 26]. This shuttle vector facilitated our transformation operation from the E. coli system to the Pichia pastoris system. yCsCBs showed active enzyme activity with a wide range of pHs, while peak enzymatic activity was assayed at pH 5.0–5.5, suggesting that yCsCBs were functional enzymes under acidic conditions. The hypothesis that CsCBs are acidic enzymes located in gut of flukes could be supported by previous reports [23, 27, 28], considering the fact that the pH of the gut lumen of Fasciola hepatica has been suggested to be pH 5.5 [29]. Enzymatic activities of yCsCBs could be completely inhibited by cathepsin B specific inhibitors or cysteine protease specific inhibitors, while serine protease specific inhibitors and trypsin specific inhibitor showed only a weak inhibition effect. These results helped us confirm that our obtained yCsCBs belong to the typical cathepsin B cysteine protease family. In addition to typical enzymatic activities, yCsCBs could degrade all tested host proteins such as human serum albumin, human fibronectin, human hemoglobin and human IgG. Those host proteins have been used in other parasites to test the digestive effect of cathepsins [19]. For instance, FhCB2 could cleave serum albumin and IgG, indicating a role in the digestion of protein substrates for nutritional purposes [30]. Recombinant FgCB3 was recently shown to digest fibronectin, consistent with a role in digesting connective tissue and host invasion [31]. The digestive effect of yCsCBs supports our hypothesis that CsCBs serve as key virulence factors for C. sinensis, it is most likely that CsCBs are involved in the pathogenesis of clonorchiasis. The biological role of CsCB could be implied by immunolocalization results, showing that CsCBs were localized in the excretory vesicle, oral sucker and intestinal tract of C. sinensis worms. Immunolocalization of CsCB is similar to C. sinensis cathepsin F, which is also a secreted protein in the intestine of C. sinensis [32]. These two enzymes were expressed throughout developmental stages of the parasite. Given that CsCBs and CsCFs are from the same protease family, it is reasonable to assume they are synthesized in epithelial cells lining the parasite intestine followed by secretion into the intestinal lumen of the parasite, to play a role for nutrient uptake in the parasite [33–35].
As the key component of secreted products, many proteins have been connected with hepatobiliary diseases observed in individuals infected with liver flukes [36, 37]. It was suggested that secreted products released by liver flukes could lead to pathologic changes in biliary epithelial cells [38, 39]. Human cells exposed to ESPs from liver flukes (C. sinensis, Fasciola hepatica, and Opisthorchis viverrini) showed diverse pathophysiological responses including proliferation, apoptosis and inflammation [40–42]. In human diseases, experimental and clinical evidence have linked cathepsin B with tumor invasion and metastasis. Cathepsin B expression increases in many human cancers at mRNA, protein and activity levels [43]. In this study, we found that CsCB4 was detected in liver tissues from infected mice or liver cancer patients induced by clonorchiasis. To gain a better understanding of CsCBs-associated human diseases, we measured the biological effects of yCsCB4 protein on human cancer cells. The results from different approaches demonstrated that yCsCB4 could promote cell proliferation, cell migration and cell invasion of human hepatocellular carcinoma cells and human cholangiocarcinoma cells. Our observed results could be supported by our previous report that severin protein from CsESPs had an anti-apoptotic role in hepatocarcinoma PLC cells [44]. Given that four CsESPs have similar biochemical properties, it is conceivable that CsCBs are involved in the pathogenesis of clonorchiasis during C. sinensis infection. However, further investigations are required in order to identify precise mechanisms to provide therapeutic strategies for clonorchiasis. With RNA interference applications in helminth [45, 46], it is feasible to perform a CsCBs-mediated intervention in C. sinensis associated diseases.
Conclusion
In summary, we expressed and purified four CsCBs in yeast and demonstrated that CsCBs can degrade various human proteins. CsCBs could be detected at a high expression level in clonorchiasis-induced liver cancer tissues. In addition, our results indicate that CsCBs could confer proliferative and invasive role in human cancer cells. The present study supports the involvement of CsCBs in the pathogenesis of clonorchiasis.
Acknowledgements
This work was supported by Science and Technology Plan in Guangdong Province (No.: 2012ZX10004-220, 2013B010404010, 2014B020203001), National Key Basic Research and Development Project (973 project; No. 2010CB530000) and National Natural Science Foundation of China (No. 81171602) to XBY. This work was supported in part by National Natural Science Foundation of China (No. 81101270) to YH. We appreciate Prof. Lifang Jiang (Department of Microbiology in Zhongshan School of Medicine at Sun Yat-sen University) for providing us shuttle vector pPICZαB and Pichia pastoris X33 cells.
Additional file
Identification of recombinant plasmids by PCR amplification and restriction enzyme digestion. Figure S2. Identification of extracellular expression of yCsCBs by 12 % SDS-PAGE. Figure S3. Identification of extracellular expression of yCsCBs by Western Blotting. Figure S4. Identification of purified protein of CsCBs by SDS-PAGE. Figure S5. Representative cell cycle analysis by flow cytometry. (DOCX 5346 kb)
Footnotes
Wenjun Chen, Dan Ning, Xiaoyun Wang and Tingjin Chen contributed equally to this work.
Competing interests
The authors declare that they have no competing interests.
Authors’ contributions
WC, DN, XW, JX and XY conceived and designed the experiments; WC, DN, XW, TC, XL, JS and DW performed the experiments; WC, DN, XW and YH analyzed the data; WC, DN, XW and XY wrote the manuscript. All authors read and approved the final manuscript.
Contributor Information
Wenjun Chen, Email: fannie_1985@hotmail.com.
Dan Ning, Email: 364574522@qq.com.
Xiaoyun Wang, Email: xwang13@uchicago.edu.
Tingjin Chen, Email: 710387301@qq.com.
Xiaoli Lv, Email: 510099864@qq.com.
Jiufeng Sun, Email: 395252689@qq.com.
De Wu, Email: 445831343@qq.com.
Yan Huang, Email: huang66@mail.sysu.edu.cn.
Jin Xu, Phone: +86-20-87331838, Email: xujinteam@163.com.
Xinbing Yu, Phone: +86-20-87331838, Email: yuhxteam@163.com.
References
- 1.Lun ZR, Gasser RB, Lai DH, Li AX, Zhu XQ, Yu XB, et al. Clonorchiasis: a key foodborne zoonosis in China. Lancet Infect Dis. 2005;5:31–41. doi: 10.1016/S1473-3099(04)01252-6. [DOI] [PubMed] [Google Scholar]
- 2.Lin J, Qu H, Chen G, He L, Xu Y, Xie Z, et al. Clonorchis sinensis acetoacetyl-CoA thiolase: identification and characterization of its potential role in surviving in the bile duct. Parasit Vectors. 2015;8:125. doi: 10.1186/s13071-015-0728-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sripa B, Kaewkes S, Intapan PM, Maleewong W, Brindley PJ. Food-borne trematodiases in Southeast Asia epidemiology, pathology, clinical manifestation and control. Adv Parasitol. 2010;72:305–50. doi: 10.1016/S0065-308X(10)72011-X. [DOI] [PubMed] [Google Scholar]
- 4.Hong ST, Fang Y. Clonorchis sinensis and clonorchiasis, an update. Parasitol Int. 2012;61:17–24. doi: 10.1016/j.parint.2011.06.007. [DOI] [PubMed] [Google Scholar]
- 5.Huang SY, Zhao GH, Fu BQ, Xu MJ, Wang CR, Wu SM, et al. Genomics and molecular genetics of Clonorchis sinensis: current status and perspectives. Parasitol Int. 2012;61:71–6. doi: 10.1016/j.parint.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 6.Wang X, Chen W, Huang Y, Sun J, Men J, Liu H, et al. The draft genome of the carcinogenic human liver fluke Clonorchis sinensis. Genome Biol. 2011;12:R107. doi: 10.1186/gb-2011-12-10-r107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoo WG, Kim DW, Ju JW, Cho PY, Kim TI, Cho SH, et al. Developmental transcriptomic features of the carcinogenic liver fluke, Clonorchis sinensis. PLoS Negl Trop Dis. 2011;5:e1208. doi: 10.1371/journal.pntd.0001208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen TT, Arimatsu Y, Hong SJ, Brindley PJ, Blair D, Laha T, et al. Genome-wide characterization of microsatellites and marker development in the carcinogenic liver fluke Clonorchis sinensis. Parasitol Res. 2015;114:2263–72. doi: 10.1007/s00436-015-4419-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sithithaworn P, Yongvanit P, Duenngai K, Kiatsopit N, Pairojkul C. Roles of liver fluke infection as risk factor for cholangiocarcinoma. J Hepatobiliary Pancreat Sci. 2014;21:301–8. doi: 10.1002/jhbp.62. [DOI] [PubMed] [Google Scholar]
- 10.Dvorak J, Delcroix M, Rossi A, Vopalensky V, Pospisek M, Sedinova M, et al. Multiple cathepsin B isoforms in schistosomula of Trichobilharzia regenti: identification, characterisation and putative role in migration and nutrition. Int J Parasitol. 2005;35:895–910. doi: 10.1016/j.ijpara.2005.02.018. [DOI] [PubMed] [Google Scholar]
- 11.Chen W, Wang X, Li X, Lv X, Zhou C, Deng C, et al. Molecular characterization of cathepsin B from Clonorchis sinensis excretory/secretory products and assessment of its potential for serodiagnosis of clonorchiasis. Parasit Vectors. 2011;4:149. doi: 10.1186/1756-3305-4-149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen W, Wang X, Lv X, Tian Y, Xu Y, Mao Q, et al. Characterization of the secreted cathepsin B cysteine proteases family of the carcinogenic liver fluke Clonorchis sinensis. Parasitol Res. 2014;113:3409–18. doi: 10.1007/s00436-014-4006-6. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Liang C, Chen W, Fan Y, Hu X, Xu J, et al. Experimental model in rats for study on transmission dynamics and evaluation of Clonorchis sinensis infection immunologically, morphologically, and pathologically. Parasitol Res. 2009;106:15–21. doi: 10.1007/s00436-009-1622-7. [DOI] [PubMed] [Google Scholar]
- 14.Garg N, Bieler N, Kenzom T, Chhabra M, Ansorge-Schumacher M, Mishra S. Cloning, sequence analysis, expression of Cyathus bulleri laccase in Pichia pastoris and characterization of recombinant laccase. BMC Biotechnol. 2012;12:75. doi: 10.1186/1472-6750-12-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrett AJ, Kirschke H, Cathepsin B, Cathepsin H, Cathepsin L. Methods Enzymol. 1981;80(Pt C):535–61. doi: 10.1016/S0076-6879(81)80043-2. [DOI] [PubMed] [Google Scholar]
- 16.Zhang F, Liang P, Chen W, Wang X, Hu Y, Liang C, et al. Stage-specific expression, immunolocalization of Clonorchis sinensis lysophospholipase and its potential role in hepatic fibrosis. Parasitol Res. 2013;112:737–49. doi: 10.1007/s00436-012-3194-1. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez LG, Wu X, Guan JL. Wound-healing assay. Methods Mol Biol. 2005;294:23–9. doi: 10.1385/1-59259-860-9:023. [DOI] [PubMed] [Google Scholar]
- 18.Korpal M, Lee ES, Hu G, Kang Y. The miR-200 family inhibits epithelial-mesenchymal transition and cancer cell migration by direct targeting of E-cadherin transcriptional repressors ZEB1 and ZEB2. J Biol Chem. 2008;283:14910–4. doi: 10.1074/jbc.C800074200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Smooker PM, Jayaraj R, Pike RN, Spithill TW. Cathepsin B proteases of flukes: the key to facilitating parasite control? Trends Parasitol. 2010;26:506–14. doi: 10.1016/j.pt.2010.06.001. [DOI] [PubMed] [Google Scholar]
- 20.Robinson MW, Menon R, Donnelly SM, Dalton JP, Ranganathan S. An integrated transcriptomics and proteomics analysis of the secretome of the helminth pathogen Fasciola hepatica: proteins associated with invasion and infection of the mammalian host. Mol Cell Proteomics. 2009;8:1891–907. doi: 10.1074/mcp.M900045-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckham SA, Piedrafita D, Phillips CI, Samarawickrema N, Law RH, Smooker PM, et al. A major cathepsin B protease from the liver fluke Fasciola hepatica has atypical active site features and a potential role in the digestive tract of newly excysted juvenile parasites. Int J Biochem Cell Biol. 2009;41:1601–12. doi: 10.1016/j.biocel.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Meemon K, Grams R, Vichasri-Grams S, Hofmann A, Korge G, Viyanant V, et al. Molecular cloning and analysis of stage and tissue-specific expression of cathepsin B encoding genes from Fasciola gigantica. Mol Biochem Parasitol. 2004;136:1–10. doi: 10.1016/j.molbiopara.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 23.Caffrey CR, Salter JP, Lucas KD, Khiem D, Hsieh I, Lim KC, et al. SmCB2, a novel tegumental cathepsin B from adult Schistosoma mansoni. Mol Biochem Parasitol. 2002;121:49–61. doi: 10.1016/S0166-6851(02)00022-1. [DOI] [PubMed] [Google Scholar]
- 24.Han YP, Li ZY, Li BC, Sun X, Zhu CC, Ling XT, et al. Molecular cloning and characterization of a cathepsin B from Angiostrongylus cantonensis. Parasitol Res. 2011;109:369–78. doi: 10.1007/s00436-011-2264-0. [DOI] [PubMed] [Google Scholar]
- 25.Brankamp RG, Sreekrishna K, Smith PL, Blankenship DT, Cardin AD. Expression of a synthetic gene encoding the anticoagulant-antimetastatic protein ghilanten by the methylotropic yeast Pichia pastoris. Protein Expr Purif. 1995;6:813–20. doi: 10.1006/prep.1995.0013. [DOI] [PubMed] [Google Scholar]
- 26.Clare JJ, Romanos MA, Rayment FB, Rowedder JE, Smith MA, Payne MM, et al. Production of mouse epidermal growth factor in yeast: high-level secretion using Pichia pastoris strains containing multiple gene copies. Gene. 1991;105:205–12. doi: 10.1016/0378-1119(91)90152-2. [DOI] [PubMed] [Google Scholar]
- 27.Sajid M, McKerrow JH, Hansell E, Mathieu MA, Lucas KD, Hsieh I, et al. Functional expression and characterization of Schistosoma mansoni cathepsin B and its trans-activation by an endogenous asparaginyl endopeptidase. Mol Biochem Parasitol. 2003;131:65–75. doi: 10.1016/S0166-6851(03)00194-4. [DOI] [PubMed] [Google Scholar]
- 28.Lowther J, Robinson MW, Donnelly SM, Xu W, Stack CM, Matthews JM, et al. The importance of pH in regulating the function of the Fasciola hepatica cathepsin L1 cysteine protease. PLoS Negl Trop Dis. 2009;3:e369. doi: 10.1371/journal.pntd.0000369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Halton DW. Nutritional adaptations to parasitism within the platyhelminthes. Int J Parasitol. 1997;27:693–704. doi: 10.1016/S0020-7519(97)00011-8. [DOI] [PubMed] [Google Scholar]
- 30.Wilson LR, Good RT, Panaccio M, Wijffels GL, Sandeman RM, Spithill TW. Fasciola hepatica: characterization and cloning of the major cathepsin B protease secreted by newly excysted juvenile liver fluke. Exp Parasitol. 1998;88:85–94. doi: 10.1006/expr.1998.4234. [DOI] [PubMed] [Google Scholar]
- 31.Sethadavit M, Meemon K, Jardim A, Spithill TW, Sobhon P. Identification, expression and immunolocalization of cathepsin B3, a stage-specific antigen expressed by juvenile Fasciola gigantica. Acta Trop. 2009;112:164–73. doi: 10.1016/j.actatropica.2009.07.016. [DOI] [PubMed] [Google Scholar]
- 32.Kang JM, Bahk YY, Cho PY, Hong SJ, Kim TS, Sohn WM, et al. A family of cathepsin F cysteine proteases of Clonorchis sinensis is the major secreted proteins that are expressed in the intestine of the parasite. Mol Biochem Parasitol. 2010;170:7–16. doi: 10.1016/j.molbiopara.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 33.Kang TH, Yun DH, Lee EH, Chung YB, Bae YA, Chung JY, et al. A cathepsin F of adult Clonorchis sinensis and its phylogenetic conservation in trematodes. Parasitology. 2004;128:195–207. doi: 10.1017/S0031182003004335. [DOI] [PubMed] [Google Scholar]
- 34.Li S, Chung YB, Chung BS, Choi MH, Yu JR, Hong ST. The involvement of the cysteine proteases of Clonorchis sinensis metacercariae in excystment. Parasitol Res. 2004;93:36–40. doi: 10.1007/s00436-004-1097-5. [DOI] [PubMed] [Google Scholar]
- 35.Na BK, Kang JM, Sohn WM. CsCF-6, a novel cathepsin F-like cysteine protease for nutrient uptake of Clonorchis sinensis. Int J Parasitol. 2008;38:493–502. doi: 10.1016/j.ijpara.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 36.Smout MJ, Laha T, Mulvenna J, Sripa B, Suttiprapa S, Jones A, et al. A granulin-like growth factor secreted by the carcinogenic liver fluke, Opisthorchis viverrini, promotes proliferation of host cells. PLoS Pathog. 2009;5:e1000611. doi: 10.1371/journal.ppat.1000611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pinlaor P, Kaewpitoon N, Laha T, Sripa B, Kaewkes S, Morales ME, et al. Cathepsin F cysteine protease of the human liver fluke, Opisthorchis viverrini. PLoS Negl Trop Dis. 2009;3:e398. doi: 10.1371/journal.pntd.0000398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cantacessi C, Mulvenna J, Young ND, Kasny M, Horak P, Aziz A, et al. A deep exploration of the transcriptome and “excretory/secretory” proteome of adult Fascioloides magna. Mol Cell Proteomics. 2012;11:1340–53. doi: 10.1074/mcp.M112.019844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mulvenna J, Sripa B, Brindley PJ, Gorman J, Jones MK, Colgrave ML, et al. The secreted and surface proteomes of the adult stage of the carcinogenic human liver fluke Opisthorchis viverrini. Proteomics. 2010;10:1063–78. doi: 10.1002/pmic.200900393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kim YJ, Choi MH, Hong ST, Bae YM. Proliferative effects of excretory/secretory products from Clonorchis sinensis on the human epithelial cell line HEK293 via regulation of the transcription factor E2F1. Parasitol Res. 2008;102:411–7. doi: 10.1007/s00436-007-0778-2. [DOI] [PubMed] [Google Scholar]
- 41.Techasen A, Loilome W, Namwat N, Duenngai K, Cha’on U, Thanan R, et al. Opisthorchis viverrini-antigen induces expression of MARCKS during inflammation-associated cholangiocarcinogenesis. Parasitol Int. 2012;61:140–4. doi: 10.1016/j.parint.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 42.Ninlawan K, O’Hara SP, Splinter PL, Yongvanit P, Kaewkes S, Surapaitoon A, et al. Opisthorchis viverrini excretory/secretory products induce toll-like receptor 4 upregulation and production of interleukin 6 and 8 in cholangiocyte. Parasitol Int. 2010;59:616–21. doi: 10.1016/j.parint.2010.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Reiser J, Adair B, Reinheckel T. Specialized roles for cysteine cathepsins in health and disease. J Clin Invest. 2010;120:3421–31. doi: 10.1172/JCI42918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chen X, Li S, He L, Wang X, Liang P, Chen W, et al. Molecular characterization of severin from Clonorchis sinensis excretory/secretory products and its potential anti-apoptotic role in hepatocarcinoma PLC cells. PLoS Negl Trop Dis. 2013;7:e2606. doi: 10.1371/journal.pntd.0002606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sripa J, Pinlaor P, Brindley PJ, Sripa B, Kaewkes S, Robinson MW, et al. RNA interference targeting cathepsin B of the carcinogenic liver fluke, Opisthorchis viverrini. Parasitol Int. 2011;60:283–8. doi: 10.1016/j.parint.2011.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wang X, Chen W, Tian Y, Huang Y, Li X, Yu X. RNAi-mediated silencing of enolase confirms its biological importance in Clonorchis sinensis. Parasitol Res. 2014;113:1451–8. doi: 10.1007/s00436-014-3785-0. [DOI] [PubMed] [Google Scholar]


