Abstract
Objectives:
To evaluate and analyze the clinical and laboratory parameters that were predictive of the development of shock in children with dengue fever.
Subjects and Methods:
Retrospective study carried out from August 2012 to July 2014 at a tertiary care hospital in Puducherry.
Results:
Two hundred and fifty-four children were admitted with dengue fever and among them dengue fever without shock was present in 159 children (62.5%) and dengue fever with shock was present in 95 cases (37.4%). Various clinical and laboratory parameters were analyzed using univariate and multivariate logistic regression between the two groups and a P value of <0.05 was taken as significant. The most common risk factors for shock on univariate analysis were headache, retro-orbital pain, palmar erythema, joint pain, facial flush, splenomegaly, lymphadenopathy, bleeding, giddiness, persistent vomiting, pleural effusion, ascites, hematocrit >20% with concomitant platelet count <50,000/mm3 on admission, deranged liver function tests, and gallbladder wall edema. On multivariate analysis, it was seen that in age >6 years, hepatomegaly, pain in the abdomen, and oliguria were the most common risk factors associated with shock in children with dengue fever. There were six deaths (2.4%) and out of them four presented with impaired consciousness (66.6%) at the time of admission.
Conclusion:
Age >6 years, hepatomegaly, abdomen pain, and oliguria were the most common risk factors for shock in children with dengue fever. Impaired consciousness at admission was the most ominous sign for mortality in dengue fever. Hence, these features should be identified early, monitored closely, and managed timely.
Keywords: Dengue shock syndrome, encephalopathy, risk factors
Introduction
Dengue fever is the most rapidly spreading mosquito-borne viral disease worldwide with an unpredictable clinical course and outcome.[1,2] If case detection and management is delayed, the morbidity and mortality from shock and hemorrhage is very high.[3,4] The objective of this study was to the determine the risk factors for shock at an early stage of illness in children admitted with dengue fever at a tertiary care hospital.
Subjects and Methods
After approval by the Institute Ethics Committee, case records of all children admitted with dengue fever at a tertiary care hospital at Puducherry from August 2012 to July 2014 were reviewed and only confirmed cases of dengue fever were included in the study. They were categorized as dengue fever without shock (nondengue shock syndrome [DSS] group) and dengue fever with shock (DSS group), and the risk factors associated with them were retrospectively analyzed. The case definition, clinical diagnosis, and management of dengue fever in children were as per the 2011 World health Organization (WHO) revised guidelines.[1] Details of clinical and laboratory profile, risk factors, and the treatment given were recorded in a predesigned pro forma. The diagnosis of dengue fever was confirmed by NS1 antigen-based ELISA test (J. Mitra kit, India) and/or dengue serology for immunoglobulin M, and IgG antibodies (Kit from National Vector Borne Disease Control Programme, Puducherry and National Institute of Virology, Pune, India).
Statistical analysis
The SPSS (IL Chicago) 16.0 software statistical software was used for data analysis. Categorical data were analyzed using Chi-square or Fisher's exact test. Continuous data were analyzed using Student's t-test or Mann–Whitney U-tests. The risk factors of shock among children with dengue fever were determined by univariate analysis. Multivariate analysis and coefficient of binary logistic regressions were used in children who were statistically significant on univariate analysis. The results were presented as unadjusted odds ratio (OR), adjusted OR, with 95% confidence interval (CI). P < 0.05 was taken as significant.
Results
Two hundred and fifty-four children with confirmed cases of dengue fever were retrospectively analyzed. Dengue fever without shock was present in 159 (62.5%) cases, and dengue fever with shock was present in 95 (37.5%) cases. The most common affected age group was 6–12 years (58.2%) and the mean age at presentation was 7.0 (3.3) years. Male to female ratio was 1.2:1.
The clinical variables most commonly associated with shock on univariate analysis were age >6 years, myalgia, headache, retro-orbital pain, palmar erythema, joint pain, facial flush, splenomegaly, and lymphadenopathy [Table 1]. The early clinical warning signs at the time of admission significantly associated with shock on univariate analysis were bleeding, persistent vomiting, giddiness, oliguria, pain in abdomen, right hypochondriac pain, epigastric tenderness, hepatomegaly, pleural effusion, and ascites [Table 1]. The common neurological manifestations at the time of admission were impaired consciousness in six children, and all belonged to DSS group and seizures in seven cases (5 in the DSS group and 2 in the non-DSS group). Bleeding manifestations were seen in 51 cases (20.1%), and the common forms of bleeding were petechiae, gum bleeding, hematemesis, and melena. Petechiae was the most common form of bleeding in nonsevere dengue infection whereas melena was the common form in severe-dengue infection. Tourniquet test was positive in 33 cases (12.9%) and showed significant association with shock (P < 0.001) [Table 1]. The laboratory parameters most commonly associated with shock on univariate analysis were thrombocytopenia at admission, hematocrit (HCT) >20% with concomitant platelet count <50,000/mm3 on admission, severe thrombocytopenia (platelet count <20,000/mm3), HCT >40, deranged liver function tests (LFTs), gallbladder wall edema, secondary infection (positive dengue IgG antibody), and hyponatremia [Table 1].
Table 1.
Univariate analysis of risk factors for shock in dengue fever in children
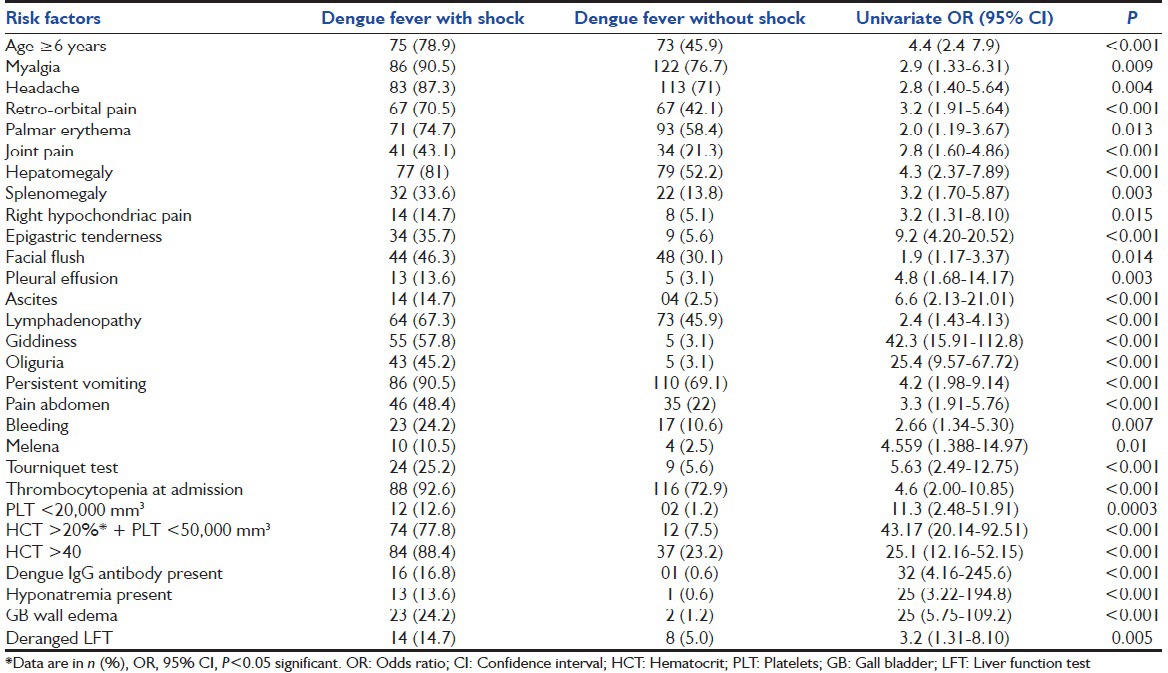
The mean HCT in the DSS and non-DSS groups were 43.4 (4.4) and 38.9 (4.4) respectively. The clinical and laboratory variables that were more common in children with shock on univariate analysis and were not statistically significant were Fever >7 days, rash, conjunctival congestion, diarrhea, pedal edema, jaundice, coagulopathy, epistaxis, petechiae, hematemesis, and leucopenia. Coryza and pharyngeal congestion were more common in nonsevere dengue infection but were not statistically significant.
Ninety-six children (37.7%) required intravenous fluids, 14 children (5.5%) required platelet transfusion, 6 children (2.3%) required fresh frozen plasma, 10 children (3.9%) required packed cell transfusion, colloids in 3 cases (1.1%), and the requirement was more in DSS group than in non-DSS group. Six children (2.3%) expired, and all were in DSS group with mean time to death being 2.7 (2.3) days. Four out of six children who had expired had impaired consciousness at the time of admission (66%).
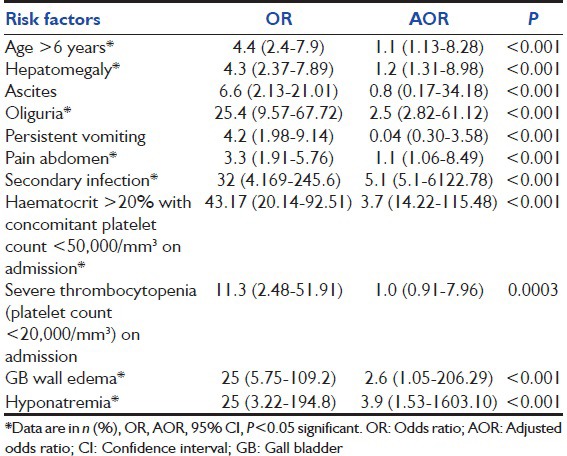
On multivariate analysis, it was seen that age > 6 years, hepatomegaly, pain in abdomen, oliguria, secondary infection, HCT > 20% on admission, gallbladder wall edema, and hyponatremia were most common risk factors associated with shock in children with dengue fever [Table 2].
Table 2.
Multivariate analysis of risk factors for shock in dengue fever in children
Discussion
This study was an attempt to predict the various risks factors associated with shock. We found that the most commonly affected group were children >6 years of age and were significantly at increased risk to develop shock. As per the 2011 WHO revised guidelines, infants and children are high risk group to develop severe dengue infection in the form of shock and hemorrhage that is contrary to our findings.[1] The probable factors for higher age to be affected were increased exposure to mosquito bites, active viral replication, and secondary infection as was with few previous studies.[5,6]
Bleeding manifestations were significantly associated with shock in our study and were more common in children >6 years of age, similar to the previous studies.[2,4] Melena was the most common form of internal bleeding in our study in comparison to the previous studies where hematemesis was the most common form of internal bleeding and tourniquet test positivity was much lower in comparison to the previous studies.[7,8] There was poor correlation between tourniquet test, bleeding manifestations, and thrombocytopenia but was significantly associated with shock. The signs of plasma leakage in the form of pleural effusion, ascites, and HCT >20% with concomitant platelet count <50,000/mm3 were found to be significant predictors of shock as previously reported by Chacko and Subramanian.[2] The early clinical warning signs in children with plasma leakage that were significantly associated with shock were persistent vomiting, giddiness, and oliguria. Hepatomegaly and pain in abdomen were the other clinical warning signs that were significantly associated with severe dengue infection were significantly associated with shock and is similar to the few previous studies.[2,3,4] Abnormal LFTs were significantly associated with shock in this study that is contrary to the previous studies.[3,5,7]
Altered sensorium at the time of admission was an ominous sign for mortality in children with shock in our study as four out of six cases (66%) had expired. Pancharoen, in his study, reported altered sensorium as the most common neurological finding followed by seizures and observed these findings in 75% of patients with DSS.[9] Dengue infection can cause neurological manifestations secondary to cerebral hypoperfusion due to shock, encephalopathy, hepatic dysfunction, metabolic derangements such as hyponatremia and hypoglycemia, or rarely due to acute disseminated encephalomyelitis or Guillain-Barre syndrome.[10]
Thrombocytopenia at the time of admission and platelet count < 20,000 mm3 (P < 0.001) were significant predictors of shock and similar to the previous studies by Chacko, Subramanian, and Dhooria et al.[2,11] Hyponatremia was significantly associated with shock in our study similar to the study by Chacko and Subramanian.[2] Gallbladder edema on ultrasound in our study was significantly associated with shock and was similar to the previous studies.[2,12]
Secondary infection was significantly associated with shock in our study. Wichmann et al. in their study showed that secondary infection was significantly associated with shock. During secondary infection T-cells become activated due to interactions with infected monocytes which induce plasma leakage by release of cascade of cytokines such as interferon-gamma, interleukin 2, and tumor necrosis factor-alpha therefore predisposing to shock.[5]
The predominant presentation in children with shock was peripheral circulatory failure without bleeding which made it difficult to classify dengue hemorrhagic fever as per the older guidelines given by WHO.[1,13,14] We used the WHO revised guidelines 2011 for dengue fever and we termed them as severe dengue with peripheral circulatory failure without bleeding rather than dengue hemorrhagic fever.[1] It also indicated a change in the pattern of presentation of dengue fever during the recent epidemics at Puducherry. We derived the risk factors of shock in children with dengue fever by univariate and multivariate analysis and coefficient of binary logistic regression to overcome the confounding variables. The limitation of the study was that many variables had statistical significance with wider CIs indicating a relatively smaller sample size and only involved a single center. A larger multicentric prospective study with wider sample size and population in this direction would be ideal.
Conclusion
Age >6 years, hepatomegaly, pain in abdomen, and oliguria were the most common risk factors for shock in children with dengue fever. Impaired consciousness at admission was the most ominous sign for mortality in dengue fever. Hence, these features should be identified early, monitored closely, and managed timely.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Geneva: WHO; 2011. WHO. Dengue Guidelines for Diagnosis, Treatment, Prevention and Control, New Edition. [PubMed] [Google Scholar]
- 2.Chacko B, Subramanian G. Clinical, laboratory and radiological parameters in children with dengue fever and predictive factors for dengue shock syndrome. J Trop Pediatr. 2008;54:137–40. doi: 10.1093/tropej/fmm084. [DOI] [PubMed] [Google Scholar]
- 3.Gupta V, Yadav TP, Pandey RM, Singh A, Gupta M, Kanaujiya P, et al. Risk factors of dengue shock syndrome in children. J Trop Pediatr. 2011;57:451–6. doi: 10.1093/tropej/fmr020. [DOI] [PubMed] [Google Scholar]
- 4.Tantracheewathorn T, Tantracheewathorn S. Risk factors of dengue shock syndrome in children. J Med Assoc Thai. 2007;90:272–7. [PubMed] [Google Scholar]
- 5.Wichmann O, Hongsiriwon S, Bowonwatanuwong C, Chotivanich K, Sukthana Y, Pukrittayakamee S. Risk factors and clinical features associated with severe dengue infection in adults and children during the 2001 epidemic in Chonburi, Thailand. Trop Med Int Health. 2004;9:1022–9. doi: 10.1111/j.1365-3156.2004.01295.x. [DOI] [PubMed] [Google Scholar]
- 6.Pham TB, Nguyen TH, Vu TQ, Nguyen TL, Malvy D. Predictive factors of dengue shock syndrome at the children hospital no 1, Ho-chi-Minh City, Vietnam. Bull Soc Pathol Exot. 2007;100:43–7. [PubMed] [Google Scholar]
- 7.Narayanan M, Aravind MA, Thilothammal N, Prema R, Sargunam CS, Ramamurty N. Dengue fever epidemic in Chennai – a study of clinical profile and outcome. Indian Pediatr. 2002;39:1027–33. [PubMed] [Google Scholar]
- 8.Aggarwal A, Chandra J, Aneja S, Patwari AK, Dutta AK. An epidemic of dengue hemorrhagic fever and dengue shock syndrome in children in Delhi. Indian Pediatr. 1998;35:727–32. [PubMed] [Google Scholar]
- 9.Pancharoen C, Thisyakorn U. Neurological manifestations in dengue patients. Southeast Asian J Trop Med Public Health. 2001;32:341–5. [PubMed] [Google Scholar]
- 10.Murthy JM. Neurological complication of dengue infection. Neurol India. 2010;58:581–4. doi: 10.4103/0028-3886.68654. [DOI] [PubMed] [Google Scholar]
- 11.Dhooria GS, Bhat D, Bains HS. Clinical profile and outcome in children of dengue haemorrhagic fever in North India. Iran J Pediatr. 2008;18:222–8. [Google Scholar]
- 12.Colbert JA, Gordon A, Roxelin R, Silva S, Silva J, Rocha C, et al. Ultrasound measurement of gallbladder wall thickening as a diagnostic test and prognostic indicator for severe dengue in pediatric patients. Pediatr Infect Dis J. 2007;26:850–2. doi: 10.1097/INF.0b013e3180619692. [DOI] [PubMed] [Google Scholar]
- 13.Basuki PS, Budiyanto, Puspitasari D, Husada D, Darmowandowo W, Ismoedijanto, et al. Application of revised dengue classification criteria as a severity marker of dengue viral infection in Indonesia. Southeast Asian J Trop Med Public Health. 2010;41:1088–94. [PubMed] [Google Scholar]
- 14.Balasubramanian S, Ramachandran B, Amperayani S. Dengue viral infection in children: A perspective. Arch Dis Child. 2012;97:907–12. doi: 10.1136/archdischild-2012-301710. [DOI] [PubMed] [Google Scholar]


