Abstract
Purpose:
To review the changing pattern of donor, corneal utilization in an eye bank at a Tertiary Care Center in Northern India by analyzing the trend in the years 2003, 2008, and 2011.
Methods:
A retrospective review of eye bank records for 3 years (2003, 2008, and 2011) was performed at the National Eye Bank. Details including a clinical grade of donor cornea, indication of corneal transplantation (therapeutic or optical), type of procedure (penetrating or lamellar keratoplasty [LK]), and clinical diagnosis of the graft recipients were recorded. Primary outcome measure was to observe any preference toward LK, judicious usage of donor corneal tissue, and impact of lamellar corneal transplant in the usage of donor corneas. Secondary outcomes included overall utilization rate and change in trend of indication for keratoplasty.
Results:
A total of 673, 745, and 864 corneas were retrieved in the years 2003, 2008, and 2011, respectively. The percentage of donor corneal utilization increased significantly over time with the rate being 65.08%, 70.06%, and 68.29%, respectively, in the years 2003, 2008, and 2011 (P = 0.014); however, this change was reflected only in the usage of nonoptical grade corneas and not for the optical grade corneas. There was an overall increase in lamellar corneal procedures for any clinical grade of cornea (P = 0.0019); number of Descemet's stripping automated endothelial keratoplasty (DSAEK) procedures increased significantly (P < 0.001), particularly for pseudophakic corneal edema (PCE) (P = 0.0085) and failed graft (P = 0.002). Significant increase in the utilization of nonoptical grade corneas was observed over the years (P = 0.005), though the utilization did not increase significantly for optical purposes viz., LK (P = 0.08).
Conclusions:
Utilization rate of donor corneas increased over the years, primarily due to increase in usage of nonoptical grade corneas for therapeutic purposes. There was a procedural shift toward DSAEK for PCE and failed graft. However, an increase in usage of nonoptical grade corneas for LK, a single donor corneal tissue for two recipients, and retrieval or utilization of optical grade cornea was not observed.
Keywords: Donor corneal utilization, eye bank, keratoplasty, split corneal surgery, trend
The World Health Organization identified in 2010 that globally 4.9 million people were bilaterally corneal blind, which accounted for 12% of the total burden of global blindness (39 million).[1] Most of the corneal blindness is preventable, and the results of corneal transplantation in many of these conditions may be suboptimal.[2] Though penetrating keratoplasty (PKP) remains the gold standard for corneal transplantation, the outcome in these cases may be marred by immune rejection, chronic endothelial cell loss, and increased intraocular pressures, all of which eventually lead to graft failure.[3] As a result of this, introduction of lamellar keratoplasty (LK) has led to a major shift in preferred practice pattern of most ophthalmologists in the world. Besides being advantageous in places with shortage of optimum quality donor tissue, targeted tissue corneal transplantation avoid problems such as astigmatism, suture-related complications, intraocular inflammation, rise in intraocular pressure, immune rejection, chronic corneal allograft dysfunction, and expulsive choroidal hemorrhage.[4,5,6] It started with the development of procedures for the removal of diseased corneal anterior stroma up to the Descemet's membrane that became popular as deep anterior LK (DALK).[7]
At National Eye Bank, cornea is procured from local collection, hospital corneal retrieval program and its collection centers in nearby areas. Procured cornea is transported in McCarey-Kaufman medium, which is being prepared locally at our Department of Ocular Pharmacology. All donors are screened for HIV and hepatitis B before the utilization of retrieved corneas. In cases, where serology cannot be performed or is positive, the retrieved cornea is not transplanted and is used for research purposes. Absolute contraindications for the use of donor cornea include poor quality cornea, serology positive for HIV, or hepatitis B virus, or if serology cannot be performed and culture positivity from the donor eye while relative contraindications include death enucleation time more than 12 h, age of patient >90 years.
Keratoplasties are performed according to the centralized waiting list, where recipients are categorized according to the need for transplantation. They are categorized as emergency, top priority, priority, and general [Annexure 1].
Annexure 1.
Categories of corneal transplant recipients

To establish the pattern of donor, corneal utilization, spectrum of corneal disorders requiring transplant, and track paradigm shift in the procedural preference at our tertiary care center, we conducted this study. We retrospectively reviewed the records of corneal replacement surgeries from our database at National Eye Bank in the years 2003, 2008, and 2011; in an interrupted time series to observe the trend of donor, corneal utilization over the 8 years.
Methods
A retrospective review was performed of eye bank records at a tertiary referral center of North India during the years 2003, 2008, and 2011. Internal record monitoring of corneal usage began in year 2003, thus explaining the selection of 2003. Year 2008 was selected to be able to gauge the trend over at least 5 years duration since the inception of internal chart monitor in 2003. Eye bank registry was formed in the year 2011, and hence the next year for analysis was chosen to be 2011. Chart data regarding clinical grade of donor cornea (assessed on slit lamp biomicroscopy) [Annexure 2], indication for corneal replacement (therapeutic or optical), type of procedure (PKP or LK), and clinical diagnosis of patients were entered in a Microsoft Excel sheet. Therapeutic keratoplasty was performed in the cases of corneal perforations, impending corneal perforations, corneal melting, and nonresponding corneal ulcers. The corneal diseases were categorized into keratoconus, corneal dystrophy or degeneration, aphakic/pseudophakic corneal edema (PCE), healed keratitis (viral/bacterial/fungal), suppurative keratitis (fungal, bacterial, and amoebic infections), failed graft, and others [congenital corneal opacity, dermoid, posttraumatic/surgical (e.g., posttrabeculectomy corneal decompensation)]. Transplantation rates for optical grade (Grade A and B+) and nonoptical grade (Grade B, B−, and C) corneas were separately analyzed.
Annexure 2.
Grading of donor eyes

Data were analyzed, and comparison was drawn between these three points in time to determine the changing trend of corneal utilization. The χ2 test was used for statistical analysis with SPSS 15.0 (SPSS Inc., Chicago, IL, USA). A P ≤ 0.05 was considered statistically significant.
Results
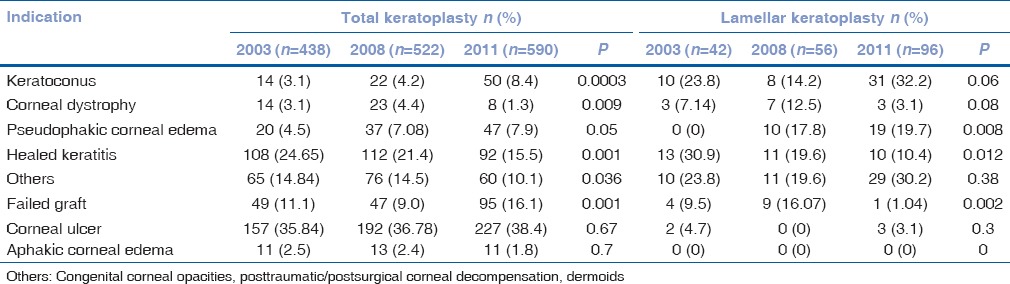
A total of 673, 745, and 864 corneas were harvested in the years 2003, 2008, and 2011, respectively, at our center. Of the total tissues harvested, 438 (65.08%), 522 (70.06%), and 590 (68.29%) were transplanted in the respective years [Table 1]. Significant difference was observed in the proportion of overall cornea transplanted over the past 8 years (P = 0.014) and use of nonoptical grade corneas (Grade B) (P = 0.005) [Table 1]. There was an increase in the number of keratoplasties for indications such as keratoconus, failed graft, PCE, and “others” category. Corneal transplantation rate decreased significantly for corneal dystrophy and healed keratitis. However, no change was observed in the rate of keratoplasty performed for indications such as aphakic corneal edema and corneal ulcer [Table 2]. The indications for keratoplasty did not differ majorly between the years 2003 and 2011. The indications for keratoplasty in decreasing order in the year 2011 were corneal ulcer, failed graft, healed keratitis, others, keratoconus, PCE, aphakic corneal edema, and corneal dystrophy [Table 2].
Table 1.
Utilization percentage of optical and nonoptical grade corneas in the 3 years
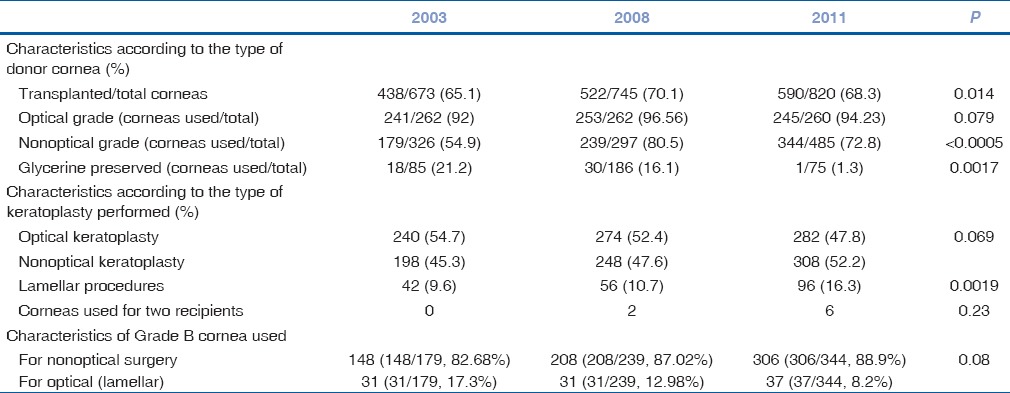
Table 2.
Indications for total and lamellar corneal procedures in 2003, 2008, and 2011
In the year 2003, of the 438 transplanted corneas, majority were B+ (51.37%) followed by nonoptical grade tissue (179/40.87%). Of the transplanted corneas, 9.6% (42 eyes) were used for lamellar corneal transplantation; the majority of these corneas were nonoptical grade (31/42; 73.8%) and suggesting their optical usage for anterior LK [Table 1].
In the year 2008, of 522 transplanted corneas, majority belonged to nonoptical grade corneas (239/45.79%) followed next by optical grade tissue (41.19%). Remarkably, of 56 corneas used for lamellar surgery, 31 belonged to nonoptical grade and the rest were optical grade, which were used for host endothelial replacement [Table 1].
In the year 2011, a total of 590 corneal caps were transplanted, of which the majority were nonoptical grade (353) followed by optical grade tissue, B+ (233) and A (3) in that order. A total of 96 corneas (16.27%) were used for lamellar surgery, of which 37 corneas were B grade and 59 were B+. Three corneas, in the year 2011, were used for two recipients. The indications for performing lamellar surgery are listed in Table 2.
In the years 2008 and 2011, an increase in the rate of lamellar corneal procedures (P = 0.0019) was observed. The rate of Descemet's stripping automated endothelial keratoplasty (DSAEK) increased significantly as compared to previous years (P = 0.001), particularly for PCE (P = 0.0085). There was a statistically significant decrease in lamellar surgery performed for healed keratitis (P = 0.012) and failed graft (P = 0.002). The indications for penetrating and lamellar tissue transplant for each year are tabulated [Table 2]. Though lamellar corneal procedures increased over years, this increase was not reflected in the usage of nonoptical grade corneas [P = 0.08; Table 1]. There was no change in the percentage of optical grade cornea retrieval or in the rate of optical keratoplasty performed over the years.
Discussion
Knowledge of regional trends of keratoplasty with respect to indications and utilization rate is important for optimal utilization of donor corneal tissue. This study is the only study from this part of the world that reports a changing trend over the course of the last few years with respect to lamellar corneal transplantation, and its effect on donor tissue utilization during the time when it was most crucial to determine the same due to changing trends over the world. With the advent of lamellar surgeries such as anterior LK or DALK for the selective removal of diseased anterior corneal layers, and endothelial keratoplasty for the replacement of deep stromal and endothelial layers, there has been procedural shift in the preference of keratoplasty procedure in most parts of the world.[8] From 1999 to 2008, the rate of DALK increased from 91 to 327 per year, whereas EK increased from 2 to 569 per year in the United Kingdom.[8] This was because while anterior LK decreased the incidence of graft rejection offered greater wound strength and stability, faster rehabilitation postsuture removal, increased the donor pool as the requirement of a healthy donor endothelium was circumvented,[4,5,6,10,11] endothelial keratoplasty eliminated the problems related to corneal sutures and surface incisions, corneal denervation, astigmatism, wound instability, prolonged recovery times, and devastating complications such as endophthalmitis, expulsive hemorrhage, and graft dehiscence often seen with PKP.[12,13,14]
Looking at any change in indications of keratoplasty, data from this study indicates that even today, infectious keratitis and healed keratitis remain the most common causes of corneal replacement in India, as was observed 15 years ago.[9] Therefore, despite the introduction of safer procedures such as LK, PKP dominated in our setup due to a high load of indications requiring therapeutic transplant. Moreover, a high proportion of therapeutic surgeries being performed reflects a greater incidence of acute infectious and corneal melting disorders in contrast to reports from developed countries.[8] A similar high incidence of therapeutic keratoplasty is reported from other Southeast Asian countries, as well in the past.[15,16]
We observed an increased utilization rate of corneas in a trend reflected over the 8 years span. However, there was no change in the rate of optical keratoplasty over the past decade; though utilization of nonoptical grade corneas increased substantially. An increase in the rate of therapeutic and LK being performed might contribute toward this rise. A host of factors could have contributed to better corneal tissue utilization. Previously, the guidelines for procuring donor corneas were not very stringent; hence, all tissues were harvested without ruling out contraindications for transplant such as septicemia and hepatitis C. Currently, donor's family is informed about all these contraindications at the time of consent. Families of donors, who do not meet tissue safety eligibility criteria, are informed that in the event of unsuitability for keratoplasty; tissue might be used for medical education and research. The families are given the option of withdrawing their decision to donate. Therefore, the harvesting of cornea not suitable for use has declined considerably. Moreover, instead of whole globe retrieval, there has been a shift toward in situ corneal excision resulting in shorter death preservation time, thus minimizing endothelial cell loss of recovered corneas. The practice of procurement of corneas from far off eye collection centers has been reduced by our eye bank due to tissue degradation during transit. The introduction of intermediate term storage media has helped in maintaining the quality of harvested corneas, implementation of an internal monitoring, evaluation system for optimal tissue tracking, and utilization of corneas with documentation of reasons for nonutilization of cornea in 2002 has helped in increasing the utilization percentage of excised corneas over the past years. These measures can also be adopted by other eye banks in developing countries to increase corneal utilization rate in their respective centers.
Nonincrease in the quantity of optical grade corneas procured is reflected by no change in the rates of optical keratoplasty performed over the years. This indicates that though tissue procurement has increased over the years, the quality of the tissue remains average. Obtaining high-quality donor cornea is essential to tackle corneal blindness in this country. Performance analysis of corneal donation at our center revealed that meager 1.9% of eligible donor pool undergoing trauma related deaths could be excised for organ transplantation, partly due to inappropriate awareness and counseling.[17,18] This occurred despite the existence of a hospital cornea retrieval program that takes into consideration several inherent advantages of procuring the corneas from within the deaths occurring at the hospital premises including availability of medical history, younger individuals, reduced time interval between death and corneal excision, and cost-effectiveness.
Due to the distinct advantages of lamellar corneal tissue replacement, we found an obvious increase in the rate of lamellar corneal transplant, especially endothelial keratoplasty. There was an overall increase in the total number of transplanted corneas with significant increase in DSAEK for conditions such as PCE for which full thickness keratoplasties were being done previously. However, no change in rate of keratoplasty was observed for aphakic corneal edema. DSAEK shows the best outcomes when performed in phakic or pseudophakic eyes with diseased endothelium,[19] as surgery in aphakic, vitrectomized eyes, carries a high risk of lenticule detachment/dislocation, and primary graft failure because of inadequate air tamponade.[19,20] This explains the preference for PKP in aphakic eyes over DSAEK.
Anterior LK is a useful alternative to conventional PKP for disorders restricted to anterior stroma. Though recent studies quote equivalent visual outcomes after anterior lamellar transplant and PKP,[10,11,21,22,23] graft survival rates and endothelial cell counts after PKP continue to decline until many years later,[23] thus conferring advantage to anterior LK for endothelial cell preservation. Moreover, despite a significant increase in endothelial keratoplasty, the increase in the use of nonoptical grade corneas for optical usage through anterior lamellar surgery was not found to be statistically significant. Therefore, contrary to expectations, an increased incidence of usage of a single donor cornea for multiple recipients was not evident. Sometimes in the event of inadvertent host corneal perforation during automated lamellar therapeutic keratoplasty or DALK, an optical grade cornea is required instead of nonoptical grade cornea as the procedure is then converted into PKP. This may also be related to logistic issues involving the waiting list of recipients based on requirement and between the timely availability of donor tissues. Nevertheless, use of a single cornea for multiple recipients is beneficial in a developing country like ours where there is a huge discrepancy between the annual demand of corneas (estimated to be 300,000) and the available tissue (approximately 15,000).[17,24] Perhaps introduction of systems to facilitate better coordination may help in increasing the usage of donor cornea for multiple recipients and aid in resolving the issue pertaining to scarcity of donor tissue. A suitable recipient pool of patients awaiting keratoplasty should be organized, thus facilitating transplant of a single donor cornea for two suitable recipients.
Hence, though we observed a procedural shift toward DSAEK for corneal endothelial disorders specifically for PCE, poor coordination for timing between the availability of two recipients for lamellar corneal transplantation was observed. Therapeutic PKP is still the most commonly performed keratoplasty and infectious disorders remain the most common indications. We did observe an encouraging trend of increased utilization rate of donor corneas specifically that of nonoptical grade corneas utilized during therapeutic keratoplasty and anterior lamellar corneal replacement surgeries. To conclude, this study suggests that though we have achieved better donor corneal utilization, policy changes need to be implemented to adopt the advantages of lamellar corneal transplantation universally for our patients who wait for long periods for undergoing keratoplasty.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Pascolini D, Mariotti SP. Global estimates of visual impairment: 2010. Br J Ophthalmol. 2012;96:614–8. doi: 10.1136/bjophthalmol-2011-300539. [DOI] [PubMed] [Google Scholar]
- 2.Garg P, Krishna PV, Stratis AK, Gopinathan U. The value of corneal transplantation in reducing blindness. Eye (Lond) 2005;19:1106–14. doi: 10.1038/sj.eye.6701968. [DOI] [PubMed] [Google Scholar]
- 3.Armitage WJ, Dick AD, Bourne WM. Predicting endothelial cell loss and long-term corneal graft survival. Invest Ophthalmol Vis Sci. 2003;44:3326–31. doi: 10.1167/iovs.02-1255. [DOI] [PubMed] [Google Scholar]
- 4.Han DC, Mehta JS, Por YM, Htoon HM, Tan DT. Comparison of outcomes of lamellar keratoplasty and penetrating keratoplasty in keratoconus. Am J Ophthalmol. 2009;148:744–51.e1. doi: 10.1016/j.ajo.2009.05.028. [DOI] [PubMed] [Google Scholar]
- 5.Sögütlü Sari E, Kubaloglu A, Ünal M, Piñero Llorens D, Koytak A, Ofluoglu AN, et al. Penetrating keratoplasty versus deep anterior lamellar keratoplasty: Comparison of optical and visual quality outcomes. Br J Ophthalmol. 2012;96:1063–7. doi: 10.1136/bjophthalmol-2011-301349. [DOI] [PubMed] [Google Scholar]
- 6.Kim MH, Chung TY, Chung ES. A retrospective contralateral study comparing deep anterior lamellar keratoplasty with penetrating keratoplasty. Cornea. 2013;32:385–9. doi: 10.1097/ICO.0b013e318254be4e. [DOI] [PubMed] [Google Scholar]
- 7.Anwar M, Teichmann KD. Big-bubble technique to bare Descemet's membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002;28:398–403. doi: 10.1016/s0886-3350(01)01181-6. [DOI] [PubMed] [Google Scholar]
- 8.Keenan TD, Jones MN, Rushton S, Carley FM National Health Service Blood and Transplant Ocular Tissue Advisory Group and Contributing Ophthalmologists (Ocular Tissue Advisory Group Audit Study. Trends in the indications for corneal graft surgery in the United Kingdom: 1999 through 2009. Arch Ophthalmol. 2012;130:621–8. doi: 10.1001/archophthalmol.2011.2585. [DOI] [PubMed] [Google Scholar]
- 9.Dandona L, Ragu K, Janarthanan M, Naduvilath TJ, Shenoy R, Rao GN. Indications for penetrating keratoplasty in India. Indian J Ophthalmol. 1997;45:163–8. [PubMed] [Google Scholar]
- 10.Bahar I, Kaiserman I, Srinivasan S, Ya-Ping J, Slomovic AR, Rootman DS. Comparison of three different techniques of corneal transplantation for keratoconus. Am J Ophthalmol. 2008;146:905–12.e1. doi: 10.1016/j.ajo.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 11.Jafarinasab MR, Feizi S, Esfandiari H, Kheiri B, Feizi M. Traumatic wound dehiscence following corneal transplantation. J Ophthalmic Vis Res. 2012;7:214–8. [PMC free article] [PubMed] [Google Scholar]
- 12.Bahar I, Kaiserman I, McAllum P, Slomovic A, Rootman D. Comparison of posterior lamellar keratoplasty techniques to penetrating keratoplasty. Ophthalmology. 2008;115:1525–33. doi: 10.1016/j.ophtha.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Terry MA. Deep lamellar endothelial keratoplasty (DLEK): Pursuing the ideal goals of endothelial replacement. Eye (Lond) 2003;17:982–8. doi: 10.1038/sj.eye.6700614. [DOI] [PubMed] [Google Scholar]
- 14.Lee WB, Jacobs DS, Musch DC, Kaufman SC, Reinhart WJ, Shtein RM. Descemet's stripping endothelial keratoplasty: Safety and outcomes: A report by the American Academy of Ophthalmology. Ophthalmology. 2009;116:1818–30. doi: 10.1016/j.ophtha.2009.06.021. [DOI] [PubMed] [Google Scholar]
- 15.Wang JY, Xie LX, Song XS, Zhao J. Trends in the indications for penetrating keratoplasty in Shandong, 2005-2010. Int J Ophthalmol. 2011;4:492–7. doi: 10.3980/j.issn.2222-3959.2011.05.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen WL, Hu FR, Wang IJ. Changing indications for penetrating keratoplasty in Taiwan from 1987 to 1999. Cornea. 2001;20:141–4. doi: 10.1097/00003226-200103000-00004. [DOI] [PubMed] [Google Scholar]
- 17.Tandon R, Verma K, Vanathi M, Pandey RM, Vajpayee RB. Factors affecting eye donation from postmortem cases in a tertiary care hospital. Cornea. 2004;23:597–601. doi: 10.1097/01.ico.0000121706.58571.f6. [DOI] [PubMed] [Google Scholar]
- 18.Kumar A, Kumar A, Bali SJ, Tandon R. Performance analysis of efforts towards promotion of corneal donation at a tertiary care trauma center in India. Cornea. 2012;31:828–31. doi: 10.1097/ICO.0b013e318240011a. [DOI] [PubMed] [Google Scholar]
- 19.Eguchi H, Miyamoto T, Hotta F, Tomida M, Inoue M, Mitamura Y. Descemet-stripping automated endothelial keratoplasty for vitrectomized cases with traumatic aniridia and aphakic bullous keratopathy. Clin Ophthalmol. 2012;6:1513–8. doi: 10.2147/OPTH.S36850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hsu M, Jorgensen AJ, Moshirfar M, Mifflin MD. Management and outcomes of descemet stripping automated endothelial keratoplasty with intraocular lens exchange, aphakia, and anterior chamber intraocular lens. Cornea. 2013;32:e64–8. doi: 10.1097/ICO.0b013e31826ef43b. [DOI] [PubMed] [Google Scholar]
- 21.Alio JL, Shah S, Barraquer C, Bilgihan K, Anwar M, Melles GR. New techniques in lamellar keratoplasty. Curr Opin Ophthalmol. 2002;13:224–9. doi: 10.1097/00055735-200208000-00006. [DOI] [PubMed] [Google Scholar]
- 22.Melles GR, Remeijer L, Geerards AJ, Beekhuis WH. The future of lamellar keratoplasty. Curr Opin Ophthalmol. 1999;10:253–9. doi: 10.1097/00055735-199908000-00006. [DOI] [PubMed] [Google Scholar]
- 23.Teichmann KD. Lamellar keratoplasty – A comeback? Middle East J Ophthalmol. 1999;7:59–60. [Google Scholar]
- 24.Krishnaiah S, Kovai V, Nutheti R, Shamanna BR, Thomas R, Rao GN. Awareness of eye donation in the rural population of India. Indian J Ophthalmol. 2004;52:73–8. [PubMed] [Google Scholar]


