Abstract
Background:
Intraoperative diagnosis of central nervous system (CNS) lesions is of utmost importance for neurosurgeons to modify the approach at the time of surgery and to decide on further plan of management. The intraoperative diagnosis is challenging for neuropathologists.
Aims:
The study was undertaken to determine the accuracy of cytological techniques (crush smears and touch imprints), frozen sections of space occupying lesions of the CNS and compare it with histopathological diagnosis.
Materials and Methods:
A total of 75 specimens received intraoperatively were subjected to cytology and frozen section study.
Results:
Neoplastic lesions formed the major group with 62 (82.7%) cases while 13 (17.3%) were nonneoplastic. The diagnostic accuracy of “squash smears” was found to be 89.2%. “Touch imprints” showed diagnostic accuracy of 78.4%. The low accuracy of touch imprints was attributed to poor cellular yield. The diagnostic accuracy of “frozen section” was 75.7%. However, the overall diagnostic accuracy was 96%.
Conclusion:
We believe that the cytololgical methods and frozen sections are complimentary to each other and both should be used to improve the intraoperative diagnostic accuracy in the CNS lesion.
Keywords: Central nervous system (CNS), frozen section, intraoperative diagnosis, squash smears, touch imprints
Introduction
Intraoperative diagnosis of space-occupying lesions of the central nervous system (CNS) is of utmost importance in order to plan the treatment of patients. The techniques available are squash smears, touch imprints, and frozen section study. Intraoperative cytologic techniques were introduced in the 1920s when Eisenhardt and Cushing reported squash smear cytology. Very few centers continued the use of smears primarily in the rapid intraoperative diagnosis while other pathologists preferred frozen sections. However, in the past two decades, the increased need for rapid diagnosis and the advent of stereotactic procedures have resulted in either supplementing or replacing the use of frozen section study by the cytology technique in intraoperative consultations.[1,2] This study was undertaken to evaluate the diagnostic utility of cytological techniques and frozen section study in the rapid intraoperative diagnosis of space occupying lesions of the CNS.
Materials and Methods
During the 18 months prospective study, fresh unfixed material was received intraoperatively. The material was subjected to cytological methods (squash smears and touch imprints) and frozen section study. The remaining tissue was processed for routine paraffin section study.
Squash smears
A tiny bit of tissue was gently crushed on a labeled clean glass slide by a second slide held at right angle.
Touch imprints
The tissue was held by forceps and gently touched on to the clean glass slide. Air dried smears and 95% ethyl alcohol fixed imprints were stained using toludine blue, May-Grünwald-Giemsa (MGG), and hematoxylin and eosin (H and E) stains as for squash smears.
Frozen section study
The tissue was processed in cryostat at −10° C to −15° C. Thin sections of 3-5 μm thickness were cut and then stained using routine H and E.
Results
During the study of the total 75 specimens, 73 were received intraoperatively while stereotactic biopsies were performed in two of the cases. The age of the patients ranged from 2 years to 84 years with a slight male predisposition (male:female-1.1:1). The majority of the cases (66.6%) were seen in the cerebral hemispheres, with the frontotemporal region being the commonest site followed by the spinal cord in 10 cases (13.3%). Neoplastic lesions formed the major group with 62 (82.7%) cases while 13 (17.3%) cases were nonneoplastic.
The overall diagnostic accuracy of “frozen section” was found to be 75.7%, whereas that of “cytological methods” was 89.2%. Frozen sections were considered better in diagnosing firmer lesions such as meningioma and schwannoma as poor cellular yield in cytological methods hampered the diagnosis. The two cases of tuberculosis showed granulomas, which were not evident on cytomorphology. In the individual lesions, the percentage accuracy was low in oligodendrogliomas (33.3%), astrocytomas (47.3%), metastatic tumors (60%), and pituitary adenomas (66.6%) as compared to cytological methods, which showed a high accuracy in oligodendrogliomas (100%) astrocytomas (100%), metastatic tumors (80%), and pituitary adenomas (100%). A combination of cytological methods, along with frozen sections, increased the percentage diagnostic accuracy to 96%. The diagnostic accuracy of the individual modalities used was much lower, viz., 89.3% in squash smears, 78.4% in touch imprints, and 75.7 % in frozen sections [Table 1 and Figures 1–3].
Table 1.
Table showing the comparative diagnostic accuracy of squash smears, touch imprints, frozen section, and combined intraoperative methods in various CNS lesions
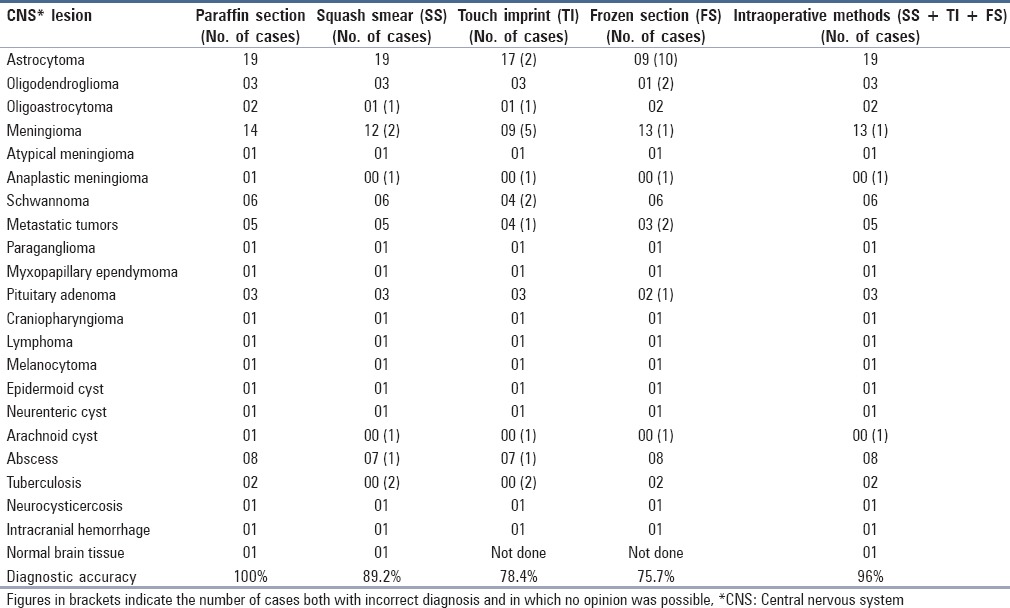
Figure 1.
(a) Low grade astrocytoma exhibiting cells with mild nuclear pleomorphism in fibrillary background (H and E, ×200) (b) “Squash smear” of high grade astrocytoma show moderate to markedly pleomorphic tumor cells including giant forms (arrow). [H and E, ×400] (c) “Frozen section” of high grade astrocytoma exhibit prominent endothelial proliferation. (H and E, ×200) (d) “Paraffin section” of the same case with pleomorphic tumor cells and bizarre forms (arrow). (H and E, ×200)
Figure 3.
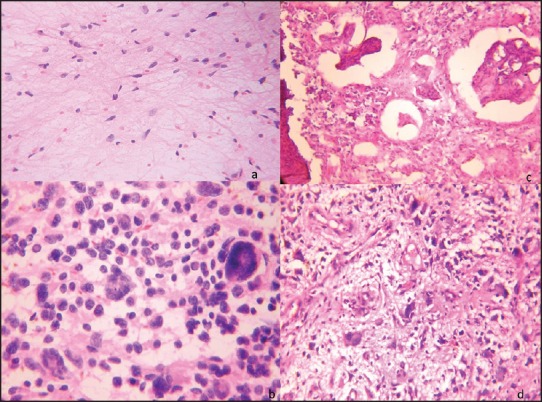
(a) Pituitary adenoma: “Squash smear”: Monomorphic tumor cells with round nuclei and moderate cytoplasm. Numerous red blood cells (RBCs) in the background (H and E, ×400) (b) “Frozen section”: Round cells with moderate cytoplasm (H and E, ×400) (c) “Frozen section”: Columnar tumor cells arranged in sinusoidal pattern (H and E, ×400) (d) “Paraffin section” of the above case showing similar cells in sinusoidal pattern (H and E, ×200)
Figure 2.
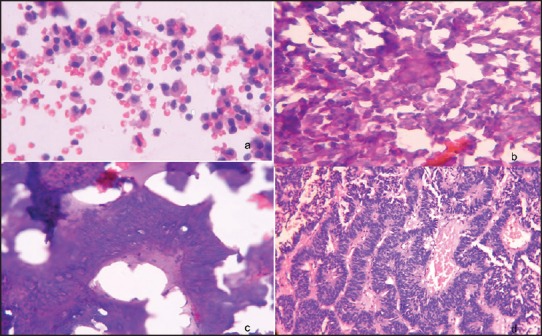
(a) Meningothelial meningioma: Smears show ovoid to elongated tumor cells with round to ovoid nuclei with fine chromatin and moderate cytoplasm. [MGG, ×400] (b) Nuclear pseudoinclusion (arrow). (MGG, ×400) (c) Fibroblastic meningioma: Crush smears show spindly cells with elongated nuclei and moderate cytoplasm. Ratio of longest to shortest diameter of nuclei is less than 2. (d) Psammomatous meningioma: Frozen section shows numerous psammoma bodies. (H and E, ×200)
The diagnosis was deferred in 17 cases on frozen section, 12 cases on touch imprints, and 4 cases on squash smears as no opinion was possible. Freezing artifacts was the main reason on frozen sections, which affected the morphology of different lesions. Poor cellular yield and sampling error were the main factors in squash smears and touch imprints.
Wrong diagnosis was made in one case of anaplastic meningioma on frozen section due to the presence of only rare mitotic figures. A wrong diagnosis was given on squash smear and touch imprints in four cases, which included two cases of tuberculosis (diagnosed as inflammatory lesions due to the absence of definite granulomas), one case of anaplastic meningioma (diagnosed as atypical meningioma as only rare mitotic figures could be identified), and a case of low grade oligoastrocytoma (diagnosed as high grade glioma probably due to thick smear giving wrong impression of high cellularity and nuclear pleomorphism on squash smears).
Discussion
Intraoperative diagnosis of space occupying lesions of the CNS helps neurosurgeons to modify the approach at surgery. The ideal intraoperative method used should be accurate, rapid, and should allow the preservation of tissue for paraffin section study. Frozen sections give the best architectural details.[3,4] However, the inherent soft nature and high water content of the nervous tissue often renders poor quality frozen sections. Hence, alternative methods such as squash smears and touch imprint cytology are often resorted to.[5,6]
“Squash smear” and “touch imprints” are cheap and technically easier to make. No special equipment is required and a very small amount of tissue can be utilized, which allows enough tissue to be preserved for paraffin section study. Inability to control thickness, crushing artifacts, and difficult smearing were some of the drawbacks of squash smears. Folkerth also pointed out the abovementioned advantages and disadvantages of squash smear cytology.[5,7,8,9]
Softer lesions such as astrocytomas, oligodendrogliomas, and pituitary adenomas smeared well. Lesions such as meningiomas and schwannomas were difficult to smear due to the increased fibrous component or calcification often rendering smears of poor quality. Folkerth[5] and Asha et al.[10] also mentioned in their study that cytology techniques are inferior to frozen sections in meningiomas and schwannomas.
Touch imprints exhibit better morphological details without any smearing artifact as compared to squash smears, especially in cases of metastatic adenocarcinoma and lymphoma. However, overall in this study touch imprints had poorer cellular yield, which hampered their diagnostic accuracy. Lui et al. emphasized that factors such as poor cellularity affect the diagnostic accuracy of touch preparation.[6,11]
“Frozen sections” are better in diagnosing firmer lesions and offered better architectural features as compared to cytology. Freezing artifacts were the major drawback, which limited the diagnostic accuracy. In the present study, no opinion was made in 22.9% cases due to marked freezing artifacts. It was also stated in other studies that frozen section was more suitable for firmer lesions and that freezing artifacts usually hampered its diagnostic utility.[6,7]
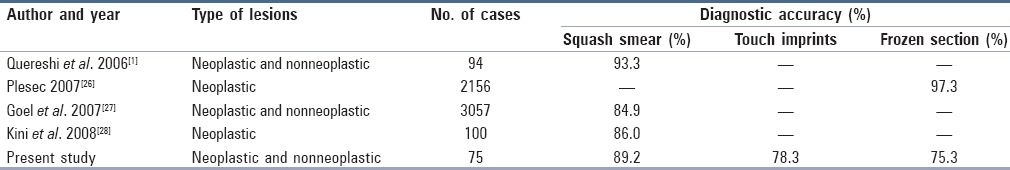
The diagnostic accuracy of “squash smears and touch imprints” was found to be in concordance with other authors. However, diagnostic accuracy of “frozen section” in the present study was 75.7%, which was far lower than that of other studies [Table 2].
Table 2.
Comparison of diagnostic accuracy of squash smear, touch imprint, and frozen section study in various studies
Low grade astrocytoma exhibited moderate cellularity with mild nuclear pleomorphism on cytology. No mitosis, necrosis, or endothelial proliferation was evident. Low grade astroctyomas are sometimes difficult to differentiate from reactive gliosis but features such as low cellularity and low nucleocytoplasmic ratio are more in favor of reactive gliosis.[8,11]
High grade astrocytomas on cytology exhibited high cellularity and moderate-to-marked nuclear pleomorphism. An attempt was made to differentiate between grade III (anaplastic) and grade IV [glioblastoma multiforme (GBM)] astrocytomas on the basis of the presence or absence of necrosis. Asha et al. also emphasize that a high degree of caution should be taken before making an attempt to categorize gliomas into astrocytomas, oligodendrogliomas, or ependymomas as mixture of all these is very frequent.[10,12]
Oligodendrogliomas exhibited characteristic monotonous round cells, mildly pleomorphic nucleus, and an absent fibrillary background. Less distinct cytoplasmic processes and calcification are characteristic of oligodendroglioma, which help in differentiating it from low grade astrocytoma.[12,13] Oligodendroglioma can also be confused with lymphoma and ependymoma on cytology. Lymphomas show dark nuclei, prominent nucleoli, chromatin clumping, and often nuclear indentation.[13,14]
Oligoastrocytoma could be correctly made out on cytology in one case. The error on cytology was attributed to wrong sampling and inappropriate smearing. It has been pointed out that sampling error can affect the grading of astrocytic neoplasms and the lesion can be over diagnosed or misdiagnosed.[4,15,16,17] Myxopapillary ependymoma showed characteristic cytology with cellular papillary processes laced by round to polygonal tumor cells and pale staining acellular globular material. Myxopapillary ependymoma can be confused with chordoma and metastatic adenocarcinoma. Differentiation from chordoma is done by the absence of physaliferous cells while metastatic adenocarcinoma can be differentiated on the basis of morphology.[13,16,18]
Meningiomas were difficult to smear and yielded poor cellularity on touch imprints. These features were also noted by other authors.[4,8,9] Frozen sections best exhibited architectural features. Cytology revealed in addition to the whorling pattern, prominent nucleoli and pseudoinclusions. It is often difficult to differentiate between fibroblastic meningioma and schwannoma as both have spindly cells. Absence of whorling in schwannoma is the differentiating feature.[18,19,20] Kobayashi[21] also pointed out that in schwannoma, the length to breadth ratio of nuclei is greater than 2, whereas in fibroblastic meningioma it is less than 2. The case of atypical meningioma could be diagnosed both on cytology and frozen section based on high cellularity, moderate nuclear pleomorphism, and presence of scattered mitotic figures. However, the case of anaplastic meningioma was diagnosed as atypical meningioma because only infrequent mitotic figures were noted in cytology and frozen section. Brain infiltration was evident on frozen section in this case. Ali et al.[22] state that it is usually difficult to grade meningiomas on cytology and suggests differentiation between atypical and anaplastic meningioma by features such as necrosis and mitosis.
Difficulty in smearing can be a drawback in schwannomas. The characteristic features of fasciculating bundles of oval to elongated cells arranged in a parallel fashion having oval to elongated nuclei were well-seen in both cytology and frozen sections. However, differentiating schwannoma from fibrous meningioma can be difficult.[9,19]
Metastatic tumors (five cases) were diagnosed better on cytology. Touch imprints were cellular and offered better morphological details as compared to squash smears. Metastatic carcinoma could also be difficult to distinguish from glioblastoma multiforme. The tumor cells in carcinomas have abundant dense cytoplasm and they lack the fibrillary background of glial tumors.[15,16,17] Powell[23] states that the squamous and adenocarcinomatous nature of metastatic epithelial malignancies can be picked up better on frozen section.
Pituitary adenomas (three cases) were diagnosed with 100% accuracy on cytology as compared to 66% accuracy rate in frozen sections. Smears and imprints revealed characteristic features of the papillary pattern of relatively monomorphic cells with nuclei having stippled chromatin and micronucleoli. In one case, the cells appeared more columnar. Frozen section also mirrored similar features. Bi- and multinucleated cells may be evident on cytology. The differential diagnosis includes normal pituitary parenchyma and ependymoma. Normal pituitary parenchyma is firmer in consistency and thus, difficult to smear. Cells of normal parenchyma show greater variation in size due to presence of different populations of cells. The lack of glial background distinguishes pituitary adenoma from ependymoma.[13,14]
Craniopharyngioma (one case) was easily recognized both on cytology and frozen section. The differential diagnosis of pituitary adenoma includes normal pituitary parenchyma and ependymoma. Normal pituitary parenchyma is firmer in consistency and thus, difficult to smear. Cells of normal parenchyma show greater variation in size due to presence of different populations of cells. The lack of glial background distinguishes pituitary adenoma from ependymoma.[8,13,14,16] Lesions such as Rathke's pouch, epidermoid cyst, and dermoid cyst lack the calcification seen in craniopharyngioma.[13,14,22,23]
Melanocytoma on smears and frozen sections showed abundant extra and intracellular melanin, which obscured the finer details of the ovoid to spindly tumor cells. Though these lesions are cellular even on histopathology, their benign nature is confirmed by the absence of mitosis, pleomorphism, and necrosis.[13] One case each of neurenteric cyst and epidermoid cyst were diagnosed. Scanty cellular smears and imprints in neurenteric cyst was a problem; hence, the diagnosis of neurenteric cyst with differential dermoid cyst was given intraoperatively. The diagnosis of a case of arachnoid cyst was missed intraoperatively due to sampling error on cytology and freezing artifacts in frozen sections. Epidermoid cyst can be confused with craniopharyngioma but characteristic cytology features and clinicoradiological data can help in the differentiation.[13,14,24,25]
Nonneoplastic lesions comprised mainly abscess (eight cases), tuberculosis (two cases), and neurocysticercosis (one case). All the cases of abscess except for one, which had a poor cellular yield were diagnosed on cytology. Both the cases of tuberculosis were not diagnosed on cytology as no granulomas were evident while frozen sections did show definite granulomas.
A case of neurocysticercosis was diagnosed on cytology and frozen sections. Although smears were poorly cellular, the presence of calcareous corpuscles clinched the diagnosis[28].
Calcareous corpuscles can be confused with psammoma bodies. Calcareous corpuscles appear naked, whereas psammoma bodies remain surrounded by meningothelial cells. Moreover, the laminations in psammoma bodies are irregular and wavy, whereas calcareous corpuscles have uniform concentric rings.[10]
Conclusion
Squash smears and touch imprints are rapid, cost-effective, and allow preservation of cellular details better than frozen section. Touch imprints exhibit better morphological details than squash smear as crush artifacts are avoided. Although frozen sections offered the best architectural details, freezing artifacts was a major drawback. Freezing artifacts can be avoided to a large extent by optimal temperature control and rapid freezing of tissue. Cytological methods are often superior to frozen sections in softer lesions. Overall, it can be said that cytology and frozen section are complimentary to each other and increased familiarity with cytomorphological features of the lesions, adequate clinical history, neuroimaging details, and intraoperative findings can help the pathologist to improve diagnostic accuracy.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Qureshi IA, Jamal S, Mamoon N, Luqman M, Sharif MA. Usefulness of crush smears intraoperative consultaion of neurological biopsis. J Coll Physicians Surg Pak. 2006;16:590–3. [PubMed] [Google Scholar]
- 2.Jaiswal S, Vij M, Jaiswal AK, Behari S. Intraoperative squash cytology of central nervous system lesions: A single center study of 326 cases. Diagn Cytopathol. 2010;40:104–12. doi: 10.1002/dc.21506. [DOI] [PubMed] [Google Scholar]
- 3.Gal AA. The centennial anniversary of the frozen section technique at the mayo clinic. Arch Pathol Lab Med. 2005;129:1532–5. doi: 10.5858/2005-129-1532-TCAOTF. [DOI] [PubMed] [Google Scholar]
- 4.Din NU, Memon A, Idress R, Ahmad Z, Hasan S. Central nervous system lesions: Correlation of intraoperative and final diagnoses, six year experience at a referral centre in a developing country, Pakistan. Asian Pac J Cancer Prev. 2011;12:1435–7. [PubMed] [Google Scholar]
- 5.Folkerth RD. Smears and frozen sections in the intraoperative diagnosis of central nervous system lesions. Neurosug Clin N Am. 1994;5:1–18. [PubMed] [Google Scholar]
- 6.Shah AB, Bhagwati SN, Mishra M. Rapid diagnosis of CNS tumours by smear technique. Acta Cytol. 1992;40:81–5. [Google Scholar]
- 7.Raisanen J, Goodman HS, Ghougassian DF, Harper CG. Role of cytology in the interaoperative diagnosis of central demylinating disease. Acta Cytol. 1998;42:907–12. doi: 10.1159/000331966. [DOI] [PubMed] [Google Scholar]
- 8.Savargaonkar P, Farmer PM. Utility of intra-operative consultations for the diagnosis of central nervous system lesions. Ann Clin Lab Sci. 2001;31:133–9. [PubMed] [Google Scholar]
- 9.Di Stefano D, Scucchi LF, Cosentino L, Bosman C, Vecchione A. Introperative diagnosis of nervous system lesions. Acta Cytol. 1998;42:346–56. doi: 10.1159/000331614. [DOI] [PubMed] [Google Scholar]
- 10.Asha T, Shankar SK, Rao TV, Das S. Role of squash-smear technique for rapid diagnosis of neurosurgical biopsies-a cytomorphological evaluation. Indian J Pathol Microbiol. 1989;32:152–60. [PubMed] [Google Scholar]
- 11.Liu Y, Silverman JF, Sturgis CD, Brown HG, Dabbs DJ, Raab SS. Utility of intraoperative consultation touch preparations. Diagn Cytopathol. 2002;26:329–33. doi: 10.1002/dc.10102. [DOI] [PubMed] [Google Scholar]
- 12.Mitra S, Kumar M, Sharma V, Mukhopadhyay D. Squash preparation: A reliable diagnostic tool in the intraoperative diagnosis of central nervous system tumors. J Cytol. 2010;27:81–5. doi: 10.4103/0970-9371.71870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ironside JW. Update on central nervous system cytopathology. II. Brain smear technique. J Clin Pathol. 1994;47:683–8. doi: 10.1136/jcp.47.8.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams HJ, Grahm DI, Doyle D. Biopsy pathology series. London: Chapman and Hill Publishers; 1981. Brain biopsy. The smear technique for neurosurgical biopsies; p. 18. [Google Scholar]
- 15.Jha B, Patel V, Patel K, Aggarwal A. Role of squash smear technique in intraoperative diagnosis of CNS tumors. Int J Med Sci Public Health. 2013;2:889–92. [Google Scholar]
- 16.Shukla K, Parikh B, Shukla J, Trivedi P, Shah B. Accuracy of cytologic diagnosis of Central Nervous system tumors in crush preparation. Indian J Pathol Microbiol. 2006;49:483–6. [PubMed] [Google Scholar]
- 17.Cahill EM, Higvegi DF. Crush preparations of lesions of the central nervous system. A useful adjunct to the frozen section. Acta Cytol. 1985;29:279–85. [PubMed] [Google Scholar]
- 18.Nguyen GK, Johnson ES, Mielke BW. Comparative cytomorphology of pituitary adenomas and oligodendrogliomas in intraoperative crush preparations. Acta Cytol. 1992;36:661–7. [PubMed] [Google Scholar]
- 19.Chen KT. Crush cytology of Rosai-Dorfman disease of the central nervous system. A report of 2 cases. Acta Cytol. 2003;47:1111–5. doi: 10.1159/000326659. [DOI] [PubMed] [Google Scholar]
- 20.Ortega L, Jiménez-Heffernan JA, Sanz E, Ortega P. Squash cytology of intradural myxopapillary ependymoma. Acta Cytol. 2002;46:428–30. [PubMed] [Google Scholar]
- 21.Kobayashi S. Meningioma, neurilemmoma and astrocytoma specimens obtained with the squash method for cytodiagnosis. A cytological and immunochemical study. Acta Cytol. 1993;37:913–22. [PubMed] [Google Scholar]
- 22.Ali S, Nassar A, Siddiqui MT. Crush preparations of meningiomas: Can grading be accomplished. Diagn Cytopathol. 2008;36:827–31. doi: 10.1002/dc.20929. [DOI] [PubMed] [Google Scholar]
- 23.Powell SZ. Intraoperative consultation, cytologic preparations and frozen section in the central nervous system. Arch Pathol Lab Med. 2005;129:1635–52. doi: 10.5858/2005-129-1635-ICCPAF. [DOI] [PubMed] [Google Scholar]
- 24.Brommeland T, Lindal S, Straume B, Dahl IL, Hennig R. Does imprint cytology of brain tumors improve intraoperative diagnosis? Acta Neurol Scand. 2003;108:153–6. doi: 10.1034/j.1600-0404.2003.00115.x. [DOI] [PubMed] [Google Scholar]
- 25.Rosenblum MK, Bilbao JM, Ang LC. Neuromuscular System. In: Rosai J, editor. Rosai and Ackerman's Surgical Pathology. 9th ed. Vol. 2. New York: Mosby; 2004. pp. 2490–1. [Google Scholar]
- 26.Plesec T, Prayson RA. Frozen section discrepancy in the evaluation of central nervous system tumours. Arch Pathol Lab Med. 2007;131:1532–40. doi: 10.5858/2007-131-1532-FSDITE. [DOI] [PubMed] [Google Scholar]
- 27.Goel D, Sundaram C, Paul R, Uppin SG, Prayaga AK, Panigrahi MK, et al. Intraoperative cytology (squash smear) in neurosurgical practice-pitfalls in diagnosis experience based on 3057 samples from a single institution. Cytopathology. 2007;18:300–8. doi: 10.1111/j.1365-2303.2007.00484.x. [DOI] [PubMed] [Google Scholar]
- 28.Kini JR, Jeyraj V, Jayaprakash CS, Indra S, Naik CNR. Intraoperative consultation and smear cytology in the diagnosis of brain tumors. Kathmandu Univ Med J (KUMJ) 2008;6:453–7. doi: 10.3126/kumj.v6i4.1734. [DOI] [PubMed] [Google Scholar]


