Abstract
Introduction:
Vyaghriharitaki Avaleha (VHA), a polyherbal classical Ayurvedic formulation has been used in Kasa (cough), Swasa (asthma), Rajayakshma (tuberculosis) etc., conditions.
Aim:
To evaluate the clinical efficacy of VHA in the management of chronic bronchitis.
Materials and Methods:
Patients of chronic bronchitis were given 10 g of VHA twice a day with lukewarm water before meals for 12 weeks. Improvement in clinical symptoms of chronic bronchitis as the primary outcome measures and St. George's Respiratory Questionnaire scores as secondary outcome measure was studied.
Results:
Out of 66 enrolled patients, 61 completed the treatment schedule. The results show that VHA provides statistically significant improvement with P < 0.001 in both primary and secondary outcome measures.
Conclusion:
VHA can be considered as a safe and effective formulation in the management of chronic bronchitis.
Keywords: Chronic bronchitis, Kasa, Vyaghriharitaki Avaleha
Introduction
Chronic bronchitis is a well-defined clinical condition in contemporary medical science classified under the broader heading of chronic obstructive pulmonary diseases (COPD) that is a progressive preventable condition, without cure. In modern medicine; antibiotics, anti-histaminics, bronchodilators, expectorants etc., are commonly used for the management of chronic bronchitis. Although, they are effective in reducing the severity of the disease and suppressing the symptoms; none of these modalities of treatment provide a permanent cure and have limitations owing to their unwanted effects.
Chronic obstructive pulmonary diseases hitherto under-diagnosed in India is now recognized in 4–10% of adult male population of India and other Asian countries.[1] Chronic bronchitis is more common in middle-aged males than in females. Approximately 20% of adult males and 5% of adult women are affected.[1] Global initiative for chronic obstructive lung disease estimates suggests that COPD will rise from the sixth to the third most common cause of death worldwide by 2020.[2]
Vyaghriharitaki Avaleha (VHA),[3] a classical Ayurvedic formulation has been in use for ages and has been found to be useful in treating respiratory disorders and promoting health. The present study has been undertaken to generate scientific data and validate the effect of this Ayurvedic formulation in chronic bronchitis.
Materials and Methods
Clinically diagnosed patients of chronic bronchitis were selected from Outdoor and Indoor patient department (OPD/IPD) of IPGT and RA Hospital, Gujarat Ayurved University, Jamnagar. The formulation, VHA, supplied by Central Council for Research in Ayurvedic Sciences (CCRAS), New Delhi, manufactured at Arya Vaidya Sala Factory, Kanjikode, Palakkad, Kerala, specially prepared for the present clinical trial using the ingredients and method of preparation given in Ayurvedic Pharmacopoeia of India (API). This work has been cleared by Institutional Ethics Committee (IEC) vide Ref. PGT/7-A/Ethics/2010-2011/3381 dated 10/01/2011 and was registered with Clinical Trial Registry of India (CTRI) vide CTRI/2011/11/002142. It was an interventional, open label, prospective type of clinical trial with the purpose of treatment with the endpoints of efficacy and safety of the trial drug, VHA with proper arrangements for withdrawals. No control group was used.
Inclusion criteria
Patients of either sex aged between 16 and 70 years with a history of uncomplicated chronic bronchitis who were willing and able to participate in the study for 16 weeks.
Exclusion criteria
Patients suffering from acute bronchitis having peak expiratory flow rate (PEFR) <50% of the predicted value and having other pulmonary diseases like emphysema, cor-pulmonale, cyanosis, pneumonia, asthma, cystic fibrosis, tuberculosis, lung cancer etc
Patients with poorly controlled diabetes mellitus (HbA1c > 10%) and poorly controlled hypertension (>160/100 mm of Hg), patients on prolonged (>6 weeks) medication with corticosteroids, bronchodilators, mast cell stabilizers, antidepressants, anticholinergics, etc., or other drugs that may have an influence on the outcome of the study
Patients suffering from major systemic illness necessitating long term drug treatment (rheumatoid arthritis, tuberculosis, psycho-neuro-endocrinal disorders, etc.) and patients with clinical evidence of heart failure.
Posology
The test drug Vyaghriharitaki Avaleha was administered orally in the dose of 10 g twice a day before meals with lukewarm water for a period of 12 weeks. Patients were guided with dos and don’ts regarding diet and lifestyle. Patients were followed-up for next 4 weeks of active treatment.
Assessment parameters
The primary outcome measure was change in the clinical symptoms of chronic bronchitis, and the secondary outcome measure was change in St. George's Respiratory Questionnaire (SGRQ) scores.[4] The changes in the disease state of chronic bronchitis were recorded and analyzed. Improvement was assessed by adopting standard method and scoring. Functional efficiency of the respiratory system (PEFR and forced expiratory volume in 1 s [FEV1]) were assessed both before and after the intervention using Electronic Lung Health Meter (Brand name: PIKO-1; manufactured by nSpire Health, USA) and functional ability is assessed with the help of the SGRQ every 14 days of intervention and at the end of the follow-up period. Clinical assessment was done on the basis of six chief complaints related to chronic bronchitis (productive cough, dyspnea, wheezing, chest pain, sore throat and nasal congestion). Each symptom was graded as - 0 for absence of symptom and 1 for presence of symptom.
Statistical analysis
The data generated in the clinical study was analyzed by applying Student's t-test using statistical software - SigmaStat 3.5, SYSTAT Software, USA.
Interpretation of results
≤25% - Unchanged
26–50% - Mild positive response
51–75% - Moderate positive response
>75% - Marked positive response.
Observations and Results
A total of 66 subjects were enrolled in this trial with the purpose of treatment, out of which 61 (92.42%) completed the treatment schedule, and 05 (7.58%) were dropped out. The details are summarized and depicted in Flow Chart 1.
Flow Chart 1.
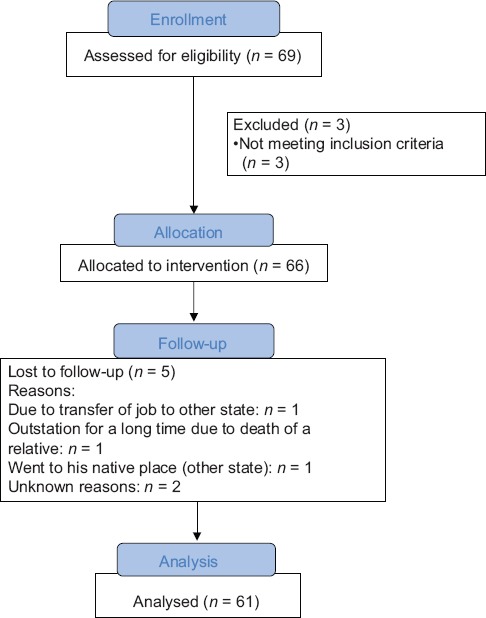
Summarizing the enrollment of patients
Distribution of patients according to age, gender and allergic factors as experienced by the patients are depicted at the Tables 1–3 respectively. The cardinal symptom of chronic bronchitis is a productive cough that was present in 100% patients. Dyspnoea in 62.12%, wheezing in 57.58%, chest pain in 34.85%, sore throat in 56.06% and nasal congestion in 71.21% patients was observed. Maximum numbers of subjects included in the trial (54.55%) were suffering from chronic bronchitis for 2 to 5 years [Table 4]. Around 25.76% of enrolled patients had been chronic smokers with a history of >5 years [Table 5]. About 40.91% patients had irregular bowel habits and 39.39% patients had constipation.
Table 1.
Distribution of 66 patients according to age
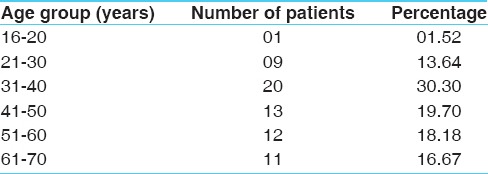
Table 3.
Distribution of patients according to allergy
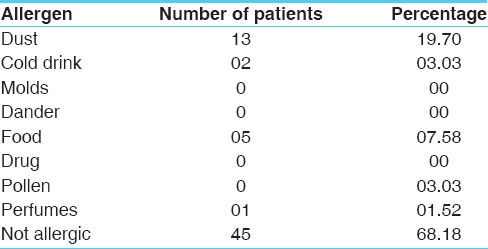
Table 4.
Distribution of subjects according to chronicity
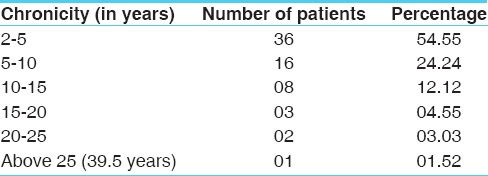
Table 5.
Distribution of subjects according to duration of smoking (regular + ex-smoker)

Table 2.
Distribution of 66 patients according to their gender

Results
Effect of therapy on primary outcome
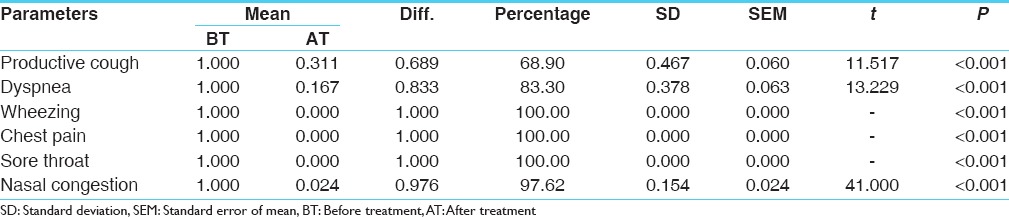
Maximum 100% result was found in wheezing, chest pain and sore throat, 97.62% relief in nasal congestion, 83.3% in dyspnoea and 68.9% relief in productive cough was noticed after treatment. This improvement is statistically highly significant (P < 0.001) [Table 6].
Table 6.
Effect on chief complaints
Effect of therapy on secondary outcome
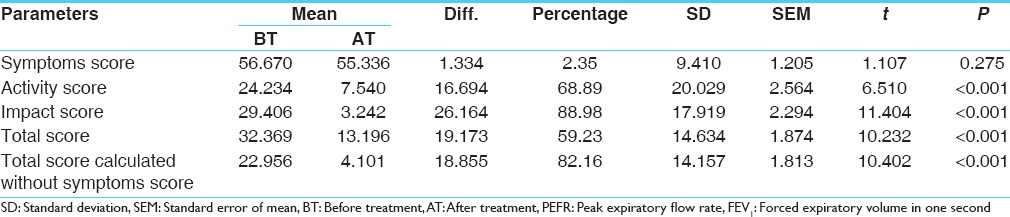
Change in total score of SGRQ was 59.23% that was statistically highly significant (P < 0.001). The improvement in the modified total score (calculated on the basis of activity and impact scores, excluding the symptoms scores) was 82.14%, which was statistically highly significant (P < 0.001) [Table 7].
Table 7.
Effect on the SGRQ scores
Effect of therapy on FEV1 and PEFR
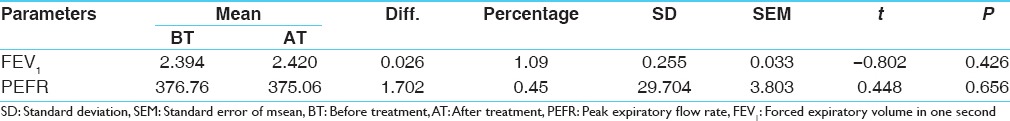
There was a slight increase of 1.09% in mean FEV1 value and a slight decrease of 0.45% in the mean PEFR value. Both the changes were statistically insignificant [Table 8].
Table 8.
Effect on FEV1 and PEFR
Overall effect of therapy on the basis of Total Score of SGRQ
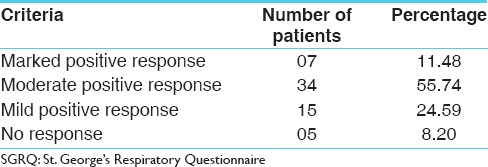
On the basis of changes in the total Score of SGRQ also, the overall effect of the drug on Chronic bronchitis was assessed. It was observed that 07 (11.48%) patients got marked positive response with the treatment, whereas 34 (55.74%) patients got moderate (50-75%) positive response, whereas 15 (24.59%) patients got mild positive response. The remaining 05 (8.20%) patients did not observe any significant change in their condition [Table 9].
Table 9.
Overall effect of therapy on the basis of total score of SGRQ
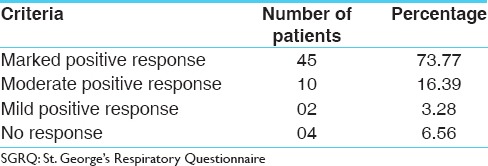
The 12-month version of SGRQ was used which is the original validated version. The patient has to give the answers to the part-1 (which is related with Symptom score) by recalling his perception for the whole last year, there was no much difference expected in Symptoms score during the 12 weeks of clinical study. So, to get the appropriate picture of the effect of therapy the change in the Total Score was calculated on the basis of changes in the Activity and Impact Scores. The modified calculation shows that 45 (73.77%) patients got marked positive response with the treatment, whereas 10 (16.39%) patients got moderate positive response, whereas 02 (3.28%) patients got mild positive response. The remaining 04 (6.56%) patients did not observed any significant change in their condition [Table 10]. This is much similar to the overall effect of the therapy calculated on the basis of symptomatic relief to the patient.
Table 10.
Overall effect of therapy on the basis of total score of SGRQ calculated without symptom score
Overall effect of therapy on the basis of symptomatic relief
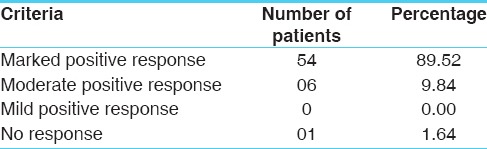
Overall effect on symptomatic relief is placed at Table 11. It provided marked positive improvement in 89.52% patients whereas 9.84% patients got moderate positive response. Only one patient did not get any response to the treatment in whom the disease was prevalent for the past 20 years.
Table 11.
Overall effect of therapy on the basis of symptomatic relief
Effect on hematological and bio-chemical parameters
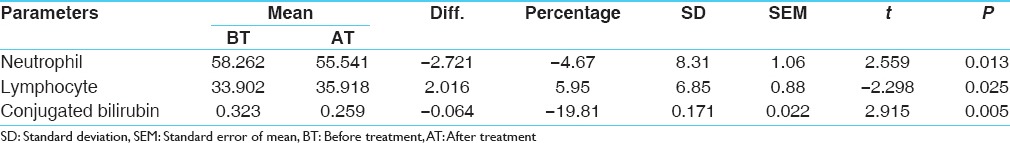
Comparative effect of therapy on hematological investigations was statistically insignificant except on differential counts of neutrophils and lymphocytes which were statistically significant. This may be an indication of positive improvement in the endogenous factors like deranged immunity. The comparative effect of therapy on bio-chemical parameters was statically insignificant except on conjugated bilirubin that was statistically significant (P = 0.005). This signifies the positive effect of the drug on liver function and general metabolism [Table 12].
Table 12.
Effect on hematological and biochemical parameters
All patients maintained the “status quo” during the follow-up period. No patient showed either aggression in the status or recurrence. No adverse drug reaction to the trial drug was reported during the study. No rescue medications were used during the study as it was not warranted. None of the subjects were required to be withdrawn from the study as there was no serious health condition requiring referral occurred during the study period.
Discussion
On the basis of relief in the symptoms to the patients, the overall effect of the drug on chronic bronchitis was assessed. It was observed that 89.52% patients got marked positive response with the treatment, whereas 9.84% got moderate (50–75%) positive response. The remaining 1 (1.64%) patient did not get any significant change in his condition.
The mean total score in SGRQ prior to treatment was 32.369 and after the treatment was 13.196, with a difference of 59.23% that is statistically highly insignificant with P < 0.001. The improvement in the modified Total score (calculated on the basis of activity and impact scores) was 82.14% that was statistically highly significant (P < 0.001).
This overall effect of the therapy shows that Vyaghriharitaki is very effective in the management of chronic bronchitis showing better improvements in both primary and secondary outcome measures.
Probable mode of action of Vyaghriharitaki Avaleha
Vyaghriharitaki Avaleha a polyherbal Ayurvedic formulation having major ingredients - Kantakari (Solanum xanthocarpum Schrad. and Wendl.), Haritaki (Terminalia chebula Retz.). Studies on S. xanthocarpum confirm its traditional use in bronchial asthma. The clinical efficacy of two herbs S. xanthocarpum and Solanum trilobatum Linn. in a dose of 300 mg tds for 3 days was investigated in mild to moderate bronchial asthma. Their effect was compared with standard bronchodilator drugs, salbutamol (4 mg) and deriphylline (200 mg). S. xanthocarpum and S. trilobatum produced a progressive improvement in the vantilatory function of asthmatic individuals over 3 days. The scores for ronchi, cough, breathlessness and sputum were decreased by these drug treatments. The improvement in PEFR and the reduction in other symptom scores clearly indicate a bronchodialator effect, a decrease of edema and secretions in the airway lumen. The response of these drugs can be considered to be that of deriphylline but less than salbutamol.[5]
Immunostimulatory activity of aqueous extract of S. xanthocarpum fruits on mice gives strong evidence that the plant is an immunostimulating agent.[6]
Haritaki (T. chebula) has been mentioned as the best Rasayana drug. T. chebula is having immunomodulatory activity.[7] With the help of various Samsakaras, Haritaki has been mentioned to be effective in various diseases with entirely different pathophysiology. This is possible due to the Sanskaranuvartana and Rasayana property. The Rasayana property is due to its Doshashamaka, Srotoshodhana and Vatanulomana property. This is the prime condition for the Rasayana effect.[8] With these inherited property (Prakriti of Dravya) when combined and processed with other drugs (Samskara), this Haritaki shows the result accordingly.[9]
The contents of Trikatu (Shunthi [Zingiber officinale Roxb.], Maricha [Piper nigrum Linn.] and Pippali [Piper longum Linn.]) and Chaturjat (Tvak [Cinnamomum zeylanicum Blume.], Ela [Elettaria cardamomum Maton.], Dalchini [Cinnamomum tamala Ness.] and Nagakeshara [Mesua ferrea Linn.]) are also effective in Kasa. But, when these drugs are used as Prakshepa, the main purpose remains to be Deepana, Pachana effect and helps in improving the bioavailability of the drugs with which they are used in. Madhu (honey) and Guda (jaggery) do also possess Kaphahara and Kasahara property.[10,11]
In nutshell, the formulation VHA is effective in chronic bronchitis by acting on the Samavayi Karanas (Doshas) and Asamavayi Karanas (Vishamashana, Vegdharana, Kshaya etc.). Its effect on the Asamavayi Karanas is the additional benefit over all the treatment modalities in the modern medical science.
For the sake of simplification and easy understanding, the manifestation of the disease can be summarized as the end result of three factors - Doshaprakopa, Agnidushti and Srotodushti (Khavaigunya). Agnidushti, in itself, can take place as an effect of Vishamashana, Vegadharana and Dhatukshaya. There is a vicious cycle of Agnidushti and Doshaprakopa (either can be cause or effect of each other).
The Doshaprakopa is corrected by the Doshashamana property of VHA. The effect of Vishamashana is corrected by the Srotoshodhana and Agnivardhana property of VHA. The effect of Vega-Vidharana is corrected by Anulomana property of VHA. The Srotodushti is corrected with the Srotoshodhana and Vishadikarana property. With its Brihana property, VHA helps in correcting the Dhatukshaya.
Prakrita Kapha is essential for proper functioning of the Pranavaha Srotasa (and all other functions in the body that depends on Kapha). When the Agni and digestion are corrected, quality Rasa Dhatu is produced and this results in the production of Prakrita Shleshama (as Mala of Rasa Dhatu). Thus, VHA helps not only in correcting the three main factors of the disease-Doshaprakopa, Agnidushti and Khavaigunya, but also helps in maintaining the proper functioning of the system, which results in sustained positive effect on the Srotasa (Prakriti sthapana).
Further, Pranavaha Srotasa is Vata-Kapha-Sthana. Its function is mainly affected by vitiation of Kapha and Vata. Therefore, the Avastha of any respiratory disease can be as either Vatavritta-Kapha or Kaphavritta-Vata (Prana). If it is Kaphavritta Avastha, then Doshashamana property of VHA helps in relieving the symptoms and if it's Vatavritta Avastha, then Vatashamana and Vatanulomana property helps in relieving the symptoms. Therefore, VHA is a drug that may be effective in all the respiratory diseases as mentioned in its Phalashruti.
Vyaghriharitaki Avaleha, as a whole, corrects the effect of Asamavayi Nidana of the disease. Theoretically, it is a better drug for Kasa of Kshayaja type. But, at the same time, Haritaki with its Srotoshodhana and Tridosh-Hara property will be effective in all types of Kasa (chronic bronchitis). The only difference is that Doshika variety of Kasa can be better handled in relatively shorter duration with the specific treatment procedures indicated for individual Doshika variations. VHA can be helpful in all types of Kasa or Chronic bronchitis.
Conclusion
Vyaghriharitaki Avaleha gives a significant improvement in both primary and secondary outcome measures of chronic bronchitis.
Financial support and sponsorship
CCRAS, New Delhi and I.P.G.T. and R.A., Gujarat Ayurved University, Jamnagar.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Golwalla AS, Golwalla SA. 20th ed. Mumbai, Churchgate: Dr. A F Golwalla Publishsers; 2003. Golwalla Medicine for Students, A Reference Book for Family Physicians; pp. 236–7. [Google Scholar]
- 2.Kasper D, Braunwald E, Fausi AS, Hausar SL, Longo D, Jameson JL, editors. Harrisons's Principles of Internal Medicine. 16th ed. New York: McGraw-Hill Medical Publishing Division; 2005. p. 1547. [Google Scholar]
- 3.Anonymous. Ayurvedic Pharmacopoeia of India, Part-II. 1st ed. I. New Delhi: Govt. of India, Ministry of Health of Family Welfare; 2008. pp. 35–7. [Google Scholar]
- 4. Available from: http://www.healthstatus.sgul.ac.uk/SGRQ_download/SGRQ%20Manual%20June%202009.pdf .
- 5.Govindan S, Viswanathan S, Vijayasekaran V, Alagappan R. Further studies on the clinical efficacy of Solanum xanthocarpum and Solanum trilobatum in bronchial asthma. Phytother Res. 2004;18:805–9. doi: 10.1002/ptr.1555. [DOI] [PubMed] [Google Scholar]
- 6.Sultana R, Khanam S, Devi K. Evaluation of immunomodulatory activity of Solanum xanthocarpum fruits aqueous extract. Pharm Lett. 2011;3:247–53. [Google Scholar]
- 7.Aher V, Wahi AK. Immunomodulatory activity of alcohol extract of Terminalia Chebula Retz. Combretaceae. Trop J Pharm Res. 2011;10:567–75. [Google Scholar]
- 8.Acharya VJ. Reprint ed. Varanasi: Chaukhamba Orientalia; 2011. Charaka Samhita of Agnivesha, Chikitsa Sthana; Vatavyadhi Chikitsa: Chapter 28, Verse 4; p. 616. [Google Scholar]
- 9.Acharya VJ, editor. Varanasi: Chaukhamba Orientalia; 2011. (Reprint ed.) Ayurved-Dipika commentary of Chakrapanidatta on Charaka Samhita of Agnivesha, Chikitsa Sthana, Rasayana, Chapter 1/1, Verse 30; p. 377. [Google Scholar]
- 10.Chunekar KC, Pandey GS, editors. Varanasi: Chaukhambha Bharati Academy; 2010. (Revised ed.) Bhavaprakasha Nighantu of Bhavamishra; p. 772. [Google Scholar]
- 11.Chunekar KC, Pandey GS, editors. Varanasi: Chaukhambha Bharati Academy; 2010. (Revised ed.) Bhavaprakasha Nighantu of Bhavamishra; p. 779. [Google Scholar]


