Abstract
Introduction:
Ashwagandha (Withania somnifera [L.] Dunal) has been traditionally used for various actions ranging from vitalizer, improve endurance and stamina, promote longevity, improve immunity, and male and female fertility. However, clinical studies are needed to prove the clinical efficacy of this herb, especially in cardiovascular endurance and physical performance.
Aims:
This prospective, double-blind, randomized, and placebo-controlled study evaluated the efficacy of Ashwagandha roots extract in enhancing cardiorespiratory endurance and improving the quality of life (QOL) in 50 healthy male/female athletic adults.
Materials and Methods:
Cardiorespiratory endurance was assessed by measuring the oxygen consumption at peak physical exertion (VO2 max) levels during a 20 m shuttle run test. The World Health Organization self-reported QOL questionnaire (physical health, psychological health, social relationships, and environmental factors) was used to assess the QOL. Student's t-test was used to compare the differences in a mean and change from baseline VO2 max levels, whereas Wilcoxon signed-rank test was used to assess changes in QOL scores from baseline in the two groups.
Results:
There was a greater increase from baseline (P < 0.0001) in the mean VO2 max with KSM-66 Ashwagandha (n = 24) compared to placebo (n = 25) at 8 weeks (4.91 and 1.42, respectively) and at 12 weeks (5.67 and 1.86 respectively). The QOL scores for all subdomains significantly improved to a greater extent in the Ashwagandha group at 12 weeks compared to placebo (P < 0.05).
Conclusion:
The findings suggest that Ashwagandha root extract enhances the cardiorespiratory endurance and improves QOL in healthy athletic adults.
Keywords: Ashwagandha, cardiorespiratory endurance, healthy athletics adults, quality of life, VO2 max
Introduction
Ashwagandha (Withania somnifera [L.] Dunal), also known as Indian Winter Cherry, is extensively used in Ayurveda, the traditional health care system in India. This herb is used as a general tonic and “adaptogen,” helping the body to adapt to stress. Also, it has been shown to possess antioxidant properties as well as an ability to support a healthy immune system.[1,2,3]
Ashwagandha is a popular medicinal plant in the South East Asia and Southern Europe. Many people use this herb for general vitality. It is popularly called “Indian Ginseng” due to its rejuvenating effects.[2,4] In addition, it causes relaxation, increases energy and promotes weight loss through stress reduction. In Ayurveda, certain herbal formulae known as Rasayana are considered to be rejuvenating.[1,2,5,6,7,8,9] and are taken as a remedy for general weakness, exhaustion, and stress. Ashwagandha is valued for its ability to increase vitality, energy, endurance and stamina, promote longevity and strengthen the immune system without stimulating the body's reserves. Numerous studies suggest Ashwagandha can directly and indirectly prevent and treat a number of diseases.[8,10,11]
Earlier studies clearly indicated that the traditional use of Ashwagandha had a logical and scientific basis.[1,2] Nonetheless; clinical studies are needed to prove the clinical efficacy of this herb, especially in cardiovascular endurance and physical performance. Hence, this study was conducted with a two-fold objective: To evaluate the efficacy of a high concentration root extract of Ashwagandha in enhancing cardiorespiratory endurance, and to improve the quality of life (QOL) in healthy athletic adults.
Materials and Methods
This randomized, double-blind, and placebo-controlled study was approved by Suraksha Independent Ethics Committee, Vishnu Institute of Pharmaceutical Education and Research, Andhra Pradesh, India (ref. no. SIEC/04/01/0014; dt. 13.04.2012) and the study was conducted in compliance with good clinical practice guidelines, Declaration of Helsinki and all other applicable regulations.
Study subjects
A total of 50 subjects were assessed with regards to eligibility for inclusion in the study at the Department of Orthopedics, Hyderabad Spine Clinics, Secunderabad, India. This being an exploratory study, the sample size was not based on any assumptions and calculations. Healthy athletic male and/or female adult subjects aged between 20 and 45 years and subjects within the body mass index range of 18.5–24.9 kg/m2 were enrolled in the study after obtaining informed written consent. Subjects with previous history of significant renal or hepatic impairment, asthma, urticarial, or other allergic reactions, severe gastrointestinal disorders such as malabsorption syndrome, diabetes, coronary artery disease, and hypertension with or without complications, any chronic physical, hormonal or psychiatric disorder, morbid obesity (body fat percent >40%), and any medical condition where exercise is contraindicated and recent surgery or trauma that incapacitates the subject for exercise, subjects with known hypersensitivity to Ashwagandha and related herbal product extracts, and those subjects who were on any kind of herbal preparations (such as other formulations containing Ashwagandha, Ginseng, Brahmi, and Ginkgo biloba) were also excluded.
Randomization
The enrolled subjects were randomized to either placebo (control) group (n = 25), or Ashwagandha (study) group (n = 25). Randomization was done using a computer based predetermined randomization chart (Rando 1.2, R. Raveendran, 2014). After the subjects were enrolled, they were provided a study serial number in chronological order. The study and placebo capsules were provide in containers without revealing the contents of the capsule and were numbered as per the randomization chart. Only after the subjects were assigned the study serial number, the corresponding container was given to the subject.
The study subjects in the Ashwagandha group were administered one capsule of KSM-66 Ashwagandha (containing 300 mg of a high concentration full-spectrum root extract of the Ashwagandha plant, Ixoreal Bio-med Private Ltd.,) orally, twice daily for a period of 12 weeks, whereas the subjects in placebo group received identical capsules containing sucrose.
The Ashwagandha root extract (KSM-66 Ashwagandha from Ixoreal Biomed, Hyderabad, India) used in this study has been standardized to 5% withanolides as ascertained by high-performance liquid chromatography (HPLC). It is manufactured using a proprietary water-based process, without the use of alcohol or any chemical solvents. The analysis of withanolides was performed with a Waters 515 HPLC System, using a Sunfire C18 column of dimension 250 mm × 4.6 mm, 5 µm at a flow rate of 1 ml/min. The solvent system was based on methanol: water (60:40). The column temperature was 30°C, and the injection volume was 20 µl.
Study visits and assessments
During the study period, the subjects visited the study center at screening and enrollment and then after 4, 8, and 12 weeks. All enrolled subjects underwent a complete physical examination at all visits.
The efficacy of Ashwagandha on cardiorespiratory endurance was evaluated by conducting a 20-m Shuttle Run Test at baseline, week 8 and week 12. Oxygen consumption at peak physical exertion (VO2 max) is one of the most widely used indicator of cardiorespiratory endurance and shuttle run test[12] is found to be a reliable substitute to assess VO2 max, when laboratory-based assessments of VO2 max are not feasible.[13]
The QOL was assessed by a self-reported World Health Organization-QOL (WHO-QOL) questionnaire. This QOL had subdomains for assessment of physical health, psychological health, social relationships, and environmental factors. Compliance was assessed using the capsule count and those who consumed over 80% of tablets were classified as compliant. All data were recorded in the source documents and data collection forms for 12 weeks.
Statistical analysis
The data were analyzed using windows based statistical program Medcalc® (Version 12.7.0.0). The VO2 max and QOL scores are expressed as a means with SD. Mann–Whitney U-test was used to compare the VO2 max values with 20-m Shuttle Run Test between the two groups. Similarly, Wilcoxon signed-rank test was used to compare the two groups for differences in the QOL scores and change from baseline in QOL scores for different subdomains of QOL questionnaire.
Results
One subject from the Ashwagandha group withdrew consent from the study and was not included for analysis. Thus, data of 49 subjects were used for analysis [Flow chart 1]. The subjects from the two groups were comparable and had a matching demographic profile. The compliance was satisfactory in both the groups.
Flow Chart 1.
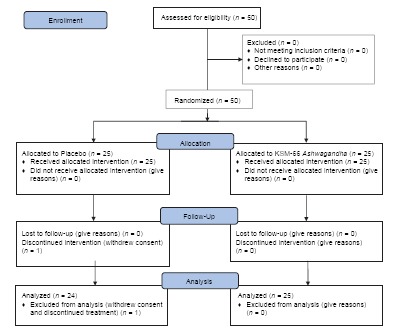
Consort 2010 flow diagram
Treatment with the root extract of Ashwagandha resulted in a significantly greater increase (P < 0.0001) in mean VO2 max at week 8 and week 12 compared to placebo subjects [Table 1]. The QOL sub-domain scores are presented in Table 2. Ashwagandha has shown to exert a greater improvement in all the QOL sub-domain scores as compared to placebo.
Table 1.
Assessment of VO2max by 20-m shuttle run test
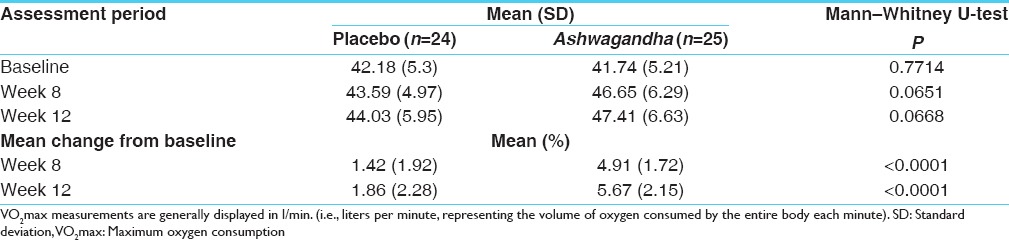
Table 2.
Assessments of WHO-QOL questionnaire
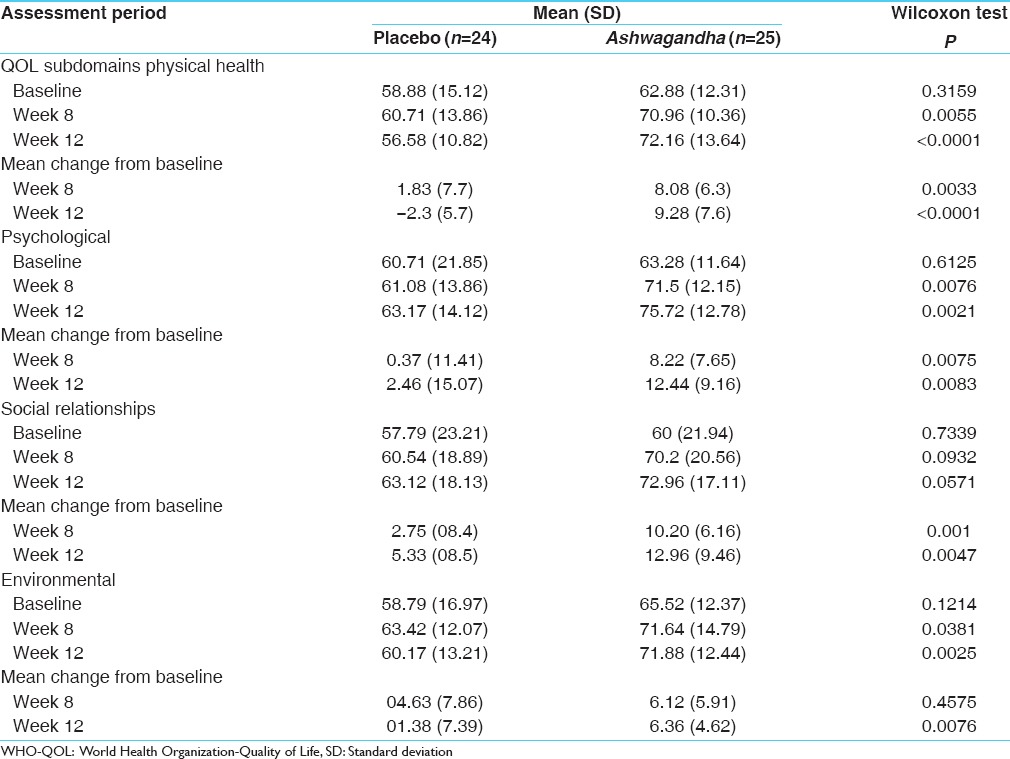
The data of this study showed a significant (P < 0.05) improvement in the physical and psychological health and the social relationship status of the study subjects by the end of 12 weeks of the study. However, the status of the environmental impact on the QOL of the study subjects was found to have increased substantially; albeit, not significant at 8 weeks and significant (P < 0.01) at 12 weeks of the study period. There were no changes in the vital parameters such as pulse rate, blood pressure (systolic and diastolic), and respiratory at resting state in the subjects of both groups.
Discussion
This study aimed to assess the effects of root extract of Ashwagandha on cardiorespiratory endurance in healthy athletic adults and to evaluate the QOL of these subjects. Isometric exercise involves daily and athletic activity, which significantly increases blood pressure, heart rate, myocardial contractility, and cardiac output; this enhanced physiological activity requires a fit cardiorespiratory system. A study in children and adolescents suggests that low cardiorespiratory fitness is strongly associated with the clustering of cardiovascular disease risk factors in children, independent of country, age, and sex.[14,15] Cardiorespiratory endurance is generally recognized as a major component of physical fitness. The maximum oxygen consumption (VO2 max) is considered by many as the most valid measure of cardiorespiratory fitness.[16,17]
The test for VO2 max is perhaps the most commonly employed procedure in exercise physiology. This measurement determines an athlete's ability to take in, transport, and utilize oxygen and is probably the best assessments of the athlete's endurance capabilities. The maximum oxygen consumption (VO2 max) is a measure of long-term aerobic and cardiovascular endurance parameters. VO2 max represents a long-term aerobic and cardiovascular endurance and is considered to be a gold standard for measuring the cardiorespiratory fitness level.[16,18] Endurance activities characteristically require high repetitions and low resistance.[16] A study states that 20 m shuttle run test is valid and reliable for prediction of the VO2 max of male and female adults.[19] The 20 m shuttle run test is as a valid test of cardiorespiratory endurance as other distance run tests in American Students of 12–15 years old.[20] In this study, 20 m shuttle run test was used to measure VO2 max levels which in turn depicted the cardiorespiratory endurance. It was observed that there was a significant increase in VO2 max by the end of 8 weeks and by the end of 12 weeks as compared to placebo group. The data of the present study defined an excellent range of VO2 max following administration of Ashwagandha.
Ashwagandha is known as an “adaptogen,” as it increases resistance to physical, chemical, and biological stressors, builds energy and general vitality.[2,7,18,21] Previous studies assessing physical and cardiorespiratory endurance of healthy adult subjects have also reported similar beneficial results with the use of Ashwagandha, underscoring the significant increase in VO2 max and muscle strengthening.[18,21] W. somnifera administered in the form of aqueous extract in capsules with gradual escalating doses from 750 to 1250 mg/day was found to be safe and well tolerated. The study also demonstrated marked muscle strengthening, exercise tolerance, and lipid-lowering potential of Ashwagandha, along with improved quality of sleep and QOL.[21]
Adenosine triphosphate is responsible for the maintenance of the energy processes at the cellular level. Ashwagandha has been shown to exert significant effects on the energy levels and mitochondrial health. It beneficially influences Mg2+ dependent ATPase activity and reduces the succinate dehydrogenase enzyme activity in the mitochondria of granulation tissue of carrageen induced air pouch granuloma as demonstrated in an experimental study.[22] In agreement with the fact that exercise endurance capacity is largely determined by the functional mitochondrial content of muscle, this study confirms the energizing effect of Ashwagandha. It has been shown that Ashwagandha increased both the red blood corpuscles (RBC) and hemoglobin count. The increase in RBC results in an increased capacity of the blood to transport oxygen directly to the exercising muscles; thus, enhancing the aerobic capacity of the athletes.[23,24] These findings suggest a possible mechanism of the ergogenic effect of Ashwagandha root extract.
An animal study reported a 2–3-fold increase in free radical (R•) concentrations of muscle and liver following exercise to exhaustion. Exhaustive exercise also resulted in decreased mitochondrial respiratory control and increased levels of oxidative stress.[25] Ashwagandha on a long period of administration markedly augmented antioxidants and significantly reduced ischemia reperfusion induced myocardial injury as reported in an earlier experimental study.[26,27] These observations are indicative of its cardio protective potential.
Ashwagandha as an herbal drug finds its mention in various reference books/literature on traditional medicines and is being used widely and since long. There are no reported Phase-I or Phase-II studies being conducted due to its known knowledge of safety and efficacy. In view of the fact that Ashwagandha is an herb which is extensively used traditionally in Ayurveda, Phase-I and Phase-II trials may not be necessary. The herb under investigation is being clinically evaluated for the same indication for which it is being used or as has been described in ancient texts. Furthermore, the design of the trial is appropriate to provide the expected outcomes for the traditional medicine.
The WHO-QOL questionnaire is used to evaluate a given subject's QOL. It comprises 26 items, which measures the following broad domains: Physical health, psychological health, social relationships, and the environment (WHO-QOL Questionnaire, 1996).[28] The procedure entails each question being read out to the subjects, along with the response options. In this study, QOL was checked by self-reported WHO-QOL Questionnaire. Ashwagandha was shown to improve all subdomains of the QOL (physical health, psychological health, social relationships, and environmental factors) of the healthy athletic adult subjects as compared to placebo. The data of the present study showed significant (P < 0.05) improvement in physical and psychological health and these domains have a greater contribution in the QOL than the other domains of social relationships, and environmental factors. This study was first of its kind and limited by a small sample size. Further studies with a larger sample with an additional aims of understanding the underlying mechanism of action of Ashwagandha may further validate the findings of this study.
Conclusions
Ashwagandha an important herb in Ayurveda, the traditional Indian medicine system is considered to be a Rasayana. Ashwagandha root extract has been used for several 1000 years in Ayurveda as a tonic, prophylactic agent and “restorative.” It has been used by athletes for improved muscular strength, resistance to fatigue, recovery from exercise, and as an ergogenic aid for many years. In this study, oral administration of a high concentration root extract of Ashwagandha led to increased VO2 max, enhanced cardiorespiratory endurance, and improved QOL in healthy athletic adults. Findings of this study suggest that Ashwagandha root extract improves the cardiovascular dynamics by increasing the VO2 max levels thereby enhancing the cardiorespiratory endurance, and also brings an improvement in QOL in healthy adults. However, due to the limited cross-section of the population considered in this study, the findings may not generalize to all populations. Further studies are needed to validate these findings.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Singh N, Nath R, Lata A, Lata A, Singh SP, Kohli RP, et al. Withania somnifera (Ashwagandha), a rejuvenating herbal drug which enhances survival during stress (an adaptogen) Int J Crude Drug Res. 1982;20:29–35. [Google Scholar]
- 2.Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (Ashwagandha): a review. Altern Med Rev. 2000;5:334–46. [PubMed] [Google Scholar]
- 3.Provino R. The role of adaptogens in stress management. Aust J Med Herbalism. 2010;22:41–9. [Google Scholar]
- 4.Weiner MA, Weiner J. Herbs that Heal. Mill Valley, CA: Quantum Books; 1994. Ashwagandha (Indian ginseng) pp. 70–2. [Google Scholar]
- 5.Shastri B, editor. Bhavprakash of Bhava Mishra, Guduchyadi Varg. 9th ed. Varanasi: Chowkhamba Sanskrit Sansthan; 1999. pp. 393–4. [Google Scholar]
- 6.Andrade C, Aswath A, Chaturvedi SK, Srinivasa M, Raguram R. A double-blind, placebo-controlled evaluation of the anxiolytic efficacy of an ethanolic extract of Withania somnifera. Indian J Psychiatry. 2000;42:295–301. [PMC free article] [PubMed] [Google Scholar]
- 7.Dhuley JN. Adaptogenic and cardioprotective action of Ashwagandha in rats and frogs. J Ethnopharmacol. 2000;70:57–63. doi: 10.1016/s0378-8741(99)00177-4. [DOI] [PubMed] [Google Scholar]
- 8.Mirjalili MH, Moyano E, Bonfill M, Cusido RM, Palazón J. Steroidal lactones from Withania somnifera, an ancient plant for novel medicine. Molecules. 2009;14:2373–93. doi: 10.3390/molecules14072373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh N, Bhalla M, de Jager P, Gilca M. An overview on Ashwagandha: a Rasayana (rejuvenator) of Ayurveda. Afr J Tradit Complement Altern Med. 2011;8(5 Suppl):208–13. doi: 10.4314/ajtcam.v8i5S.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malhotra CL, Das PK, Dhalla NS, Prasad K. Studies on Withania Ashwagandha, Kaul. III. The effect of total alkaloids on the cardiovascular system and respiration. Indian J Med Res. 1981;49:448–60. [Google Scholar]
- 11.Sukanya DH, Lokesha AN, Datta G, Himabindu K. Phytochemical diversity in Ashwagandha (Withania somnifera) J Med Aromat Plants. 2010;1(2):TS2–p26. Abstract: National Conference on Biodiversity of Medicinal and Aromatic Plants: Collection, Characterization and Utilization, Held at Anand, India; 24-25 November, 2010. [Google Scholar]
- 12.Leger LA, Lambert J. A maximal multistage 20m shuttle run test to predict VO2 max. Eur J Appl Physiol. 1982;49:1–5. doi: 10.1007/BF00428958. [DOI] [PubMed] [Google Scholar]
- 13.Cairney J, Hay J, Veldhuizen S, Faught B. Comparison of VO2 maximum obtained from 20 m shuttle run and cycle ergometer in children with and without developmental coordination disorder. Res Dev Disabil. 2010;31:1332–9. doi: 10.1016/j.ridd.2010.07.008. [DOI] [PubMed] [Google Scholar]
- 14.Longhurst JC, Stebbins CL. The power athlete. Cardiol Clin. 1997;15:413–29. doi: 10.1016/s0733-8651(05)70349-0. [DOI] [PubMed] [Google Scholar]
- 15.Anderssen SA, Cooper AR, Riddoch C, Sardinha LB, Harro M, Brage S, et al. Low cardiorespiratory fitness is a strong predictor for clustering of cardiovascular disease risk factors in children independent of country, age and sex. Eur J Cardiovasc Prev Rehabil. 2007;14:526–31. doi: 10.1097/HJR.0b013e328011efc1. [DOI] [PubMed] [Google Scholar]
- 16.Jones GL, Killian KJ, Summers E, Jones NL. Inspiratory muscle forces and endurance in maximum resistive loading. J Appl Physiol. 1985;58:1608–15. doi: 10.1152/jappl.1985.58.5.1608. [DOI] [PubMed] [Google Scholar]
- 17.Zamunér AR, Moreno MA, Camargo TM, Graetz JP, Rebelo AC, Tamburús NY, et al. Assessment of subjective perceived exertion at the anaerobic threshold with the borg CR-10 Scale. J Sports Sci Med. 2011;10:130–6. [PMC free article] [PubMed] [Google Scholar]
- 18.Sandhu JS, Shah B, Shenoy S, Chauhan S, Lavekar GS, Padhi MM. Effects of Withania somnifera (Ashwagandha) and Terminalia arjuna (Arjuna) on physical performance and cardiorespiratory endurance in healthy young adults. Int J Ayurveda Res. 2010;1:144–9. doi: 10.4103/0974-7788.72485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leger LA, Lambert J. A maximal multistage 20m shuttle run test to predict VO2 max. Eur J Appl Physiol. 1982;49:1–12. doi: 10.1007/BF00428958. [DOI] [PubMed] [Google Scholar]
- 20.Liu NY, Plowman SA, Looney MA. The reliability and validity of the 20-meter shuttle test in American students 12 to 15 years old. Res Q Exerc Sport. 1992;63:360–5. doi: 10.1080/02701367.1992.10608757. [DOI] [PubMed] [Google Scholar]
- 21.Raut AA, Rege NN, Tadvi FM, Solanki PV, Kene KR, Shirolkar SG, et al. Exploratory study to evaluate tolerability, safety, and activity of Ashwagandha (Withania somnifera) in healthy volunteers. J Ayurveda Integr Med. 2012;3:111–4. doi: 10.4103/0975-9476.100168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Begum VH, Sadique J. Effect of Withania somnifera on glycosaminoglycan synthesis in carrageenin-induced air pouch granuloma. Biochem Med Metab Biol. 1987;38:272–7. doi: 10.1016/0885-4505(87)90091-0. [DOI] [PubMed] [Google Scholar]
- 23.Ziauddin M, Phansalkar N, Patki P, Diwanay S, Patwardhan B. Studies on the immunomodulatory effects of Ashwagandha. J Ethnopharmacol. 1996;50:69–76. doi: 10.1016/0378-8741(95)01318-0. [DOI] [PubMed] [Google Scholar]
- 24.McArdle WD, Katch FI, Katch VL. Exercise Physiology: Energy, Nutrition and Human Performance. 4th ed. Philadelphia: Lea and Febiger; 1996. [Google Scholar]
- 25.Davies KJ, Quintanilha AT, Brooks GA, Packer L. Free radicals and tissue damage produced by exercise. Biochem Biophys Res Commun. 1982;31(107):1198–205. doi: 10.1016/s0006-291x(82)80124-1. [DOI] [PubMed] [Google Scholar]
- 26.Gupta SK, Mohanty I, Talwar KK, Dinda A, Joshi S, Bansal P, et al. Cardioprotection from ischemia and reperfusion injury by Withania somnifera: a hemodynamic, biochemical and histopathological assessment. Mol Cell Biochem. 2004;260:39–47. doi: 10.1023/b:mcbi.0000026051.16803.03. [DOI] [PubMed] [Google Scholar]
- 27.Mohanty I, Arya DS, Dinda A, Talwar KK, Joshi S, Gupta SK. Mechanisms of cardioprotective effect of Withania somnifera in experimentally induced myocardial infarction. Basic Clin Pharmacol Toxicol. 2004;94:184–90. doi: 10.1111/j.1742-7843.2004.pto940405.x. [DOI] [PubMed] [Google Scholar]
- 28.Geneva: World Health Organization; 1996. [Last retrieved on 2013 Sep 06]. WHO-QOL-BREF Introduction. Administration, Scoring and Generic Version of the Assessment: Field Trial Version; December, 1996.WHO/MSA/MNH/PSF/97. from http://www.who.int/mental_health/media/68.pdf . [Google Scholar]


