Abstract
Purpose:
To evaluate cortical activity using pattern visual evoked potentials (PVEPs) in patients with mild and moderate amblyopia (esotropic and anisometropic).
Methods:
PVEP was recorded in 43 unilateral amblyopic patients, including 15 esotropic (ET) and 28 anisometropic (AM) patients, selected from three different medical centers in the city of Shiraz, Iran and compared to that obtained from 15 age and sex matched normal subjects who served as controls. Visual acuity (VA) in amblyopic eyes was equal to or less than 0.7 LogMAR. The latency of P100 was recorded monocularly using two check sizes of 15 and 60 min of arcs at two different levels of contrasts (30% and 100%).
Results:
P100 latency in amblyopic eyes was significantly increased compared to the normal group (P < 0.001). There was a significant difference (P < 0.001) in P100 latency in anisometropic and esotropic amblyopic eyes as compared to normal subjects, using high spatial frequency and with both levels of contrast. A significant difference was observed with large check sizes and high contrast between anisometropic amblyopic and normal eyes (P = 0.03). However, there was no significant difference between these two groups and the control group with other stimuli.
Conclusion:
The neural response based on p100 latency in PVEP was different between amblyopic groups and normal subjects. PVEP may be valuable for diagnosis, prognosis and treatment of amblyopia.
Keywords: Amblyopic, Anisometropic, Esotropic, Visual Evoked Potential
INTRODUCTION
Amblyopia affects 2.5% of the population and is one of the most important causes of vision loss in children; not surprising it has been the subject of many studies.[1] Any vision reduction because of retinal image blur and suppression, such as anisometropia, strabismus or light deprivation, is considered as amblyopia.[2] Studies have shown that amblyopia is not simple vision reduction; it is a complicated mechanism of developmental malfunction in the brain[3,4] Therefore, untimely treatment and diagnosis of the disorder may cause irreversible dysfunction and seriously affect the patient's quality of life in future.[5] Based on the neural plasticity theory, treatment of amblyopia before six years of age is very important, as it will entail better results.[6]
Anisometropic and esotropic amblyopia have a higher incidence as compared to other causes of the condition.[5] Anisometropic amblyopia involves a primary sensory disorder, whereas esotropic amblyopia is due to motor–sensory defects, therefore the neurological pattern of these types of amblyopia may be different. Failure of some parts of the visual cortex to receive neural input is responsible for incorrect visual information processing ultimately leading to amblyopia.[5] Neurological studies evaluating glucose metabolism and cerebral blood flow have also proven functional impairment in the amblyopic visual cortex.[7]
Visually evoked potential (VEP), which is the electrophysiological response of brain activity recorded to visual stimuli, is one of the current techniques to understand the complicated mechanism of amblyopia.[8,9] VEP test responses are the result of primary visual cortex activity located at the occipital lobe known as V1.[10] Central visual pathways, including the two main anatomical pathways, i.e. parvocellular and magnocellular, transmit visual inputs from the retina to the cortex in parallel.[11] Parvocellulars are sensitive to high spatial frequencies/low temporal frequencies, while magnocellulars are more sensitive to low spatial frequencies/high temporal frequencies.[12] Therefore, the function of these pathways could be evaluated by changes in spatial and temporal frequency properties, and it also seems possible to examine the effect of amblyopia on these two pathways.[4]
Amblyopia has been one of the controversial challenges in vision science and many studies have been carried out based on the difference of neural sensitivity in these patients; in some cases, the results justify each other[13,14,15,16,17] while in others, they contradict one another.[18,19,20,21,22] The current study was aimed to compare P100 wave latency using PVEP in the amblyopic eye of esotropic and anisometropic amblyopic patients as compared to a normal group.
METHODS
A total of 58 subjects aged four to fourteen years were evaluated by convenience sampling. These included 43 unilateral amblyopic patients (28 anisometropic and 15 esotropic) and 15 normal subjects. Amblyopia was defined by two criteria: (1) Visual acuity at least two lines worse than the sound eye and (2) visual acuity of 0.3 log MAR or worse in the amblyopic eye. In the presence of more than 2.00 D of anisometropia and absence of other amblyogenic factors, the condition was considered as anisometropic amblyopia. The presence of esotropia without other amblyogenic factors was necessary to categorize the patient with esotropic amblyopia. The samples were recruited from governmental ophthalmological centers such as Poostchi and Motahari and a private center, Maaliabad Optometry and Vision Therapy, in Shiraz, Iran.
The exclusion criteria included pathological complications in terms of ophthalmological evaluations, having a history of neurological diseases, vertical deviations secondary to surgery, and a previous amblyopic therapy program. The fixation of patients was carefully evaluated using a direct ophthalmoscope.
The study procedures were explained to the parents of all study subjects and informed consent was obtained. All patients underwent dry and cycloplegic refraction using Cyclopentolate 0.5%. In strabismic cases, suppression was checked with the worth 4-dot test (WFDT) using the chart projector (NIDEK CO., LTD., CP770, Tokyo, Japan) and anomalous retinal correspondence was tested using the Bagolini test.
Visual acuity was evaluated by standard Snellen Distance Chart (Abtahi Medical Co., Tehran, Iran) and Yang Vision Tester (SIFI Diagnostic S.P.A-Via Castellana, 70/e-31100 Treviso, Italy). Esotropic patients had noticeable esotropia without optical correction and also having esotropia or esophoria or microtropia or eccentric fixation with optical correction.
The control group had visual acuity of 0.0 log MAR and spherical equivalent refractive error less than +1.50 diopters.
Pattern reversal VEPs were recorded using the Roland RETI system (Roland Consult, Brandenburg an der Havel, Germany), with check sizes of 15 and 60 min of arc, and contrast levels of 30 and 100% in one eye of each patient. Temporal frequency was 1.5 Hertz for all tests. These spatial frequencies are most commonly used for checker board visual stimulation in PVEP recording machines and according to the temporal frequency of check sweeps, they provide effective visual discrimination for amblyopic eyes. Attachment electrodes were placed according to International Society of Electrophysiological Vision (ISCEV) instructions. The reference, ground and active electrodes were located on the frontal (Fpz), the vertex (Cz) and on the occipital area (Oz), respectively. The responses were recorded with optical correction. The patients were instructed to maintain fixation at the center of the stimulus located at a distance of 100cm on a 20 × 30 cm black-and-white video display monitor. The stimulus was displayed with a pattern reversal rate of 1.5 times per second. VEP recordings were repeated three times when cooperation was poor. Fixation stability of the eyes was monitored closely by an experienced technician. The same conditions were considered for all VEP recordings. A connected computer analyzed the data. P100 latency was measured with each check size using two different levels of contrast for each eye. In each recording, 200 sweeps were averaged. All VEP tests were performed at the Electrophysiology Laboratory at Poostchi Eye Center, Shiraz, Iran.
Data were analyzed using SPSS software (version 15.0, IBM Co., Chicago, IL, USA). A P value of 0.05 was considered as statistically significant. Statistical analysis was performed using one-way ANOVA. Least Significant Difference (LSD) tests for comparison of three groups and independent t-test for comparison between groups were used, respectively.
RESULTS
The current study included 58 subjects, consisting of 35 female and 23 male subjects. Twenty-eight anisometropic subjects, 15 patients with esotropic amblyopia (most of whom were partially accommodative), and 15 normal cases completed the VEP test with different stimuli. P100 latencies of amblyopic eyes in both groups were markedly longer than those of the normal group (P < 0.001).
P100 Latency in Anisometropic Amblyopic Versus Normal Eyes
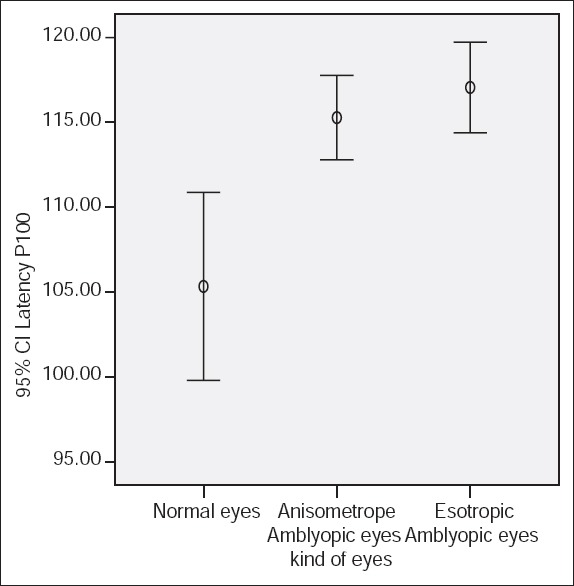
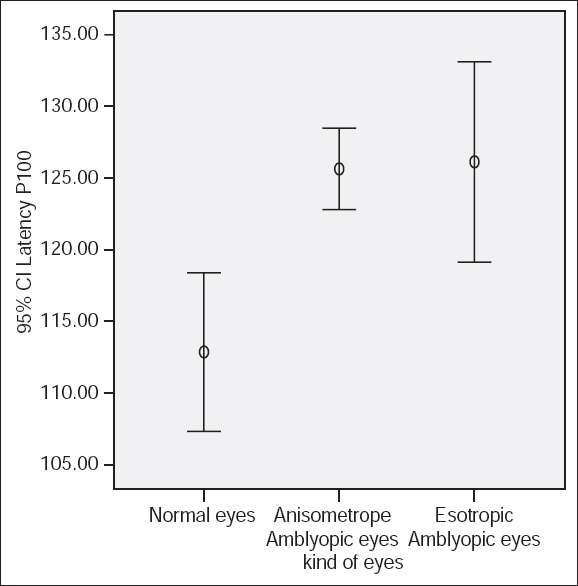
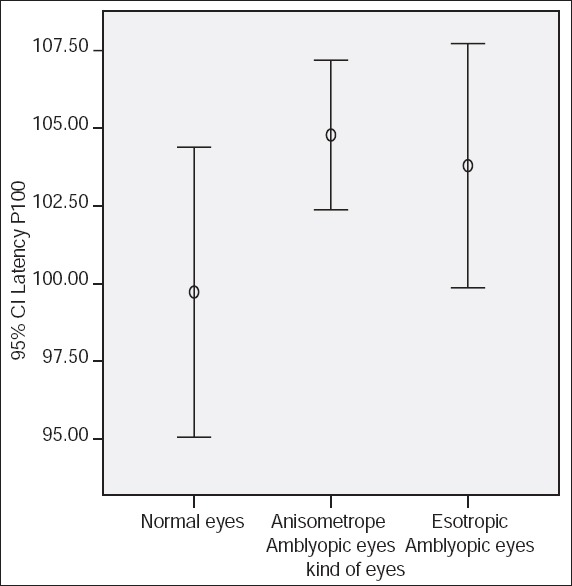
P100 latencies in anisometropic amblyopic eyes were longer than normal eyes, using small check sizes and at both levels of contrasts, (P < 0.001) [Table 1, Figures 1 and 2]. P100 latency in anisometropic amblyopic eyes using large check sizes was longer only with high contrast (P = 0.03) [Table 1, Figure 3]. In spite of an observed difference, P100 latencies using large check size with low contrast, did not differ significantly (P = 0.085) [Table 1, Figures 3 and 4].
Table 1.
Mean and SD of P100 latency (ms) in anisometropic amblyopic eyes versus normal controls
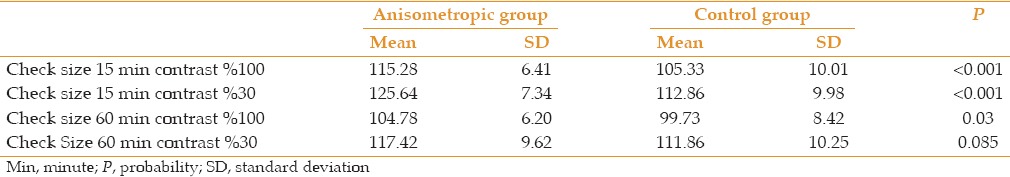
Figure 1.
P100 latency using small check size (15 min of arc) and 100% contrast.
Figure 2.
P100 latency using small check size (15 min of arc) and 30% contrast.
Figure 3.
P100 latency using large check size (60 min of arc) and 100% contrast.
Figure 4.
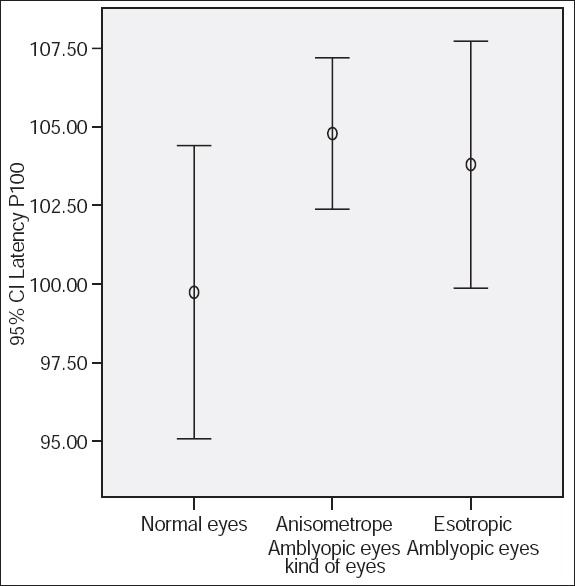
P100 latency using large check size (60 min of arc) and 30% contrast.
P100 Latency in Esotropic Amblyopic Versus Normal Eyes
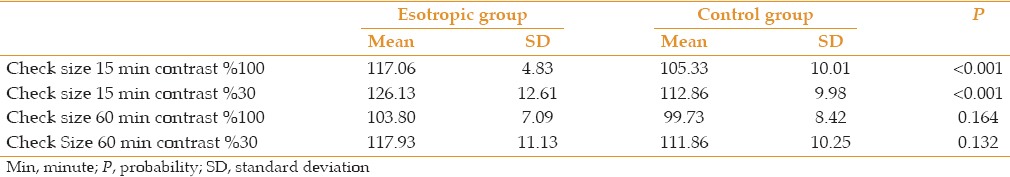
P100 latencies in esotropic amblyopic eyes were significantly longer than normal eyes using small check sizes at both levels of contrasts (P < 0.001) [Table 2, Figures 1 and 2]. Using large check size and with both levels of contrast, mean P100 latencies were not significantly longer than normal (P = 0.164) [Table 2, Figures 3 and 4].
Table 2.
Mean and SD of P100 latency (ms) esotropic amblyopic eyes versus normal controls
P100 Latency in Anisometropic Versus Esotropic Amblyopic Eyes
No significant difference was observed in P100 latency between these two amblyopic groups across all check sizes and contrast levels [Tables 3 and 4].
Table 3.
Mean and SD of P100 latency (ms) in esotropic versus anisometropic amblyopic eyes
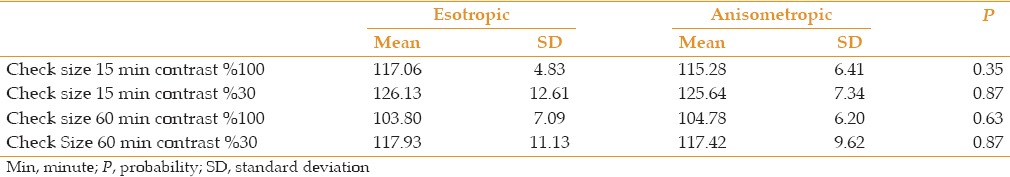
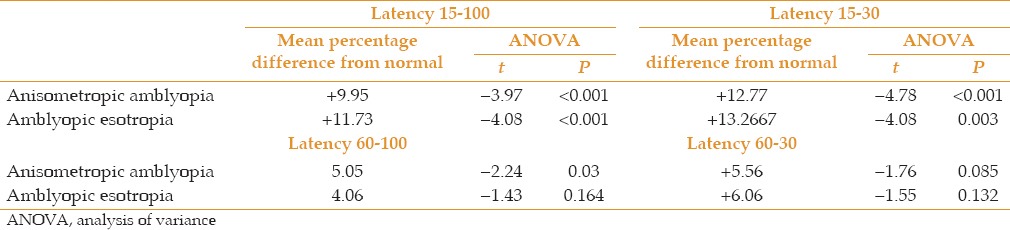
Table 4.
The mean percentage of difference from normal values in P100 latency (ms) in anisometropic versus esotropic amblyopic eyes
DISCUSSION
As previously mentioned, amblyopia is considered as a disorder of cortical function.[8] Although studies have not precisely shown the level at which these deficits occur, valuable studies performed over many years employing different tests, such as, electrophysiology, functional MRI and positron emission tomography (PET) have approximated the location of these deficits.[7,16,17,22,23,24,25]
In the present study, PVEP in amblyopic patients showed noticeable changes in P100 latency using some stimuli, in comparison to normal patients which is consistent with previous studies.[13,25,26,27]
As detailed in Table 1, P100 latencies were significantly increased in anisometropic amblyopic eyes using small-check size at both levels of contrasts. However, when exposed to large-check size stimuli, abnormal results were recorded only with high contrast. It is well known that high spatial frequencies preferentially activate short axon neurons with slow neural conduction (attributed to the parvocellular system) whereas, at lower spatial frequencies, fast neural conduction is carried out by large axon neurons, (referred to as the magnocellular pathway).[19,27,28,29]
It seems that both parvocellular and magnocellular pathways are damaged in anisometropic amblyopia and there have been similar findings showing that anisometropic patients respond abnormally to this test at both high and low spatial frequencies.[13] Some other studies also showed differences in cellular function among anisometropic patients.[21] Functional magnetic resonance imaging (MRI) results also advocate functional differences in the cortex.[16]
On the other hand, there was a significant difference in P100 latency between esotropic amblyopic and normal eyes when they were exposed to small-check size stimuli (the parvocellular system is characterized with %100 contrast and high spatial frequency). Even though P100 latencies at low spatial frequencies were not significantly different, recorded mean P100 latencies were somewhat longer than normal, possibly signifying functional differences in these two groups. According to these findings, it appears that using these stimuli could better record parvocellular defects in esotropic amblyopia than magnocellular defects. On the other hand, It defined these stimuli could show the cortical anisometropic defects well.
Previous studies have reported functional disorders in the parvocellular pathway in patients with esotropia whereas no difference was observed in their responses to large-check stimuli.[18,26] However using colorful and motion stimuli, Davies et al reported disorders in both pathways.[30] Levi et al believed that amblyopia had no influence on P100 latency, whereas, the amplitude of VEP was affected by amblyopia.[31]
According to this study, differences in the results displayed in Tables 1 and 2 can help choose appropriate stimuli and conditions for evaluating visual function in amblyopia. Low spatial frequency stimuli in high contrast could record cortical anisometropic defects in compare with normal group whereas; they could not show cortical esotropic amblyopia disorders in comparison with normal group. This finding could signify unparalleled visual information processing in these two groups of subjects. Our findings summarized in Table 3 showed no significant difference in response to any stimuli between the two amblyopic groups, which could have been the result of the small number of cases.
Our findings also confirm previous studies indicating obvious damage in the parvocellular but healthy magnocellular pathway in esotropic patients according to electrophysiological methods and PET.[7,18,21] In other words, sensitivity deficit at high spatial frequencies proves damage in the parvocellular pathway, while sensitivity and activation defects over the entire frequency range suggests both parvocellular and magnocellular pathway deficits.[27,32]
In summary, differences in electrophysiological functions of anisometropic and esotropic amblyopic patients, in comparison with the control group, raises the possibility of cellular dysfunction in the visual system. It may be hypothesized that by studying a larger group of subjects and using a variety of different stimuli, significantly different responses may be elicited in anisometropic and esotropic amblyopic subjects. It seems that these two kinds of amblyopia, while showing some similarities in reacting to stimuli in VEP, may have dissimilarities which may be important in terms of both diagnosis and treatment.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
Acknowledgments
The authors would like to thank Dr. Nasrin Shokrpour at Center for Development of Clinical Research of Nemazee Hospital for editorial assistance.
REFERENCES
- 1.Press LJ, editor. Applied Concepts in Vision Therapy. USA: Mosby-Year Book, Inc; 2008. Amblyopia: A microcosm of visual disorders; p. 76. [Google Scholar]
- 2.Schmidt PP. Eye Care for Infants and Young Children. Oxford: Butterworth-Heineman; 1997. Screening for the vision problems of young childeren; p. 175. [Google Scholar]
- 3.Press LJ, Kohl P. Eye Care for Infants and Young Children. USA: Butterworth-Heineman; 1997. Vision therapy for amblyopia; p. 155. [Google Scholar]
- 4.Sloper J. Amblyopia beyond acuity. J AAPOS. 2008;12:3–4. doi: 10.1016/j.jaapos.2007.10.011. [DOI] [PubMed] [Google Scholar]
- 5.Satterfield D, Keltner JL, Morrison TL. Psychosocial aspects of strabismus study. Arch Ophthalmol. 1993;111:1100–1105. doi: 10.1001/archopht.1993.01090080096024. [DOI] [PubMed] [Google Scholar]
- 6.Cool J. The Roots of Illiteracy in Developmental Neurobiology. A Piece to the Puzzle: Acooperative Attack on Literacy., In Optometry Extention Program Conference 1988: George Washington University. 1988 [Google Scholar]
- 7.Demer JL. Positron emission tomographic studies of cortical function in human amblyopia. Neurosci Biobehav Rev. 1993;17:469–476. doi: 10.1016/s0149-7634(05)80125-3. [DOI] [PubMed] [Google Scholar]
- 8.Halfeld Furtado de Mendonça R, Abbruzzese S, Bagolini B, Nofroni I, Ferreira EL, Odom JV. Visual evoked potential importance in the complex mechanism of amblyopia. Int Ophthalmol. 2013;33:515–519. doi: 10.1007/s10792-013-9734-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sokol S. Visually evoked potentials: Theory, techniques and clinical applications. Surv Ophthalmol. 1976;21:18–44. doi: 10.1016/0039-6257(76)90046-1. [DOI] [PubMed] [Google Scholar]
- 10.Boyd JM. Kuafman P. Adler's Physiology of the Eye. London: Mosby; 2003. Overview of the central visual pathway; p. 641. [Google Scholar]
- 11.Davis AR, Sloper JJ, Neveu MM, Hogg CR, Morgan MJ, Holder GE. Differential changes of magnocellular and parvocellular visual function in early- and late-onset strabismic amblyopia. Invest Ophthalmol Vis Sci. 2006;47:4836–4841. doi: 10.1167/iovs.06-0382. [DOI] [PubMed] [Google Scholar]
- 12.Lalor EC, Foxe JJ. Visual evoked spread spectrum analysis (VESPA) responses to stimuli biased towards magnocellular and parvocellular pathways. Vision Res. 2009;49:127–133. doi: 10.1016/j.visres.2008.09.032. [DOI] [PubMed] [Google Scholar]
- 13.Campos EC, Prampolini ML, Gulli R. Contrast sensitivity differences between strabismic and anisometropic amblyopia: Objective correlate by means of visual evoked responses. Doc Ophthalmol. 1984;58:45–50. doi: 10.1007/BF00140897. [DOI] [PubMed] [Google Scholar]
- 14.Levi DM, Harwerth RS. Contrast evoked potentials in strabismic and anisometropic amblyopia. Invest Ophthalmol Vis Sci. 1978;17:571–575. [PubMed] [Google Scholar]
- 15.Feng LX, Zhao KX. Study on anisometropic amblyopia by simultaneously recording multifocal VEP and multifocal ERG. Zhonghua Yan Ke Za Zhi. 2005;41:41–46. [PubMed] [Google Scholar]
- 16.Bonhomme GR, Liu GT, Miki A, Francis E, Dobre MC, Modestino EJ, et al. Decreased cortical activation in response to a motion stimulus in anisometropic amblyopic eyes using functional magnetic resonance imaging. J AAPOS. 2006;10:540–546. doi: 10.1016/j.jaapos.2006.07.008. [DOI] [PubMed] [Google Scholar]
- 17.Heravian J, Daneshvar R, Dashti F, Azimi A, Ostadi Moghaddam H, Yekta AA, et al. Simultaneous pattern visual evoked potential and pattern electroretinogram in strabismic and anisometropic amblyopia. Iran Red Crescent Med J. 2011;13:21–26. [PMC free article] [PubMed] [Google Scholar]
- 18.Demirci H, Gezer A, Sezen F, Ovali T, Demiralp T, Isoglu-Alkoc U. Evaluation of the functions of the parvocellular and magnocellular pathways in strabismic amblyopia. J Pediatr Ophthalmol Strabismus. 2002;39:215–221. doi: 10.3928/0191-3913-20020701-09. [DOI] [PubMed] [Google Scholar]
- 19.Levi DM, Harwerth RS. Spatio-temporal interactions in anisometropic and strabismic amblyopia. Invest Ophthalmol Vis Sci. 1977;16:90–95. [PubMed] [Google Scholar]
- 20.McKee SP, Schor CM, Steinman SB, Wilson N, Koch GG, Davis SM, et al. The classification of amblyopia on the basis of visual and oculomotor performance. Trans Am Ophthalmol Soc. 1992;90:123–144. [PMC free article] [PubMed] [Google Scholar]
- 21.Shan Y, Moster ML, Roemer RA, Siegfried JB. Abnormal function of the parvocellular visual system in anisometropic amblyopia. J Pediatr Ophthalmol Strabismus. 2000;37:73–78. doi: 10.3928/0191-3913-20000301-05. [DOI] [PubMed] [Google Scholar]
- 22.Wang X, Cui D, Zheng L, Yang X, Yang H, Zeng J. Combination of blood oxygen level-dependent functional magnetic resonance imaging and visual evoked potential recordings for abnormal visual cortex in two types of amblyopia. Mol Vis. 2012;18:909–919. [PMC free article] [PubMed] [Google Scholar]
- 23.Sokol S. Abnormal evoked potential latencies in amblyopia. Br J Ophthalmol. 1983;67:310–314. doi: 10.1136/bjo.67.5.310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Li C, Cheng L, Yu Q, Xie B, Wang J. Relationship of visual cortex function and visual acuity in anisometropic amblyopic children. Int J Med Sci. 2012;9:115–120. doi: 10.7150/ijms.9.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kubová Z, Kuba M, Juran J, Blakemore C. Is the motion system relatively spared in amblyopia? Evidence from cortical evoked responses. Vision Res. 1996;36:181–190. doi: 10.1016/0042-6989(95)00055-5. [DOI] [PubMed] [Google Scholar]
- 26.Levi DM, Walters JW. Visual evoked responses in strabismic and anisometropic amblyopia: Effects of check size and retinal locus. Am J Optom Physiol Opt. 1977;54:691–698. doi: 10.1097/00006324-197710000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Krzystkowa KM, Kubatko-Zielinska A, Wójcik E, Strek W, Lebiedz J. Changes observed in electrophysiological investigations in amblyopia and strabismus. Klin Oczna. 1998;100:229–234. [PubMed] [Google Scholar]
- 28.Parisi V, Scarale ME, Balducci N, Fresina M, Campos EC. Electrophysiological detection of delayed postretinal neural conduction in human amblyopia. Invest Ophthalmol Vis Sci. 2010;51:5041–5048. doi: 10.1167/iovs.10-5412. [DOI] [PubMed] [Google Scholar]
- 29.Jones R, Keck MJ. Visual evoked response as a function of grating spatial frequency. Invest Ophthalmol Vis Sci. 1978;17:652–659. [PubMed] [Google Scholar]
- 30.Davis AR, Sloper JJ, Neveu MM, Hogg CR, Morgan MJ, Holder GE. Differential changes in color and motion-onset visual evoked potentials from both eyes in early- and late-onset strabismic amblyopia. Invest Ophthalmol Vis Sci. 2008;49:4418–4426. doi: 10.1167/iovs.07-1437. [DOI] [PubMed] [Google Scholar]
- 31.Levi DM. Patterned and unpatterned visual evoked responses in strabismic and anisometropic amblyopia. Am J Optom Physiol Opt. 1975;52:455–464. doi: 10.1097/00006324-197507000-00002. [DOI] [PubMed] [Google Scholar]
- 32.Hess RF, Howell ER, Kitchin JE. On the relationship between pattern and movement perception in strabismic amblyopia. Vision Res. 1978;18:375–377. doi: 10.1016/0042-6989(78)90046-9. [DOI] [PubMed] [Google Scholar]


