Abstract
Purpose:
To determine the proportion of patients with central serous chorioretinopathy (CSCR) mistaken for posterior uveitis and to identify the deleterious consequences.
Methods:
Charts of 1,657 patients admitted in the section of inflammatory eye diseases at the Center for Ophthalmic Specialized Care (COS) in Lausanne, Switzerland from 1995 to 2013 were reviewed. CSCR cases misdiagnosed as posterior uveitis or those with superimposed disease due to steroid therapy for uveitis were studied. Delay in diagnosis, specific erroneous uveitis diagnosis and evolution of the disease were also evaluated. Retrospectively, the most useful means for a correct diagnosis of CSCR were the original fluorescein angiography (FA), indocyanine green angiography (ICGA) and optical coherence tomography (OCT) when available.
Results:
Out of a total of 1,657 patients, 15 (0.9%) cases with CSCR were identified. These included 12 subjects misdiagnosed as posterior uveitis and 3 uveitis subjects with superimposed CSCR following corticosteroid therapy for uveitis. The presentation of the disease was largely influenced by improper and continued use of corticosteroids.
Conclusion:
CSCR is a rare but not negligible misdiagnosis in posterior uveitis representing approximately 1% of subjects from a collective series of uveitis cases at a referral center. Investigative measures such as FA, ICGA and OCT are crucial for reaching a correct diagnosis and avoiding disease aggravation due to corticosteroid therapy.
Keywords: Central Serous Chorioretinopathy, Fluorescein Angiography, Indocyanine Green Angiography, Optical Coherence Tomography, Uveitis
INTRODUCTION
Central serous chorioretinopathy (CSCR) is a common non-inflammatory condition affecting young adults, preponderantly men, and is characterized by neurosensory retinal detachment with or without pigment epithelial detachment.[1,2] Common thinking is that the condition is caused by dysfunction in choroidal circulation and retinal pigment epithelium (RPE) with secondary damage to the outer retina. Acute episodes cause visual loss due to subretinal fluid leakage and macular neurosensory detachment; in chronic cases, irreversible visual loss due to widespread RPE damage and retinal atrophy may occur.
It is now well known that in the majority of CSCR cases, the use of corticosteroids triggers the disease.[3,4] In a large proportion of patients, exogenous corticosteroids, either oral or other forms, and in rare instances, endogenous hypercortisolism, is the origin of CSCR and accounts for the development of disease.[5,6,7,8,9,10,11,12]
One of the pitfalls in the approach to posterior inflammatory disorders is to misdiagnose CSCR as posterior uveitis. Thus, it is of great significance to identify CSCR among patients who are seen at or referred to a uveitis clinic, especially because most of the time, the first line of therapy in posterior uveitis is corticosteroids delivered by periocular or systemic routes. Such a scenario will induce a vicious circle of aggravation of the condition leading to escalation of corticosteroid administration and further deterioration of CSCR.
Fluorescein angiography (FA) and indocyanine green angiography (ICGA) signs of CSCR are fairly well characterized. FA signs include leaking pinpoints (ink dot leakage) with or without a smoke-stack configuration, pigment epithelium detachment, RPE changes with atrophy and diffuse epitheliopathy in chronic forms with bullous detachment of the neurosensory retina in advanced forms. ICGA signs comprise hyperfluorescent leaking points and bilateral increased choroidal permeability causing diffuse late choroidal hyperfluorescence.[13,14]
Diagnosis has been substantially assisted by optical coherence tomography (OCT) showing subretinal fluid (neurosensory retinal detachment) and small pigment epithelium detachments (PEDs).[15,16] These characteristics should be kept in mind and sought for in cases of unexpected clinical and angiographic aggravation of presumed posterior uveitis.
In rare instances, uveitis itself can be complicated by CSCR due to corticosteroid therapy and may represent a substantial challenge since it has to be distinguished from worsening of the uveitis.[17]
The current study was aimed to determine the frequency of CSCR misdiagnosed as uveitis, identify causes of erroneous diagnosis and analyze the outcome of such cases at a uveitis referral center.
METHODS
Charts of patients admitted to the uveitis clinic at the Center for Ophthalmic Specialized Care (COS), Lausanne, Switzerland between 1995 and 2013 were reviewed. Patients who were referred with a diagnosis for posterior uveitis and later identified as CSCR were included and their files were studied retrospectively.
All patients underwent a complete routine work-up for posterior uveitis. At each major visit, a routine and complete ocular examination was performed including visual acuity measured in decimal notations, slit lamp examination, applanation tonometry and dilated fundus examination. In addition, the following procedures were performed: Laser flare photometry Kowa FM-500 (Kowa Company, Ltd., Electronics and Optics Division, Tokyo, Japan), OCT (OTI-Spectral OCT/SLO; OTI Inc., Toronto, Canada) or Heidelberg Spectralis OCT system (Heidelberg Engineering, Heidelberg, Germany), and dual FA and ICGA. A Topcon 50 IA camera (Tokyo, Japan) coupled to ImageNet software (Topcon Inc. Tokyo, Japan), a digital imaging system or a Heidelberg Spectralis Retinal Angiography system (Heidelberg Engineering, Heidelberg, Germany) were used to acquire FA and ICGA images.
The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
Time interval from erroneous diagnosis to recognition of CSCR was noted and mean time for delayed diagnosis was recorded. When possible, documents related to clinical examination and investigations at the time of erroneous diagnosis were reviewed and signs leading to a correct diagnosis of CSCR which had been missed were probed. Elements leading to a correct diagnosis of CSCR were analyzed. Evolution of disease before and after the correct diagnosis was compared.
RESULTS
Of a total of 1,657 uveitis patients seen from 1995 to 2013 at the COS, 15 patients (0.9%) actually had CSCR but had been misdiagnosed as uveitis. These included 12 non-uveitis cases and 3 uveitis subjects with superimposed CSCR. Out of 12 non-uveitis cases, 3 patients were erroneously diagnosed with ocular tuberculosis, 3 cases with choriocapillaritis (2 multifocal choroiditis and one acute multifocal placoid pigment epitheliopathy [AMPPE]), 2 cases with Vogt-Koyanagi-Harada (VKH) disease, 1 case with lupus erythematosus disseminatus (LED) and 3 cases with undetermined uveitis. All the three patients misdiagnosed as ocular tuberculosis had negative interferon gamma release assays (IGRAs) (QuantiFERON-TB, Gold In-Tube, Cellestis, Carnegie, Australia). In two patients, IGRA had not been performed at presentation but both received dual antitubercular antibiotics with systemic corticosteroids. Despite the fact that IGRAs were negative twice, the third patient was also put on antitubercular antibiotics and systemic corticosteroids in order to “control inflammation”. In all three subjects, visual acuity deteriorated progressively.
In 3 cases, CSCR developed following corticosteroid therapy for uveitis and was misdiagnosed as worsening of the underlying inflammatory disease.
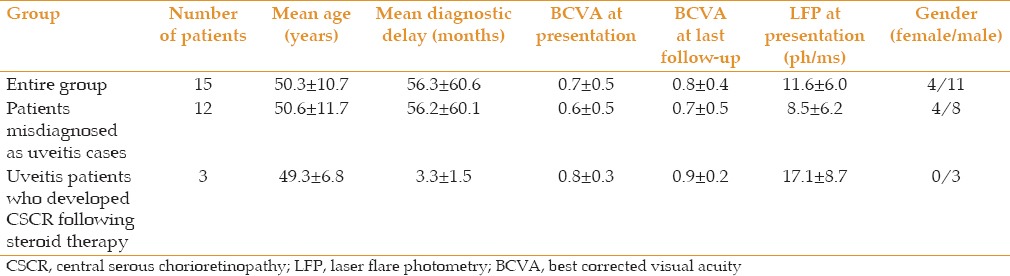
Table 1 represents demographic and clinical data of CSCR patients. Mean age of the patients was 50.3 ± 10.7 years overall, 50.6 ± 11.7 years in the group misdiagnosed as uveitis and 49.3 ± 6.8 years in the uveitis group who developed CSCR after corticosteroid therapy. Mean diagnostic delay was 56.3 ± 60.6 months in the entire group, 56.2 ± 60.1 months in subjects initially misdiagnosed as uveitis and 3.3 ± 1.5 months in the uveitis group with superimposed CSCR. In the entire group, mean best corrected visual acuity (BCVA) was 0.7 ± 0.5 at presentation, 0.6 ± 0.5 in group with misdiagnosed uveitis and 0.8 ± 0.3 in the uveitis group in whom CSCR was superimposed.
Table 1.
Demographic and clinical data of patients CSCR
All patients were under systemic corticosteroid therapy but none of the subjects who had been misdiagnosed with posterior uveitis presented with inflammatory signs using FA (disk hyperfluorescence retinal vasculitis) or ICGA (choroiditis or dark dots). In contrast all patients in whom CSCR was the consequence of corticosteroid therapy for uveitis, showed signs of inflammation.
Anterior segments were within normal limits in all patients and laser flare photometry (LFP) revealed slight subclinical blood-ocular barrier disruption (mean, 11.6 ± 6.0 ph/ms; normal values <6.5 ph/ms). In the subgroup of uveitis patients, mean LFP value was 17.1 ± 8.7 ph/ms, which was slightly higher than the non-uveitis group (8.5 ± 6.2 ph/ms) and the difference was statistically significant (P = 0.02).
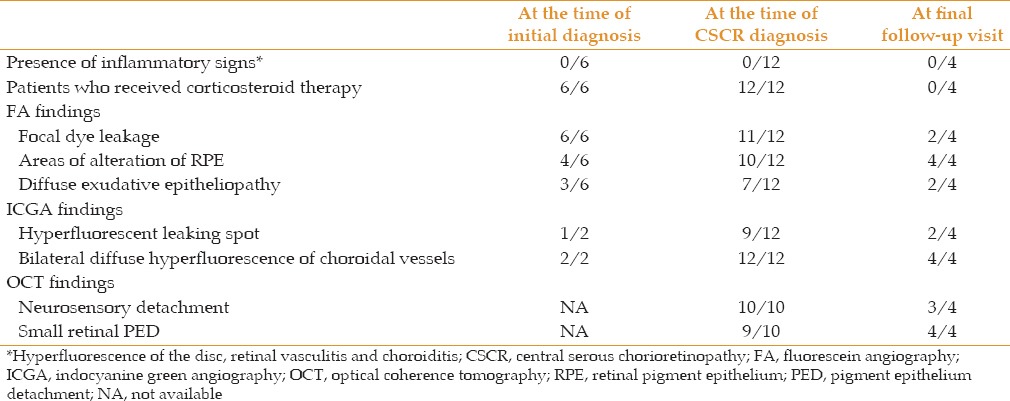
Features of CSCR cases misdiagnosed as uveitis at the time of initial diagnosis, at the time of correct CSCR diagnosis, and at final follow-up are presented in Table 2.
Table 2.
Features of CSCR cases misdiagnosed as uveitis at the time of initial diagnosis, at the time of correct diagnosis and at final follow-up
Features at Referral
Out of 12 patients with missed diagnoses, mean delay was 56 ± 60.1 (range, 2-120) months. BCVA at referral in patients with delayed diagnosis (later than 24 months) was significantly worse than those with “early diagnosis” (earlier than 24 months), (0.3 ± 0.3 versus 0.7 ± 0.2, P < 0.05). All patients were receiving corticosteroid therapy for non-ocular diseases (one patient was affected by psoriasis, 2 by polychondritis, one by lupus, 2 had orthopedic pathologies requiring steroid infiltrations, 3 had rhinitis and 3 used steroids to relieve articular pain).
Using FA or ICGA, none of the subjects showed signs of inflammation. LFP values confirmed the absence blood-ocular barrier disruption (8.5 ± 6.2 ph/ms). FA revealed focal dye leakage (ink blot or smoke-stack appearance) in 11 patients, pigmentary changes or atrophy in 10 subjects and diffuse exudative epitheliopathy or bullous serous detachment in 7 out of 12 cases [Figure 1]. On ICGA, all patients demonstrated diffuse bilateral hyperfluorescence of choroidal vessels and in 9 subjects, a hyperfluorescent leaking spot was identified.
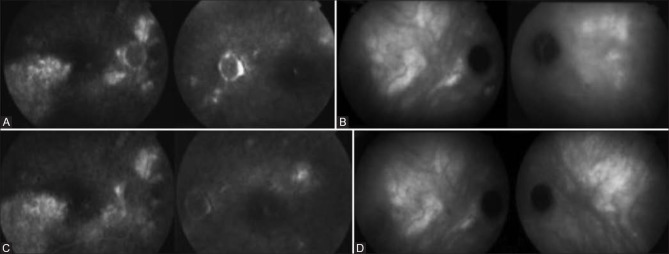
Figure 1.
Fluorescein angiography (FA) and indocyanine green angiography (ICGA) images of a patient with central serous chorioretinopathy (CSCR) at the time of the diagnosis (A and B) and one year after stopping systemic steroid therapy (C and D). A, on FA, the presence of diffuse exudative epitheliopathy indicates long-standing disease. C, One year after stopping steroid therapy, diffuse exudative epitheliopathy is reduced but still present (the lack of disc leakage and vasculitis on FA should be stressed). B, on ICGA, diffuse bilateral hyperfluorescence of choroidal vessels is seen at the time of diagnosis and (D) one year after stopping steroid therapy.
OCT which was available in 10 patients revealed neurosensory retinal detachment and small pigment epithelium detachments (PEDs) in 10 and 9 cases, respectively [Figure 2].
Figure 2.
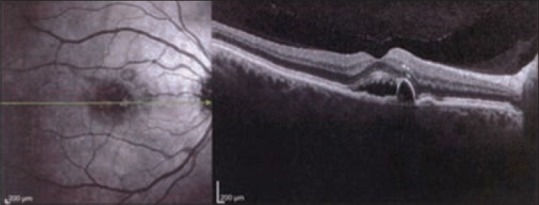
Typical optical coherence tomography findings in a patient with central serous chorioretinopathy (CSCR). Note the pigment epithelium detachment (PED) in the context of a neurosensory serous detachment.
The features of misdiagnosed cases at the time of referral (diagnosis of CSCR) are detailed in Table 2.
Features at Correct Diagnosis
In 6 patients historical documents were available and no sign of inflammation had been present on FA or ICGA. However, focal points of dye leakage, RPE changes or atrophy and diffuse exudative epitheliopathy were evident in 6, 4 and 3 patients, respectively. ICGA was available in only 2 cases which showed diffuse bilateral hyperfluorescence of choroidal vessels along with a hyperfluorescent leaking point in one case [Table 2].
Follow-up after Discontinuation of Corticosteroids
Four patients had regular follow-up at least one year after discontinuation of corticosteroids (mean follow-up, 27 ± 18 months). BCVA in CSCR patients misdiagnosed as uveitis was 0.69 ± 0.48 at final follow-up. None of the patients showed signs of inflammation after discontinuation of corticosteroids. Pigmentary alterations or atrophy remained on FA in all cases; focal dye leakage and exudative epitheliopathy persisted in two patients. In all 4 patients with complete follow-up, bilateral diffuse hyperfluorescence of choroidal vessels was still present on ICGA. Out of 3 patients with hyperfluorescent spots at presentation, only 2 cases still had detectable lesions at last follow-up. OCT revealed PEDs in all patients; in 3 patients serous neurosensory retinal detachment persisted [Table 2].
Features of CSCR in uveitis patients treated with steroids were detailed in Tables 1 and 2.
All 3 uveitis patients who developed CSCR due to corticosteroid therapy showed signs of inflammation on FA or ICGA. BCVA at the time of CSCR diagnosis was 0.8 ± 0.3 and reached 0.9 ± 0.2, indicating less impaired vision than the misdiagnosed group. LFP values in this group (17.1 ± 8.7 ph/ms) were significantly higher than the non-uveitis group (8.5 ± 6.2 ph/ms, P < 0.02), confirming disruption of the ocular-blood barrier already observed on FA. Mean diagnostic delay in this group of patients was relatively short, only 3.3 ± 1.5 months [Table 1]. On FA, a focal point of dye leakage was identified in all 3 cases, along with areas of pigmentary alterations in 2 cases, whereas no case presented with diffuse exudative epitheliopathy. On ICGA, diffuse bilateral hyperfluorescence of choroidal vessels was recorded in all 3 patients and in one patient a hyperfluorescent leaking spot could also be identified. OCT was available in only one patient who only had small PEDs with no serous neurosensory retinal detachment [Table 2].
DISCUSSION
To the best of our knowledge, this is the first report on the frequency of CSCR in a uveitis collective resulting from misdiagnoses or due to corticosteroid therapy. CSCR in such a setting is rare but not negligible, as it amounts to approximately 1% of all cases. Out of 15 CSCR patients identified among 1,647 uveitis referrals, 12 were misdiagnosed as posterior uveitis and 3 were uveitis patients who developed CSCR during follow-up, always linked to corticosteroid therapy.
Although misdiagnosis of CSCR as posterior uveitis is rare, special care should be given to avoid it, as the consequences are usually deleterious and may even be catastrophic. A misdiagnosis of CSCR as uveitis and prolonged corticosteroid therapy often aggravates the condition leading to a vicious cycle of intensified treatment and lack of clinical response.[4,18]
The majority of our patients had already been seen by a uveitis specialist before being referred to our center and this fact, in itself, possibly constitutes the strongest factor inducing the misdiagnosis. Since the patients were referred for uveitis, differential diagnosis was biased towards uveitis and only considered within a restricted range of related entities. If all of these cases had been sent to a retina center, they would probably not have been misdiagnosed. Our study indicated several clear findings which allowed the diagnosis of CSCR at referral; however, these findings had been present earlier and could have allowed a correct diagnosis. The sex ratio was strongly in biased toward men (74%), a feature characteristic of CSCR.[1,19] The strong implication of corticosteroids in the development of CSCR was confirmed in our series as all patients were under systemic corticosteroid therapy both at initial diagnosis and upon referral.
The single most important factor that should have allowed exclusion of posterior uveitis as a diagnosis was the lack of objective signs of inflammation such as retinal vasculitis and optic disc hyperfluorescence on FA and/or choroiditis on ICGA that were absent both at initial diagnosis and upon referral. Low to near normal LFP values also confirmed the absence of inflammation.
The strongest positive feature speaking in favour of a diagnosis of CSCR was choroidal hyperfluorescence due to hyperpermeable choroidal vessels on ICGA which was present bilaterally in all patients at initial diagnosis and referral, and persisted after discontinuation of corticosteroids.
OCT findings were also found to be strong elements helping the diagnosis of CSCR; neurosensory retinal detachment was present in all cases at referral and small pigment epithelial detachments were found in 90% of subjects. Albeit, OCTs were not available at the time of initial erroneous diagnosis in our series.
Classic FA signs, such as dye leakage, were almost constantly seen both at initial diagnosis and upon referral. Diffuse exudative epitheliopathy, the more severe form of CSCR, with or without bullous detachment were already present in half of the patients whose records at initial diagnosis were available, indicating that corticosteroids had already been given for a long period of time. More than 50% of patients had diffuse exudative epitheliopathy at referral, all of whom had received higher doses of corticosteroids and/or prolonged administration as compared with the 5 cases without diffuse epitheliopathy. Our series also shows that once diffuse exudative epitheliopathy has developed, discontinuation of corticosteroids does not lead to improved function.
The subgroup of uveitis patients showed higher LFP values as compared to the non-uveitis subgroup, indicating that LFP might be an additional modality useful to exclude an inflammatory origin in borderline cases. LFP was also slightly elevated in the non-uveitis group, possibly because prolonged and inappropriate corticosteroid therapy leads to breakdown of the aqueous-blood barrier resulting in mild subclinical inflammation.
For the group of uveitis patients who developed CSCR as a complication of corticosteroid therapy, the diagnostic delay was very short. The hint leading to a correct diagnosis was improvement of inflammatory signs including retinal vasculitis, cystoid macular edema, disc hyperfluorescence and LFP.
In summary, combining FA, ICGA, OCT and LFP facilitated the diagnosis of CSCR in a group of CSCR patient misdiagnosed as posterior uveitis, although sufficient signs were already present at the time of misdiagnosis. We believe that the single most important factor leading to misdiagnosis was the fact that all patients were seen by a uveitis specialist who did not consider the diagnosis of non-inflammatory conditions and overlooked signs clearly supporting a diagnosis of CSCR.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Liew G, Quin G, Gillies M, Fraser-Bell S. Central serous chorioretinopathy: A review of epidemiology and pathophysiology. Clin Experiment Ophthalmol. 2013;41:201–214. doi: 10.1111/j.1442-9071.2012.02848.x. [DOI] [PubMed] [Google Scholar]
- 2.Montero JA, Ruiz-Moreno JM. Optical coherence tomography characterisation of idiopathic central serous chorioretinopathy. Br J Ophthalmol. 2005;89:562–564. doi: 10.1136/bjo.2004.049403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Stoffelns BM, Kramann C, Schoepfer K. Central serous chorioretinopathy (CSC) and corticosteroids. Klin Monbl Augenheilkd. 2008;225:370–375. doi: 10.1055/s-2008-1027271. [DOI] [PubMed] [Google Scholar]
- 4.Khairallah M, Kahloun R, Tugal-Tutkun I. Central serous chorioretinopathy, corticosteroids, and uveitis. Ocul Immunol Inflamm. 2012;20:76–85. doi: 10.3109/09273948.2011.650776. [DOI] [PubMed] [Google Scholar]
- 5.Gass JD, Little H. Bilateral bullous exudative retinal detachment complicating idiopathic central serous chorioretinopathy during systemic corticosteroid therapy. Ophthalmology. 1995;102:737–747. doi: 10.1016/s0161-6420(95)30960-8. [DOI] [PubMed] [Google Scholar]
- 6.Abu el-Asrar AM. Central serous chorioretinopathy complicating systemic corticosteroid therapy. Eur J Ophthalmol. 1997;7:297–300. doi: 10.1177/112067219700700317. [DOI] [PubMed] [Google Scholar]
- 7.Bouzas EA, Karadimas P, Pournaras CJ. Central serous chorioretinopathy and glucocorticoids. Surv Ophthalmol. 2002;47:431–448. doi: 10.1016/s0039-6257(02)00338-7. [DOI] [PubMed] [Google Scholar]
- 8.Karadimas P, Kapetanios A, Bouzas EA. Central serous chorioretinopathy after local application of glucocorticoids for skin disorders. Arch Ophthalmol. 2004;122:784–786. doi: 10.1001/archopht.122.5.784. [DOI] [PubMed] [Google Scholar]
- 9.Kocabora MS, Durmaz S, Kandemir N. Exacerbation of central serous chorioretinopathy following intravitreal triamcinolone injection. Graefes Arch Clin Exp Ophthalmol. 2008;246:1783–1786. doi: 10.1007/s00417-008-0932-2. [DOI] [PubMed] [Google Scholar]
- 10.Mondal LK, Sarkar K, Datta H, Chatterjee PR. Acute bilateral central serous chorioretinopathy following intra-articular injection of corticosteroid. Indian J Ophthalmol. 2005;53:132–134. doi: 10.4103/0301-4738.16181. [DOI] [PubMed] [Google Scholar]
- 11.Giovansili I, Belange G, Affortit A. Cushing disease revealed by bilateral atypical central serous chorioretinopathy: Case report. Endocr Pract. 2013;19:e129–e133. doi: 10.4158/EP12389.CR. [DOI] [PubMed] [Google Scholar]
- 12.Haimovici R, Gragoudas ES, Duker JS, Sjaarda RN, Eliott D. Central serous chorioretinopathy associated with inhaled or intranasal corticosteroids. Ophthalmology. 1997;104:1653–1660. doi: 10.1016/s0161-6420(97)30082-7. [DOI] [PubMed] [Google Scholar]
- 13.Lafaut BA, Salati C, Priem H, De Laey JJ. Indocyanine green angiography is of value for the diagnosis of chronic central serous chorioretinopathy in elderly patients. Graefes Arch Clin Exp Ophthalmol. 1998;236:513–521. doi: 10.1007/s004170050114. [DOI] [PubMed] [Google Scholar]
- 14.Shiraki K, Moriwaki M, Matsumoto M, Yanagihara N, Yasunari T, Miki T. Long-term follow-up of severe central serous chorioretinopathy using indocyanine green angiography. Int Ophthalmol 1997. 1998;21:245–253. doi: 10.1023/a:1006038621426. [DOI] [PubMed] [Google Scholar]
- 15.Quin G, Liew G, Ho IV, Gillies M, Fraser-Bell S. Diagnosis and interventions for central serous chorioretinopathy: Review and update. Clin Experiment Ophthalmol. 2013;41:187–200. doi: 10.1111/j.1442-9071.2012.02847.x. [DOI] [PubMed] [Google Scholar]
- 16.Yalcinbayir O, Gelisken O, Akova-Budak B, Ozkaya G, Gorkem Cevik S, Yucel AA. Correlation of spectral domain optical coherence tomography findings and visual acuity in central serous chorioretinopathy. Retina. 2014;34:705–712. doi: 10.1097/IAE.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 17.Schalenbourg A, Leys A, De Courten C, Coutteel C, Herbort CP. Corticosteroid-induced central serous chorioretinopathy in patients with ocular inflammatory disorders. Klin Monbl Augenheilkd. 2002;219:264–267. doi: 10.1055/s-2002-30660. [DOI] [PubMed] [Google Scholar]
- 18.Arantes TE, Garcia CR, Rossi MR, Muccioli C. Spectral domain optical coherence tomography and angiographic findings in central serous chorioretinopathy complicated by bilateral nonrhegmatogenous retinal detachment associated with systemic corticosteroids. Ocul Immunol Inflamm. 2009;17:316–318. doi: 10.3109/09273940903030888. [DOI] [PubMed] [Google Scholar]
- 19.Todd KC, Hainsworth DP, Lee LR, Madsen RW. Longitudinal analysis of central serous chorioretinopathy and sex. Can J Ophthalmol. 2002;37:405–408. doi: 10.1016/s0008-4182(02)80043-6. [DOI] [PubMed] [Google Scholar]