Abstract
Purpose:
To report the use of intraoperative spectral domain optical coherence tomography (SD-OCT) for detecting anatomical changes during macular surgery.
Methods:
In a consecutive case series, 32 eyes of 32 patients undergoing concurrent pars plana vitrectomy and intraoperative SD-OCT for macular hole (MH), epiretinal membrane (ERM) and vitreomacular traction (VMT) were enrolled. Intraoperative changes in retinal thickness and dimensions of the macular hole were measured in patients with ERM and VMT following surgical manipulation using a hand-held SD-OCT device (iVue, Optovue Inc., Fremont, CA, USA).
Results:
SD-OCT images of sixteen eyes with macular hole were subjected to quantitative and qualitative analysis. All MH dimensions remained stable during consecutive stages of surgery except for MH apex diameter, which showed a significant decrease after internal limiting membrane (ILM) peeling (P=0.025). Quantitative analysis of ten patients with ERM showed a significant decrease in retinal thickness after membrane removal (P=0.018) which did not remain significant until the end of the procedure (P=0.8). In three cases, subretinal fluid was formed after ILM peeling. Quantitative analysis of five patients with VMT showed a decrease in retinal thickness during consecutive steps of the surgery, although these changes were not significant. In two cases, subretinal fluid was formed after ILM peeling.
Conclusion:
Intraoperative SD-OCT is a useful imaging technique which provides vitreoretinal surgeons with rapid awareness of changes in macular anatomy during surgery and may therefore result in better anatomical and visual outcomes.
Keywords: Intraoperative Optical Coherence Tomography, Macular Pathology, Macular Surgery
INTRODUCTION
Optical coherence tomography has gained an important role in the diagnosis, management and follow-up of vitreoretinal disorders.[1] The introduction of spectral-domain optical coherence tomography (SD-OCT), as an improved modality, has provided vitreoretinal surgeons with larger scan areas, faster acquisition time and higher resolution.[2] Numerous investigations have been performed analyzing SD-OCT images before and after surgical intervention in patients with macular and hyaloid-retinal interface disease. All emerging data have fruitfully shown significant correlations between anatomical changes in areas of interest, especially the inner segment-outer segment (IS-OS) junction, and visual outcomes in patients with macular hole (MH), vitreomacular traction (VMT) and epiretinal membrane (ERM).[3,4,5,6,7,8,9,10] However, accurate image acquisition with this modality needs a compliant patient who can sit in an upright position. Hence, a handheld SD-OCT device was designed to overcome the problem in special situations, such as retinopathy of prematurity and determining the source of leakage in a patient with optic pit maculopathy. It can also use in different situations to evaluate latent anatomical changes that may affect treatment and prognosis.[11,12]
Dayani et al first described the intraoperative use of SD-OCT for macular surgery in 2009.[13] The device provides the surgeon with immediate information about anatomical changes during surgery, such as the presence of subretinal fluid, fluid dynamics and amount of surgical trauma. Few reports exist reporting the intraoperative use of SD-OCT for retinal surgeries with small sample sizes.[14,15]
In this study, we evaluated the role of intraoperative hand-held SD-OCT in detecting changes in retinal anatomy during different stages of macular surgeries in 32 eyes suffering from MH, ERM and VMT syndrome. To the best of our knowledge, this is the largest study reporting concurrent pars plana vitrectomy and intraoperative SD-OCT.
METHODS
In a consecutive case series, patients undergoing macular surgery with concurrent intraoperative SD-OCT imaging for full thickness MH, VMT or ERM from September 2012 to March 2013 were enrolled. The study protocol was reviewed and approved by the Institutional Ethics Committee at Tehran University of Medical Sciences and all participants provided written informed consent prior to inclusion.
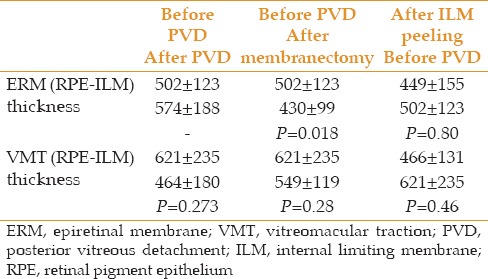
All eyes were operated using 23-gauge, three-port pars plana deep vitrectomy. Vitreous and membranes were stained with triamcinolone and the internal limiting membrane (ILM) was stained with brilliant blue-G (BBG). SD-OCT was performed intraoperatively using the iVue hand-held SD-OCT (Optovue Inc., Fremont, CA, USA). During surgeries for ERM and VMT, images were obtained before posterior vitreous detachment (PVD) induction, after PVD induction, following residual ERM peeling (if applicable) and after ILM peeling [Figure 1a–c respectively]. During surgeries for MH, images were taken in three steps: Before PVD formation, after PVD formation, and following ILM peeling.
Figure 1.
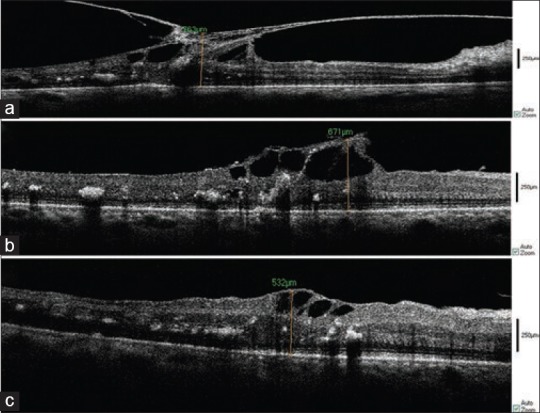
Intraoperative SD-OCT of a 61-year-old diabetic man with VMT. Before vitrectomy (a), after PVD induction (b), after ILM peeling (c), The significant decrease in the size of retinal cyst is demonstrated.
Images were analyzed for qualitative and quantitative characteristics. Quantitative measurements were performed using the InVivoVue Clinic version 1.2 software employing the available caliper. Regarding MH surgeries, parameters included MH height (calculated by measuring the highest point on either side of the hole, from the ILM to the retinal pigment epithelium [RPE] and taking average values), MH base diameter, MH apex diameter and distance from the smallest diameter of the hole to the base center [Figure 2]. Regarding surgeries performed for VMT and ERM, retinal thickness (distance from ILM to RPE) was measured, and residual ERM or ILM thickness were also measured after each peeling.
Figure 2.
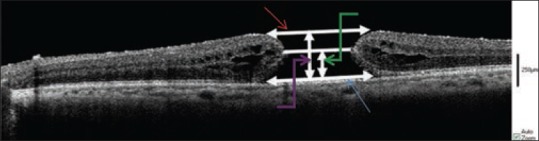
Apex diameter (red arrow), base diameter (blue arrow), height (purple arrow), distance through the thinnest diameter of the hole to the base center (green arrow).
Qualitative findings, including foveal contour and appearance of corrugations on the retinal surface, were also compared at different time points. Any significant qualitative finding, such as new hypo- or hyper-reflective lesions, changes in foveal depression and development of intra- or subretinal fluid or space was documented.
Data analysis was performed using SPSS version 18.0 software (SPSS, Inc, Chicago, IL, USA) and P values < 0.05 were considered as significant. Analysis in each group was performed using the Wilcoxon test.
RESULTS
Macular Hole
Sixteen eyes from sixteen patients underwent pars plana vitrectomy for MH. One patient suffered from traumatic macular hole while the other fifteen had idiopathic MHs.
Qualitative findings
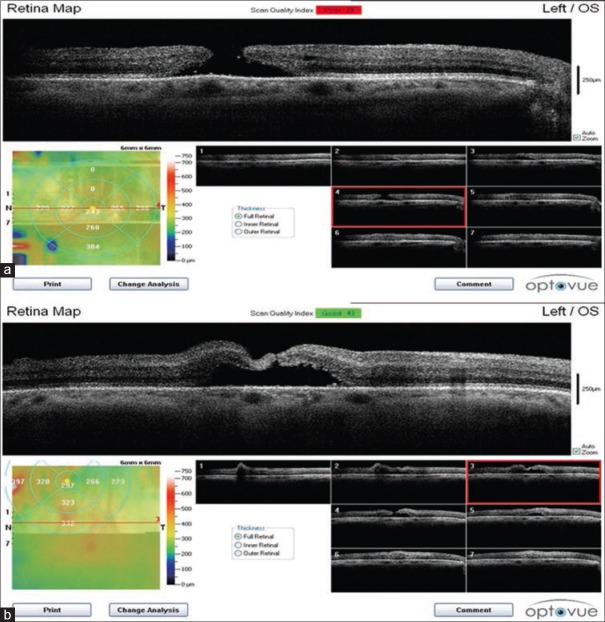
Triamcinolone is readily seen as hyper-reflective dots on SD-OCT while blood can be seen as a hyper-refletive area at the floor of the hole. There was no subretinal fluid during and after surgery [Figure 3].
Figure 3.
Spectral domain optical coherence tomography of macular hole. Triamcinolone is seen as hypereflective dots. Blood is seen as a hypereflective area at the floor of the hole.
Quantitative measures
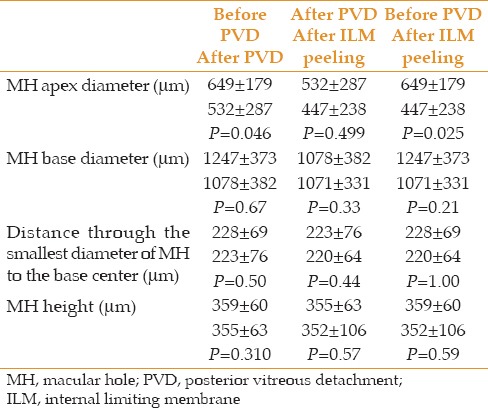
Table 1 summarizes quantitative changes. No significant change was noted in MH height during different stages of surgery and this parameter remained stable. In a similar manner, no significant change was noted in the distance from the smallest diameter of the hole to the base center during surgery. Also, the base diameter of MH remained stable during surgery. However, the apex diameter of MH showed a significant decrease after ILM peeling as compared to prior stages of surgery (P=0.025).
Table 1.
MH descriptive data and test statistics
Epiretinal Membrane
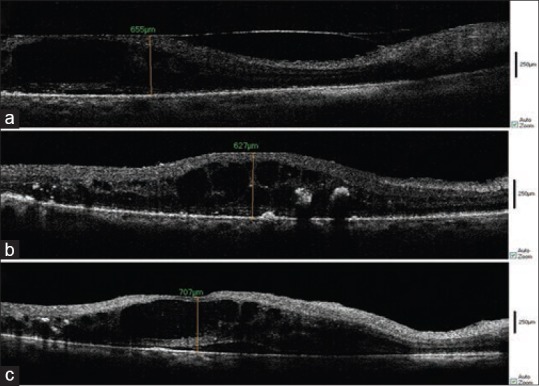
Ten eyes of ten patients underwent pars plana vitrectomy for ERM [Figure 4]. Eight of these patients suffered from diabetes mellitus.
Figure 4.
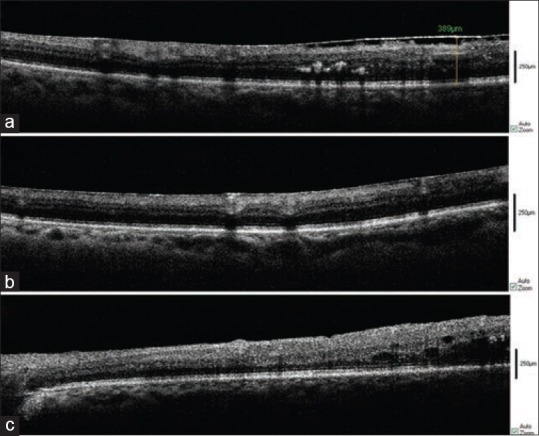
Intraoperative spectral domain optical coherence tomography (SD-OCT) of epiretinal membrane (ERM) in a diabetic patient. Before vitrectomy (a), after membrane removal (b) and after ILM peeling (c).
Qualitative findings
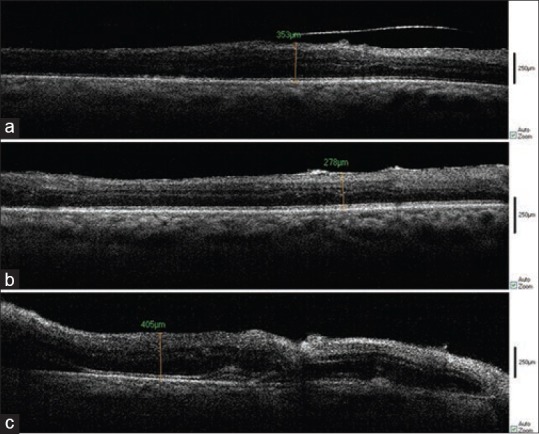
In three cases, following uncomplicated membrane removal or ILM peeling, subretinal fluid was created which appeared as a hyporeflective area on OCT images, an event which might be formed during surgery but was ignored by the surgeon [Figure 5].
Figure 5.
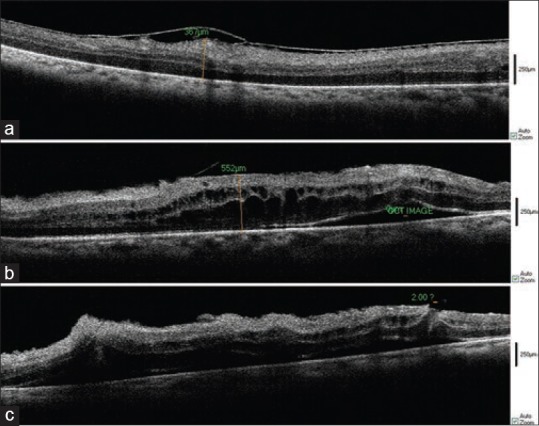
Intraoperative SD-OCT of a 50 year-old-patient with ERM. Before PVD creation (a), after membrane removal (b), after ILM peeling (c), Subretinal fluid formation is easily seen. A break is found on the left side of the image.
In one case, an unroofed retinal cyst was seen after membrane removal during surgery [Figure 6]; but in this case, shallow subretinal fluid was invisible to the surgeon. Residual ERM or ILM were obvious on OCT images in four eyes without the need for any staining.
Figure 6.
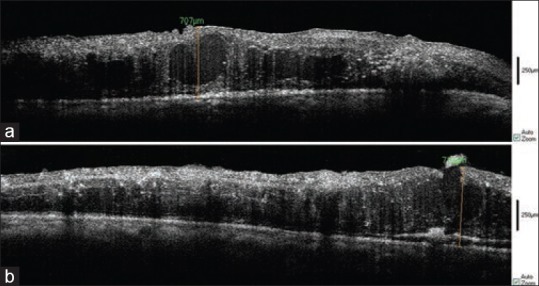
SD-OCT of ERM in a large retinal cyst readily seen before vitrectomy (a), after membranectomy, the cyst was unroofed and subretinal fluid was created (b).
Quantitative measures
Retinal thickness (from the ILM to RPE) was measured during different stages of surgery [Table 2]. Retinal thickness was increased after PVD creation but the difference was not statistically significant (P=0.2). Thickness was decreased significantly after membrane removal (430±99 µm) as compared to values before PVD induction (502±123 µm) (P=0.018). However, when we performed ILM peeling, retinal thickness was increased. Post ILM peeling, retinal thickness (449±155 µm) was comparable to primary thickness (before PVD creation) (P=0.8).
Table 2.
ERM and VMT descriptive data and test statistics
Vitreomacular Traction Syndrome
Six eyes of six patients underwent pars plana vitrectomy for VMT. Of these patients, five (83.3%) were diabetic.
Qualitative findings
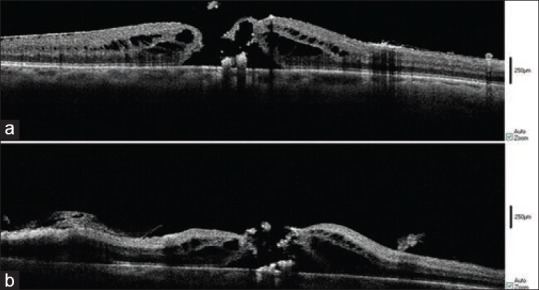
In two cases, subretinal fluid was detected intraoperatively [Figures 7 and 8] and in two eyes, there were significant ILM or ERM remnants after the operation.
Figure 7.
Intraoperative SD-OCT of a 73-year-old diabetic patient with VMT. After PVD induction (a), after membranectomy (b), after ILM peeling (c), Subretinal fluid formation which was ignored by the surgeon, was detected. No retinal break was found.
Figure 8.
Intra-operative SD-OCT of 65-year-old patient with VMT. After PVD induction (a), after membrane removal (b), after ILM peeling (c), Subretinal fluid formation is seen. No break was found.
Quantitative measures
Retinal thickness before PVD creation, after membrane removal and after ILM peeling was 621±235 µm, 549±119 µm and 466±131 µm, respectively [Table 2]. Although retinal thickness was decreased throughout consecutive stages of surgery, the differences were not significant.
Overall for all cases, in 5 of 32 operated eyes (15.65%), subretinal fluid was formed during surgery, which was ignored by the eye surgical microscope but was detected by intraoperative SD-OCT and further actions were taken. Based on intraoperative SD-OCT findings in six eyes there were significant remnants of ERM or ILM and in two eyes there was a small retinal.
Based on intraoperative OCT findings, surgery plans were changed and additional maneuvers were done to overcome these changes.
Four cases (12.5%) had low-quality images due to old corneal opacity, stromal corneal edema after phacoemulsification, posterior capsule opacity and being silicone-filled, and were thus not included in the final analysis.
DISCUSSION
SD-OCT has offered new insights into the pathogenesis, diagnosis and management of macular disease. However, dependency of the instrument on patient compliance and his/her ability to sit in an upright position has limited its use to pre- and post-operative clinical settings.[16] With the advent of intraoperative SD-OCT in 2009, a new era has begun in vitreoretinal surgery. Binder et al in their study, demonstrated the feasibility of this imaging technique and its influence on surgical decisions, and intraoperative judgments and techniques which could result in improved outcomes.[15] Since that time, few authors have investigated the role of intraoperative SD-OCT during macular surgeries.[11,17]
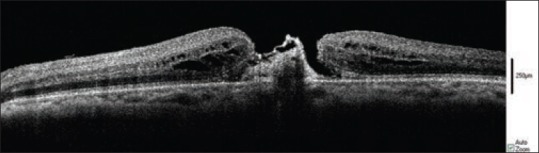
In our sixteen cases of macular hole, while we observed a decrease in MH apex diameter after ILM peeling while no significant change was noted in MH base diameter and height. These findings are in contrast to those reported by Ray et al who found an increase in MH base diameter and found no change in MH apex diameter.[14] They believe that the increase in the hyporeflective area at the MH base corresponds to a shallow neurosensory retinal detachment. The possible explanation of this discrepancy could be the nature of our sample mix which included fifteen eyes with idiopathic and only one eye with traumatic MH. In fact, the absence of ERM in the vast majority of our cases might have required less surgical manipulation and therefore the probability of subretinal fluid formation and retinal detachment was probably minimal in our series. Successful closure of the hole may be predicted by a decrease in macular hole dimensions on OCT images during surgery [Figure 9].[18]
Figure 9.
Closure of the macular hole apex after ILM peeling. Before ILM peeling (a), after ILM peeling (b).
The study by Ehlers et al showed that with different types of retinal abnormalities, intraoperative SD-OCT can detect subretinal fluid formations or ERM or ILM remnants that may go unrecognized by the surgeon during the operation. Therefore, this imaging modality helps to decide for additional membrane removal or ILM peeling without re-staining or altering the surgical procedure (e.g., gas tamponade) for addressing the subretinal fluid [Figure 10].[19,20]
Figure 10.
Intraoperative imaging of macular hole before ILM peeling. Triamcinolone dots are seen as hyper-reflective dots. A flap is seen at roof of the hole (a), The same patient after ILM peeling. The lifted edge of the ILM can be seen at the peripheral margin of the peel. A pinch is seen. Subretinal fluid is seen as a hyporeflective area (b).
In the present study, we performed concurrent intraoperative SD-OCT and pars plana vitrectomy for ten eyes with ERM. Although we noticed a significant decrease in retinal thickness after membrane removal, thickness was increased after ILM peeling despite a good retinal surface configuration. Hence, the difference between pre- and post-operative retinal thickness was not statistically significant. Ray et al detected an increase in retinal thickness less than 2% after ILM peeling. They also observed the appearance of a subretinal hyporeflective area consistent with shallow retinal detachment after ILM peeling. We noticed subretinal fluid formation in three of our cases.
The tighter the junction between ERM and the underlying retina, the greater the possibility of creation of subretinal fluid after surgical manipulation. We can observe this correlation in diabetics, especially eyes with a history of macular photocoagulation, as a subretinal hyporeflective area on intraoperative OCT images without any break or complication. It has been reported that patients with diabetic maculopathy have thickened ILM with abundant cellular proliferation on its vitreous side.[21] Since 80% of our patients in the ERM group were diabetic, these alterations in retinal thickness could be the result of histopathologic changes in this group.
We performed pars plana vitrectomy concurrent with intraoperative SD-OCT for six diabetic patients with VMT and observed an average decrease of 24.5% in retinal thickness. The difference was not statistically significant because of small sample size (5 cases). Intra-operative SD-OCT images showed small changes in retinal cysts [Figures 1 and 6].
Our study has some limitations. The most relevant is the use of a hand-held intraoperative SD-OCT device, which necessitated the surgeon to halt surgical maneuvers during imaging. Difficult registration of the images by the device is another limitation. Air or gas in the eye precluded imaging, so we could not see retinal anatomical changes after air-fluid exchange. Despite these limitations, intra-operative hand-held SD-OCT provided valuable additional information on retinal anatomy in macular disease and provided insight into microstructural changes during different steps of macular surgery. These observations may prove fruitful in improving the outcomes of surgery and eliminating the need for reoperations.
Financial Support and Sponsorship
Nil.
Conflicts of Interest
There are no conflicts of interest.
REFERENCES
- 1.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, et al. Optical coherence tomography. Science. 1991;254:1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Srinivasan VJ, Wojtkowski M, Witkin AJ, Duker JS, Ko TH, Carvalho M, et al. High-definition and 3-dimensional imaging of macular pathologies with high-speed ultrahigh-resolution optical coherence tomography. Ophthalmology. 2006;113:2054e1–14. doi: 10.1016/j.ophtha.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sano M, Shimoda Y, Hashimoto H, Kishi S. Restored photoreceptor outer segment and visual recovery after macular hole closure. Am J Ophthalmol. 2009;147:313–318.e1. doi: 10.1016/j.ajo.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 4.Oh J, Smiddy WE, Flynn HW, Jr, Gregori G, Lujan B. Photoreceptor inner/outer segment defect imaging by spectral domain OCT and visual prognosis after macular hole surgery. Invest Ophthalmol Vis Sci. 2010;51:1651–1658. doi: 10.1167/iovs.09-4420. [DOI] [PubMed] [Google Scholar]
- 5.Chalam KV, Murthy RK, Gupta SK, Brar VS, Grover S. Foveal structure defined by spectral domain optical coherence tomography correlates with visual function after macular hole surgery. Eur J Ophthalmol. 2010;20:572–577. doi: 10.1177/112067211002000306. [DOI] [PubMed] [Google Scholar]
- 6.Inoue M, Watanabe Y, Arakawa A, Sato S, Kobayashi S, Kadonosono K. Spectral-domain optical coherence tomography images of inner/outer segment junctions and macular hole surgery outcomes. Graefes Arch Clin Exp Ophthalmol. 2009;247:325–330. doi: 10.1007/s00417-008-0999-9. [DOI] [PubMed] [Google Scholar]
- 7.Chang LK, Koizumi H, Spaide RF. Disruption of the photoreceptor inner segment-outer segment junction in eyes with macular holes. Retina. 2008;28:969–975. doi: 10.1097/IAE.0b013e3181744165. [DOI] [PubMed] [Google Scholar]
- 8.Baba T, Yamamoto S, Arai M, Arai E, Sugawara T, Mitamura Y, et al. Correlation of visual recovery and presence of photoreceptor inner/outer segment junction in optical coherence images after successful macular hole repair. Retina. 2008;28:453–458. doi: 10.1097/IAE.0b013e3181571398. [DOI] [PubMed] [Google Scholar]
- 9.Arichika S, Hangai M, Yoshimura N. Correlation between thickening of the inner and outer retina and visual acuity in patients with epiretinal membrane. Retina. 2010;30:503–508. doi: 10.1097/IAE.0b013e3181bd2d65. [DOI] [PubMed] [Google Scholar]
- 10.Falkner-Radler CI, Glittenberg C, Hagen S, Benesch T, Binder S. Spectral-domain optical coherence tomography for monitoring epiretinal membrane surgery. Ophthalmology. 2010;117:798–805. doi: 10.1016/j.ophtha.2009.08.034. [DOI] [PubMed] [Google Scholar]
- 11.Ehlers JP, Kernstine K, Farsiu S, Sarin N, Maldonado R, Toth CA. Analysis of pars plana vitrectomy for optic pit-related maculopathy with intraoperative optical coherence tomography: a possible connection with the vitreous cavity. Arch Ophthalmol. 2011;129:1483–1486. doi: 10.1001/archophthalmol.2011.316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chavala SH, Farsiu S, Maldonado R, Wallace DK, Freedman SF, Toth CA. Insights into advanced retinopathy of prematurity using handheld spectral domain optical coherence tomography imaging. Ophthalmology. 2009;116:2448–2456. doi: 10.1016/j.ophtha.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dayani PN, Maldonado R, Farsiu S, Toth CA. Intraoperative use of handheld spectral domain optical coherence tomography imaging in macular surgery. Retina. 2009;29:1457–1468. doi: 10.1097/IAE.0b013e3181b266bc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ray R, Baranano DE, Fortun JA, Schwent BJ, Cribbs BE, Bergstrom CS, et al. Intraoperative microscope-mounted spectral domain optical coherence tomography for evaluation of retinal anatomy during macular surgery. Ophthalmology. 2011;118:2212–2217. doi: 10.1016/j.ophtha.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 15.Binder S, Falkner-Radler CI, Hauger C, Matz H, Glittenberg C. Feasibility of intrasurgical spectral-domain optical coherence tomography. Retina. 2011;31:1332–1336. doi: 10.1097/IAE.0b013e3182019c18. [DOI] [PubMed] [Google Scholar]
- 16.Wolf S, Wolf-Schnurrbusch U. Spectral-domain optical coherence tomography use in macular diseases: A review. Ophthalmologica. 2010;224:333–340. doi: 10.1159/000313814. [DOI] [PubMed] [Google Scholar]
- 17.Lee LB, Srivastava SK. Intraoperative spectral-domain optical coherence tomography during complex retinal detachment repair. Ophthalmic Surg Lasers Imaging. 2011;42:e71–e74. doi: 10.3928/15428877-20110804-05. Online. [DOI] [PubMed] [Google Scholar]
- 18.Hoerauf H. Predictive values in macular hole repair. Br J Ophthalmol. 2007;91:1415–1416. doi: 10.1136/bjo.2007.119594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ehlers JP, Tam T, Kaiser PK, Martin DF, Smith GM, Srivastava SK. Utility of intraoperative optical coherence tomography during vitrectomy surgery for vitreomacular traction syndrome. Retina. 2014;34:1341–1346. doi: 10.1097/IAE.0000000000000123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ehlers JP, Tao YK, Srivastava SK. The value of intraoperative optical coherence tomography imaging in vitreoretinal surgery. Curr Opin Ophthalmol. 2014;25:221–227. doi: 10.1097/ICU.0000000000000044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsunaga N, Ozeki H, Hirabayashi Y, Shimada S, Ogura Y. Histopathologic evaluation of the internal limiting membrane surgically excised from eyes with diabetic maculopathy. Retina. 2005;25:311–316. doi: 10.1097/00006982-200504000-00010. [DOI] [PubMed] [Google Scholar]