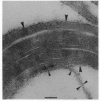
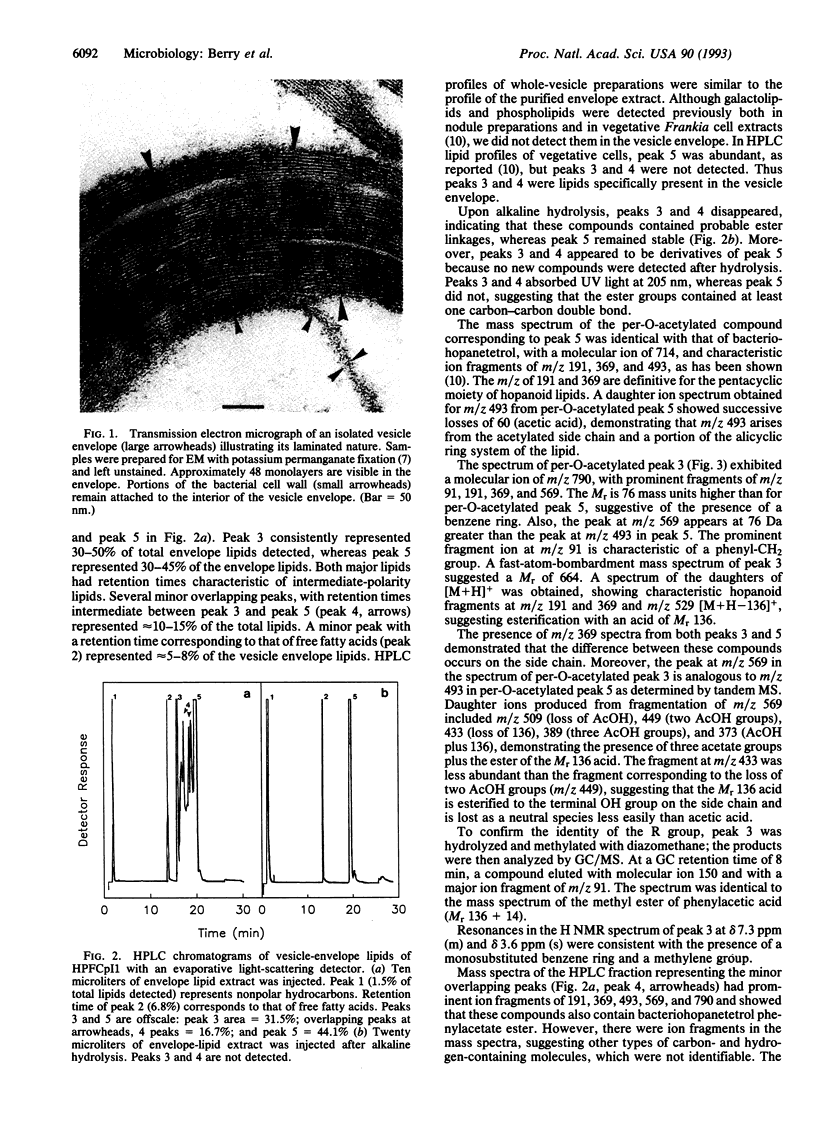
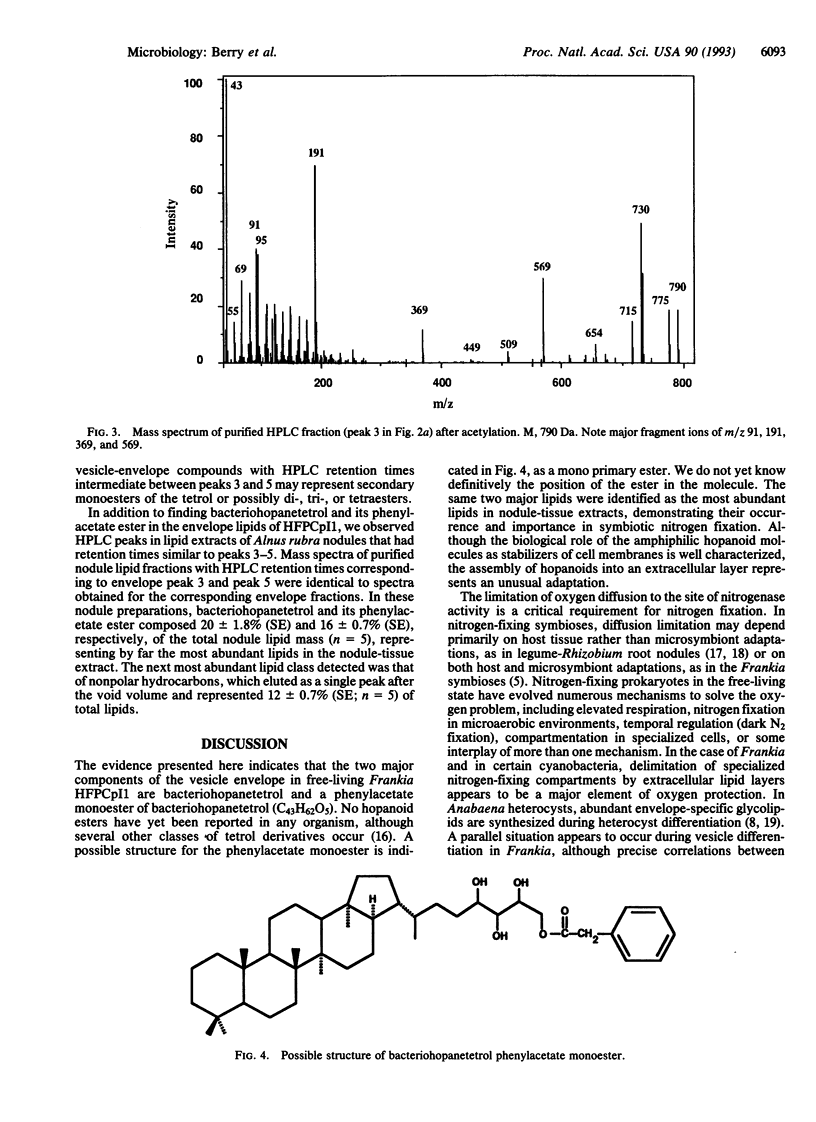
Abstract
Biological nitrogen fixation in aerobic organisms requires a mechanism for excluding oxygen from the site of nitrogenase activity. Oxygen exclusion in Frankia spp., members of an actinomycetal genus that forms nitrogen-fixing root-nodule symbioses in a wide range of woody Angiosperms, is accomplished within specialized structures termed vesicles, where nitrogen fixation is localized. The lipidic vesicle envelope is apparently a functional analogue of the cyanobacterial heterocyst envelope, forming an external gas-diffusion barrier around the nitrogen-fixing cells. We report here that purified vesicle envelopes consist primarily of two hopanoid lipids, rather than of glycolipids, as is the case in cyanobacteria. One envelope hopanoid, bacteriohopanetetrol phenylacetate monoester, is vesicle-specific. The Frankia vesicle envelope thus represents a layer specific to the locus of nitrogen fixation that is biosynthetically uniquely derived.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Berry A. M., Moreau R. A., Jones A. D. Bacteriohopanetetrol: abundant lipid in frankia cells and in nitrogen-fixing nodule tissue. Plant Physiol. 1991 Jan;95(1):111–115. doi: 10.1104/pp.95.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Callaham D., Deltredici P., Torrey J. G. Isolation and Cultivation in vitro of the Actinomycete Causing Root Nodulation in Comptonia. Science. 1978 Feb 24;199(4331):899–902. doi: 10.1126/science.199.4331.899. [DOI] [PubMed] [Google Scholar]
- Harriott O. T., Khairallah L., Benson D. R. Isolation and structure of the lipid envelopes from the nitrogen-fixing vesicles of Frankia sp. strain CpI1. J Bacteriol. 1991 Mar;173(6):2061–2067. doi: 10.1128/jb.173.6.2061-2067.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambein F., Wolk C. P. Structural studies on the glycolipids from the envelope of the heterocyst of Anabaena cylindrica. Biochemistry. 1973 Feb 27;12(5):791–798. doi: 10.1021/bi00729a002. [DOI] [PubMed] [Google Scholar]
- Murry M. A., Fontaine M. S., Tjepkema J. D. Oxygen protection of nitrogenase in Frankia sp. HFPArI3. Arch Microbiol. 1984 Oct;139(2-3):162–166. doi: 10.1007/BF00401993. [DOI] [PubMed] [Google Scholar]
- Murry M. A., Zhongze Z., Torrey J. G. Effect of O2 on vesicle formation, acetylene reduction, and O2-uptake kinetics in Frankia sp. HFPCcI3 isolated from Casuarina cunninghamiana. Can J Microbiol. 1985 Sep;31(9):804–809. doi: 10.1139/m85-151. [DOI] [PubMed] [Google Scholar]
- Parsons R., Silvester W. B., Harris S., Gruijters W. T., Bullivant S. Frankia vesicles provide inducible and absolute oxygen protection for nitrogenase. Plant Physiol. 1987 Apr;83(4):728–731. doi: 10.1104/pp.83.4.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tunlid A., Schultz N. A., Benson D. R., Steele D. B., White D. C. Differences in fatty acid composition between vegetative cells and N(2)-fixing vesicles of Frankia sp. strain CpI1. Proc Natl Acad Sci U S A. 1989 May;86(9):3399–3403. doi: 10.1073/pnas.86.9.3399. [DOI] [PMC free article] [PubMed] [Google Scholar]