Abstract
Introduction
Low-, intermediate- and high-risk categories have been defined to help guide the treatment of patients with NMIBC (Ta, T1, CIS). However, while low- and high-risk disease have been well-classified, the intermediate-risk (IR) category has traditionally comprised a heterogeneous group of patients that do not fit into either of these categories. As a result, many urologists remain uncertain about the categorization of patients as ‘intermediate-risk’ as well as the selection of the most appropriate therapeutic option for this patient population.
Purpose
To examine current literature and clinical practice guidelines on IR NMIBC and, based on this review, provide urologists with a better understanding of this heterogeneous risk group as well as practical recommendations for the management of IR patients.
Materials and Methods
The IBCG analyzed published clinical trials, meta-analyses and current clinical practice guidelines that examined IR NMIBC available as of September 2013. The definition of IR, patient outcomes and guideline recommendations were considered, as were the limitations of the available literature and additional parameters that may be useful in guiding treatment decisions in IR patients.
Results
Currently, definitions and management recommendations for IR NMIBC vary. The most simple and practical definition is that proposed by the IBCG and the AUA: multiple and/or recurrent low-grade Ta tumors. The IBCG proposes that the following factors be considered to aid in clinical decisions in IR disease: number (>1) and size (> 3cm) of tumors, timing (recurrence within 1 year) and frequency (> 1 per year) of recurrences, and previous treatment. In patients without these risk factors, a single, immediate instillation of chemotherapy is advised. In those with 1–2 risk factors, adjuvant intravesical therapy (intravesical chemotherapy or maintenance BCG) is recommended, and previous intravesical therapy should be considered when choosing between these adjuvant therapies. For those with 3–4 risk factors, maintenance BCG is recommended. It is also important that all IR patients are accurately risk stratified both at initial diagnosis and during subsequent follow-up. This requires an appropriate TURBT, vigilance to rule out CIS or other potential high-risk tumors, and review of histological material directly with the pathologist.
Conclusions
IR disease is a heterogeneous group and there is paucity of independent studies comparing therapies and outcomes in the subgroups of IR patients. The IBCG has proposed a management algorithm to assist in this regard that considers tumor characteristics, timing and frequency of recurrences and previous treatment. Subgroup analyses of the IR subjects in pivotal EORTC trials and meta-analyses will be important to validate the proposed algorithm and support clear evidence-based recommendations for the subgroups of IR patients.
Keywords: non-muscle invasive bladder cancer, definition, bacillus Calmette-Guérin, intravesical chemotherapy, intermediate-risk, low-grade Ta bladder carcinoma, recurrent tumors, multiple tumors
Introduction
NMIBC includes Ta, T1 tumors and CIS and is therefore, by definition, a heterogeneous disease with varying oncological outcomes. Recently, low-, intermediate- and high-risk categories have been defined that can be used to help predict prognosis and guide the treatment of patients with NMIBC. While low-risk (i.e., solitary, primary low-grade [G1] Ta) and high-risk (i.e., any T1, high-grade [G3] or CIS) disease have been well-defined using the TMN staging system and the 1973 and 2004 WHO grading classifications, the intermediate-risk (IR) category has traditionally comprised all patients not included in either of these categories. Hence, IR disease consists of a heterogeneous group of patients ranging from those with a solitary, but recurrent, low-grade Ta tumor to those with multiple, frequently recurrent, intravesical-treatment-refractory low-grade Ta tumors. Therefore, the IBCG has previously suggested that the IR category be subdivided into those with “low-intermediate-risk disease” and those with “high-intermediaterisk disease”.1,2
Given the heterogeneity of IR NMIBC, urologists are often uncertain about which patients fall into this risk category as well as the most appropriate intravesical treatment option (i.e., BCG or chemotherapy) for these patients. A recent on-line chart review involving 102 urologists and 971 patients with NMIBC from Europe and North America found that the treatment of IR NMIBC (defined as multiple or recurrent low-grade tumors; n=197) varied substantially; 24% of subjects were treated with TURBT alone, 42% with an immediate post-operative chemotherapeutic instillation, 29% with intravesical chemotherapy, 7% with BCG induction only, 11% with BCG induction plus maintenance, and 7% with “other” therapies (e.g., surveillance, intravesical EMDA with MMC, outpatient laser fulguration, etc.).3 Even current clinical practice guidelines vary with respect to recommended therapeutic options for IR patients, with some advising active surveillance and office fulguration4 and others recommending intravesical chemotherapy or maintenance BCG.5,6 The objectives of this review are to provide a better understanding of this heterogeneous risk group as well as practical recommendations for the management of IR disease based on the available literature.
Materials and Methods
A comprehensive Medline search was conducted to identify published clinical trials, systematic reviews, clinical practice guidelines and meta-analyses that examined IR NMIBC between 1980 and 2013. Keywords included bladder cancer, non-muscle invasive, intermediate-risk, low-grade Ta, G1–2, recurrent tumors, multiple tumors, BCG, intravesical chemotherapy, and TURBT. Reference lists of guidelines, meta-analyses and original papers were also reviewed to identify additional applicable literature.
The members of the IBCG (the authors) met on three occasions throughout 2012–2013 to critically review the identified literature and form consensus on practical recommendations for the management of IR NMIBC. Data were stratified based on the expert opinion of group members and articles were included if they focused primarily on IR disease (i.e., multiple or recurrent low-grade [G1–2] Ta tumors). Articles focusing specifically on low- (solitary, primary low-grade Ta tumors) or high-risk (T1, high-grade Ta, CIS) NMIBC were excluded. Recommendations provided are based on group consensus.
Results
Current Definitions and Treatment Outcomes/Recommendations in IR NMIBC
Clinical Practice Guidelines
Table 1 reviews the current definitions and treatment recommendations for IR NMIBC proposed by the EAU, AUA, ICUD, NCCN and IBCG.4–10 Note that the definitions used vary and, in some instances, are cumbersome for use in routine clinical practice (e.g., see EAU definition shown in Table 1). The simplest and most practical definition is that proposed by the IBCG and AUA: multiple or recurrent low-grade Ta tumors.7–9
Table 1.
| Guidelines | Definition of IR | Recommended Treatment Options |
|---|---|---|
| EAU (2013)5,6 | All tumors not defined as low-risk (primary, solitary, Ta, G1 [low grade], < 3 cm, no CIS) or high-risk (any of the following: T1 tumor, G3 [high grade] tumor, CIS, multiple and recurrent and large [> 3 cm] Ta G1–2 tumors [all conditions must be presented in this point]) |
|
| NCCN (2013)10 | No specific definition of IR disease provided; however, treatment recommendations for low-grade Ta tumors and post-treatment persistent or recurrent disease are provided (see right) |
|
| ICUD (2012)4 | No specific definition of IR disease provided; however, treatment recommendations for recurrent, low-grade tumors are provided (see right) |
|
| IBCG (2011)9 | Multiple or recurrent low-grade tumors |
|
| AUA (2007)7,8 | Multifocal and/or large volume, histologically confirmed, lowgrade Ta or recurrent low-grade Ta bladder cancer (high risk of recurrence, low risk of progression) |
|
Note: Please refer to respective guidelines for the specific categories of consensus or evidencebased grading systems used by each of the individual guideline panels.
Although most of the guidelines agree that adjuvant therapy with either BCG or chemotherapy is indicated in IR disease, the strength of this recommendation varies and controversy exists about whether BCG induction plus maintenance or induction alone should be utilized. The EAU recommends one immediate instillation of chemotherapy post TURBT followed by 1 year of full-dose BCG treatment, or by further chemotherapeutic instillations for a maximum of 1 year.5,6 Similar to the EAU guidelines, the IBCG recommends BCG induction plus maintenance or intravesical chemotherapy after complete TURBT. Recent evidence suggests that the effects of a single immediate chemotherapeutic instillation appear to be most pronounced in low-risk NMIBC, with no clear advantage in patients with recurrent or multiple tumors,11,12 or in those scheduled to receive further treatment with BCG.13
The AUA recommends an induction course of BCG or MMC for IR disease. Although maintenance BCG or MMC is considered optional in these patients, the AUA acknowledges that maintenance is more effective in decreasing recurrences than induction alone.7,8 The ICUD and the NCCN do not specifically define an IR category but do provide some guidance on recurrent, low-grade tumors (which are considered as IR by the EAU, AUA and IBCG).4,10 Unlike some of the other guideline groups, the ICUD considers observation and/or office fulguration as appropriate therapeutic strategies for recurrent low-grade Ta tumors, but emphasizes that these should not replace formal TURBT as primary treatment of initial tumors or for recurrences that are suspected to represent a change in tumor stage or grade.4 The ICUD also states that intravesical BCG could potentially be used in patients with recurrent, low-grade Ta disease who have not responded to intravesical chemotherapy. The NCCN recommends TURBT plus observation or a single post-operative chemotherapeutic dose, and/or induction intravesical chemotherapy in low-grade Ta tumors. For post-treatment recurrences, adjuvant intravesical therapy is recommended. 10
Major Clinical Trials and Meta-Analyses
Table 2 summarizes the definitions used in some of the more recent clinical trials and meta-analyses examining IR NMIBC as well as outcomes noted in these trials.14–19 Note that the definition of IR disease used in most of these trials is broad, i.e., any patient without primary, solitary, low-grade NMIBC (low-risk) or high-grade T1 or CIS (high-risk) disease. Other published trials have examined IR patients but have failed to provide a definition of what constituted “intermediate risk” and, therefore, have not been included in Table 2. Hendricksen et al., for example, compared a standard epirubicin treatment schedule (4 weekly and 5 monthly instillations) to this standard schedule plus either an early instillation or maintenance instillations in 731 patients with IR and high-risk NMIBC.20 The investigators failed to provide a specific definition of IR disease, but did note that the majority of patients had multiple (80%), Ta (79%), and G1–2 tumors (89%). At the 5-year follow-up, no significant difference in recurrence rates were noted between the treatment groups.
Table 2.
Definitions of IR NMIBC and treatment outcomes in major clinical trials, systematic reviews and meta-analyses14–19
| Study/Meta-Analysis | Definition of IR | % (n) of IR patients in study cohort | Outcomes |
|---|---|---|---|
| Bóhle et al.(2003)14 | TaT1, G1–2, multifocal, >3 cm diameter (i.e., all tumors not considered low-risk [single TaG1, ≤ 3 cm diameter] or highrisk [T1, G3, multifocal or highly recurrent, CIS]) | 72% (1984/2749) |
|
| Ojea et al. (2007) 15 | Stages TaG2 and T1G1–2 bladder tumors, without CIS | 100% (430) |
|
| Hinotsu et al., (2011) 16 | EORTC recurrence score of 1–9 (intermediate-risk) | 84% (97/115) |
|
| Malmstróm et al. (2009)17 | All tumors not low-risk (single, primary, Ta G1 tumors) or high-risk (G3 tumors or CIS) | 74% (1983/2820) |
|
| Sylvester et al. (2010) [EORTC 30911]18 | Neither T1 nor G3 tumors | 61% (497/837) |
|
| Oddens et al. (2013) [EORTC 30962]19 | Multiple pTa, G1–2 tumors | 58% (789/1355) |
|
As mentioned earlier, most current clinical practice guidelines agree that adjuvant therapy with either BCG or chemotherapy is necessary in IR NMIBC. Data from a number of recent trials (Table 2) suggest that BCG with maintenance is superior to maintenance chemotherapy in this population.17–19,21 The EORTC 30911 trial compared the long-term efficacy of six weekly intravesical instillations of epirubicin, BCG and BCG plus isoniazid followed by three weekly maintenance instillations of epirubicin or BCG at months 3, 6, 12, 18, 24, 30, and 36 after TURBT (SWOG maintenance schedule) in 837 patients with IR and high-risk NMIBC.18 Approximately 60% of patients included in this trial were classified as IR; patients with CIS were excluded from the study. After a median follow-up of 9.2 years, time to first recurrence, time to distant metastases, and disease-specific and overall survival were all significantly prolonged with BCG vs. epirubicin (see Table 2). Further analysis showed that the observed treatment benefits with maintenance BCG were larger in IR vs. high-risk patients (see Table 3).
Table 3.
EORTC 30911 results: comparison of epirubicin and BCG in the intermediate- and high-risk groups and the total study population18
| Intermediate risk | High risk | Total | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Events/patients (%) | HR (95% CI) | P value | Events/patients (%) | HR (95% CI) | P value | Events/patients (%) | HR (95% CI) | P value | |
|
| |||||||||
| Recurrence | |||||||||
| Epirubicin | 100/170 (58.8) | 1 | - | 46/104 (44.2) | 1 | - | 147/279 (52.7) | 1 | - |
| BCG | 131/327 (40.1) | 0.59 (0.45–0.76) | <0.001 | 82/219 (37.4) | 0.69 (0.48–1.05) | 0.09 | 213/558 (38.2) | 0.62 (0.50–0.76) | <0.001 |
|
| |||||||||
| Progression or distant metastases | |||||||||
| Epirubicin | 23/170 (13.5) | 1 | - | 16/104 (15.4) | 1 | - | 39/279 (14.0) | 1 | - |
| BCG | 17/327 (5.2) | 0.39 (0.21–0.73) | 0.002 | 34/219 (15.5) | 0.80 (0.44–1.45) | 0.70 | 51/558 (9.1) | 0.63 (0.41–0.95) | 0.031 |
|
| |||||||||
| Death: bladder cancer | |||||||||
| Epirubicin | 12/170 (7.1) | 1 | - | 7/104 (6.7) | 1 | - | 19/279 (6.8) | 1 | - |
| BCG | 8/327 (2.4) | 0.35 (0.14–0.86) | 0.020 | 11/219 (5.0) | 0.60 (0.23–1.56) | 0.39 | 19/558 (3.4) | 0.47 (0.25–0.89) | 0.026 |
Adapted from Sylvester RJ, et al., Eur Urol 2010.18
An IPD meta-analysis of 9 trials that included 2820 NMIBC patients (74% of which had IR disease) found a 32% reduction in the risk of recurrence with BCG maintenance (schedules ranged from 3 months to 2 years) vs. maintenance MMC (maintenance schedules varied from 3 months to 3 years) (p<0.0001) (see Table 2).17 Although no significant difference in progression and death were noted between treatment groups, the low number of these events in this primarily IR cohort precluded such an analysis.
A subgroup analysis of the prospective, randomized FinnBladder I study examined the long-term efficacy of maintenance BCG vs. maintenance MMC (both regimens involved five weekly instillations followed by monthly instillations for 2 years) in patients with frequently recurrent TaT1 tumors without CIS (67% of patients had Ta tumors and 60% PUNLMP or low-grade disease); median follow-up was 19.4 years.21 The recurrence rate was significantly lower in the BCG maintenance group (59%; 26 of 44 subjects) vs. the MMC group (80%; 36 of 45 subjects) (p=0.005). A trend towards fewer progressions and cancer-specific deaths was also observed in patients treated with BCG.
Recent evidence from the EORTC 30962 trial provides further guidance on the appropriate BCG maintenance schedule in IR patients.19 In this study, 1355 patients with IR and high-risk NMIBC were randomly assigned to full-dose BCG for 1 year, one-third dose BCG for 1 year, one-third dose BCG for 3 years, or full-dose BCG for 3 years. BCG maintenance instillations were administered as per the SWOG schedule used in EORTC 30911 and the primary endpoint was the duration of the disease-free interval. Full-dose BCG for 1-year was associated with the best outcomes in IR subjects, with no further improvement in outcomes noted when maintenance was continued to 3 years (Table 2).
Factors to Consider in IR NMIBC
To date, there are no independent studies comparing outcomes and treatment options for the heterogeneous spectrum of IR patients. Until such data becomes available, various factors (as outlined below) need to be considered to aid in clinical decisions in IR disease, including whether or not the patient truly belongs in this risk category, the number and size of tumors, the timing and frequency of recurrences, and previous treatment.
Although recent evidence suggests that novel molecular and genetic markers (e.g., FGFR3 and P53 tumor suppressor gene mutations, Ki-67 protein and CK20 expression, ezrin expression), may help improve the staging, prognosis, and selection of therapeutic options for patients with NMIBC,22 none of these markers are ready for integration into routine clinical practice and, therefore, they are not discussed in detail here. While factors such as age, comorbidities, socioeconomic status and gender need to be considered in all patients with NMIBC, these parameters generally do not influence risk classification and, hence, are also not reviewed in this manuscript.
Is it Really Low-Grade or IR Disease?
Accurate initial classification of IR disease is essential for ensuring optimal patient outcomes. TURBT is the standard for the initial diagnosis and treatment of all risk levels of NMIBC,5,6 and a complete procedure is required to achieve a good prognosis.23–25 It is also imperative that the presence or absence of CIS (a high-grade tumor) be properly determined at baseline in order to accurately risk stratify the patient. Overall, the frequency of CIS in patients with IR disease is likely low (<4%),26 but this may increase with current use of image enhancement technologies (e.g., PDD or NBI). A retrospective study of 289 patients with Ta bladder cancer who underwent multiple bladder biopsies found the incidence of CIS to be 6% in TaG1 and 9% in TaG2 tumors when using the 1973 WHO grading system. When using the 2004 WHO grading classification, the incidence of CIS was approximately 4% in low-grade Ta tumors.27
Urinary and bladder wash cytology as well bladder biopsies can aid in the diagnosis of CIS. Because cytology has a high sensitivity and specificity for the detection of high-grade urothelial carcinoma,5 the authors suggest that it be considered in all NMIBC patients pre-TURBT (including those with suspected low-grade disease) to help rule out high-risk disease and guide further testing.
If CIS is suspected because of abnormal cytology or previous history, PDD or NBI can aid in the detection of CIS.28–30 Two recent meta-analyses found that PDD using ultraviolet light after intravesical instillation of 5-ALA or HAL detected significantly more CIS cases and more tumor-positive patients than white-light cystoscopy alone.28,29 Another meta-analysis of eight randomized trials found NBI to provide higher diagnostic precision for the identification of CIS than white light cystoscopy.30
Appropriate risk stratification of IR NMIBC also relies on expert pathologic interpretation. Unfortunately, there exists inherent subjectivity in the interpretation of histopathological material (particularly in IR disease), with interobserver variability in staging and grading ranging between 50–60%.5 The 2004 WHO grading system, which categorizes tumors as either low- or high-grade, has been shown to reduce interobserver variability in grading interpretation compared to the WHO 1973 G1–G3 classification system.31,32 However, clinical practice guidelines recommend that tumors be graded using both the 1973 and 2004 WHO classifications until the 2004 system has been further validated.4–6,9 These guidelines also recommend close cooperation between urologists and pathologists, and suggest that slides be reviewed directly with the pathologist whenever possible.
Natural History of IR Disease
The natural history of IR disease is difficult to predict given the heterogeneity of tumors in this risk category. To the best of our knowledge, there are no studies comparing the disease course of patients with single, recurrent low-grade Ta tumors to that of patients with multiple, recurrent low-grade tumors. Nonetheless, risk tables developed by the EORTC and CUETO33,34 can assist urologists in predicting individual risks of tumor recurrence and progression in IR patients, and may help guide the clinician in deciding when intravesical chemotherapy or BCG may be warranted. According to the EORTC risk calculator, the 5-year probabilities of recurrence and progression are 46% and 6%, respectively, for a single, recurrent, low-grade Ta tumor compared to 78% and 17%, respectively, in frequent (> 1 per year) recurrences of multiple, large, low-grade Ta tumors.33 Given the higher risks of recurrence and progression in this latter group, therapy with BCG maintenance may be warranted in these patients. One of the major limitations of the EORTC risk tables is that they are based on a patient population treated predominantly with intravesical chemotherapy (rather than BCG); hence, these tables tend to overestimate the risk of recurrence and progression in patients treated with BCG. Recently, CUETO proposed a modified scoring system for BCG–treated patients that significantly decreases the probability of overestimation of recurrence and progression risk in this group of patients.34
Multiplicity and Tumor Size
Patients with multiple tumors may be at increased risk for poor outcomes because the chances of incomplete resection increase with the number of tumors. Furthermore, multifocality indicates the susceptibility of the entire urothelium to tumor development (i.e. the field effect).35
Several older studies have shown tumor multiplicity to be a significant predictor of recurrence in low-grade Ta tumors.36–38 In a multivariate analysis examining risk factors for recurrence and progression in 1529 patients with primary NMIBC (31% of this cohort had stage Ta, G1–2 disease), Millan-Rodrıguez et al. found that both recurrence and progression were nearly twice as high when > 1 tumor was present.38 Although not specific to patients with IR disease, a more recent retrospective analysis examining the clinical and pathologic data of 112 patients with primary NMIBC treated with TURBT and BCG found tumor multiplicity to be the only independent predictor of disease recurrence.39
A large tumor size increases the risk of undetected invasion of the lamina propria, especially in cases where no second resection has been performed.35 Evidence suggests that tumor size is an important predictive factor of recurrence and progression to muscle-invasive disease.37,38 In the multivariate analysis by Millan-Rodriguez et al., the risk of both tumor recurrence and progression was found to be 1.7 times higher for patients with tumors > 3 cm in diameter.38 Therefore, multiplicity and tumor size are factors suggestive of “higher-risk” IR NMIBC that may warrant BCG maintenance therapy. It is important to note that literature showing an association between tumor size and outcomes has focused on tumor diameters > 3 cm. However, in actual practice, it is uncommon to encounter tumors of this size in IR disease, unless the patient has been lost to follow-up. Thus, some experts have suggested that a tumor diameter > 1 cm be considered as a cut-off (rather than > 3 cm); however, this remains to be studied prospectively.
Timing and Frequency of Recurrences
Recurrence at 3-month cystoscopy has been found to be strongly correlated with later recurrences in older studies as well as more current studies.36,40–42 In a study of 414 patients with Ta G1–2 tumors, 80% were recurrence-free during the 5-year follow-up period if no recurrence occurred at the first 3-month follow-up cystoscopy; however, in subjects who experienced a recurrence at 3 months, only 10% were recurrence-free.41 Kurth et al. assessed factors affecting recurrence, progression and death from malignant disease in 576 patients with NMIBC from two EORTC studies.43 Multivariate analysis showed that previous recurrence rate, in addition to tumor size, tumor grade and positive 3-month cystoscopy, were the most powerful predictors of these three outcomes. A more recent CUETO analysis of 1062 patients with NMIBC treated with BCG found recurrence at 3-month cystoscopy to be highly associated with an increased risk of disease progression (HR=4.6).44
Findings from a number of studies suggest that a recurrence rate of >1 per year is also strongly associated with future recurrences, particularly in low-grade Ta disease.38,40,45,46 The combined analysis of individual patient data from 2596 NMIBC patients that was used to develop the EORTC risk tables found prior recurrence rate to be one of the most important prognostic factors for future recurrences (HR, 1.35, 95% CI, 1.24, 1.46; p<0.0001 for ≤1 recurrence/year vs. >1 recurrence/year).33 Given this evidence, early and frequent recurrences are suggestive of “higher risk” IR disease that may also warrant more aggressive treatment.
Previous Treatment
Another important consideration in IR NMIBC is the patient’s previous treatment regimen. Although most of the evidence examining outcomes in NMIBC patients who recur following intravesical therapy tends to be focused on high-risk patients, some evidence exists to suggest that BCG may be superior to chemotherapy for the management of treatment failures in IR patients as well. A randomized trial of 261 patients with NMIBC (89 with multiple, recurrent Ta/T1 G1–2 disease without CIS) found that, at 5-year follow-up, crossover treatment was successful in 39% of patients receiving second-line BCG compared to 19% receiving second-line MMC.47 Another randomized trial comparing intravesical BCG and MMC with planned cross-over following failure of initial therapy in patients with low-grade, recurrent Ta or T1 disease found that 32% (19/39) of subjects who failed MMC and received rescue BCG remained disease-free at follow-up compared to 19% (4/21) of those receiving MMC following BCG failure.48 In addition, the Malmström et al. IPD meta-analysis that included a predominantly IR cohort found that BCG maintenance was not only more effective than MMC in reducing tumors recurrences in chemotherapy-naive subjects, but also in those previously treated with intravesical chemotherapy (p=0.03).17
The IBCG thus recommends that for the appropriate management of recurrences or treatment failures in IR disease, the previous treatment received by the patient should be considered (i.e., intravesical chemotherapy or BCG).9 For IR patients failing chemotherapy, the IBCG recommends TURBT plus BCG induction plus maintenance (ideally the SWOG 3-week maintenance protocol); an alternative intravesical chemotherapy may also be considered. For BCG failures, TURBT plus risk stratification is key, and either continued BCG with maintenance or radical cystectomy are recommended; an alternative intravesical therapy49 (e.g., chemotherapy such as gemcitabine or combination with interferon) or inclusion in a clinical trial may also be considered. Although gemcitabine, thermochemotherapy and combination therapy with interferon have been shown to be effective in these patients, additional studies are needed before these therapies can be routinely recommended for BCG failures.49
Classification of IR Disease and Recommendations for Management
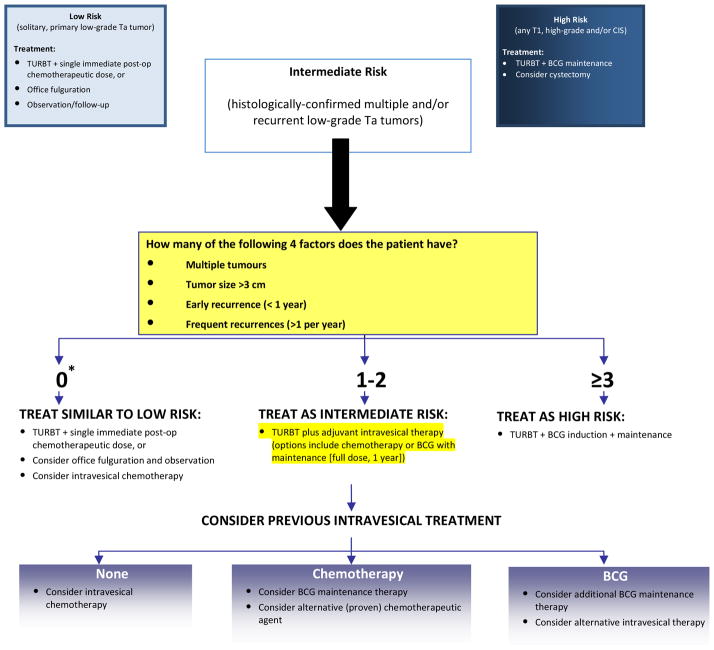
Based on the evidence and clinical practice guidelines reviewed, the IBCG has developed the algorithm in Figure 1 to assist urologists in better stratifying IR patients into those at higher risk for disease recurrence/progression who may benefit from BCG maintenance therapy vs. those at lower risk in whom intravesical chemotherapy may be sufficient. The authors would like to emphasize that this algorithm is based on expert opinion. As mentioned earlier, there is a paucity of independent clinical trials comparing outcomes and treatment options for the subgroups of IR patients; subgroup analyses of IR cohorts from key trials and meta-analyses will be needed for validation of the recommendations proposed in this algorithm.
Figure 1. Algorithm for the management of IR NMIBC.
Note: The recommendations provided in this algorithm have been simplified for ease of use and will need to be customized to each individual patient, taking into account the patient’s diagnosis, histology, age, previous history, and his/her overall condition. For example, a 75-year-old male with numerous comorbidities who experiences two small (< 1 cm) low-grade recurrences more than 1-year after initial therapy, may be a candidate for office fulguration and observation rather than BCG maintenance or intravesical chemotherapy as suggested in this algorithm.
*A score of 0 refers to a solitary, recurrent (> 1 year) low-grade tumor
The definition of IR used in this algorithm is that originally proposed by the IBCG and AUA7–9: multiple or recurrent low-grade Ta tumors. The group believes this to be a simple and practical definition for use in routine clinical practice.
Choice of therapy for patients with IR disease needs to be considered on a case-by-case basis. To guide treatment decisions, the algorithm takes into account the key factors discussed previously: tumor size; tumor multiplicity; timing and frequency of recurrences; and previous treatment. Patients with none of these factors (i.e., those with a solitary, late [>1 year] lowgrade recurrence) are at low-risk of disease recurrence and progression and, therefore, can be treated similar to low-risk patients (i.e., TURBT plus a single, immediate chemotherapeutic instillation or, in select cases, even office fulguration and observation). For those with 1–2 factors, both intravesical chemotherapy and BCG maintenance (SWOG 3-week protocol; full-dose for 1 year) are appropriate options. As discussed previously, the patient’s previous treatment regime should be considered to help determine the relative advantages of BCG vs. chemotherapy in these patients. IR patients with 3 or more factors are at the highest risk of recurrence and progression based on both the CUETO and EORTC risk tables33,34 and, therefore, these subjects would likely benefit most from BCG maintenance therapy. Some urologists may be reluctant to use BCG, particularly maintenance therapy, in patients with IR disease due to side effects that have been associated with BCG use in the past. However, recent results from the EORTC 30962 trial found no significant differences in toxicity according to dose or duration of BCG treatment; 7.6% of patients receiving one-third dose BCG stopped treatment for toxicity vs. 8.0% who received full-dose BCG; 7.1% of subjects randomized to 1 year of maintenance stopped BCG due to adverse events compared to 8.6% randomized to 3 years of maintenance.50 Therefore, the additional 2 years of maintenance were not associated with an appreciable increase in toxicity. These results are better than those observed in previous BCG trials and are likely associated with improvements in BCG administration practices and increased knowledge on the prevention and management of BCG-associated adverse events.
Upon each tumor recurrence, risk category should be reconsidered. For example, IR patients who recur with a high-grade or T1 tumor, or CIS, should be reclassified as high-risk. Also, high-risk patients who experience a low-grade recurrence following treatment are not considered IR, but continue to be high-risk.
Conclusions
While low- and high-risk NMIBC have been well-defined, the IR group has traditionally comprised all patients excluded from either of these categories. Currently, definitions of IR NMIBC in both the available literature and in clinical practice guidelines vary, as do treatment recommendations for these patients. Similar to the AUA, the IBCG defines IR NMIBC as multiple or recurrent low-grade Ta tumors. The group recommends that a number of factors be considered to aid in clinical decisions in IR disease, including: the number (> 1) and size (> 3cm) of tumors, the timing (recurrence within 1 year) and frequency (> 1 per year) of recurrences, and previous treatment (intravesical BCG or chemotherapy). Patients with none of these factors can be treated similar to low-risk patients (i.e., TURBT plus a single, immediate chemotherapeutic instillation or, in select cases, office fulguration and observation), while those with 3 or more factors should be treated as high-risk (i.e., TURBT plus BCG with maintenance). For those with 1–2 factors, both intravesical chemotherapy and BCG maintenance are appropriate options, and the patient’s previous treatment regime should be considered to help guide choice of intravesical therapy.
The IBCG further acknowledges that a correct initial diagnosis and accurate risk stratification are essential for guiding management decisions and ensuring an optimal prognosis in IR subjects. Therefore, a complete TURBT is critical, with appropriate vigilance towards ruling out high-grade disease (including CIS) with the use of urinary cytology, PDD, NBI or random biopsies, as appropriate. Clinicians should also review histological material directly with pathologists whenever possible.
Acknowledgments
The authors thank Julie Tasso and Sandra Steele from Complete Medical Communications, whose administrative and editorial support were made possible through an unrestricted educational grant from Sanofi Pasteur.
Abbreviations and Acronyms
- AUA
American Urological Association
- 5-ALA
5-aminolevulinic acid
- BCG
bacillus Calmette-Guérin
- CI
confidence interval
- CIS
carcinoma in situ
- CUETO
Club Urologico Espanol de Tratamiento Oncologico
- EAU
European Association of Urology
- EMDA
electromotive drug administration
- EORTC
European Organization for the Research and Treatment of Cancer
- FGFR3
fibroblast growth factor receptor 3
- HAL
hexylaminolevulinate
- HR
hazard ratio
- IBCG
International Bladder Cancer Group
- ICUD
International Consultation on Urological Diseases
- IR
intermediate-risk
- MMC
mitomycin C
- NBI
narrow band imaging
- NCCN
National Comprehensive Cancer Network
- NMIBC
non-muscle invasive bladder cancer
- OR
odds ratio
- PDD
photodynamic diagnosis
- PUNLMP
papillary urothelial neoplasm of low malignant potential
- RFS
recurrence-free survival
- TURBT
transurethral resection of the bladder tumor
- WHO
World Health Organization
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lamm D, Persad R, Colombel M, et al. Maintenance bacillus Calmette-Guérin: the standard of care for the prophylaxis and management of intermediate- and high-risk non-muscle-invasive bladder cancer. Eur Urol Suppl. 2010;9:715. [Google Scholar]
- 2.Brausi MA. Challenging the EAU guidelines on non–muscle-invasive bladder cancer (NMIBC): single instillation of chemotherapy after transurethral resection of NMIBC and chemotherapy versus bacillus Calmette-Guérin in treatment of intermediate-risk tumours. Eur Urol Suppl. 2010;9:406. [Google Scholar]
- 3.Witjes JA, Palou J, Soloway M, et al. Current clinical practice gaps in the treatment of intermediate- and high-risk non-muscle-invasive bladder cancer (NMIBC) with emphasis on the use of bacillus Calmette-Guérin (BCG): results of an international individual patient data survey (IPDS) BJU Int. 2013;112:742. doi: 10.1111/bju.12012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Konety B, Oosterlinck W, Chang S, et al. Low-grade Ta urothelial carcinoma of the bladder. In: Soloway M, Khoury S, editors. Bladder Cancer. 2. Vienna: ICUD-EAU; 2012. pp. 231–246. [Google Scholar]
- 5.Babjuk M, Burger M, Zigeuner R, et al. Guidelines on Non-Muscle-Invasive Bladder Cancer (TaT1 and CIS) Arnhem, the Netherlands: European Association of Urology; 2013. [Accessed July 24, 2013]. Available at: http://www.uroweb.org/gls/pdf/05_TaT1_Bladder_Cancer_LR.pdf. [Google Scholar]
- 6.Babjuk M, Burger M, Zigeuner R, et al. EAU guidelines on non–muscle-invasive urothelial carcinoma of the bladder: update 2013. Eur Urol. 2013;64:639. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Hall MC, Chang SS, Dalbagni G, et al. Guideline for the Management of Nonmuscle Invasive Bladder Cancer (Stages Ta,T1, and Tis): 2007 update. Linthicum, Maryland: American Urological Association; 2007. [Accessed August 28, 2013]. Available at http://www.auanet.org/education/guidelines/bladder-cancer.cfm. [Google Scholar]
- 8.Hall MC, Chang SS, Dalbagni G, et al. Guidelines for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178:2314. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]
- 9.Brausi M, Witjes JA, Lamm D, et al. A review of current guidelines and best practice recommendations for the management of nonmuscle invasive bladder cancer by the International Bladder Cancer Group. J Urol. 2011;186:2158. doi: 10.1016/j.juro.2011.07.076. [DOI] [PubMed] [Google Scholar]
- 10.National Comprehensive Cancer Network. Clinical Practice Guidelines in Oncology: Bladder Cancer. Version 1. Jenkintown, Pennsylvania: National Comprehensive Cancer Network; 2014. [Accessed January 22, 2014]. Available at http://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf. [Google Scholar]
- 11.Gudjónsson S, Adell L, Merdasa F, et al. Should all patients with non-muscle-invasive bladder cancer receive early intravesical chemotherapy after transurethral resection? The results of a prospective randomised multicentre study. Eur Urol. 2009;55:773. doi: 10.1016/j.eururo.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 12.Dobruch J, Herr H. Should all patients receive single chemotherapeutic agent instillation after bladder tumour resection? BJU Int. 2009;104:170. doi: 10.1111/j.1464-410X.2009.08654.x. [DOI] [PubMed] [Google Scholar]
- 13.Cai T, Nesi G, Tinacci G, et al. Can early single dose instillation of epirubicin improve bacillus Calmette-Guerin efficacy in patients with nonmuscle invasive high risk bladder cancer? Results from a prospective, randomized, double-blind controlled study. J Urol. 2008;180:110. doi: 10.1016/j.juro.2008.03.038. [DOI] [PubMed] [Google Scholar]
- 14.Böhle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guérin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169:90. doi: 10.1016/S0022-5347(05)64043-8. [DOI] [PubMed] [Google Scholar]
- 15.Ojea A, Nogueira JL, Solsona E, et al. A multicentre, randomised prospective trial comparing three intravesical adjuvant therapies for intermediate-risk superficial bladder cancer: low-dose bacillus Calmette-Guérin (27 mg) versus very low-dose bacillus Calmette-Guérin (13. 5 mg) versus mitomycin C. Eur Urol. 2007;52:1398. doi: 10.1016/j.eururo.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 16.Hinotsu S, Akaza H, Naito S, et al. Maintenance therapy with bacillus Calmette-Guérin Connaught strain clearly prolongs recurrence-free survival following transurethral resection of bladder tumor for non-muscle-invasive cancer. BJU Int. 2011;108:187. doi: 10.1111/j.1464-410X.2010.09891.x. [DOI] [PubMed] [Google Scholar]
- 17.Malmström PU, Sylvester RJ, Crawford DE, et al. An individual patient data meta-analysis of the long-term outcome of randomised studies comparing intravesical mitomycin C versus bacillus Calmette-Guérin for non-muscle-invasive bladder cancer. Eur Urol. 2009;56:247. doi: 10.1016/j.eururo.2009.04.038. [DOI] [PubMed] [Google Scholar]
- 18.Sylvester RJ, Brausi MA, Kirkels WJ, et al. Long-term efficacy results of EORTC Genito-Urinary Group randomized phase 3 study 30911 comparing intravesical instillations of epirubicin, bacillus Calmette-Guérin and bacillus Calmette-Guérin plus isoniazid in patients with intermediate- and high-risk stage Ta T1 urothelial carcinoma of the bladder. Eur Urol. 2010;57:766. doi: 10.1016/j.eururo.2009.12.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Oddens J, Brausi M, Sylvester R, et al. Final results of an EORTC-GU Cancer Group randomized study of maintenance bacillus Calmette-Guérin in intermediate- and high-risk Ta, T1 papillary carcinoma of the urinary bladder: one-third dose versus full dose and 1 year versus 3 years of maintenance. Eur Urol. 2013;63:462. doi: 10.1016/j.eururo.2012.10.039. [DOI] [PubMed] [Google Scholar]
- 20.Hendricksen K, Witjes WP, Idema JG, et al. Comparison of three schedules of intravesical epirubicin in patients with non-muscle-invasive bladder cancer. Eur Urol. 2008;53:984. doi: 10.1016/j.eururo.2007.12.033. [DOI] [PubMed] [Google Scholar]
- 21.Järvinen R, Kaasinen E, Sankila A, Rintala E. Long-term efficacy of maintenance bacillus Calmette-Guérin versus maintenance mitomycin C instillation therapy in frequently recurrent TaT1 tumours without carcinoma in situ: a subgroup analysis of the prospective, randomised FinnBladder I study with a 20-year follow-up. Eur Urol. 2009;56:260. doi: 10.1016/j.eururo.2009.04.009. [DOI] [PubMed] [Google Scholar]
- 22.Kamat AM, Hegarty PK, Gee JR, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: screening, diagnosis, and molecular markers. Eur Urol. 2013;63:4. doi: 10.1016/j.eururo.2012.09.057. [DOI] [PubMed] [Google Scholar]
- 23.Brausi M, Collette L, Kurth K, et al. Variability in the recurrence rate at first follow-up cystoscopy after TUR in stage Ta T1 transitional cell carcinoma of the bladder: a combined analysis of seven EORTC studies. Eur Urol. 2002;41:523. doi: 10.1016/s0302-2838(02)00068-4. [DOI] [PubMed] [Google Scholar]
- 24.Mariappan P, Zachou A, Grigor KM, et al. Detrusor muscle in the first, apparently complete transurethral resection of bladder tumour specimen is a surrogate marker of resection quality, predicts risk of early recurrence, and is dependent on operator experience. Eur Urol. 2010;57:843. doi: 10.1016/j.eururo.2009.05.047. [DOI] [PubMed] [Google Scholar]
- 25.Mariappan P, Finney SM, Head E, et al. Good quality white-light transurethral resection of bladder tumours (GQ-WLTURBT) with experienced surgeons performing complete resections and obtaining detrusor muscle reduces early recurrence in new non-muscle-invasive bladder cancer: validation across time and place and recommendation for benchmarking. BJU Int. 2012;109:1666. doi: 10.1111/j.1464-410X.2011.10571.x. [DOI] [PubMed] [Google Scholar]
- 26.van der Meijden A, Oosterlinck W, Brausi M, et al. Significance of bladder biopsies in Ta,T1 bladder tumors: a report from the EORTC Genito-Urinary Tract Cancer Cooperative Group. EORTC-GU Group Superficial Bladder Committee. Eur Urol. 1999;35:267. doi: 10.1159/000019859. [DOI] [PubMed] [Google Scholar]
- 27.Sos L, Palou J, Huguet J, et al. Comparison of WHO 1973 to WHO 2004 grading system in bladder cancer related to association to CIS, recurrence and progression in Ta tumours. Eur Urol Suppl. 2009;8:289, abstract no. 674. [Google Scholar]
- 28.Burger M, Grossman HB, Droller M, et al. Photodynamic diagnosis of non-muscle-invasive bladder cancer with hexaminolevulinate cystoscopy: a meta-analysis of detection and recurrence based on raw data. Eur Urol. 2013;64:846. doi: 10.1016/j.eururo.2013.03.059. [DOI] [PubMed] [Google Scholar]
- 29.Kausch I, Sommerauer M, Montorsi F, et al. Photodynamic diagnosis in non-muscle-invasive bladder cancer: a systematic review and cumulative analysis of prospective studies. Eur Urol. 2010;57:595. doi: 10.1016/j.eururo.2009.11.041. [DOI] [PubMed] [Google Scholar]
- 30.Zheng C, Lv Y, Zhong Q, Wang R, Jiang Q. Narrow band imaging diagnosis of bladder cancer: systematic review and meta-analysis. BJU Int. 2012;110:E680. doi: 10.1111/j.1464-410X.2012.11500.x. [DOI] [PubMed] [Google Scholar]
- 31.May M, Brookman-Amissah S, Roigas J, et al. Prognostic accuracy of individual uropathologists in noninvasive urinary bladder carcinoma: a multicentre study comparing the 1973 and 2004 World Health Organisation classifications. Eur Urol. 2010;57:850. doi: 10.1016/j.eururo.2009.03.052. [DOI] [PubMed] [Google Scholar]
- 32.Burger M, van der Aa MN, van Oers JM, et al. Prediction of progression of non muscle-invasive bladder cancer by WHO 1973 and 2004 grading and by FGFR3 mutation status: a prospective study. Eur Urol. 2008;54:835. doi: 10.1016/j.eururo.2007.12.026. [DOI] [PubMed] [Google Scholar]
- 33.Sylvester RJ, van der Meijden AP, Oosterlinck W, et al. Predicting recurrence and progression in individual patients with stage Ta T1 bladder cancer using EORTC risk tables: a combined analysis of 2596 patients from seven EORTC trials. Eur Urol. 2006;49:466. doi: 10.1016/j.eururo.2005.12.031. [DOI] [PubMed] [Google Scholar]
- 34.Fernandez-Gomez J, Madero R, Solsona E, et al. The EORTC tables overestimate the risk of recurrence and progression in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guérin: external validation of the EORTC risk tables. Eur Urol. 2011;60:423. doi: 10.1016/j.eururo.2011.05.033. [DOI] [PubMed] [Google Scholar]
- 35.Thalmann GN, Birkhäuser F, Roth B. Management of pT1G3 bladder cancer. Eur Urol Suppl. 2011;10:e1. [Google Scholar]
- 36.Parmar MK, Freedman LS, Hargreave TB, et al. Prognostic factors for recurrence and followup policies in the treatment of superficial bladder cancer: report from the British Medical Research Council Subgroup on Superficial Bladder Cancer (Urological Cancer Working Party) J Urol. 1989;142(2 Pt 1):284. doi: 10.1016/s0022-5347(17)38731-1. [DOI] [PubMed] [Google Scholar]
- 37.Heney NM, Ahmed S, Flanagan MJ, et al. Superficial bladder cancer: progression and recurrence. J Urol. 1983;130:1083. doi: 10.1016/s0022-5347(17)51695-x. [DOI] [PubMed] [Google Scholar]
- 38.Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri, et al. Multivariate analysis of the prognostic factors of primary superficial bladder cancer. J Urol. 2000;163:73. doi: 10.1016/s0022-5347(05)67975-x. [DOI] [PubMed] [Google Scholar]
- 39.Ajili F, Manai M, Darouiche A, et al. Tumor multiplicity is an independent prognostic factor of non-muscle-invasive bladder cancer treated with Bacillus Calmette-Guerin immunotherapy. Ultrastruct Pathol. 2012;36:320. doi: 10.3109/01913123.2012.681833. [DOI] [PubMed] [Google Scholar]
- 40.Larsson P, Wijkström H, Thorstenson A, et al. A population-based study of 538 patients with newly detected urinary bladder neoplasms followed during 5 years. Scand J Urol Nephrol. 2003;37:195. doi: 10.1080/00365590310008037. [DOI] [PubMed] [Google Scholar]
- 41.Fitzpatrick JM, West AB, Butler MR, et al. Superficial bladder tumors (stage pTa, grades 1 and 2): the importance of recurrence pattern following initial resection. J Urol. 1986;135:920. doi: 10.1016/s0022-5347(17)45923-4. [DOI] [PubMed] [Google Scholar]
- 42.Ali-El-Dein B, Sarhan O, Hinev A, et al. Superficial bladder tumours: analysis of prognostic factors and construction of a predictive index. BJU Int. 2003;92:393. doi: 10.1046/j.1464-410x.2003.04360.x. [DOI] [PubMed] [Google Scholar]
- 43.Kurth KH, Denis L, Bouffioux C, et al. Factors affecting recurrence and progression in superficial bladder tumours. Eur J Cancer. 1995;31A:1840. doi: 10.1016/0959-8049(95)00287-s. [DOI] [PubMed] [Google Scholar]
- 44.Fernandez-Gomez J, Solsona E, Unda M, et al. Prognostic factors in patients with non-muscle-invasive bladder cancer treated with bacillus Calmette-Guérin: multivariate analysis of data from four randomized CUETO trials. Eur Urol. 2008;53:992. doi: 10.1016/j.eururo.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 45.Oosterlinck W, Solsona E, Akaza H, et al. Low-grade Ta (noninvasive) urothelial carcinoma of the bladder. Urology. 2005;66(Suppl 6A):75. doi: 10.1016/j.urology.2005.07.063. [DOI] [PubMed] [Google Scholar]
- 46.Millán-Rodríguez F, Chéchile-Toniolo G, Salvador-Bayarri J, et al. Primary superficial bladder cancer risk groups according to progression, mortality and recurrence. J Urol. 2000;164:680. doi: 10.1016/s0022-5347(05)67280-1. [DOI] [PubMed] [Google Scholar]
- 47.Malmström P-U, Wijkström H, Lundholm C, et al. 5-year followup of a randomized prospective study comparing mitomycin C and bacillus Calmette-Guérin in patients with superficial bladder carcinoma. Swedish-Norwegian Bladder Cancer Study Group. J Urol. 1999;161:1124. [PubMed] [Google Scholar]
- 48.Mangiarotti B, Trinchieri A, Del Nero A, Montanari E. A randomized prospective study of intravesical prophylaxis in non-muscle invasive bladder cancer at intermediate risk of recurrence: mitomycin chemotherapy vs BCG immunotherapy. Arch Ital Urol Androl. 2008;80:167. [PubMed] [Google Scholar]
- 49.Burger M, Oosterlinck W, Konety B, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: non-muscle-invasive urothelial carcinoma of the bladder. Eur Urol. 2013;63:36. doi: 10.1016/j.eururo.2012.08.061. [DOI] [PubMed] [Google Scholar]
- 50.Brausi M, Oddens J, Sylvester R, et al. Side effects of bacillus Calmette-Guérin (BCG) in the treatment of intermediate- and high-risk Ta, T1 papillary carcinoma of the bladder: results of the EORTC Genito-Urinary Cancers Group randomised phase 3 study comparing one-third dose with full dose and 1 year with 3 years of maintenance BCG. Eur Urol. 2014;65:69. doi: 10.1016/j.eururo.2013.07.021. [DOI] [PubMed] [Google Scholar]