Abstract
Consensus practice guidelines and the implementation of clinical therapeutic advances are usually based on the results of large, randomized clinical trials (RCTs). However, RCTs generally inform us on an average treatment effect for a presumably homogeneous population, but therapeutic interventions rarely benefit the entire population targeted. Indeed, multiple RCTs have demonstrated that interindividual variability exists both in drug response and in the development of adverse effects. The field of pharmacogenomics promises to deliver the right drug to the right patient. Substantial progress has been made in this field, with advances in technology, statistical and computational methods, and the use of cell and animal model systems. However, clinical implementation of pharmacogenetic principles has been difficult because RCTs demonstrating benefit are lacking. For patients, the potential benefits of performing such trials include the individualization of therapy to maximize efficacy and minimize adverse effects. These trials would also enable investigators to reduce sample size and hence contain costs for trial sponsors. Multiple ethical, legal, and practical issues need to be considered for the conduct of genotype-based RCTs. Whether pre-emptive genotyping embedded in electronic health records will preclude the need for performing genotype-based RCTs remains to be seen.
Introduction
The aim of individualized medicine is to deliver the right drug to the right patient. Pharmacogenomics is a critical component of individualized medicine, and is the study of the role of inheritance in individual variation in drug response.1,2 The clinical goals of pharmacogenomics are to maximize drug efficacy, avoid adverse drug effects, and target responsive patients. The research goals are to enhance our understanding of disease by discovery of new pathways and mechanisms of action. The field has evolved considerably with advances in DNA genotyping and sequencing technology, new bioinformatics tools and statistical methodology, use of electronic health records and biobanks, and functional validation of genetic signals using in vitro cell systems and animal models. Clinical implementation of pharmacogenomics is, however, still nascent, especially in cardiovascular disease.3-9 The FDA have included details of 119 drug–gene pair associations in drug labelling; however, the information relating to only 15% of these associations is based on convincing randomized clinical trial (RCT) data.10 Of the small number of cardiovascular drug–gene pairs described in drug labels, two had adequate or convincing clinical validity, but none had convincing clinical utility. Interest in using biomarkers to stratify or target patients in cardiovascular RCTs is increasing,11 but genotype-based RCTs have been initiated only over the past 6 years.12-14 Using genotype as a biomarker of drug response or toxicity in RCTs enables the use of smaller sample sizes, a decrease in costs, an increase in the likelihood of success, and the minimization of adverse events.
In this Review, we provide an overview of the efforts to implement pharmacogenomics into clinical practice using the gold-standard approach of RCTs. We first discuss the current approaches used to identify pharmacogenetic markers, the evidence supporting their clinical validity, and the rationale for their use in RCTs. We then describe the various RCT designs in which pharmacogenetic markers could be used, with specific examples of cardiovascular studies related to drug–gene pairs.
Identifying pharmacogenetic markers
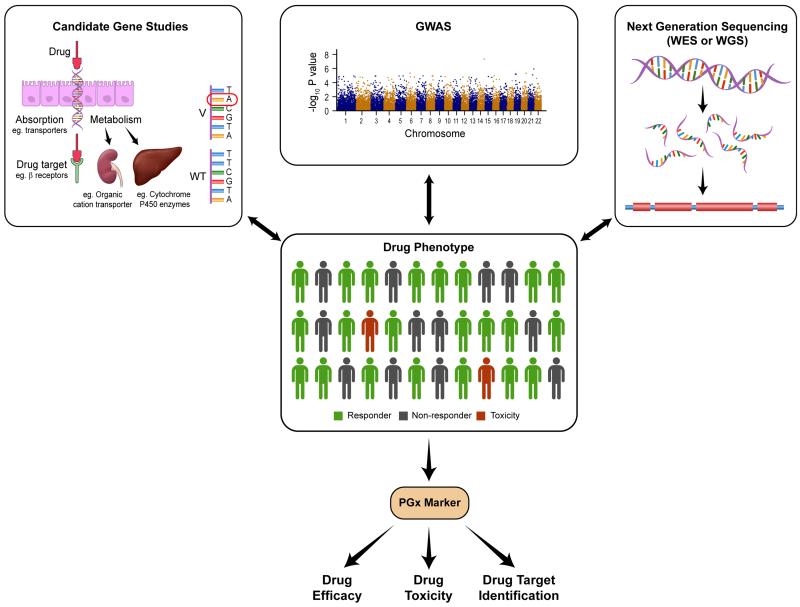
Genetic variation that is associated with a particular drug-response phenotype is considered a pharmacogenetic marker. Studies linking genotype with drug-response phenotype are performed to identify relevant pharmacogenetic markers that can subsequently be used in clinical practice (Figure 1). Pharmacogenetic markers can be used to identify individuals who do or do not respond to drugs, as well as individuals who are at risk of drug toxicity.
Figure 1.
Genotyping and sequencing strategies to identify pharmacogenetic markers associated with drug efficacy (response versus no response) and drug toxicity. Abbreviations: GWAS, genome-wide association study; WES, whole exome sequencing; WGS, whole genome sequencing.
Drug-response phenotype
Interindividual variability in drug dosage (for example, with warfarin15), response or lack of response to a drug (for example, with β-blockers in heart failure16), and drug toxicity (for example, statin-induced muscle toxicity17) are considered classic drug-response phenotypes. However, with the advent of more-expensive, ‘next-generation’ sequencing technology, extreme drug-response phenotypes—defined typically as those individuals with drug response ≤5th percentile, or ≥95th percentile—are more commonly used to identify novel pharmacogenetic markers (for example, in warfarin dosing).18
The concept of pharmacogenetics has also been extended to functional genetic variation in potential drug targets that supports the use of a drug in a certain disease process (for example, variation in Niemann–Pick C1-like protein 1 and use of ezetimibe in patients with coronary artery disease19). The identification of mutations in potential drug targets has enabled the development of novel drugs for various types of cancer previously considered untreatable (for example, vemurafenib, a BRAF kinase inhibitor for metastatic melanoma that is positive for a BRAF V600E mutation20).
Genotyping or sequencing studies
The three broad research strategies that have been adopted to identify pharmacogenetic markers are candidate gene studies, genome-wide association studies (GWAS), and next-generation sequencing approaches (Figure 1).
Candidate gene studies
An altered drug-response phenotype can result from genetic variation in the known pharmacokinetic or pharmacodynamic pathway of a drug. The pharmacokinetic pathway consists of factors that influence the drug’s absorption, distribution, metabolism, or excretion, thereby influencing the concentration of the drug that reaches the target. The pharmacodynamic pathway consists of the drug’s target itself and/or signalling pathways downstream that mediate drug effect. Therefore, candidate gene studies involve DNA genotyping or sequencing of individuals for common or rare variation in genes that comprise the known pharmacokinetic and/or pharmacodynamic pathways of the drug. A genetic association study is then performed to identify the pharmacogenetic marker that is significantly associated with the drug phenotype. Candidate gene studies have resulted in successful identification of various pharmacogenetic markers, including CYP2C19 for clopidogrel use (pharmacokinetic pathway), CYP2C9 for warfarin use (pharmacokinetic pathway),21 VKORC1 for warfarin use (pharmacodynamic pathway),22 and ADRB1 for buncindolol use (pharmacodynamic pathway).16 The disadvantage of candidate gene studies, however, is their inability to identify genetic variants in as-yet-undiscovered pathways that might influence the drug-response phenotype.
GWAS
Millions of single nucleotide polymorphisms (SNPs) across the entire genome are assayed in GWAS, and these studies, therefore, have the potential to identify variants in genes that might not have been previously considered to influence the drug-response phenotype. An example of the success of this approach for drug toxicity is the identification of the noncoding SNP rs4363657 in the SLCO1B1 gene on chromosome 12, which was identified as being significantly associated with statin myopathy (P = 2.0 × 10−9; OR 16.9 for CC homozygotes and 4.5 for CT heterozygotes).17 SLCO1B1 encodes OATP1B1, a solute carrier organic transporter responsible for the active transport of statins into hepatocytes and, therefore, plasma clearance and subsequent metabolism of the drug.
In addition to the identification of genetic variants not previously considered to potentially influence drug response, GWAS have also been used to confirm the role of candidate genes thought to influence drug-response phenotypes. The first GWAS performed to assess variability in warfarin dosing to achieve therapeutic anticoagulation confirmed the role of SNPs in the warfarin drug target VKORC1 (rs9923231, P = 5.4 × 10−78) and the cytochrome P450 metabolizing enzyme CYP2C9 (rs1057910, P = 4.5 × 10−17; rs1799853, P = 8.8 × 10−13).15 Moreover, the association between the CYP4F2 gene and warfarin metabolism was previously controversial;23 however, after adjusting for age, sex, and the known genes that influence warfarin dosing (VKORC1 and CYP2C9), the single coding SNP rs2108622 in the CYP4F2 gene was found to have genome-wide significance (P = 8.3 × 10−10).15
The limitations of GWAS are the inability to identify variants with small effect size, especially when associated with a drug-response phenotype that is not directly related to drug effect. For example, compared with variability in clopidogrel inhibition of platelet aggregation, a GWAS assessing death as the end point is less likely to result in identification of a CYP2C19 variant that is of genome-wide significance. Genetic variants with small effect size require large sample sizes and replication of findings is essential to infer significant and clinically valid associations between the genotype and the drug-response phenotype. Finding an appropriately powered replication study to assess such associations can be extremely challenging.24,25 GWAS identify common genetic variants and, therefore, can miss rare variants with potentially large effect size that might account for the missing heritability of complex drug-response phenotypes.26
Next-generation sequencing
Next-generation sequencing technology is increasingly being used in the field of pharmacogenomics to identify rare genetic variants that are associated with drug response.18,27 In response to decreasing costs of sequencing, whole genome and whole exome sequencing studies are being used to identify clinically relevant, rare genetic variants.28 Whole exome sequencing has identified rare coding variants in KCNE1 (a potassium channel gene) and ACN9 (a gluconeogenesis pathway gene) as being risk factors for drug-induced long QT syndrome.27 Individuals at risk were also found to have an increased prevalence of rare variants that have been implicated in congenital long QT syndrome. Whole exome sequencing has also been performed to identify genetic variants that contribute to extreme warfarin dose variability (≤35 mg per week or ≥49 mg per week) in African-American individuals.18 For the first time, an association between genetic variation in the folate homeostasis pathway (rs7856096 in the folate homeostasis gene FPGS) and warfarin dose variability (in this case, lower warfarin doses) was demonstrated in African-American individuals (P = 1.8 ×10−8).
Despite being a new technology with the capacity to identify rare culprit variants, next-generation sequencing is not a panacea for identifying all inheritance patterns in pharmacogenomics. Copy number and long insertion–deletion variants are examples of genetic variant types that are not reliably detected by whole exome or whole genome sequencing.29 Additionally, ordering clinicians might not have the necessary expertise to interpret incidental genetic results that are highly medically actionable.30 The increased sensitivity of next-generation sequencing in detecting multiple rare and common genetic variants of uncertain functional relevance, and the small sample sizes used in these type of studies, make the interpretation of these associations with drug-response phenotypes challenging.31
Rationale for genotype-based RCTs
The implementation of pharmacogenetics in clinical practice faces many challenges, including the availability of genotyping or sequencing that can be performed in a Clinical Laboratory Improvements Amendments (CLIA)-approved laboratory in a timely manner, user-friendly decision support tools and, perhaps most importantly, the lack of demonstration of clinical utility in well-designed RCTs.3 The rationale for performing such genotype-based RCTs is outlined below.
Variable drug response and toxicity
The role of inheritance in variable drug response has been anecdotally observed for many years.32 A 20-fold variation between individuals is observed in those trying to achieve therapeutic anticoagulation using warfarin, with genetic factors contributing up to 30% of this dose variability.1,18,33 Genetic factors also have a role in the pharmacokinetics of newer anticoagulants such as dabigatran; the presence of each CES1 rs2244613 minor allele resulted in a 15% decrease in dabigatran trough levels and a lower risk of bleeding with the drug.34 The widely used antiplatelet drug clopidogrel is converted to an active metabolite by the cytochrome P450 enzyme CYP2C19, a protein that is encoded by a highly polymorphic gene. Variation in CYP2C19 has been associated with significantly reduced active clopidogrel metabolite levels, high residual platelet reactivity, and an increased risk of major adverse cardiovascular events, especially after percutaneous coronary intervention (PCI).35,36
However, genetic variation that could alter drug pharmacokinetics or pharmacodynamics is not always associated with a predictable drug response. An example is azathioprine, an important immunosuppressant drug used in heart transplantation. Azathioprine is metabolized by thiopurine S-methyltransferase (TPMT). Loss-of-function genetic variation in TPMT could result in higher levels of active azathioprine metabolite, and carriers could be at an increased risk of life-threatening leucopenia when treated with azathioprine.37,38 Heart transplantation recipients who are carriers of loss-of-function TPMT genetic variants with higher azathioprine levels should, theoretically, also have a lower likelihood of cardiac rejection. Conversely, in vitro lymphocyte proliferation assays and assessment of cardiac rejection by endomyocardial biopsy in patients with inactive TPMT alleles have suggested that, compared with wild-type patients, azathioprine use in these individuals might result in a higher risk of rejection.39,40 Therefore, although studies might demonstrate convincing associations between genotype and drug-response phenotype, rigorously conducted RCTs are needed before we start considering these drug–gene pairs in clinical practice—altering therapy on the basis of these associations might not always result in the desired outcomes as seen in trials based on platelet function testing.41,42
Success in other medical specialties
A few pivotal genotype-based RCTs have resulted in change to clinical practice. The drug–gene pair abacavir–HLA-B*5701 was successfully assessed in an RCT and is a good example of how pharmacogenetic markers can be used to prevent toxicity and thereby improve outcomes. Abacavir is a nucleoside reverse-transcriptase inhibitor that is used to treat patients with HIV, but can result in a potentially fatal hypersensitivity reaction in 5–8% of patients taking the drug.43-47 The association between HLA–B*5701 and abacavir-induced hypersensitivity reaction was demonstrated and replicated in multiple gene-association studies.43-47 Approximately 6 years after the initial report, patients with HIV were randomly assigned to prospective HLA-B*5701 screening before abacavir treatment (n = 980) or to the control group (n = 976) in a double-blind, prospective study.48 Immunologically confirmed hypersensitivity reactions occurred in none of the patients in the prospective genetic screening group versus 2.7% of those in the control group (P <0.001), thus demonstrating the utility of a pharmacogenetic test in reducing the incidence of drug toxicity.48 The trial findings led to the use of HLA-B*5701 screening before abacavir use in the clinical setting. The large effect size (approximately 50% of HLA-B*5701 carriers develop hypersensitivity when exposed to abacavir) and the relatively high prevalence of the associated minor allele contributed to the successful outcome of this trial.48
The G551D mutation in the cystic fibrosis transmembrane conductance regulator gene (CFTR) is an example of a pharmacogenetic marker that can be successfully used to improve treatment outcomes. An RCT demonstrated the benefit of ivacaftor therapy in 161 patients with cystic fibrosis who were carriers of the G551D mutation.49 The success of this trial highlights the possibility of using a small sample size if the RCT is enriched with potential responders who are genetically identified.
FDA labelling
The FDA has provided pharmacogenetic information in drug labels for a small number of cardiovascular drugs—metoprolol, carvedilol, propranolol, propafenone, statins, isosorbide, hydralazine, clopidogrel, and warfarin—in an attempt to guide physicians who prescribe these drugs.50 The pharmacogenetic information provided for metoprolol, carvedilol, propranolol, and propafenone are primarily based on the effect of CYP2D6 metabolizer status on the pharmacokinetics of the drug. The only cardiovascular drugs that have specific pharmacogenetic drug-label recommendations are warfarin (relating to dose adjustments) and clopidogrel (for the use of alternative antiplatelet drug therapy in poor metabolizers). Groups such as the Clinical Pharmacogenetics Implementation Consortium (CPIC) have provided prescribing guidelines for these drugs on the basis of findings reported in the available literature as well as FDA recommendations.33,51 Clinical validity (defined as the capacity to predict drug response) and clinical utility (defined as improvement of clinical outcomes) have been examined for various drug–gene pairs identified in drug labels.10 The warfarin–CYP2C9/VKORC1 and clopidogrel–CYP2C19 drug–gene pairs demonstrated clinical validity, but did not fulfil criteria for clinical utility.10 “A systematic review or meta-analysis of RCTs showing consistency in results or at least one large RCT”10 was required for the demonstration of clinical utility, highlighting the importance of performing RCTs for the clinical implementation of pharmacogenetics.10,52
Point-of-care genotyping
A short turnaround time is often important for genotype-based therapeutic interventions to be implemented in clinical trials and clinical practice. The availability of point-of-care genotyping has overcome the limitations of a time delay caused by traditional genotyping, and allows genotype-guided therapy to be initiated in a timely manner. An example, which has been effectively utilized in a genotype-based clinical trial,53 is the FDA-approved point-of-care Spartan RX™ CYP2C19 genotyping platform (Spartan Biosciences, Canada) that uses a buccal swab to provide results of a patient’s CYP2C19*2 and *3 carrier status within 1 h. The importance of providing genotyping results in a timely manner was also seen in the warfarin pharmacogenetic trials. In EU-PACT,12 a point-of-care genetic test provided results within 2 h to guide warfarin dosing for all patients randomly assigned to the genotyping group. However, in COAG,13 genetic results were available for only 45% of individuals on the first day after randomization, resulting in the use of a clinical algorithm without the genotype data in the genotype-guided dosing strategy group.
Pharmacoeconomic considerations
The administration of the right drug to the right patient could reduce health-care costs by enhancing drug efficacy and reducing drug toxicity, and thereby resulting in reduced hospital admissions and utilization of health-care services. Generic clopidogrel is approximately one-sixth of the cost of the newer antiplatelet drugs, and a strategy of using this therapy in wild-type CYP2C19 patients could result in considerable cost savings compared with the universal use of newer antiplatelet drugs. Indeed, cost-effectiveness analyses have demonstrated that substantial cost savings could be achieved with a genotype-based strategy compared with empirical use of the newer P2Y12 inhibitors for all patients after PCI to overcome ‘genetic clopidogrel resistance’.54,55 According to one study, compared with prasugrel use in all patients, the use of prasugrel and clopidogrel in a genotype-guided strategy resulted in an incremental cost-effectiveness ratio of US$30,200 per quality-adjusted life year.55 The major limitation of these studies is that cost assumptions are based on outcomes from post hoc analyses of RCTs. The true value of a genotype-based approach can be determined only from prospective genotype-based RCTs.
Phase II trial pharmacogenomics
Performing GWAS and whole exome or whole genome sequencing in a small phase II trial might enable early identification of common or rare variants associated with drug response or toxicity that have a large effect size.51 Such an approach might then allow enrichment of phase II trials with responders and/or exclusion of patients at risk of adverse events. The benefits of such an approach would be a smaller sample size, lower costs of performing such a trial, and greater likelihood of a positive result.
Genomic clinical trial design
Over the past several decades, phase III RCTs have often required multiple thousands of patients to demonstrate clinically meaningful, but modest, benefits in an unselected patient population. In the past decade, however, some investigators have proposed RCT protocols that attempt more accurately and selectively to identify patients who might benefit from treatment. In many cases, especially in the field of oncology, these RCT protocols are now in use. The refined RCT designs fall into two general categories—retrospective and prospective design. Prospective designs can be further divided into various possible approaches (Figure 2). The examples cited below are primarily from the oncology field, but could be applied to cardiovascular disease.
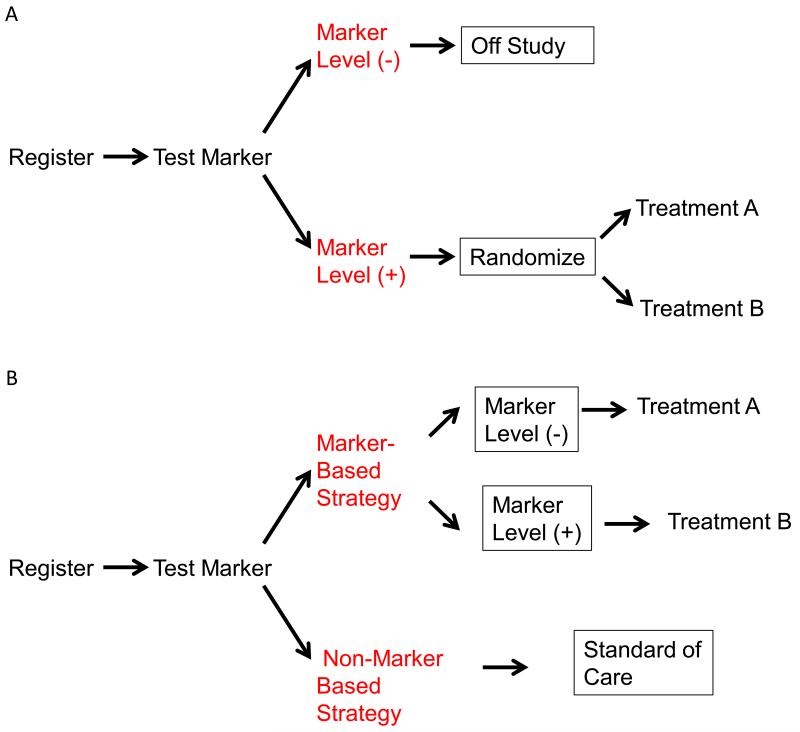
Figure 2.
Some possible prospective biomarker-based clinical trial designs. a | In the enrichment design, patients are tested for presence of the required biomarker, and only those who have the marker present are randomized. B | in the treatment by pharmacogenetic maker interaction design, all patients are tested for the biomarker, but then all patients are randomized (stratified by marker status) to the same two treatment arms to allow determination of the value of the investigational treatment separately in the different biomarker based groups. C | In the marker-based strategy design, patients are randomized to either have treatment defined by their biomarker status, or by a non-marker based method such as physician choice d | In the umbrella trial design, all enrolled patients are assigned to a substudy on the basis of information about multiple biomarkers, and are then randomly assigned to either experimental therapy or standard-of-care treatment. Experimental therapy will differ, and standard-of-care treatment might differ, by substudy.
Retrospective approach
Retrospective study designs aim rigorously and formally to identify a potential predictive marker from an already completed RCT. This approach is desirable when an RCT has already been completed (at least through the enrolment period) and a potential pharmacogenetic marker is identified from a source outside of the RCT. In this circumstance, if banked specimens are available from the completed RCT, a prospectively defined analysis plan can be developed to test whether the treatment effect differs by the level of a pharmacogenetic marker. To be able to address the pharmacogenetic marker hypothesis, this ‘prospective–retrospective’ strategy requires biospecimens to be available from a large proportion of all patients enrolled in the RCT (to prevent selection bias) and not to have been used in the development of the assay under investigation. A prospective analysis plan completed before any pharmacogenetic marker analyses, and sufficient statistical power with the existing sample size of the RCT, are also necessary.56 Moreover, to be considered ‘definitive’, the entire validation process should be duplicated on a second patient cohort from an independent trial. This independent validation is crucial because, although prespecification adds substantial credibility to the initial validation, multiple markers could be tested from a single trial, which increases the risk of a false-positive finding.
This approach has been successfully used to establish the predictive capacity of RAS mutations as a marker for lack of benefit of inhibitors of the epidermal growth factor receptor in patients with colorectal cancer.57 Retrospective DNA analysis has been performed in two RCTs demonstrating the superiority of alternative antiplatelet therapy to clopidogrel in reducing major adverse cardiovascular events in carriers of the CYP2C19 loss-of-function allele, but the stringent criteria described above were not met in these genetic substudies.58,59 For example, DNA analysis was performed in only 21% of the TRITON-38 trial59 participants and 55% of the PLATO study58 population, none of these genetic substudies was prospectively powered to address the hypothesis definitively, and no duplicate trials have been available to validate these findings.
Prospective approach
Prospective validation remains the gold standard for any therapeutic approach, including biomarker-based treatment. Multiple prospective strategies have been developed to test for treatment efficacy in the context of a biomarker.
Enrichment designs
The most statistically straightforward prospective approach is the enrichment design (Figure 2a), in which only patients who have the feature thought to predict clinical benefit (that is, a specific mutation, blood type, or disease characteristic) are included in the RCT. Examples of cardiovascular genotype-based RCTs that have an enrichment design are TAILOR-PCI60 and GENETIC-AF.61 If the marker used to select patients is truly predictive of differential benefit, this approach can substantially reduce the required sample size compared with the unselected design, which would also include the cohort of patients with little or no likelihood of benefit.62 This approach, however, leaves multiple questions unanswered: does the treatment have benefit in excluded patients, could the biomarker used to select patients be further optimized and, if the trial produces negative findings, are they a result of a failure of the biomarker or of the treatment itself? Nevertheless, when justified by appropriate preliminary data, the use of enrichment designs offers the potential for smaller, more-efficient trials and, equally importantly, the avoidance of treating patients who have little or no likelihood of benefiting from the therapy.
In the absence of clear data to support an enrichment design, prospective pharmacogenetic marker-based hypotheses can still be tested within an unselected design. In this situation, patients are enrolled regardless of pharmacogenetic marker status (although availability of an appropriate biologic specimen might be a prerequisite), but the planned analysis incorporates the biomarker as an important component.
One such approach is to include all patients in the trial, but to prespecify multiple hypotheses within the statistical design with a careful strategy to limit the overall risk of false-positive error. If the treatment is thought to be of potential benefit to all patients, but of greater benefit to a specific subpopulation, the RCT might be designed to test the overall population level effect first at a reduced α level from the usual 0.05 (for example test at 0.04). Then, if the overall finding is negative, the remaining α can be used to test for a treatment effect in just the biomarker-positive population. An alternative approach is first to use a portion of the α to test for the treatment effect in the biomarker-positive population then, if the finding is positive, to test for activity in the biomarker-negative patients using a prespecified α split.63
The treatment by pharmacogenetic marker interactive design (Figure 2b) is a different ‘unselected’ design approach in which all patients are enrolled, but the pharmacogenetic marker is tested and used as a stratification factor before patients are randomly assigned to treatment.64 The trial is then powered to test independently the treatment effect in the two pharmacogenetic marker-based populations. These two groups can be monitored and analysed separately, with independent rules; for example, with aggressive monitoring for futility in the patients who do not have the pharmacogenetic marker, if the hypothesis is that the treatment benefit might be reduced or absent (or adverse events increased) in those patients.
When the pharmacogenetic marker is not binary, or when there are multiple pharmacogenetic markers from which to choose for potentially several treatments, the pharmacogenetic-marker-based strategy design (Figure 2c) can be used. In this design, patients are randomly assigned to either their treatment determined by the pharmacogenetic marker or by physician choice or to standard of care. The pharmacogenetic-marker-based strategy design is inefficient if only one biomarker and treatment is being tested, but continues to be used in trials such as SHIVA,65 an attempt to validate a genomic-guided treatment strategy in metastatic cancer.
Adaptive designs
The use of ‘adaptive’ designs, which use the data accumulated during the course of a trial to change study features such as treatment groups, randomization ratio, or end points, has generated much attention among investigators designing biomarker-based RCTs. An example of a cardiovascular RCT incorporating an adaptive design is the GENETIC-AF trial.61 Several approaches have been proposed to identify a predictive biomarker within the population used to test a new therapy, if the primary complete population test is negative. This strategy is possible only if the type I error (α) has been partitioned, with some α reserved for the subgroup identification. All such approaches have been criticized as having an over-reliance on the data from a single trial both to identify and to validate a biomarker, as well as for the lack of a biological, knowledge-driven algorithm. Other types of adaptation have been proposed and used in biomarker-based trials, such as in BATTLE66 and ISPY-2.67 In these studies, patients were randomly assigned to multiple potential treatments, and activity of each treatment assessed within several predefined biomarker-based groups on an ongoing basis. Substantial controversy exists in the use of adaptive randomization within these trials, where the ratio of patient randomization between the treatment groups is altered to increase the number of patients being treated with agents that are showing the most-promising early efficacy. A vigorous debate exists about whether such adaptive randomization is desirable from either a statistical or ethical perspective.68
Biomarker-based all-comers designs
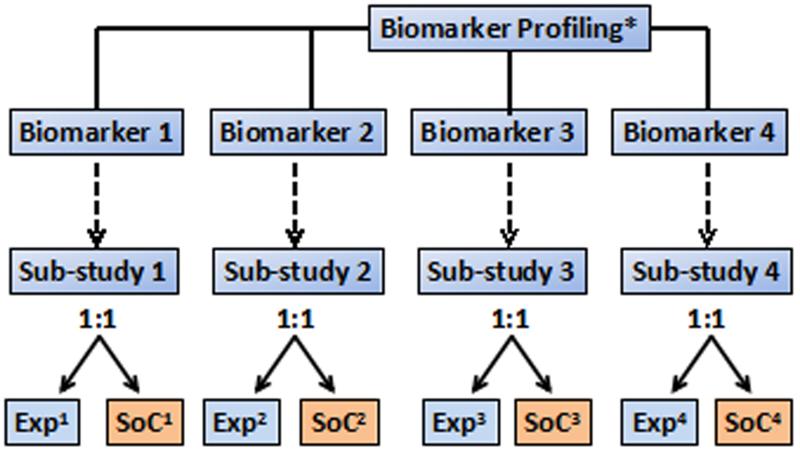
A biomarker-based RCT strategy that has garnered increasing attention over the past 2–3 years is the umbrella trial design (Figure 2d). In this design, all enrolled patients have their pharmacogenetic marker status tested and are then, on the basis of their pharmacogenetic marker status, assigned to a substudy in which they are randomly allocated to a specific treatment protocol. Assignment can be to a single treatment, as is planned in the NCI Match trial,50 with the goal of generating a signal for future trials, or via randomization, which is occurring in the phase II/III LUNG-Map study.50
Hybrid approaches of all of these designs are also possible, for example within the ALCHEMIST trial50 in adjuvant non-small cell lung cancer, in which all patients are screened, and those with specific mutations (ALK or EGFR) are assigned to an RCT, whereas the remaining patients are genomically profiled and followed up for disease outcomes and survival, but are treated off protocol.
Application in cardiovascular diseases
Despite convincing altered pharmacokinetic and pharmacodynamic drug profiles with genetic variation, the clinical implementation of pharmacogenetics in cardiovascular disease has been limited by the lack of demonstration of incremental benefit with the use of pharmacogenetic markers in well-designed and adequately powered RCTs. Over the past 6 years, however, the landscape in cardiovascular pharmacogenetics has changed with the initiation of well-designed RCTs using genotyping strategies to individualize antiplatelet, β-blocker, statin, and warfarin therapy (Table 1). Prospective RCTs to assess the use of a pharmacogenetic biomarker to guide prescription of an alternative to standard therapy are underway for β-blocker (bucindolol instead of metoprolol) and antiplatelet (ticagrelor instead of clopidogrel) therapy. Clinical outcomes in each of these trials are being directly compared in the carriers of the pharmacogenetic markers. By contrast, alternative dosing strategies for one drug were evaluated in RCTs to assess the use of the pharmacogenetic markers for warfarin sensitivity, and outcomes were compared for the genotyping versus the nongenotyping groups, rather than by directly assessing the effect of dosing strategy in carriers of the minor allele of the pharmacogenetic markers.
Table. Cardiovascular Genotype-based Clinical Trials.
| Trial | TAILOR PCI |
POPular Genetics Study |
COAG | EU-PACT | Genetic AF |
|---|---|---|---|---|---|
| Study Population | Post-PCI ACS, Stable CAD, STEMI |
Post-PCI, STEMI | Any indication for warfarin initiation |
AF or venous thromboembolism |
Persistent AF, recent cardioversion to sinus |
| Sample Size | 5,270 | 2,700 | 1,022 | 455 | 620 |
| Pharmacogenetic Marker |
CYP2C19*2 or *3 | CYP2C19*2 or *3 |
CYP2C9 and VKORC1 |
CYP2C9*2 or *3 and VKORC1 (−1639G→A) |
ADRB1 (389 Arg/Arg) |
| Treatment Arms | Genotyping arm (clopidogrel in WT + ticagrelor in CYP2C19*2 or *3 carriers) vs Non- genotyping arm (clopidogrel) |
Genotyping arm (clopidogrel in WT + ticagrelor or prasugrel in CYP2C19*2 or *3 carriers) vs non- genotyping arm (ticagrelor) |
Genotyping arm (Warfarin dose based on genotype + clinical variables) vs non-genotyping arm (Warfarin dose based on clinical variables) |
Genotyping arm (Warfarin dose based on genotype and clinical variables) vs non- genotyping arm (fixed Warfarin dosing) |
Bucindolol vs Metoprolol in B1 389 Arg/Arg carriers |
| Trial Design | Pragmatic, open label, randomized prospective, modified- enrichment superiority study |
Open label, randomized, prospective, non- inferiority marker- based strategy study |
Double-blind, randomized, prospective, marker-based- strategy superiority trial |
Pragmatic, single- blind, randomized, marker-based strategy superiority trial |
Double-blind, 2–arm, active-controlled, adaptive–designed, enriched superiority study |
| Primary Endpoints | Composite of CV death, stroke, MI, ST, urgent revascularization |
Composite of death, stroke, MI, ST, urgent revascularization, major bleeding. |
Percentage of time in therapeutic INR |
Percentage of time in therapeutic INR |
Time to first event of symptomatic AF/atrial flutter or all-cause mortality. |
| Results | Currently recruiting | Currently recruiting | Genotyping guided dosing not superior to conventional warfarin dosing |
Genotyping guided dosing superior to conventional fixed warfarin dosing |
Currently recruiting |
| References | Clinicaltrials.gov: NCT01742117 |
Clinicaltrials.gov: NCT01761786 |
12 | 13 | Clinicaltrials.gov: NCT01970501 |
β-Blockers
The use of β-blockers (such as bisoprolol, carvedilol, metoprolol, and succinate) has resulted in a significant reduction in mortality and hospitalizations, and improvement in functional capacity and cardiac structure and function in patients with heart failure.69-71 However, one β-blocker tested in an RCT did not show a significant survival benefit compared with placebo—in the BEST,72 bucindolol and placebo were associated with 30% and 33% mortality, respectively, in patients with NYHA class III–IV heart failure (P = 0.13). Genetic variation might have a role in the variable clinical response observed with β-blocker therapy. The nonsynonymous SNP Arg389Gly, in the gene encoding the β1-adrenergic receptor ADBR1, is present in 30% of European and Chinese individuals and 40% of individuals with African-American ancestry.73 This SNP has differential effects on the function of the β1-adrenergic receptor in vitro, resulting in a greater reduction in norepinephrine-stimulated cAMP production with bucindolol in Arg389-transfected cells, compared with Gly389-transfected cells.16 The increased in vitro β-blocker efficacy of bucindolol observed in the β1Arg389 genotype translated into a 38% reduction in mortality in β1Arg389 carriers treated with bucindolol compared with placebo in BEST.16 The β1Gly389 carriers had no survival benefit with bucindolol therapy, raising the possibility that the overall negative results of BEST were caused by an altered drug response owing to underlying genetic variation. This concept is now being tested in GENETIC-AF.61 The underlying hypothesis of GENETIC-AF is that bucindolol used in carriers of the β1Arg389 allele is superior to metoprolol therapy in the prevention of the recurrence of symptomatic atrial fibrillation or flutter in patients with heart failure.61 This double-blind, genotype-directed RCT incorporates an interim analysis and adaptive design element. If the Data and Safety Monitoring Board determines that phase IIb data for the initial 200 randomly allocated patients is favourable, the trial will proceed to the random assignment of a total of 620 patients in the phase III component of the RCT. GENETIC-AF is an example of a prospective, pharmacogenetic trial based on the retrospective discovery of a candidate pharmacodynamic-based genetic marker of drug response in a negative phase III RCT, with the new trial enriched for ‘genetic responders’.
Statins
Genetic variation in SLCO1B1, a gene that encodes a transport protein that facilitates the hepatic uptake of statins, has been associated with skeletal myopathy (OR 4.5 in heterozygotes and 16.9 in homozygotes) with simvastatin use.17 Retrospective DNA analysis was performed in two separate RCTs, in patients with statin-induced skeletal myopathy and in controls, to demonstrate this significant association.17 The genetic variant results in altered SLCO1B1 transporter function,74 leading to decreased clearance of simvastatin and increased simvastatin plasma levels.75 These data have led to a recommendation of using a lower dose of simvastatin or considering alternative statins, such as pravastatin or rosuvastatin, in carriers of the SLCO1B1 minor allele.76 A genetic substudy of JUPITER, in which investigators evaluated the effect of SLCO1B1 variants on clinically reported myalgia in individuals receiving rosuvastatin, did not demonstrate an increased risk attributable to the SLCO1B1 variants.77 The data for an association between SLCO1B1 genetic variants and myopathy with other statins are either not available or are inconclusive, primarily owing to these studies being underpowered.76,78,79 Clinical implementation of simvastatin pharmacogenetics has remained challenging, owing to the wide therapeutic index of statins, availability of low-cost alternative statins, and the challenge of accurately defining the phenotype of myalgia in clinical practice. In an attempt to make statin pharmacogenetics more relevant to clinical practice, investigators in the Genetically Guided Statin Therapy to Improve Medication Adherence RCT80 are randomly assigning 375 patients, who have perceived statin intolerance without previous biochemical evidence of severe myopathy, to a genetically guided statin therapy group or to usual care. The trial is designed to test the hypothesis that genetically guided statin therapy will result in greater statin adherence and lower LDL-cholesterol levels when compared with a non-genotype-guided usual care strategy.
Warfarin
The wide individual variability in warfarin dosing, with its narrow therapeutic index, results in warfarin being the cause of one-third of emergency room visits for adverse drug reactions.81 Consequently, pharmacogenetic-based warfarin dosing has been extensively studied, and the FDA has included dosing recommendations for warfarin in the drug labelling information to reduce dose variability and minimize adverse effects of thrombosis or bleeding. This dosing information is based on genetic variation in CYP2C9, the cytochrome P450 enzyme that metabolizes S-warfarin, and VKORC1, which encodes the enzyme target of warfarin.
No adequately powered RCTs have been performed to assess whether dosing algorithms incorporating pharmacogenetics are associated with fewer adverse clinical end points than usual consideration of clinical variables. EU-PACT12 and COAG13 were designed to examine the role of pharmacogenetics in maintaining patients within a therapeutic international normalized ratio (INR) range (2–3) and were both completed in 2013. EU-PACT12 was an RCT involving 455 patients. One trial group received standard dosing (individuals aged ≤75 years received 10 mg on day 1, and 5 mg on days 2 and 3, whereas patients aged >75 years received 5 mg daily on days 1–3), and the other group received genotype-guided dosing. The primary outcome—the percentage of time that the trial participants were in therapeutic INR over a 12-week period—was 67.4% in the genotyping group and 60.3% in the standard-dosing group (P <0.001).12 Fewer patients in the genotyping group compared with the standard-dosing group had an INR >4 (27% versus 37%; P = 0.03). The median time to achieving therapeutic INR was also shorter in the genotyping group than in patients receiving the standard dosing (21 days versus 29 days; P <0.001). Therefore, the EU-PACT trial12 indicated that a pharmacogenetic-based dosing strategy might be more desirable than conventional warfarin dosing.
COAG13 was a double-blind RCT to assess the percentage of time in therapeutic INR over a 4-week period in patients who received either genotyping-guided dosing or dosing guided on the basis of clinical variables only. The primary outcome did not vary between the two groups—the percentage of time in therapeutic INR was 45.4% in the standard-dosing group and 45.2% in the genotyping-guided group (P = 0.91).13 However, in African-American individuals, who comprised one-third the study population, clinical-variable dosing was found to be superior to genotyping-based dosing (P = 0.01). Notably, though, 76% of African-American participants had no genetic variation in the two genes assessed (compared with 25% of non-African-American participants). Genetic variants that help to predict warfarin dosing variability in African-American individuals—such as CYP2C9*5, *6, *8, and *11—were not tested for and, therefore, were not used to guide dosing in the COAG trial.13
Important differences between the EU-PACT12 and COAG13 trials exist. The control group in the EU-PACT trial12 received fixed dosing, whereas the control group in the COAG trial13 used clinical variables such as age, sex, African-American ethnicity, and concomitant drug use to determine dose. One might predict the clinical-variable dosing strategy to be more precise than fixed dosing; however, at 4 weeks, the percentage of time spent in therapeutic INR was equivalent with fixed dosing in EU-PACT12 and with clinical-variable dosing in COAG.13 Another important difference was that genotyping results were made available within 2 h for individuals in the EU-PACT trial, but took longer in the COAG trial. All day 1 dosing was determined on the basis of genotype in the genotyping group of EU-PACT.12 By contrast, in the COAG trial,13 only 45% of participants had genetic information available to guide dosing on day 1, although 99% of participants had genetic information to guide dosing by day 3. Furthermore, African-American patients comprised almost one-third of the study population in the COAG trial,13 but only 1% of the population of EU-PACT12 were black patients. The presence of African-American individuals who had results opposite to the predicted effects of genotyping might have resulted in a negative outcome in the COAG study.13 Finally, when comparing a genotype-based strategy with a nongenotype-based strategy, it is important to assess the prevalence of the genetic variants in the two groups and to ensure sample size calculations are made on the basis of the effect of the genetic variants on the phenotype as well as the prevalence of these variants. In the genotyping group, the prevalence of individuals homozygous for VKORC1 was 11% in the COAG trial,13 but was 17% in the EU-PACT trial.12 Similarly, only 1% of study participants in the COAG study13 were homozygous CYP2C9 carriers, compared with 3.4% of individuals in the EU-PACT trial.12 According to FDA recommendations, warfarin dosing is typically 5–7 mg per day in individuals who do not carry these genetic variants, whereas patients who are homozygous for VKORC1 and CYP2C9*2 should be receiving doses that are almost 40% lower (3–4 mg per day), and warfarin dosing is even further reduced for carriers of the CYP2C9*3 allele (recommended to be 0.5–2 mg per day).82 Whether trial results would have been different if a greater proportion of participants with warfarin sensitivity had been identified by genotype remains unknown, especially for the COAG trial. The most important question—whether pharmacogenetic testing affects clinical outcomes such as thromboembolic or bleeding events—remains to be assessed, and in the absence of demonstrating such benefit, insurance reimbursement for pharmacogenetic testing for warfarin remains problematic.
Clopidogrel
Clopidogrel is the drug most commonly prescribed with aspirin after PCI, with >2 million prescriptions written in the USA each month.83 Clopidogrel is a thienopyridine prodrug that requires the cytochrome P450 enzyme CYP2C19 to convert it to its active thiol metabolite. Common genetic variation in CYP2C19 (*2 and *3 alleles) leads to a loss-of-functional protein. This variation occurs in approximately 30% of the white population and 50% of Asian individuals.51,84,85 Carriers of CYP2C19*2 and *3 alleles (or loss-of-function alleles) have significantly reduced clopidogrel active metabolite levels and high residual platelet reactivity. Preliminary data suggest that, when treated with clopidogrel, these carriers might be at an increased risk of major adverse cardiovascular events, especially after PCI, compared with noncarriers.35,36,86-88 As a result, the FDA have issued a black box warning advising practitioners to “consider alternative treatment in patients identified as CYP2C19 poor metabolizers” and note that these patients can be identified by genotyping.89 However, routine clinical use of genotyping to identify CYP2C19 loss-of-function alleles in patients treated with clopidogrel is not recommended in the latest guidelines published by the American College of Cardiology, American Heart Association, and Society for Cardiovascular Angiography and Interventions,90,91 owing to the lack of prospective clinical evidence that such patients with CYP2C19 loss-of-function alleles would benefit from alternative therapy.
To address this gap in clinical evidence, a large, prospective RCT of genotype-directed antiplatelet therapy compared with routine care is underway. TAILOR-PCI60 will help to determine whether prospectively identifying patients with CYP2C19 loss-of-function alleles, and prescribing these individuals alternative antiplatelet therapy (such as ticagrelor), would be clinically beneficial. A total of 5,270 patients with stable coronary artery disease or acute coronary syndromes who have undergone PCI will be enrolled to either routine care or prospective CYP2C19 genotyping using a ‘point-of-care’ FDA-approved genotyping platform. The primary analysis will be conducted only in the approximately 1,694 at-risk patients with CYP2C19*2 or *3 alleles who have either received clopidogrel (in the control group) or ticagrelor (in the genotype group). The primary end points of the trial include myocardial infarction, stroke, cardiovascular death, severe recurrent ischaemia, and stent thrombosis at 1 year. A secondary aim is to assess the incidence of major or minor bleeding in participants with CYP2C19 loss-of-function allele(s) receiving ticagrelor versus those receiving clopidogrel.
In contrast to TAILOR-PCI, a superiority trial in which investigators are prospectively comparing the use of ticagrelor with clopidogrel in CYP2C19 loss-of-function carriers, the POPular Genetics study14,92 is a noninferiority trial, in which a genotyping strategy (ticagrelor in CYP2C19 loss-of-function allele carriers and clopidogrel in wild-type patients) is being compared with the universal use of ticagrelor or prasugrel in the nongenotyping group. The POPular Genetics study investigators plan to enrol 2,700 individuals who have undergone primary PCI after a ST-segment elevation myocardial infarction; patients who have received thrombolytics will be excluded. The primary end point is a net clinical benefit end point, and consists of a composite of death, stroke, myocardial infarction, stent thrombosis, urgent revascularization, and major bleeding at 1 year.
Both these trials are designed to determine whether patients pre-emptively identified by genotyping as being clopidogrel resistant have improved clinical outcomes after PCI if they are treated with alternative therapy, such as ticagrelor or prasugrel. These trials will also hopefully inform us on whether selective use of prasugrel or ticagrelor in carriers of the CYP2C19 loss-of-function allele can minimize the bleeding risks that are usually associated with these newer P2Y12 inhibitors, especially in those who could potentially benefit most from the drug. If so, this strategy could lead to a reduction in overall costs.
Challenges and future directions
Although RCTs are considered the gold standard of medical science, whether RCTs are the best modality to enable clinical implementation of pharmacogenetics is controversial.93 For example, conducting RCTs to assess the clinical utility of testing for rare genetic variants with large effect sizes that help to predict rare, but life-threatening, adverse drug reactions is impractical. Various legal and ethical considerations to performing genotype-based RCTs also exist.94 Detection of adverse drug reactions in a genotypically homogeneous and small study population might be difficult, and this lack of detection could pose unique risks, especially when prescribing a drug for ‘off-label’ use. Furthermore, if genetic testing identifies drug ‘nonresponders’, these individuals might be viewed as economically burdensome to the health-care system, which might raise privacy concerns and stigmatization fears for the patient. Drugs prescribed on the basis of genotype might also suffer the same fate as orphan drugs, resulting in higher costs to patients and third-party payers. The increased costs might stem from the need for a financial incentive for pharmaceutical companies to want to participate in this type of research, given the substantial cost of RCTs and the potential to benefit only a select group of patients.
To reduce costs, pharmacogenetic studies designed to demonstrate clinical utility are becoming increasingly pragmatic. For example, genetic association studies have been performed using electronic health records linked to DNA biobanks.94 Warfarin doses have been electronically extracted and pharmacogenetic studies have successfully replicated the association between stable warfarin dosing and genetic variants in VKORC1 and CYP2C9.95 The utility of pharmacogenetic information pre-emptively made available in the electronic health record, with clinical decision support modules, is being assessed in the eMERGE-PGx project.97 In this study, 9,000 patients will undergo next-generation sequencing to assess variation in 84 genes related to commonly prescribed drugs, and the genetic information will be integrated into the electronic health record. Information about a clinically validated pharmacogenetic marker will automatically be made available to the physician electronically, with a decision support module, when the corresponding drug is being prescribed. Physician prescribing patterns and clinical outcomes will be tracked. Whether such pragmatic approaches using electronic health records will enable clinical implementation of pharmacogenetics and will preclude the need for genotype-based RCTs remains to be seen.
Conclusions
Although costs of DNA genotyping and sequencing have dramatically decreased over the past decade, the identification and clinical implementation of pharmacogenetic markers remains a challenge. The easy availability of DNA samples from adequately powered, well-conducted clinical trials for both discovery and replication of pharmacogenetic markers is limited. Defining an accurate drug-response phenotype is also difficult, because clinical end points are often heterogeneous and not directly related to drug effect. Currently used techniques, such as GWAS, have been unable to identify genetic variants significantly associated with drug-response phenotypes in >50% of analysed cases.98 The negative results of these studies have been attributed to ‘missing heritability’ of complex traits, and might be a consequence of the presence of rare variants.26 Next-generation sequencing allows identification of these rare variants, but the expense of performing whole exome or whole genome sequencing in large studies, the statistical challenges of associating rare variants with drug-response phenotypes, and the limited accuracy of these techniques in detecting certain types of genetic variants, limit their use in pharmacogenomics.4 Although knowledge of rare variants might enable insight into drug biology, the use of these variants as a pharmacogenetic marker might not be practical. Despite successful identification of several pharmacogenetic markers, the functional annotation of these variants—such as the combined effect of gain-of-function and loss-of-function genetic variants on the drug phenotype—is unknown and, in the context of multiple drugs that have interactions with each other, the effect of pharmacogenetic variation on overall drug phenotype is also unknown.99 Ensuring the validity of the pharmacogenetic marker is, therefore, critically important before implementation of the marker into clinical practice. Adequately powered, pragmatic, prospective RCTs are required to translate the use of pharmacogenetic markers into clinical practice.
Key points.
Substantial progress has been made in the field of pharmacogenomics, with advances in genotyping and sequencing technology, and by the routine collection of DNA samples to study the drug-response phenotype
Genetic markers associated with drug toxicity and drug efficacy can be identified by candidate gene, genome-wide association, and next-generation sequencing studies
The potential of targeting the right patient with the right drug, and FDA labelling guidance to use pharmacogenetic markers, have provided new impetus to conduct genotype-based randomized clinical trials (RCTs)
Prospective approaches using a pharmacogenetic-based strategy with enrichment or adaptive designs are being increasingly used in cardiovascular RCTs
Clinical adoption of pharmacogenetics in the practice of cardiovascular medicine will become a reality when a transition has been made from conducting genetic association studies to rigorously performed genotype-based RCTs
Figure 3.
Acknowledgements
Supported in part by Mayo Transplant Scholarly Award (N.L.P.), U01 GM61388 (The Pharmacogenetics Research Network). The TAILOR-PCI study is funded in part by the Mayo Clinic Centre for Individualized Medicine, and the Mayo Clinic Division of Cardiology. We thank Ms Luanne Wussow, Mayo Clinic Rochester, MN, USA for her assistance with the preparation of this manuscript. We thank Bryony Mearns PhD, Chief Editor, Nature Reviews Cardiology for her editorial assistance and helpful suggestions.
Abbreviation
- RCT
randomized clinical trial
Footnotes
Competing interests
The authors declare no competing interests.
Author contributions
N.L.P. researched data for the article. N.L.P., D.J.S., and C.S.R. provided substantial contributions to discussion of the content. N.L.P., D.J.S., M.E.F., and C.S.R. wrote and reviewed/edited the article before submission.
References
- 1.Pereira NL, Weinshilboum RM. Cardiovascular pharmacogenomics and individualized drug therapy. Nat. Rev. Cardiol. 2009;6:632–638. doi: 10.1038/nrcardio.2009.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang L, McLeod HL, Weinshilboum RM. Genomics and drug response. N. Engl. J. Med. 2011;364:1144–1153. doi: 10.1056/NEJMra1010600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pereira NL, Weinshilboum RM. The impact of pharmacogenomics on the management of cardiac disease. Clin. Pharmacol. Ther. 2011;90:493–495. doi: 10.1038/clpt.2011.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Koboldt DC, Steinberg KM, Larson DE, Wilson RK, Mardis ER. The next-generation sequencing revolution and its impact on genomics. Cell. 2013;155:27–38. doi: 10.1016/j.cell.2013.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bowton E, et al. Biobanks and electronic medical records: enabling cost-effective research. Sci. Transl. Med. 2014;6:234cm3. doi: 10.1126/scitranslmed.3008604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thorn C, Klein T, Altman R. PharmGKB: The Pharmacogenomics Knowledge Base. Pharmacogenomics. 2013;1015:311–320. doi: 10.1007/978-1-62703-435-7_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.MacRae CA. Cardiac arrhythmia: in vivo screening in the zebrafish to overcome complexity in drug discovery. Expert Opin. Drug Discov. 2010;5:619–632. doi: 10.1517/17460441.2010.492826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiang J, et al. Genome-wide association study for biomarker identification of Rapamycin and Everolimus using a lymphoblastoid cell line system. Front. Genet. 2013;4:166. doi: 10.3389/fgene.2013.00166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Volzke H, et al. Personalized cardiovascular medicine: concepts and methodological considerations. Nat. Rev. Cardiol. 2013;10:308–316. doi: 10.1038/nrcardio.2013.35. [DOI] [PubMed] [Google Scholar]
- 10.Wang B, Canestaro WJ, Choudhry NK. Clinical evidence supporting pharmacogenomic biomarker testing provided in US Food and Drug Administration drug labels. JAMA Intern. Med. 2014;174:1938–1944. doi: 10.1001/jamainternmed.2014.5266. [DOI] [PubMed] [Google Scholar]
- 11.Ahmad T, et al. Charting a roadmap for heart failure biomarker studies. JACC Heart Fail. 2014;2:477–488. doi: 10.1016/j.jchf.2014.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pirmohamed M, et al. A randomized trial of genotype-guided dosing of warfarin. N. Engl. J. Med. 2013;369:2294–2303. doi: 10.1056/NEJMoa1311386. [DOI] [PubMed] [Google Scholar]
- 13.Kimmel SE, et al. A pharmacogenetic versus a clinical algorithm for warfarin dosing. N. Engl. J. Med. 2013;369:2283–2293. doi: 10.1056/NEJMoa1310669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bergmeijer TO, et al. CYP2C19 genotype-guided antiplatelet therapy in ST-segment elevation myocardial infarction patients—rationale and design of the Patient Outcome after primary PCI (POPular) Genetics study. Am. Heart J. 2014;168:16–22e1. doi: 10.1016/j.ahj.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 15.Takeuchi F, et al. A genome-wide association study confirms VKORC1, CYP2C9, and CYP4F2 as principal genetic determinants of warfarin dose. PLoS Genet. 2009;5:e1000433. doi: 10.1371/journal.pgen.1000433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liggett SB, et al. A polymorphism within a conserved β1-adrenergic receptor motif alters cardiac function and β-blocker response in human heart failure. Proc. Natl Acad. Sci. 2006;103:11288–11293. doi: 10.1073/pnas.0509937103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SEARCH Collaborative Group SLCO1B1 variants and statin-induced myopathy—a genomewide study. N. Engl. J. Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- 18.Daneshjou R, et al. Genetic variant in folate homeostasis is associated with lower warfarin dose in African Americans. Blood. 2014;124:2298–2305. doi: 10.1182/blood-2014-04-568436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Myocardial Infarction Genetics Consortium Investigators Inactivating mutations in NPC1L1 and protection from coronary heart disease. N. Engl. J. Med. 2014;371:2072–2082. doi: 10.1056/NEJMoa1405386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bollag G, et al. Clinical efficacy of a RAF inhibitor needs broad target blockade in BRAF-mutant melanoma. Nature. 2010;467:596–599. doi: 10.1038/nature09454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aithal GP, Day CP, Kesteven PJL, Daly AK. Association of polymorphisms in the cytochrome P450 CYP2C9 with warfarin dose requirement and risk of bleeding complications. Lancet. 1999;353:717–719. doi: 10.1016/S0140-6736(98)04474-2. [DOI] [PubMed] [Google Scholar]
- 22.Rost S, et al. Mutations in VKORC1 cause warfarin resistance and multiple coagulation factor deficiency type 2. Nature. 2004;427:537–541. doi: 10.1038/nature02214. [DOI] [PubMed] [Google Scholar]
- 23.Zhang JE, et al. Effects of CYP4F2 genetic polymorphisms and haplotypes on clinical outcomes in patients initiated on warfarin therapy. Pharmacogenet. Genomics. 2009;19:781–789. doi: 10.1097/FPC.0b013e3283311347. [DOI] [PubMed] [Google Scholar]
- 24.Ioannidis JP. To replicate or not to replicate: the case of pharmacogenetic studies: have pharmacogenomics failed, or do they just need larger-scale evidence and more replication? Circ. Cardiovasc. Genet. 2013;6:413–418. doi: 10.1161/CIRCGENETICS.113.000106. [DOI] [PubMed] [Google Scholar]
- 25.Aslibekyan S, Claas SA, Arnett DK. To replicate or not to replicate: the case of pharmacogenetic studies establishing validity of pharmacogenomic findings: from replication to triangulation. Circ. Cardiovasc. Genet. 2013;6:409–412. doi: 10.1161/CIRCGENETICS.112.000010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manolio TA, et al. Finding the missing heritability of complex diseases. Nature. 2009;461:747–753. doi: 10.1038/nature08494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Weeke P, et al. Exome sequencing implicates an increased burden of rare potassium channel variants in the risk of drug-induced long QT interval syndrome. J. Am. Coll. Cardiol. 2014;63:1430–1437. doi: 10.1016/j.jacc.2014.01.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashley EA, et al. Clinical assessment incorporating a personal genome. Lancet. 2010;375:1525–1535. doi: 10.1016/S0140-6736(10)60452-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Biesecker LG, Green RC. Diagnostic clinical genome and exome sequencing. N. Engl. J. Med. 2014;370:2418–2425. doi: 10.1056/NEJMra1312543. [DOI] [PubMed] [Google Scholar]
- 30.Green RC, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet. Med. 2013;15:565–574. doi: 10.1038/gim.2013.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein DB, et al. Sequencing studies in human genetics: design and interpretation. Nat. Rev. Genet. 2013;14:460–470. doi: 10.1038/nrg3455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scriver CR, Childs B, editors. Garrod’s Inborn Factors in Disease. Oxford University Press; 1989. [Google Scholar]
- 33.Johnson JA, et al. Clinical Pharmacogenetics Implementation Consortium Guidelines for CYP2C9 and VKORC1 genotypes and warfarin dosing. Clin. Pharmacol. Ther. 2011;90:625–629. doi: 10.1038/clpt.2011.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Paré G, et al. Genetic determinants of dabigatran plasma levels and their relation to bleeding. Circulation. 2013;127:1404–1412. doi: 10.1161/CIRCULATIONAHA.112.001233. [DOI] [PubMed] [Google Scholar]
- 35.Mega JL, et al. Cytochrome P-450 polymorphisms and response to clopidogrel. N. Engl. J. Med. 2009;360:354–362. doi: 10.1056/NEJMoa0809171. [DOI] [PubMed] [Google Scholar]
- 36.Mega JL, et al. Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA. 2010;304:1821–1830. doi: 10.1001/jama.2010.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang L, et al. Human thiopurine S-methyltransferase pharmacogenetics: variant allozyme misfolding and aggresome formation. Proc. Natl Acad. Sci. USA. 2005;102:9394–9399. doi: 10.1073/pnas.0502352102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lennard L, Van Loon JA, Weinshilboum RM. Pharmacogenetics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clin. Pharmacol. Ther. 1989;46:149–154. doi: 10.1038/clpt.1989.119. [DOI] [PubMed] [Google Scholar]
- 39.Van Loon JA, Weinshilboum RM. Human lymphocyte thiopurine methyltransferase pharmacogenetics: effect of phenotype on 6-mercaptopurine-induced inhibition of mitogen stimulation. J. Pharmacol. Exp. Ther. 1987;242:21–26. [PubMed] [Google Scholar]
- 40.Liang JJ, et al. TPMT genetic variants are associated with increased rejection with azathioprine use in heart transplantation. Pharmacogenet. Genomics. 2013;23:658–665. doi: 10.1097/FPC.0000000000000005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Price MJ, et al. Standard- vs high-dose clopidogrel based on platelet function testing after percutaneous coronary intervention: the GRAVITAS randomized trial. JAMA. 2011;305:1097–1105. doi: 10.1001/jama.2011.290. [DOI] [PubMed] [Google Scholar]
- 42.Collet J-P, et al. Bedside monitoring to adjust antiplatelet therapy for coronary stenting. N. Engl. J. Med. 2012;367:2100–2109. doi: 10.1056/NEJMoa1209979. [DOI] [PubMed] [Google Scholar]
- 43.Martin AM, et al. Predisposition to abacavir hypersensitivity conferred by HLA-B*5701 and a haplotypic Hsp70-Hom variant. Proc. Natl Acad. Sci. 2004;101:4180–4185. doi: 10.1073/pnas.0307067101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mallal S, et al. Association between presence of HLA-B*5701, HLA-DR7, and HLA-DQ3 and hypersensitivity to HIV-1 reverse-transcriptase inhibitor abacavir. Lancet. 2002;359:727–732. doi: 10.1016/s0140-6736(02)07873-x. [DOI] [PubMed] [Google Scholar]
- 45.Hetherington S, et al. Genetic variations in HLA-B region and hypersensitivity reactions to abacavir. Lancet. 2002;359:1121–1122. doi: 10.1016/S0140-6736(02)08158-8. [DOI] [PubMed] [Google Scholar]
- 46.Hughes AR, et al. Association of genetic variations in HLA-B region with hypersensitivity to abacavir in some, but not all, populations. Pharmacogenomics. 2004;5:203–211. doi: 10.1517/phgs.5.2.203.27481. [DOI] [PubMed] [Google Scholar]
- 47.Phillips EJ, et al. Clinical and immunogenetic correlates of abacavir hypersensitivity. AIDS. 2005;19:979–981. doi: 10.1097/01.aids.0000171414.99409.fb. [DOI] [PubMed] [Google Scholar]
- 48.Mallal S, et al. HLA-B*5701 screening for hypersensitivity to abacavir. N. Engl. J. Med. 2008;358:568–579. doi: 10.1056/NEJMoa0706135. [DOI] [PubMed] [Google Scholar]
- 49.Ramsey BW, et al. A CFTR potentiator in patients with cystic fibrosis and the G551D mutation. N. Engl. J. Med. 2011;365:1663–1672. doi: 10.1056/NEJMoa1105185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abrams J, et al. National Cancer Institute’s Precision Medicine Initiatives for the new National Clinical Trials Network. Am. Soc. Clin. Oncol. Educ. Book. 2014;34:71–76. doi: 10.14694/EdBook_AM.2014.34.71. [DOI] [PubMed] [Google Scholar]
- 51.de Morais SM, et al. The major genetic defect responsible for the polymorphism of S-mephenytoin metabolism in humans. J. Biol. Chem. 1994;269:15419–15422. [PubMed] [Google Scholar]
- 52.Teutsch SM, et al. The Evaluation of Genomic Applications in Practice and Prevention (EGAPP) initiative: methods of the EGAPP Working Group. Genet. Med. 2009;11:3–14. doi: 10.1097/GIM.0b013e318184137c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Roberts JD, et al. Point-of-care genetic testing for personalisation of antiplatelet treatment (RAPID GENE): a prospective, randomised, proof-of-concept trial. Lancet. 2012;379:1705–1711. doi: 10.1016/S0140-6736(12)60161-5. [DOI] [PubMed] [Google Scholar]
- 54.Lala A, et al. Genetic testing in patients with acute coronary syndrome undergoing percutaneous coronary intervention: a cost-effectiveness analysis. J. Thromb. Haemost. 2013;11:81–91. doi: 10.1111/jth.12059. [DOI] [PubMed] [Google Scholar]
- 55.Kazi DS, et al. Cost-effectiveness of genotype-guided and dual antiplatelet therapies in acute coronary syndrome. Ann. Intern. Med. 2014;160:221–232. doi: 10.7326/M13-1999. [DOI] [PubMed] [Google Scholar]
- 56.Simon RM, Paik S, Hayes DF. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J. Natl Cancer Inst. 2009;101:1446–1452. doi: 10.1093/jnci/djp335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Douillard J-Y, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N. Engl. J. Med. 2013;369:1023–1034. doi: 10.1056/NEJMoa1305275. [DOI] [PubMed] [Google Scholar]
- 58.Wallentin L, et al. Effect of CYP2C19 and ABCB1 single nucleotide polymorphisms on outcomes of treatment with ticagrelor versus clopidogrel for acute coronary syndromes: a genetic substudy of the PLATO trial. Lancet. 2010;376:1320–1328. doi: 10.1016/S0140-6736(10)61274-3. [DOI] [PubMed] [Google Scholar]
- 59.Sorich MJ, Vitry A, Ward MB, Horowitz JD, McKinnon RA. Prasugrel vs. clopidogrel for cytochrome P450 2C19-genotyped subgroups: integration of the TRITON-TIMI 38 trial data. J. Thromb. Haemost. 2010;8:1678–1684. doi: 10.1111/j.1538-7836.2010.03923.x. [DOI] [PubMed] [Google Scholar]
- 60.US National Library of Medicine. 2015 doi: 10.1080/15360280801989377. ClinicalTrials.gov [online], https://www.clinicaltrials.gov/ct2/show/NCT01742117. [DOI] [PubMed]
- 61.US National Library of Medicine. 2014 doi: 10.1080/15360280801989377. ClinicalTrials.gov [online], https://www.clinicaltrials.gov/ct2/show/NCT01970501. [DOI] [PubMed]
- 62.Maitournam A, Simon R. On the efficiency of targeted clinical trials. Stat. Med. 2005;24:329–339. doi: 10.1002/sim.1975. [DOI] [PubMed] [Google Scholar]
- 63.Freidlin B, Korn EL, Gray R. Marker Sequential Test. (MaST) design. Clin. Trials. 2014;11:19–27. doi: 10.1177/1740774513503739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mandrekar SJ, Sargent DJ. Clinical trial designs for predictive biomarker validation: theoretical considerations and practical challenges. J. Clin. Oncol. 2009;27:4027–4034. doi: 10.1200/JCO.2009.22.3701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Le Tourneau C, et al. Randomised proof-of-concept phase II trial comparing targeted therapy based on tumour molecular profiling vs conventional therapy in patients with refractory cancer: results of the feasibility part of the SHIVA trial. Br. J. Cancer. 2014;111:17–24. doi: 10.1038/bjc.2014.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim ES, et al. The BATTLE trial: personalizing therapy for lung cancer. Cancer Discov. 2011;1:44–53. doi: 10.1158/2159-8274.CD-10-0010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barker AD, et al. I-SPY 2: an adaptive breast cancer trial design in the setting of neoadjuvant chemotherapy. Clin. Pharmacol. Ther. 2009;86:97–100. doi: 10.1038/clpt.2009.68. [DOI] [PubMed] [Google Scholar]
- 68.Korn EL, Freidlin B. Outcome-adaptive randomization: is it useful? J. Clin. Oncol. 2011;29:771–776. doi: 10.1200/JCO.2010.31.1423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.CIBIS-II Investigators and Committees The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 70.Packer M, et al. The effect of carvedilol on morbidity and mortality in patients with chronic heart failure. N. Engl. J. Med. 1996;334:1349–1355. doi: 10.1056/NEJM199605233342101. [DOI] [PubMed] [Google Scholar]
- 71.Hjalmarson Ă , et al. Effects of controlled-release metoprolol on total mortality, hospitalizations, and well-being in patients with heart failure: Tte metoprolol CR/XL randomized intervention trial in congestive heart failure (MERIT-HF) JAMA. 2000;283:1295–1302. doi: 10.1001/jama.283.10.1295. [DOI] [PubMed] [Google Scholar]
- 72.Beta-Blocker Evaluation of Survival Trial Investigators A trial of the beta-blocker bucindolol in patients with advanced chronic heart failure. N. Engl. J. Med. 2001;344:1659–1667. doi: 10.1056/NEJM200105313442202. [DOI] [PubMed] [Google Scholar]
- 73.Moore JD, Mason DA, Green SA, Hsu J, Liggett SB. Racial differences in the frequencies of cardiac β1-adrenergic receptor polymorphisms: analysis of c145A>G and c1165G>C. Hum. Mutat. 1999;14:271. doi: 10.1002/(SICI)1098-1004(1999)14:3<271::AID-HUMU14>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 74.Tirona RG, Leake BF, Merino G, Kim RB. Polymorphisms in OATP-C: identification of multiple allelic variants associated with altered transport activity among European- and African-Americans. J. Biol. Chem. 2001;276:35669–35675. doi: 10.1074/jbc.M103792200. [DOI] [PubMed] [Google Scholar]
- 75.Pasanen MK, Neuvonen M, Neuvonen PJ, Niemi M. SLCO1B1 polymorphism markedly affects the pharmacokinetics of simvastatin acid. Pharmacogenet. Genomics. 2006;16:873–879. doi: 10.1097/01.fpc.0000230416.82349.90. [DOI] [PubMed] [Google Scholar]
- 76.Ramsey LB, et al. The clinical pharmacogenetics implementation consortium guideline for SLCO1B1 and simvastatin-induced myopathy: 2014 update. Clin. Pharmacol. Ther. 2014;96:423–428. doi: 10.1038/clpt.2014.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Danik JS, et al. Lack of association between SLCO1B1 polymorphisms and clinical myalgia following rosuvastatin therapy. Am. Heart J. 2013;165:1008–1014. doi: 10.1016/j.ahj.2013.01.025. [DOI] [PubMed] [Google Scholar]
- 78.Brunham LR, et al. Differential effect of the rs4149056 variant in SLCO1B1 on myopathy associated with simvastatin and atorvastatin. Pharmacogenomics J. 2012;12:233–237. doi: 10.1038/tpj.2010.92. [DOI] [PubMed] [Google Scholar]
- 79.Voora D, et al. The SLCO1B1*5 genetic variant is associated with statin-induced side effects. J. Am. Coll. Cardiol. 2009;54:1609–1616. doi: 10.1016/j.jacc.2009.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.US National Library of Medicine. 2014 doi: 10.1080/15360280801989377. ClinicalTrials.gov [online], https://www.clinicaltrials.gov/ct2/show/NCT01894230. [DOI] [PubMed]
- 81.Budnitz DS, Shehab N, Kegler SR, Richards CL. Medication use leading to emergency department visits for adverse drug events in older adults. Ann. Intern. Med. 2007;147:755–765. doi: 10.7326/0003-4819-147-11-200712040-00006. [DOI] [PubMed] [Google Scholar]
- 82. [Accessed April 7th, 2015];Coumadin (warfarin sodium) tablets Label. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2011/009218s107lbl.pdf.
- 83.Go AS, et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.De Morais SM, et al. Identification of a new genetic defect responsible for the polymorphism of (S)-mephenytoin metabolism in Japanese. Mol. Pharmacol. 1994;46:594–598. [PubMed] [Google Scholar]
- 85.Scott SA, et al. Clinical Pharmacogenetics Implementation Consortium guidelines for cytochrome P450-452C19 (CYP2C19) genotype and clopidogrel therapy. Clin. Pharmacol. Ther. 2011;90:328–332. doi: 10.1038/clpt.2011.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Brandt JT, et al. Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J. Thromb. Haemost. 2007;5:2429–2436. doi: 10.1111/j.1538-7836.2007.02775.x. [DOI] [PubMed] [Google Scholar]
- 87.Holmes MV, Perel P, Shah T, Hingorani AD, Casas JP. CYP2C19 genotype, clopidogrel metabolism, platelet function, and cardiovascular events: a systematic review and meta-analysis. JAMA. 2011;306:2704–2714. doi: 10.1001/jama.2011.1880. [DOI] [PubMed] [Google Scholar]
- 88.Umemura K, Furuta T, Kondo K. The common gene variants of CYP2C19 affect pharmacokinetics and pharmacodynamics in an active metabolite of clopidogrel in healthy subjects. J. Thromb. Haemost. 2008;6:1439–1441. doi: 10.1111/j.1538-7836.2008.03050.x. [DOI] [PubMed] [Google Scholar]
- 89. [Accessed April 7, 2015];FDA Drug Safety Communication: reduced effectiveness of Plavix (clopidogrel) in patients who are poor metabolizers of the drug. Available at: http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm203888.htm.
- 90.Holmes DR, Jr, et al. ACCF/AHA clopidogrel clinical alert: approaches to the FDA “boxed warning”: a report of the American College of Cardiology Foundation Task Force on Clinical Expert Consensus Documents and the American Heart Association endorsed by the Society for Cardiovascular Angiography and Interventions and the Society of Thoracic Surgeons. J. Am. Coll. Cardiol. 2010;56:321–341. doi: 10.1016/j.jacc.2010.05.013. [DOI] [PubMed] [Google Scholar]
- 91.Levine GN, et al. 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J. Am. Coll. Cardiol. 2011;58:e44–e122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 92.US National Library of Medicine. 2013 doi: 10.1080/15360280801989377. ClinicalTrials.gov [online], https://www.clinicaltrials.gov/ct2/show/NCT01761786. [DOI] [PubMed]
- 93.Mrazek DA, Lerman C. Facilitating clinical implementation of pharmacogenomics. JAMA. 2011;306:304–305. doi: 10.1001/jama.2011.1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rothstein MA, Epps PG. Ethical and legal implications of pharmacogenomics. Nat. Rev. Genet. 2001;2:228–231. doi: 10.1038/35056075. [DOI] [PubMed] [Google Scholar]
- 95.Kohane IS. Using electronic health records to drive discovery in disease genomics. Nat. Rev. Genet. 2011;12:417–428. doi: 10.1038/nrg2999. [DOI] [PubMed] [Google Scholar]
- 96.Xu H, et al. Facilitating pharmacogenetic studies using electronic health records and natural-language processing: a case study of warfarin. J. Am. Med. Inform. Assoc. 2011;18:387–391. doi: 10.1136/amiajnl-2011-000208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Rasmussen-Torvik LJ, et al. Design and anticipated outcomes of the eMERGE-PGx project: a multicenter pilot for preemptive pharmacogenomics in electronic health record systems. Clin. Pharmacol. Ther. 2014;96:482–489. doi: 10.1038/clpt.2014.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Daly AK. Genome-wide association studies in pharmacogenomics. Nat. Rev. Genet. 2010;11:241–246. doi: 10.1038/nrg2751. [DOI] [PubMed] [Google Scholar]
- 99.Altman RB, Whirl-Carrillo M, Klein TE. Challenges in the pharmacogenomic annotation of whole genomes. Clin. Pharmacol. Ther. 2013;94:211–213. doi: 10.1038/clpt.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]