Abstract
Biomonitoring is a very useful tool for assessing human exposure to environmental pollutants. This review discusses recent methods for the quantitative measurement of biomarkers of exposure to different classes of chemicals used in personal-care products (e.g., musk fragrances, preservatives, UV filters, and insect repellents) and consumer products (e.g., organophosphate flame retardants, phthalate esters, perfluorinated compounds, and industrial phenols). The measurements are mainly taken in urine, blood, and breast milk. We also discuss the different procedures commonly used for sample-pretreatment, extraction, and clean up, and chromatographic techniques currently used to determine these compounds. Finally, we present data on the main biomarkers occurring in different human specimens.
Keywords: Biomarker, Blood, Breast milk, Emerging pollutant, Flame retardant, Liquid chromatography with tandem mass spectrometry (LC-MS/MS), Musk fragrance, Perfluorinated compound, Personal-care product, Urine
1. Introduction
The potential health effects of industrial chemicals continually introduced into the environment and the massive use of personal-care products (PCPs) are subjects of increasing concern. In the framework of water and environmental control, some of these chemicals have been termed “emerging pollutants.” Many are high-production volume (HPV) chemicals. Although not necessarily new, their environmental fate and toxicological effects require additional study and evaluation. Included among these emerging pollutants are a diverse group of PCP substances (e.g., UV filters, preservatives and antimicrobials, musk fragrances, and insect repellents), and industrial chemicals used in a myriad of consumer products [e.g., organophosphate flame retardants (OPFRs), phthalate esters, perfluorinated compounds (PFCs), and industrial phenols].
Considerable research has already been devoted to assessing human exposure to emerging pollutants, particularly into the occurrence of these chemicals in various environmental constituents (e.g., air, water, and food) [1]. Also, important efforts are under way to evaluate the presence of these compounds in biological specimens (e.g., urine, blood, and breast milk), also known as human biomonitoring (see Fig. 1), Human biomonitoring has become an increasingly relevant tool for not only evaluating human exposure to chemicals, but also:
assessing the potential health risks associated with exposure to these compounds;
evaluating time trends in concentrations;
determining whether technological changes can affect human exposure;
conducting epidemiological studies; and,
evaluating the efficacy of regulatory actions [2].
Figure 1.
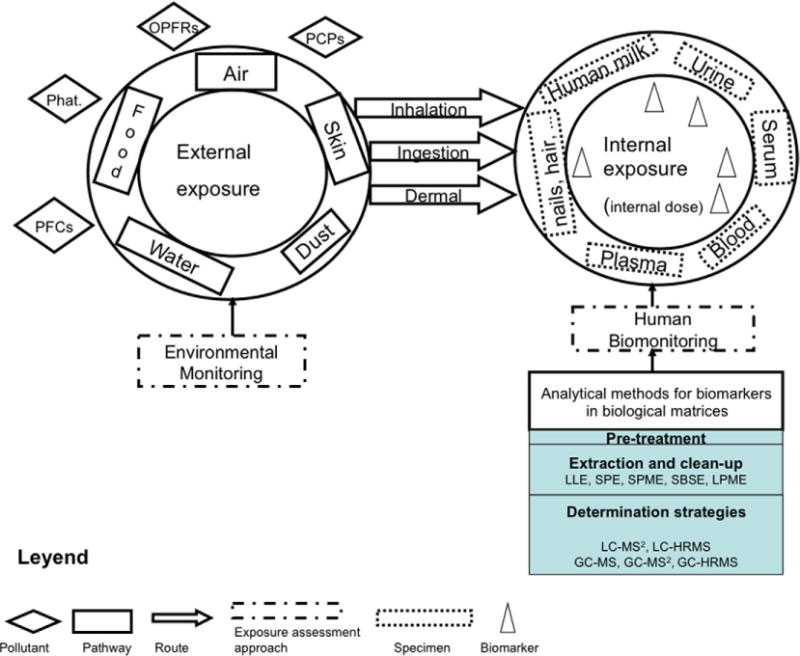
Scheme of human biomonitoring and the analytical methods (Phat, Phthalates; PFCs: Perfluorinated compounds; OPFRs, Organophospate flame retardants; PCPs, Personal-care products).
When assessing human exposure to environmental chemicals using biomonitoring, researchers need to select appropriate biomarkers and human specimens. Biomarkers can be the chemical substance itself, its metabolite(s), or the products of interaction between the chemical and target biomolecules. Biomarkers of exposure, which link the biomarker measured to specific environmental exposures, are most frequently used for the biomonitoring of environmental pollutants. Human biomonitoring has been applied in different countries as a successful tool in the exposure and risk assessment of pollutants, and human biomonitoring has been increasingly integrated into environmental and health monitoring [3–6].
Analytical capabilities are at the core of monitoring for emerging contaminants in various matrices. Analytical methods currently used for the determination of emerging pollutants in environmental compartments have been studied widely; recently, several outstanding critical reviews were published [7–9]. But not as much attention has been paid to the analytical methods employed for biomonitoring biomarkers of exposure to emerging pollutants.
The primary objective of this review is to survey the most relevant liquid chromatography (LC) and gas chromatography (GC) coupled to mass spectrometry (MS) in analytical methods published in the past five years. Our review focuses on routine measurement in human specimens (mainly urine, blood, and breast milk) of biomarkers of exposure to four groups of chemicals used in PCPs and four groups of industrial chemicals used in consumer products. We also include other considerations about the occurrence of the most relevant biomarkers.
2. Compounds
Table 1 shows the groups of chemicals used in PCPs and consumer products covered by this review. For some of the substances (i.e. synthetic musks, environmental phenols or PFCs), the biomarkers are the parent compounds, free or conjugated. However, for OPFRs or phthalates, the biomarkers of exposure are metabolites of the parent compound(s). Table 1 also shows the major sources of exposure to the different substances and the main target human specimens.
Table 1.
Biomarkers of exposure to emerging compounds and their parent compounds
| Biomarker [Formula name (Name, Other names, trade names). Abbreviation. (CAS RN)] |
Parent (CAS RN) | Some sources and pathways of exposures | Human specimen |
|---|---|---|---|
| 1. Personal care products (PCP) | |||
| 1.1. Synthetic musk fragrances | Dermal contact: deodorants, shampoos, detergents, washing and cleaning agents, fabric softeners | B, S, P, HM | |
| Polycyclic musks | |||
| 1,3,4,6,7,8-Hexahydro-4,6,6,7,8,8-hexamethylcyclopenta[l]-2-benzopyran (Galaxolide). HHCB. (1222-05-5) | * | ||
| 7-Acetyl-1,1,3,4,4,6-hexamethyl-1,2,3,4-tetrahydronaphthalene (Tonalide). AHTN. (1506-02-1) | * | ||
| 4-Acetyl-1,1-dimethyl-6-tert-butylindane (Celestolide). ADBI. (13171-00-1) | * | ||
| 5-Acetyl-1,1,2,6-tetramethyl-3-isopropyl-indane (Traseolide). ATII. (68140-48-7) | * | ||
| 6-Acetyl-1,1,2,3,3,5-hexamethyllindane (Phantolide). AHMI (15323-35-0) | * | ||
| HHCB-lactone (HHCB-l) | HHCB | ||
| Nitro musks | |||
| 1-Tert-butyl-3,5-dimethyl-2,4,6-trinitrobenzene (Musk xylene). MX (81-15-2) | * | ||
| 4-Tert-butyl-2,6-dimethyl-3,5-dinitroacetophenone (Musk ketone). MK (81-14-1) | * | ||
| 1,1,3,3,5-Pentamethyl-4,6-dinitroindan (Musk moskene). MM (116-66-5) | * | ||
| 4-Ter-butyl-3-methoxy-2,6-dinitrotoluene (Musk ambrette). MA (123-69-3) | * | ||
| 1-Ter-butyl-3,4,5-trimethyl-2,6-dinitrobenzene (Musk tibetene). MT (145-39-1) | * | ||
| 1.2. Antimicrobials and Preservatives | U | ||
|
|||
| 2,4,4′-Trichloro-2′-hydroxydiphenyl ether (Triclosan, Irgasan DP 300, Microban B). TCS. (3380-34-5) | * | ||
| 3,4,4′-Trichlorocarbanilide (Triclocarban). TCC. (101-20-2) | * | ||
| Methyl 4-hydroxybenzoate (Methylparaben).MP. (99-76-3) | * | ||
| Ethyl 4-hydroxybenzoate (Ethylparaben).EP. (120-47-8) | * | ||
| n-Propyl 4-hydroxybenzoate (n-Propylparaben).PP. (94-13-3) | * | ||
| n-Butyl 4-hydroxybenzoate (n-Butylparaben).BP. (94-26-8) | * | ||
| Benzyl 4-hydroxybenzoate (Benzylparaben).BzP. (94-18-8) | * | ||
| 1.3. UV filters | Dermal application: sunscreens and cosmetic products | U | |
| (2-Hydroxy-4-methoxyphenyl)(phenyl)methanone (Benzophenone-3, Oxybenzone, HMB). BP-3 (131-57-7) | |||
| 5-Benzoyl-4-hydroxy-2-methoxybenzenesulfonic acid [2-Hydroxy-4-Methoxy-5-sulfonylbenzophenone]. BP-4 | |||
| 2,4-Dihydroxyphenyl)(phenyl)methanone (2,4-Dihydroxibenzophenone, Aduvex 12). DHB, BP-1 | BP-3 | ||
| Bis(2,4-dihydroxyphenyl)methanone. BP-2 | BP-3 | ||
| (2-Hydroxy-4-methoxyphenyl)(2-hydroxyphenyl)methanone, [2,2′-dihydroxy-4-methoxybenzophenone, Dioxybenzone, BP-8]. DHMB (131-53-3), BP-8 Phenyl(2,3,4-trihydroxyphenyl)methanone, [2,3,4-Trihydroxybenzophenone].THB | BP-3 | ||
| 1.4. Insect repellents | Dermal contact and inhalation of aerosol formulations | U | |
| N,N-diethyl-3-methylbenzamide, [DEET].DEET. (134-62-6) | |||
| N-ethyl-3methylbenzamide. ET | DEET | ||
| N,N-diethyl-3-(hydroxymethyl)benzamide. DHMB 3-(Diethylcarbamoyl)benzoic acid. DCB. Bayrepel (119515-38-7) |
DEET | ||
| 2. Industrial chemicals | |||
| 2.1. Organophosphate flame retardants (OPFRs) | Ingestion of contaminated foods Inhalation of contaminated indoor and outdoor air |
U | |
| Bis(1,3-dichloro-2 propyl) phosphate. BDCPP | TDCPP, tris(1,3-dichloro-2-propyl) phosphate (13674-87-8 | ||
| Diphenyl phosphate. DPP | TPP, Triphenyl phosphate (115-86-6) | ||
| Bis-2 chlorethylphosphate. BCEP | TCEP, tris(2-chlorethyl)phosphate (115-96-8) | ||
| Di-m-cresylphosphate. DmCP | TmCP, tri-m-cresylphosphate | ||
| Di-p-cresylphosphate. DpCP | TpCP, tri-p-cresylphosphate | ||
| Di-b-butyl phosphate. DBP | TBP, Tri-n-butylphosphate | ||
| Bis(2-chloropropyl)phosphate. BCPP | TCPP, Tris(2-chlropropyl)phosphate | ||
| 2.2. Phthalates | Exposure through diet and life-style dependent pathways (e.g. cosmetics, body care products) | U | |
| Monoethyl phthalate. MEP (2306-33-4) | Diethyl phthalate (DEP) (84-66-2) | ||
| Mono-n-butyl phthalate. MBP (131-70-04) | Dibutyl phthalate (DBP)(84-74-2) | ||
| Mono-isobutyl phthalate. MiBP | Dibutyl phthalate (DBP)(84-74-2) | ||
| Monocyclohexyl phthalate. MCHP (7517-36-4) | Dicyclohexyl phthalate (DCHP (84-61-7) | ||
| Mono(2-ethyl-5-oxohexyl) phthalate. MEOHP | Di-2-ethylhexyl phthalate (DEHP) (117-81-7) | ||
| Mono(2-ethyl-5-hydroxyhexyl) phthalate. MEHHP | Di-2-ethylhexyl phthalate (DEHP) (117-81-7) | ||
| Mono(2-ethyl-5-carboxypentyl) phthalate. MECPP (40809-41-4) | Di-2-ethylhexyl phthalate (DEHP) (117-81-7) | ||
| Mono(2-ethylhexyl) phthalate. MEHP (4376-20-9) | Di-2-ethylhexyl phthalate (DEHP) (117-81-7) | ||
| Monobenzyl phthalate. MBzP (2528-16-7) | Benzylbutyl phthalate (BzBP) (85-68-7) | ||
| Mono-methyl phthalate. MMP (4376-18-5) | Dimethyl phthalate (DMP) (131-11-3) | ||
| Mono (3-carboxypropyl) phthalate. MCPP | Di-n-octyl phthalate (DOP) (117-84-0) | ||
| Mono-n-octyl phthalate. MOP | Di-n-octyl phthalate (DOP) (117-84-0) | ||
| Monocarboxy-isooctylphthalate. MCOP | Di-isononylphthalate (DiNP) (28553-12-0) | ||
| Mono-isononyl phthalate. MiNP | Di-isononylphthalate (DiNP) (28553-12-0) | ||
| Monooxo-isononyl phthalate. Oxo-MiNP | Di-isononylphthalate (DiNP) (28553-12-0) | ||
| Monocarboxy-isononyl phthalate. MCNP | Di-isononylphthalate (DiNP) (28553-12-0) | ||
| 2.3. Perfluorinated compounds (PFCs) | Diet Inhalation of outdoor and indoor air |
B,S,P,HM | |
| Perfluorooctane sulfonamide. PFOSA | * | ||
| N-methyl-perfluorooctane sulfonamide. Me-PFOSA | * | ||
| N-ethyl-perfluorooctane sulfonamide. Et-PFOSA | * | ||
| 2-(N-methyl-perfluorooctanesulfonamido) ethanol. Me-PFOSA-EtOH | * | ||
| 2-(N-ethyl-perfluorooctanesulfonamido) ethanol. Et-PFOSA-EtOH | * | ||
| 2-(N-methyl-perfluorooctanesulfonamido) acetic acid. Me-PFOSA-AcOH | * | ||
| 2-(N-ethyl-perfluorooctanesulfonamido) acetic acid. Et-PFOSA-AcOH | * | ||
| Perfluorobutane sulfonate. PFBuS | * | ||
| Perfluorohexane sulfonate. PFHxS | * | ||
| Perfluorooctane sulfonate. PFOS | * | ||
| Perfluoropentanoate. PFPeA | * | ||
| Perfluorohexanoate. PFHxA | * | ||
| Perfluoroheptanoate. PFHpA | * | ||
| Perfluorooctanoate. PFOA | * | ||
| Perfluorononanoate. PFNA | * | ||
| Perfluorodecanoate. PFDeA | * | ||
| Perfluoroundecanoate. PFUA | * | ||
| Perfluorododecanoate. PFDoA | * | ||
| Perfluoro-7-methyl octanoic acid. i,p-PFNA | * | ||
| 2.4. Environmental phenols (alkyl phenols (AP) and chlorophenols (CP)) | * | Diet (AP), and through chlorinated drinking water or air inhalation (CPs) | U |
| 4,4′-Propane-2,2-diyldiphenol (Bisphenol A). BPA | * | ||
| Ortho-phenyl phenol. oPP | * | ||
| 2,4-Dichlorophenol. 2,4-DCP | 2,4-Dichlorophenoxyacetic acid (2,4-D) and other chlorophenols | ||
| 2,5-Dichlorophenol. 2,5-DCP | 1,4-Dichlorobenzene | ||
| 2,4,5-Trichlorophenol. 2,4,5-TCP | Several organochlorine chemicals, including hexachlorobenzene and hexachlorocyclohexanes | ||
| 2,4,6-Trichlorophenol. 2,4,6-TCP | Several organochlorine chemicals, including hexachlorobenzene and hexachlorocyclohexanes |
The biomarker is the parent compound free o conjugated; B: Whole blood; S: Serum; P: Plasma; U: Urine; HM: Human milk.
2.1. Personal-care products
Through the use of PCPs and other household products, humans are continually exposed to synthetic musks, preservatives and antimicrobials, sunscreen filters, and insect repellents. Dermal contact can be a major route of exposure to these compounds. Synthetic musks, which scent a variety products [10], are lipophilic and persistent in the body, so they are expected to accumulate in lipid-rich tissues, human milk, and blood. Some studies suggest a half-life of several months [11].
Antimicrobials [e.g., triclosan (TCS) and triclocarban (TCC)] can be absorbed across the skin into the blood stream and are excreted over several days in bile, feces, and urine. Total (free plus conjugated) and conjugated (sulfated and glucuronidated) concentrations of TCS and TCC can serve as biomarkers of human exposure to these biocides [12,13].
Apart from dermal exposure, parabens, commonly used preservatives in cosmetics, can appear in the body through ingestion because they are also used in some food products. Parabens are hydrolyzed to p-hydroxybenzoic acid (a non-specific biomarker) and excreted in urine (free or conjugated). They can also be excreted as parent compounds in their free or conjugated form (e.g., glucuronidated) [14,15]. Researchers often use the concentration of the total urinary species of parent parabens as a specific biomarker of exposure to parabens in humans [16].
Sunscreen agent benzophenone-3 undergoes both Phase I and Phase II metabolism, and the metabolites are excreted in urine within hours after exposure [17]. The Phase I metabolites include BP-1, BP-8 and 2,3,4-trihydroxybenzophenone [18]. BP-3 or any of its metabolites can potentially be used as biomarkers of exposure to BP-3 [19].
Insect repellent N,N-diethyl-3-methylbenzamide (DEET) is metabolized in the human body and excreted in urine. Although understanding of the DEET metabolism remains incomplete, some dealkylated and oxidized metabolites have been described [20–23]. Urinary levels of DEET and its metabolites reflect recent exposures.
2.2. Industrial chemicals
After exposure, organophosphorous triesters are hydrolyzed to the corresponding dialkylphosphates and diarylphosphates in blood and urine [24].
Copper et al. [25] reported the presence in urine of bis(1,3-dichloro-2-propyl)phosphate (BDCPP) and diphenyl phosphate (DPP) – metabolites of OPFRs tris(1,3-dichloro-2-propyl)phosphate and triphenyl phosphate, respectively – that could serve as biomarkers of exposure to their parent compounds.
Schindler et al. [26] studied the presence in human urine of one chlorinated metabolite, bis-2-chlorethylphosphate, and three metabolites with aromatic groups, namely DPP, di-m-cresyl phosphate, and di-p-cresyl phosphate.
After exposure, phthalates are rapidly metabolized to their respective hydrolytic monoesters. For some phthalates, the monoesters can be further transformed to their oxidative products as free or conjugated species before excretion [27]. Urinary concentrations of all of these phthalate metabolites are used as biomarkers of exposure in urine [4].
PFCs are amphiphilic chemicals that do not accumulate preferentially in adipose tissues, but they bind easily to blood proteins and accumulate in the liver and the kidneys [28]. Humans appear to have a long half-life (3–5 years) of serum elimination of some PFCs [e.g., perfluorooctane sulfonate (PFOS), perfluorohexane sulfonate (PFHxS) and perfluorooctanonate (PFOA)]. For human biomonitoring purposes, PFCs are often measured in blood serum and breast milk. Unlike other persistent organic pollutants, PFCs are not lipophilic, so their concentrations in breast milk are much lower than in serum.
Apart from the phenols found in PCPs (e.g., triclosan, triclocarban, and BP-3), humans and wildlife are exposed to a large variety of other phenolic compounds through the production and use of certain industrial products. Bisphenol A (BPA), alkyl phenols, and chlorophenols are included among the most common of these environmental phenols.
After exposure, these phenols are metabolized and, after glucuronidation and sulfation in the liver, eliminated mainly in urine [29], so human exposure to phenolic compounds can be assessed by measuring the total (free and conjugated) compounds in urine [19].
3. Analytical methods
Generally, the quantitative measurement of biomarkers of exposure to organic pollutants in human specimens includes a sample-pretreatment step, followed by an extraction and clean-up process, and finally by a separation and detection method. Table 2 shows a selection of relevant analytical procedures proposed in recent literature.
Table 2.
Select mass spectrometry analytical methods for the determination of biomarkers of musk fragrances, insect repellents, environmental phenols, and other industrial chemicals in biological samples
| Analytes | Matrix | Pre-treatment | Extraction/Clean up | Analytical system | Analytical Column/mobile phase | Analytical performance | Ref. |
|---|---|---|---|---|---|---|---|
| ADBI, HHCB, AHTN, MM, MK, MX | B (2 mL) | PP (Eth) | LLE (Hx)/SPE (silica gel) | GC-(EI+)-MS(SIM) | HP-5MS (30 m × 0.25 mm, 0.25 μm) | LOD: 0.13–0.15 μg/L; Recovery: 77.9–118.5%; RSD: 1.5–9.5% | [30] |
| ADBI, AHMI, MA, ATII, HHCB, MX, AHTN, MM, MT, MK, MT, HHCB-l | HM (15 g) | Freeze-drying | ASE (Hx-DCM, 1:1)/GPC (EA-cHx, 1:1) + SPE(Florisil) | GC-MS2 | DB-5M (30 m × 0.25 mm, 0.25 μm) | LOD: 0.6–5.4 ng/g lipid; Recovery: >84.2%; RSD: <20% | [31] |
| HHCB, AHTN, MX, MK | HM | PP (Eth) | LLE (DE-Hx)/GPC (DCM-Hx) + SPE (silica gel, Hx, DCM, sequential) | GC(EI)-MS(SIM) | HP-5MS (30 m × 0.25 mm, 0.25 μm) | LOD: 4–5 ng/g lipid; Recovery: 71–118% | [31] |
| ADBI, HHCB, ATII, AHTN, MA, MK, MT, MX | B (9 mL) | PP (ACN) | LLE (Pe)/SPE (silica gel, Hx-EA, DCM, EA, sequential) + SPE (AlOx) | GC (NCI)-MS (SIM) | DB-624 (60 m × 0.25 × 1.4 μm) | LOD: 3–62 ng/L Recovery: 70.5–88.3%; RSD: 2–8.3% | [11] |
| DEET, DHMB, ET, DCB | U (1 mL) | ED | SPE: Oasis HLB, EE | HPLC-APCI(+)-MS/MS | Betasil phenyl (100 × 4.6 mm, 5 μm)/W-ACN (1% acetic acid) | LOD: 33–139 pg/mL. Recovery: 78–112%; RSD: <18% | [33] |
| BPA, TCS, BP-3, oPP; 2,4-DCP, 2,5-DCP; 2,4,5-TCP, MP, EP, PP, BP, BzPB | S (0.1 mL) | ED. Dilution (0.1 M FAc) | On-line SPE (LiChrosphere RP-18 ADS, 25 mm × 4 mm, 25 μm). Eluent:MeOH-W | LC-APCI(−)-MS/MS | 2 Columns Chromolith Performance RP-18 (100 mm × 4.6 mm)/MeOH-W |
LOD: 0.1–0.5 ng/mL; Recovery: 92–115%; RSD: 6.8–25.1% | [34] |
| BPA, TCS | S (1 mL), U (3 mL) | ED. FAc | SPE (Oasis HLB, MeOH-DCM); derivatization with PFBCl; SPE (AS) | GC-ECNI-MS(SIM) | DB-5 (30 m × 0.25 mm, 0.25 μm) | LOD: 0.05–0.5 ng/mL Recovery: 76–110% RSD: <20% | [35] |
| Phenols | U (1 mL) | ED | SBSE (500 μm-thick PDMS)(derivatization with BSTFA during TD step) | TD-GC(EI)-MS(SIM) | DB-5MS (30 m × 0.25 mm, 0.5 μm) | LOD: 0.01–0.11 ng/mL Recovery: 91–95% RSD: <2.5–7.1% | [36] |
| BPA | U (1 mL) | ED | HF-LPME (in situ derivatization with AAA) | GC(EI)-MS(SIM) | DB-5MS (30 m × 0.25 mm, 0.5 μm) | LOD: 0.02 ng/mL Recovery: 101% RSD: 7% | [37] |
| TCS | Urine (1 mL) | ED | SBSE (500 μm-thick PDMS) | TD-GC(EI)-MS(SIM) | DB-5MS (30 m × 0.25 mm, 0.5 μm) | LOD: 0.05 ng/mL Recovery: 102–113% RSD: 2.4–6.7% | [38] |
| BP-3, DHB, DHMB, THB | U (5 mL) Se (0.7 mL) | ED | SPE: Bond Elut C18, acetone | LC-ES(+)-MS/MS | Mediterranean SEA 18 (50 mm × 2.1 mm, 3 μm)/W (0.1% FAc)-MeOH (0.1% FAc) | LOD: 0.13–0.19 ng/mL Recovery: 105–114% RSD: 7.2–9.4% | [18] |
| BPA, BPB | U (5 mL) | ED | DLLME (ACN-T4CE), in situ derivatization with AAA | MD-GC-(EI)-MS | DB-5HT (5 m × 0.32 mm, 0.10 μm/DB-5MS (20 m × 0.18 mm, 0.18 μm) |
LOD: 0.03–0.05 ng/mL Recovery: 56–77% RSD: 7–20% | [39] |
| BP-3, DHB, HMB, BP-2 | U (0.5 mL) | ED | LLE (shaking with 50% MTBE:EA) | LC-ESI(−)-MS/MS | BETASIL C18 (100 mm × 2.1 mm × 5 μm)/methanol:water |
LOD: 0.082–0.28 ng/mL Recovery: 85.2–99.6% RSD: 1.4–4.5% | [40] |
| MP, EP, PP, BP, BP, TCS, BPA, BP-3, 2,4-DCP, 2,5DCP, 2,4,5-TCP, oPP | HM (0.1 mL) | ED; PP (MeOH); Dilution (0.1 M FAc) | On-line SPE (LiChrosphere RP-18 ADS (25 mm × 4 mm, 25 μm) | LC-APCI(−)-MS/MS | Zorbax Eclipse XDB-C8 (150 mm × 4.6 mm, 5 μm)/MeOH-W | LOD: 0.1–1 ng/mL Recovery: 84–119% RSD: 11–16.6% | [41] |
| MP, EP, PP, BP, BzP | U (0.5 mL) S (0.5 mL) | ED | Automated SPE (Strata XL; ACN, EA, sequential) | LC-ESI (−)-MS/MS | Synergi 4U fusion-RP 80A, (75 mm × 2mm × 4 μm)/0.1% AA in W-0.1 % AA in CAN | LOD: 0.03–0.41 ng/mL Recovery: 75.6–135.6% RSD: 2.5–14.8% | [42] |
| BPA, DHB, HMB, DHMB, MP, EP, PP, BP, | Pl (1.5 g) | n. a. | SLE (shaking) with EA | LC-APCI(−)-MS/MS | Gemini C18 (100 mm × 2.0 mm, 3 μm)/0.1% ammoniacal aqueous solution: 0.1% ammonia in MeOH | LOD: 0.03–0.6 ng/g; Recovery: 95–106% RSD: 2–8% | [43] |
| MP, EP, PP, BP | Pl (1.5 g) | n. a. | SLE (shaking) with EA | LC-APCI(−)-MS/MS | Gemini C18 (100 mm × 2.0 mm, 3 μm)/0.1% ammoniacal aqueous solution: 0.1% ammonia in MeOH | LOD: 0.03–0.06 ng/g; Recovery: 82–108% RSD: <14% | [44] |
| MP, EP, PP, BP | Hbct (3 g) | SLE (shaking with acetone-Hx (1:1)/SPE (silica gel). Derivatization with MSTFA | GC-MS(SIM) | DB-1 (30 m × 0.32 mm, 0.25 μm) | LOD: 1.05–3.75 ng/g Recovery: 96–113% RSD: 4.6–15.6% | [45] | |
| 14 PFCs | HM (3 mL) | LLE with acetone + SPE (Oasis HLB, MeOH-NH4OH (99:1)+ SPE (carbon graphitized) | LC-(ESI−)-HRMS (Orbitrap) | Geminis C18 (50 × 2 mm, 3 μm)/MeOH-ammonium acetate | LOD:1–50 pg/mL Recovery: %RSD: 1–14 % | [46] | |
| 14 PFCs | B (1 mL) | TBA (ion pairing) | LLE (MTBE) | UPLC(ESI−)-MS/MS | BEH C18 (50 × 2.1 mm, 1.7 μm)/2 mM ammonium acetate-MeOH | LOD: 0.009–0.34 ng/mL Recovery: 77–120% RSD: <10%, 16% | [47] |
| 21 PFCs | S (0.15 mL) | PP (MeOH) Dilution (FaC) | On-line SPE (Betasil C8, 10 × 4 mm, 5 μm) | LC(ESI−)-MS/MS | Betasil C8 (50 × 2.1 mm, 3 μm)/ACN-W | LOD: 0.002–0.05 ng/mL Recovery: 85–121% RSD: 1.2–12% | [48] |
| 7 PFCs | HM (0.4 mL). S (0.2 mL) | HM: EPH; PP (MeHO). S: PP (ACN) | On-line SPE (Oasis HLB (20 × 2.2 mm, 25 μm) | LC-(ESI−)-MS/MS | ReproSil-Pur-ODS3, (150 × 2 mm, 5 μm) | LOD: 0.02–0.15 μg/L; 0.1–0.4 μg/L (serum) Recovery: 73–112% RSD: 5–17% | [49] |
| MEP, MBP, MEHP, MINP, MBz | U (2 mL) | ED | LLE (Hx). Derivatization with Diazomethane/SPE (Florisil) | GC(EI)-(SIM)-MS | HP-5MS SV (30 m × 0.25 mm, 0.5 μm) | LOQ: 1–5 ng/mL; Recovery: 86.3–119% RSD: 0.6–9.1% | [50] |
| 22 Phthalates | U (0.1 mL) | ED Dilution (AA-ACN-W) | On-line SPE (Chromolith Flash RP-18e, 25 mm × 4.6 mm, 2 μm). Eluent: 0.1% AA in water: 0.1% AA in CAN | LC-ESI(−)-MS2 | BETASIL Phenyl (100 mm × 2.1 mm, 3 μm)/0.1% AA in water:0.1% AA in CAN |
LOD: 0.2–1.1 ng/mL; Recovery: % RSD: 3.3–14% | [51] |
| BDCPP, DPP | U (5 mL) | Dilution (AA) | SPE (StrataX-AW, ACN) | LC-(APCI−)-MS/MS | Kinetex XBC18 (fused core) (100 × 2.1 mm, 2.6 μm)/W_MeOH | LOD: 8–208 pg/mL Recovery: 71–95%RSD%: 4–23% | [25] |
| BCEP, DPP, DmCP, DpCP | U (5 mL) | Derivatization with PFBBr | SPE (Isolute ENV+, ACN); SPE (Bon ElutPSA + BonElutFl, acetone-Hx, 30:70) | GC-MS/MS | DB-35MS (35%-hexyl-ethylpolysiloxane, 60 m × 0.25 mm, 0.25 μm) | LOD: 0.1–1 μg/L Recovery: 79–113% RSD: 3.3–9.6% | [24] |
| DBP, DPhP, and other 12 phosphate compounds | U (3 mL) | PP (ACN); Dilution (MeOH-W) | LC-(ESI−)-MS/MS | Luna Phenyl-Hexyl 150 × 2 mm, 3 μm) | LOD: 0.3–11 μg/L Recovery: 69–122% RSD: 20% | [52] | |
| DBP, BCPP | U (5 mL) | Derivatization with PFBBr | SPE(Isolute ENV+, ACN); SPE (Bon ElutPSA+BonElutFl, acetone-Hx, 30:70) | GC-MS/MS | DB-35MS (35%-hexyl-ethylpolysiloxane, 60 m × 0.25 mm, 0.25 μm) | LOD: 0.25 μg/L Recovery: 97–100.6% RSD: 1.8–5.5% | [26] |
B, Whole blood; U, Urine; S, Serum; Se, Semen; Pl, Placenta; Hbct, Human breast cancerous tissue; PP, Protein precipitation; Eth, Ethanol; Hx, Hexane; EA, Ethyl acetate; SLE, Solid-liquid extraction; DCM, Dichloromethane; EA, Ethyl acetate; AlOx, Aluminum oxide; EE, Ethyl ether; W, Water; FAc, Formic acid; SBSE, Stir-bar sorptive extraction; EPH, Enzymatic protein hydrolysis; TD, Thermal desorption; PDMS, Polydimethylsiloxane; ASE, Accelerated solvent extraction; GPC, Gel-permeation chromatography; LLE, Liquid-liquid extraction; ED, Enzymatic de-conjugation; AA, Acetic acid; ACN, Acetonitrile; MSTFA, N-Methyl-N (trimethylsilyl) trifluoroacetamide; PFBCI, Pentafluorobenzoylchloride; AS, Acidified silica; AAA, Acetic acid anhydride; BSTFA, N,OBis(trimethylsilyl)acetamide; HP-LPME, Hollow-fiber liquid-phase microextraction; TBAHSO4, Tetrabutylammonium hydrogen sulfate; THF, Tetrahydrofuran; MTBE, Methyl-t-butyl ether; DLLME, Dispersive liquid-liquid microextraction; T4CE, Tetrachloroethylene; MD, Multi-dimensional; SDME, Single-drop microextraction; C6MIM][PF6], 1-hexyl-3-methylimidazolium hexafluorophosphate;; DEA, Diethylaminopropyl; LLE, Liquid-liquid extraction; LD, Liquid desorption; TBA, Tetra-n-butylammonium hydrogen sulfate; PFBBr, Pentafluorobenzylbromide.
Pre-treatment of biological samples to remove interferences or to hydrolyze the conjugated forms of the target biomarkers is often required. For biomonitoring of hydrophilic non-persistent chemicals (e.g., phenols, parabens, or phthalates), urine is the matrix of choice. Many of these chemicals are excreted as urinary glucuronide and sulfate conjugates [52]. Deconjugation is usually done by an enzymatic hydrolysis treatment (it can also be an acid hydrolysis) to deconjugate selectively glucuronides and/or sulfated conjugates. In many cases, the pretreatment is a simple dilution of the urine with water or formic acid. This dilution reduces any between-sample matrix variability that could otherwise affect analyte recovery.
Blood and its products (whole blood, plasma, and serum), and breast milk are the matrices most widely used for biomonitoring of human exposure to persistent compounds (e.g., synthetic musks). To disrupt the protein-compound binding and facilitate (e.g., prevent clogging) solid-phase extraction (SPE), researchers commonly use protein precipitation with acetonitrile [44], methanol [49] or freeze-drying [31].
3.1. Extraction and clean-up strategies
Although direct analysis of samples or pretreated samples is optimal, additional sample clean-up is usually necessary. Extraction and purification strategies range from classic liquid-liquid extraction (LLE) to recent developments in the field of miniaturized methods [e.g., solid-phase microextraction (SPME)]. LLE is still widely employed for the extraction of biomarkers from urine, human milk, and blood. Ethyl acetate, hexane and acetone are the most widely employed solvents for the extraction of different biomarkers {e.g., benzophenone derivatives [41], phthalates [51], musk fragrances [32] or PFCs [47] in biological matrices}.
Guo et al. [48] used an ion-pairing agent to permit the use of a non-polar solvent (e.g., methyl-tert-butyl ether) for the extraction of 14 PFCs in human blood. However, LLE is time consuming, solvent consuming, and labor intensive.
SPE is one of is the most important sample-preparation approach to extract and to purify analytes from liquid matrices (i.e. urine, blood, saliva, and milk), with both off-line and on-line configurations. Researchers need to select an appropriate SPE sorbent, and to optimize elution solvent, sample volume, and pH conditions.
Although some conventional bonded silica sorbents are still in use [18], these are being replaced by hydrophilic-hydrophobic balance polymeric material {e.g., Oasis HLB [36] or Strata XL [43]}. For polar anionic compounds (e.g., OPFRs and PFCs), weak-anion exchange sorbents {e.g., StrataX-AW and Oasis WAX [105]} have proved useful [25]. SPE provides better selectivity and higher recoveries, and uses much less solvent than conventional LLE. For large-scale biomonitoring and epidemiological studies, high throughput with adequate sensitivity is necessary. For this purpose, on-line SPE techniques have been proposed [50]. SPE with restricted access materials, which combine both reversed-phase separation and size-exclusion mechanisms [54], was used on-line in a column-switching configuration for the determination of five parabens and seven environmental phenols in serum [35]. Likewise, monolithic materials [55] have been used in some online SPE methods for the determination of a wide range of urinary phthalate metabolites [52].
Sorptive extraction is based on equilibrium processes between an aqueous sample and a sorbent. It includes SPME, stir-bar sorptive extraction (SBSE), and other miniaturized techniques that have acquired the generic name of liquid-phase microextraction (LPME) [56]. Unlike conventional LLE, LPME reduces the volume of organic solvents used, and has been used for the extraction of BPA and other environmental phenols from urine [37].
For the analysis of solid matrices (e.g., placenta or human breast cancerous tissues), current methods involve solid-liquid extraction (SLE) using ethyl acetate for parabens and phthalates [44] and acetone:hexane (1:1) [46]. Likewise, pressurized fluid extraction (PFE) has been proposed for the quick extraction of synthetic musks [31].
3.2. Determination strategies
As Fig. 2 illustrates, the analytical methods most commonly employed for the determination of biomarkers of exposure to environmental pollutants are LC-tandem mass spectrometry (LC-MS/MS) and GC-MS. These bio-markers belong to different families of chemicals with divergent physicochemical properties, so the choice for using LC or GC depends on the polarity, volatility, and thermal stability of the analytes. Whereas musk fragrances, due to their high volatility, are GC-amenable compounds, others (e.g., phthalates, parabens or PFCs) are generally measured by LC-MS/MS.
Figure 2.
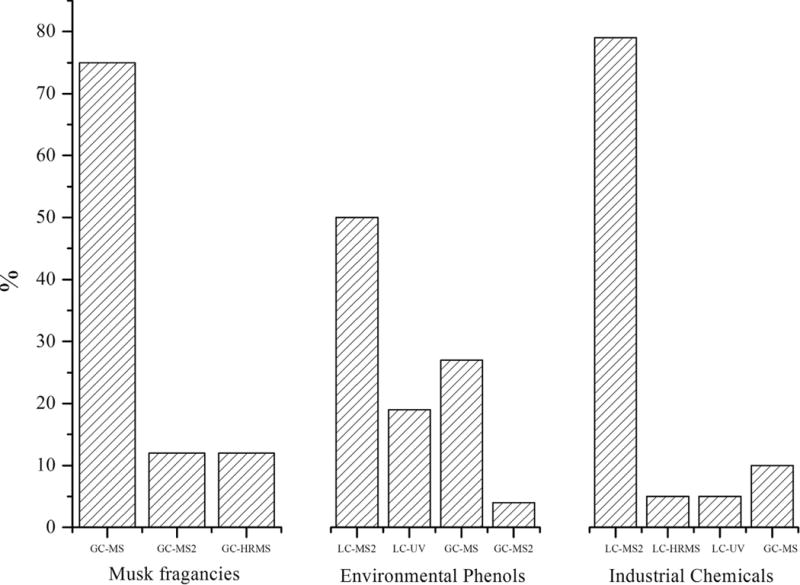
Percentage of analytical studies using LC and GC techniques for the biomarkers of the different pollutants (studies published 2005–2011; number of studies considered = 53).
Musk fragrances are determined by GC-MS with electron-impact and selected-ion-monitoring modes. The limits of detection (LODs) are at sub-ng/mL levels. Recently, Hutter et al. [11] published a method for measuring 11 synthetic musks in blood using GC-MS with negative chemical ionization. The method provides LODs of 0.003–0.062 ng/mL. Likewise, Wang et al. [31] reported a GC-MS/MS method for the quantitative determination of 13 synthetic musks in human milk, with LODs in the range 0.6–5.4 ng/g lipid. A recent publication proposed the determination of the six most important synthetic musks by ultra-performance liquid chromatography (UPLC) coupled to MS/MS with atmospheric pressure photoionization (APPI); LODs were <6 pg [57]. Although the method has only been used for environmental samples (i.e. PM 2.5), it opens the way for new analytical perspectives on the analysis of biological matrices for musks.
LC-MS/MS has become essential to achieve the determination of polar compounds, providing wide scope, high sensibility, and good selectivity [58]. Current LC approaches use reversed-phase columns. To measure biomarkers of phthalates and OPFR, some authors [52,53] proposed the use of stationary phases with phenyl rings that provide special selectivity for polar groups. The mobile phases comprise methanol, acetonitrile and water. Besides, solvent modifiers {e.g., ammonium acetate (proton acceptor) [48], formic acid [18] and acetic acid (proton donors) [34]} are added to enhance ionization efficiencies of the target compounds.
Triple quadrupole (QqQ) is the most commonly used analyzer for polar biomarkers of PCPs and industrial pollutants (e.g., OPFRs or PFCs). In general, to confirm the findings of the target analytes and to avoid false positives, two selected reaction monitoring (SRM) transitions per compound are used [59]. Electrospray ionization (ESI) is the most frequently used interface for determining phthalate metabolites. Silva et al. [52] reported a multi-residue method for quantifying 22 phthalate metabolites in urine using online SPE coupled to LC-MS/MS working in ESI negative mode, with LODs of 0.2–1.1 ng/mL. Although the ESI interface is prone to matrix effects (mainly ion suppression), they reported limited or no matrix effects, due to the use of a diluted sample.
ESI in negative mode is widely used for PFC ionization (see Table 2). Perfluorinated alkyl carboxylic acids are well suited to multiple reaction monitoring (MRM), providing a selective and sensitive response. That said, Haung et al. [49] observed a much lower response when MRM was applied to perfluorinated alkyl sulfonates and perfluorinated alkyl sulfonamides. To achieve a higher response (5–60 times higher), Haung and colleagues propose the use of the pseudo-MRM approach.
Avoiding the limited fragmentations of some PFCs in MS/MS mode is important, as is circumventing some of the quantification problems arising from co-eluting interferences reported in literature. In that regard, Kadar et al. [47] recently proposed a novel strategy for quantifying PFCs in human breast milk using high-performance LC (HPLC) coupled to an orbital-trap high-resolution (HR) mass spectrometer [60]. The source operates in negative ESI mode, and the analyzer records in full-scan mode with a mass resolution of 30,000 FWHM. This method reaches LODs in the pg/mL range, provides exceptional specificity to discriminate the target compounds from potential interferences, and has high repeatability (in the range 1–14%).
Atmospheric pressure chemical ionization (APCI) is an appropriate interface for the determination of environmental phenols (Table 2), although ESI has also been used. Cooper et al. [25] also used this ionization mode for the determination of BDCPP and DPP in urine.
The most recent ionization source for coupling LC to MS is atmospheric pressure photoionization (APPI), which is less prone to ion-suppression effects, and, for certain compounds (i.e. TCS), achieves higher sensitivity than ESI and APCI [35]. In general, the use of a dopant is required for ionization or enhancement of the ionization yield, as in the determination of environmental phenols and parabens in serum [35].
4. Occurrence in human specimens
In recent years, researchers have directed growing attention both toward biomonitoring studies of the general population and toward targeted studies on segments of the population that might have the highest exposures. Table 3 shows some relevant recent studies of biomarkers of exposure to emerging pollutants in different countries and population groups. Table 3 shows that average concentrations of phthalate urinary biomarkers range from a few to several hundred ng/mL. These studies have revealed widespread exposure to several phthalates whose metabolites have been detected frequently in the studied populations.
Table 3.
Occurrence of biomarkers in biological specimens
| Country (Sampling year) | Population | n (Age, years) | Matrix | Biomarkers | Average Concentration/Range (μg/L) | 95th (μg/L) | Detection Rate (%) | Ref. |
|---|---|---|---|---|---|---|---|---|
| China (2010) | Adults | 183 (10–50) | Urine | 14 Phthalate metabolites | 0.6–65.6 (geometric mean) | All samples have at least one | [47] | |
| Germany (2002–2008) | Adults | 240 (19–29) | Urine | 11 Phthalate metabolites | 2.1–65.4 (median) | 6.6–190.8 | [60] | |
| USA (2003–2004) | General population | 2605 (⩾ 6) | Urine | 13 Phthalate metabolites | 2.3–194.4 (geometric mean) | [61] | ||
| USA (2005–2006) | General population | 2548 (⩾ 6) | Urine | 4 Parabens (total: free + conjugated) | <LOQ to 56.4 (geometric mean) | 19.6–974 | MP: 99.1% PP: 92.7% EP: 42% BP: 47% |
[62] |
| USA (2003–2005) | Adults | 100 | Urine | 5 Parabens (total) | <LOQ-43.9 (median) | 0.4–412 | 39%(BP)-99(MP)% | [16] |
| Denmark (2006) | Adults | 60 (18.2–26.5) | Urine | 5 Parabens (total) | <LOQ-17.7 (median) | 75th: <LOQ-64.6 | BP(7%)–MP(98.3) | [42] |
| Austria | Women | 58 (>50) | Plasma | 11 Synthetic musks | 0.017–6.9 (maximum) | Gx: 89%, MX: 63%; MK:43% Td:23%, Others:<4% | [33] | |
| China (2006–2007) | Women | 100 | Milk | 4 Synthetic musks | 4–63 ng/g lipid (median) | HHCB:99%; MK: 83% AHTN:75%;MX: 60% |
[32] | |
| USA (2003–2004) | General population | 2517 (⩾ 6) | Urine | UV filter: BP-3 | 22.9 (geometric mean) | 1040 | 98.6% | [19] |
| USA (2003–2004) | General population | 2517 (⩾ 6) | Urine | BPA, 4-t-OP | BPA: 2.6; 4-t-OP:<LOQ(geometric mean) | BPA: 15.9; 4-tOP: 2.2 | BPA: 92.6%, 4-t-OP: 57.4% | [63] |
| Canada (2007–2009) | General population | 5476 (6–79) | Urine | BPA, 2,4-DCP | BPA: 1.16; 2,4-DCP:0.88 (geometric mean) | BPA: 7.01; 2,4-DCP: 8.92 | BPA: 91%; 2,4-DCP: 78% | [3] |
| Korea (2009) | Adults | 1870 (18–69) | Urine | BPA, triclosan | BPA: 1.90 ; Triclosan: 1.68 (geometric mean) | BPA: 7.74; Triclosan: 135.1 | BPA:99.8%; Triclosan: 92.6% | [64] |
| Germany (2008) | Adults | 30 (11–68) | Urine | BCEP, DPP, DmCP, DpCP | <LOQ | Range: BCEP, <LOQ-27.5; DPP, <LOQ-4.1 | BCEP: 50%, DPP30%, DmCP and DpCP: 0% | [24,65] |
| USA | Adults | Urine | BDCPP, DPP | BDCPP: 0.148 DPP: 0.410 (geometric mean) |
Range: 0.046–3.469 | [25] | ||
| USA (2003–2004) | Adults | 2094 (⩾ 12) | Serum | 12 PFCs | PFOS, 20.7 ; PFOA, 3.9; PFHxS, 1.9 ; PFNA, 1.0 (geometric mean) | 95th: PFOS, 54.6 μg/L; PFOA, 9.8 μg/L; PFHxS, 8.3 μg/L; PFNA, 3.2 μg/L | PFOS, PFOA, PFHxS, PFNA in >98%, Others: <0.5–31.3% | [66] |
| Spain (2008) | Women | 20 | Milk | 6 PFCs | PFOS: 28-865 ng/L i,p-PFNA: 21-260 ng/L |
PFOs, i,p-PFNA: 95% | [67] | |
| Canada (2007–2009) | Adults | 2880 (20–75) | Plasma | PFOS, PFOA, PFHxS | PFOS:8.85 PFOA: 2.52 PFHxS: 2.26 (geometric mean) |
PFOS:27.53 PFOA: 5.50 PFHxS: 12.49 | 97–100% | [3] |
| USA (2001–2002) | General population | 2535 | Urine | DEET | <LOD (geometric mean) | 95th: 0.180 | – | [4] |
n: number of subjects, Gx: Galaxolide, Td: Tonalide.
In the general population, some parabens (e.g., methyl paraben and propyl paraben) could be detected in almost all the urine samples studied (see Table 3), with average concentrations (free plus conjugated) in the range 10–50 ng/mL. In general, ethyl paraben, butyl paraben, and benzyl paraben were detected less frequently (30–50%), with concentrations lower than 1 ng/mL.
The presence of synthetic musks has been studied mainly in breast milk. Polycyclic musks are detected more frequently and at higher concentrations than nitro musks. Galaxolide is by far the most common of the polycyclic musks. Galaxolide could be detected in almost all the samples studied (plasma and breast milk) at average concentrations around 60 ng/g lipid. Tonalide has been reported with frequencies higher than 50%.
BPA, TCS, and BP-3 are three of the most studied environmental phenols. Exposure to BPA is widespread, with detection frequencies in several of the studies generally higher than 95% and average concentrations of few μg/L (see Table 3). These data and the relatively short half-life (~6 h) of BPA suggest that continual exposure to BPA is mainly through diet.
OPFRs are mostly measured in blood and urine. Additional research is needed to characterize the levels of the most relevant compounds from groups of people representing the general population. Apart from the analysis of some metabolites, Sundkvist et al. [68] monitored the presence of 11 parent compounds in pooled human-milk samples from Sweden. Eight compounds were present in all nine pooled samples, with median concentrations of 4.3–45 ng/g lipid.
Serum levels of PFCs tend to reflect cumulative exposure over several years. Exposure in the general population is widespread, with frequency of detection near 100% for some compounds (e.g., PFOS, PFOA, PFHxS, and perfluorononanoate) (Table 3). Fromme et al. [69] recently published a complete review on internal exposure to PFCs of the general population in different countries.
5. Conclusions
As interest grows in the value of biomonitoring for assessing human exposure to emerging pollutants, demand increases for analytical procedures to identify and to quantify a wide range of biomarkers in biological specimens. Within this field, the multi-analyte methods able to determine multiple compounds of the same class (e.g., musk fragrances, phthalates, PFCs, and OPFRs) are common. However, the multi-analyte, multi-class methods are less frequent than in other fields (e.g., environmental monitoring). This could be a future trend in this area of analytical chemistry.
Sample preparation is dominated by SPE, with polymeric materials replacing the conventional silica-bonded sorbents. Biomonitoring in epidemiologic risk assessment or in toxicological studies entails the analysis of a large number of samples, which requires high-throughput analytical techniques. One general trend for these types of study is the development of on-line techniques for increasingly automated and rapid analyses.
LC-MS/MS is the prevailing technique for quantifying and confirming the biomarkers of environmental phenols and industrial chemicals in biological matrices. Although ESI in negative mode is the most widely used ionization mode, APCI in negative mode is frequently employed, mainly for environmental phenols and parabens. As for detection, QqQ operating in SRM mode is used most often, as it offers the required sensibility, selectivity, and dynamic range for the determination and the quantification of polar metabolites with excellent accuracy and precision. For the less polar musk fragrances, GC-MS is the most frequently used technique.
Although HR-full-scan analysis has scarcely been used to date, we expect its increasing application in this field, mainly for wide-range accurate-mass screening of biomarkers. Recent advances in LC-HRMS using time-of-flight (ToF) or Orbitrap analyzers would provide a very suitable alternative to QqQ instruments. The high resolving power (>25,000–100,000 FWHM) and mass accuracy (<5 ppm) of these instruments allow the screening of targeted, as well as untargeted, analytes. Also, the capacity to maximize the information from a sample (full scan) allows retrospective analysis. However, identification and confirmation criteria require international standardization.
Acknowledgments
Vicent Yusa acknowledges the Valencia’s Ministry of Education for support through the BEST 2011 research grant.
Footnotes
Disclaimer
The findings and the conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
Contributor Information
Vicent Yusa, Public Health Research Center of Valencia (CSISP), Av. Catalunya, 21, 46020, Valencia, Spain.
Xiaoyun Ye, Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, GA, USA.
Antonia M. Calafat, Division of Laboratory Sciences, National Center for Environmental Health, Centers for Disease Control and Prevention, Atlanta, GA, USA
References
- 1.Brausch JM, Rand GM. Chemosphere. 2011;82:1518. doi: 10.1016/j.chemosphere.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 2.National Research Council. Human Biomonitoring for Environmental Chemicals. The National Academic Press; Washington, DC, USA: 2006. [Google Scholar]
- 3.Health Canada. Canadian Health Measures Cycle (2007–2009) Health Canada; Ottawa, Ontario, Canada: 2010. [Google Scholar]
- 4.Department of Health and Human Services. Centers for Disease Control and Prevention. Atlanta, GA, USA: 2009. [Google Scholar]
- 5.German Human Biomonitoring Commission. Berlin, Germany: 2006. [Google Scholar]
- 6.Buchberger WW. J Chromatogr, A. 2011;1218:603. doi: 10.1016/j.chroma.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 7.Peck AM. Anal Bioanal Chem. 2006;386:907. doi: 10.1007/s00216-006-0728-3. [DOI] [PubMed] [Google Scholar]
- 8.Pedrouzo M, Borrull F, Marce RM, Pocurull E. Trends Anal Chem. 2011;30:749. [Google Scholar]
- 9.Pietrogrande MC, Basaglia G. Trends Anal Chem. 2007;26:1086. [Google Scholar]
- 10.Roosens L, Covaci A, Neels H. Chemosphere. 2007;69:1540. doi: 10.1016/j.chemosphere.2007.05.072. [DOI] [PubMed] [Google Scholar]
- 11.Hutter H, Wallner P, Hartl W, Uhl M, Lorbeer G, Gminski R, Mersch-Sundermann V, Kundi M. Int J Hyg Environ Health. 2010;213:124. doi: 10.1016/j.ijheh.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 12.Sandborgh-Englund G, Adolfsson-Erici M, Odham G, Ekstrand J. J Toxicol Environ Health, Part A. 2006;69:1861. doi: 10.1080/15287390600631706. [DOI] [PubMed] [Google Scholar]
- 13.Ye X, Zhou X, Furr J, Ahn KC, Hammock BD, Gray EL, Calafat AM. Toxicology. 2011;286:69. doi: 10.1016/j.tox.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Soni MG, Carabin IG, Burdock GA. Food Chem Toxicol. 2005;43:985. doi: 10.1016/j.fct.2005.01.020. [DOI] [PubMed] [Google Scholar]
- 15.Boberg J, Taxvig C, Christiansen S, Hass U. Reprod Toxicol. 2010;30:301. doi: 10.1016/j.reprotox.2010.03.011. [DOI] [PubMed] [Google Scholar]
- 16.Ye X, Bishop AM, Reidy JA, Needham LL, Calafat AM. Environ Health Perspect. 2006;114:1843. doi: 10.1289/ehp.9413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez H, Farbrot A, Larko O, Wennberg AM. Br J Dermatol. 2006;154:337. doi: 10.1111/j.1365-2133.2005.07007.x. [DOI] [PubMed] [Google Scholar]
- 18.Leon Z, Chisvert A, Tarazona I, Salvador A. Anal Bioanal Chem. 2010;398:831. doi: 10.1007/s00216-010-3947-6. [DOI] [PubMed] [Google Scholar]
- 19.Calafat AM, Wong LY, Ye X, Reidy JA, Needham LL. Environ Health Perspect. 2008;116:893. doi: 10.1289/ehp.11269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Constantino L, Iley J. Xenobiotica. 1999;29:409. doi: 10.1080/004982599238588. [DOI] [PubMed] [Google Scholar]
- 21.Usmani KA, Rose RL, Goldstein JA, Taylor WG, Brimfield AA, Hodgson E. Drug Metab Dispos. 2002;30:289. doi: 10.1124/dmd.30.3.289. [DOI] [PubMed] [Google Scholar]
- 22.Hodgson E, Rose RL. Drug Metab Rev. 2005;37:1. doi: 10.1081/dmr-200046955. [DOI] [PubMed] [Google Scholar]
- 23.Selim S, Hartnagel RE, Osimitz TG, Gabriel KL, Schoenig GP. Fund Appl Toxicol. 1995;25:95. doi: 10.1006/faat.1995.1043. [DOI] [PubMed] [Google Scholar]
- 24.Schindler BK, Foerster K, Angerer J. J Chromatogr, B. 2009;877:375. doi: 10.1016/j.jchromb.2008.12.030. [DOI] [PubMed] [Google Scholar]
- 25.Cooper E, Covaci A, van Nuijs A, Webster T, Stapleton H. Anal Bioanal Chem. 2011;401:2123. doi: 10.1007/s00216-011-5294-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schindler BK, Foerster K, Angerer J. Anal Bioanal Chem. 2009;395:1167. doi: 10.1007/s00216-009-3064-6. [DOI] [PubMed] [Google Scholar]
- 27.Calafat AM, Ye XY, Silva MJ, Kuklenyik Z, Needham LL. Int J Androl. 2006;29:166. doi: 10.1111/j.1365-2605.2005.00570.x. [DOI] [PubMed] [Google Scholar]
- 28.Jones PD, Hu WY, De Coen W, Newsted JL, Giesy JP. Environ Toxicol Chem. 2003;22:2639. doi: 10.1897/02-553. [DOI] [PubMed] [Google Scholar]
- 29.Asimakopoulos AG, Thomaidis NS, Koupparis MA. Toxicol Lett. 2012;210:141. doi: 10.1016/j.toxlet.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 30.Hu Z, Shi Y, Niu H, Cai Y, Jiang G, Wu Y. Environ Toxicol Chem. 2010;29:1877. doi: 10.1002/etc.258. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, Zhang J, Gao F, Yang Y, Duan H, Wu Y, Berset JD, Shao B. J Chromatogr, B. 2011;879:1861. doi: 10.1016/j.jchromb.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 32.Zhang X, Liang G, Zeng X, Zhou J, Sheng G, Fu J. J Environ Sci Chin. 2011;23:983. doi: 10.1016/s1001-0742(10)60506-2. [DOI] [PubMed] [Google Scholar]
- 33.Bravo R, Norrgran J, Restrepo PA, Walker R, Needham LL, Barr DB. Abstracts of papers presented at the 2008 Pittsburgh Conference. In: Stockwell PB, editor. J Autom Methods Manag Chem. 2008. p. 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ye X, Tao LJ, Needham LL, Calafat AM. Talanta. 2008;76:865. doi: 10.1016/j.talanta.2008.04.034. [DOI] [PubMed] [Google Scholar]
- 35.Geens T, Neels H, Covaci A. J Chromatogr, B. 2009;877:4042. doi: 10.1016/j.jchromb.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Kawaguchi M, Ito R, Sakui N, Okanouchi N, Saito K, Seto Y, Nakazawa H. Anal Bioanal Chem. 2007;388:391. doi: 10.1007/s00216-007-1225-z. [DOI] [PubMed] [Google Scholar]
- 37.Kawaguchi M, Ito R, Okanouchi N, Saito K, Nakazawa H. J Chromatogr, B. 2008;870:98. doi: 10.1016/j.jchromb.2008.06.011. [DOI] [PubMed] [Google Scholar]
- 38.Kawaguchi M, Ito R, Honda H, Endo N, Okanouchi N, Saito K, Seto Y, Nakazawa H. J Chromatogr, B. 2008;875:577. doi: 10.1016/j.jchromb.2008.10.008. [DOI] [PubMed] [Google Scholar]
- 39.Cunha S, Fernandes J. Talanta. 2010;83:117. doi: 10.1016/j.talanta.2010.08.048. [DOI] [PubMed] [Google Scholar]
- 40.Kunisue T, Wu Q, Tanabe S, Aldous KM, Kannan K. Anal Methods. 2010;2:707. [Google Scholar]
- 41.Ye X, Bishop AM, Needham LL, Calafat AM. Anal Chim Acta. 2008;622:150. doi: 10.1016/j.aca.2008.05.068. [DOI] [PubMed] [Google Scholar]
- 42.Frederiksen H, Jorgensen N, Andersson AM. J Expos Sci Environ Epidemiol. 2011;21:262. doi: 10.1038/jes.2010.6. [DOI] [PubMed] [Google Scholar]
- 43.Vela-Soria F, Jimenez-Diaz I, Rodriguez-Gomez R, Zafra-Gomez A, Ballesteros O, Navalon A, Vilchez J, Fernandez M, Olea N. Talanta. 2011;85:1848. doi: 10.1016/j.talanta.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 44.Jimenez-Diaz I, Vela-Soria F, Zafra-Gomez A, Navalon A, Ballesteros O, Navea N, Fernandez M, Olea N, Vilchez J. Talanta. 2011;84:702. doi: 10.1016/j.talanta.2011.01.075. [DOI] [PubMed] [Google Scholar]
- 45.Shanmugam G, Ramaswamy BR, Radhakrishnan V, Tao H. Microchem J. 2010;96:391. [Google Scholar]
- 46.Kadar H, Veyrand B, Barbarossa A, Pagliuca G, Legrand A, Bosher C, Boquien CY, Durand S, Monteau F, Antignac JP, Le Bizec B. Chemosphere. 2011;85:473. doi: 10.1016/j.chemosphere.2011.07.077. [DOI] [PubMed] [Google Scholar]
- 47.Guo Y, Wu Q, Kannan K. Environ Int. 2011;37:893. doi: 10.1016/j.envint.2011.03.005. [DOI] [PubMed] [Google Scholar]
- 48.Haug LS, Thomsen C, Becher G. J Chromatogr, A. 2009;1216:385. doi: 10.1016/j.chroma.2008.10.113. [DOI] [PubMed] [Google Scholar]
- 49.Mosch C, Kiranoglu M, Fromme H, Voelkel W. J Chromatogr, B. 2010;878:2652. doi: 10.1016/j.jchromb.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 50.Kondo F, Ikai Y, Hayashi R, Okumura M, Takatori S, Nakazawa H, Izumi SI, Makino T. Bull Environ Contam Toxicol. 2010;85:92. doi: 10.1007/s00128-010-0051-8. [DOI] [PubMed] [Google Scholar]
- 51.Silva MJ, Samandar E, Preau JL, Reidy JA, Needham LL, Calafat AM. J Chromatogr, B. 2007;860:106. doi: 10.1016/j.jchromb.2007.10.023. [DOI] [PubMed] [Google Scholar]
- 52.Reemtsma T, Lingott J, Roegler S. Sci Total Environ. 2011;409:1990. doi: 10.1016/j.scitotenv.2011.01.032. [DOI] [PubMed] [Google Scholar]
- 53.Cruz-Vera M, Lucena R, Cardenas S, Valcarcel M. Anal Bioanal Chem. 2010;397:1029. doi: 10.1007/s00216-010-3476-3. [DOI] [PubMed] [Google Scholar]
- 54.Bugey A, Staub C. J Sep Sci. 2007;30:2967. doi: 10.1002/jssc.200700141. [DOI] [PubMed] [Google Scholar]
- 55.Mahugo-Santana C, Sosa-Ferrera Z, Torres-Padron M Esther, Santana-Rodriguez J Juan. Trends Anal Chem. 2011;30:731. [Google Scholar]
- 56.Lung SCC, Liu CH. Anal Chem. 2011;83:4955. doi: 10.1021/ac2006872. [DOI] [PubMed] [Google Scholar]
- 57.Petrovic M, Farre M, Lopez de Alda M, Perez S, Postigo C, Koeck M, Radjenovic J, Gros M, Barcelo D. J Chromatogr, A. 2010;1217:4004. doi: 10.1016/j.chroma.2010.02.059. [DOI] [PubMed] [Google Scholar]
- 58.European Commission. Commission Decision of 12 August 2002 (2002/657/EC) Offic J Eur Commun. 2002;221:8. [Google Scholar]
- 59.Makarov A, Scigelova M. J Chromatogr, A. 2010;1217:3938. doi: 10.1016/j.chroma.2010.02.022. [DOI] [PubMed] [Google Scholar]
- 60.Göen T, Dobler L, Koschorreckc J, Müller J, Wiesmüller GA, Drexler H, Kolossa-Gehrin M. Int J Hyg Environ Health. 2011;215:36. doi: 10.1016/j.ijheh.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 61.Centers for Disease Control and Prevention. Four National Report on Human Exposure to Environmental Chemicals. Atlanta, GA, USA: 2009. p. 258. [Google Scholar]
- 62.Calafat AM, Ye X, Wong LY, Bishop AM, Needham LL. Environ Health Perspect. 2010;118:679. doi: 10.1289/ehp.0901560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Calafat AM, Ye X, Wong LY, Reidy JA, Needham LL. Environ Health Perspect. 2008;116:39. doi: 10.1289/ehp.10753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim K, Park H, Yang W, Lee JH. Environ Res. 2011 doi: 10.1016/j.envres.2011.09.003. [DOI] [PubMed] [Google Scholar]
- 65.Voelkel W, Kiranoglu M, Fromme H. Environ Res. 2011;111:143. doi: 10.1016/j.envres.2010.10.001. [DOI] [PubMed] [Google Scholar]
- 66.Calafat AM, Wong LY, Kuklenyik Z, Reidy JA, Needham LL. Environ Health Perspect. 2007;115:1596. doi: 10.1289/ehp.10598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Llorca M, Farré M, Picó Y, Lopez-Teijon M, Álvarez J, Barcelo D. Environ Int. 2010;36:594. doi: 10.1016/j.envint.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 68.Sundkvist AM, Olofsson U, Haglund P. J Environ Monit. 2010;12:943. doi: 10.1039/b921910b. [DOI] [PubMed] [Google Scholar]
- 69.Fromme H, Tittlemier SA, Voelkel W, Wilhelm M, Twardella D. Int J Hyg Environ Health. 2009;212:239. doi: 10.1016/j.ijheh.2008.04.007. [DOI] [PubMed] [Google Scholar]


