Abstract
Oxidative stress and TGFβ-induced disturbance of cells and tissues are implicated in initiation and progression of pathophysiology of cells/tissues. Using primary human Trabecular Meshwork (TM) cells from normal and glaucomatous subjects, we demonstrated that peroxiredoxin (PRDX) 6, an antioxidant, offsets the deleterious effects of oxidative stress on TM cells by optimizing ROS and TGFβ levels. An analysis of glaucomatous TM cells revealed a reduced expression of PRDX6 mRNA and protein. Biochemical assays disclosed enhanced levels of ROS, as well as high levels of TGFβs, and these cells expressed elevated extracellular matrix (ECM) andTsp1 proteins with reduced MMP2; conditions implicated in the pathophysiology of glaucoma. Nonglaucomatous TM cells exposed to TGFβs/ROS showed similar features as in glaucomatous cells. The abnormalities induced were reversed by delivery of PRDX6. The data provide evidence that oxidative stress-induced abnormality in TM may be related to reduced PRDX6 expression, and provide a foundation for antioxidant-based therapeutics for treating glaucoma.
Keywords: Peroxiredoxin 6, reactive oxygen species, transforming growth factor β, extracellular matrix proteins, antioxidant
Introduction
Oxidative damage driven by reactive oxygen species (ROS) and reduced expression of antioxidant molecules are major factors in the pathophysiology leading to the progression of many diseases, including glaucoma [1, 2]. Ample evidence shows that a high concentration of ROS induced by physiological and/or environmental stress can modulate the cell’s defense system and activate the pathways that lead to cell death [3]. Among the various types of damaging effects, those contributing to cell injury are apoptosis, loss of homeostasis, abnormal phenotypes, cellular senescence, overshooting of organelles, and overstimulation of genes or proteins [3–5]. To cope with these insults, cells have evolved an antioxidant defense that strictly regulates cellular ROS, a prerequisite to maintenance of normal cell homeostasis. Furthermore, these cellular antioxidants play a pivotal role in correcting overproduction of ROS generated by cellular metabolism or environmental stress. Cells with reduced expression of antioxidants bear higher levels of ROS, and changes in the redox state of cells lead to the modification of cellular signal transduction including activation and expression of growth factors and proteins involved in cell defense. Recent significant advances in redox biology have contributed to our understanding of possible mechanisms underlying the ways in which cells/tissues are damaged by growth factors such as transforming growth factor β (TGFβ) and tumor necrosis factor α (TNFα), and ROS–mediated oxidative stress-induced abnormal signaling [2–4]. ROS activate TGFβ and in turn TGFβ further induces oxidative stress as well as overstimulation of gene that leads to pathophysiology of cells /tissues [3, 6, 7]. Recently, studies of the etiology and progression of glaucoma have revealed the pivotal roles of TGFβs and oxidative stress [8–11]. Our recent report [8] also supports this finding by showing that TNFα- or glutamate-induced increases in production of ROS in retinal ganglion cells (RGC), is a major cause of RGC toxicity.
Moreover, the continual rise in the incidence of glaucoma, a major cause of blindness worldwide, points to unveil the plausible underlying mechanisms involved in its progression, and potential development of therapeutic molecule(s) for treating and preventing this disease. Although oxidative stress and TGFβ have been implicated [12], little progress has been made in this area as yet. ROS-mediated oxidative stress-induced alteration of TM cell biology and the resultant increased resistance of outflow has been documented [13, 14]. Trabecular meshwork (TM), a specialized eye tissue, is essential for maintenance of homeostasis of the entire outflow system. TM is closely connected to the anterior chamber of the eye [15]. Several active stressors like superoxide anions, H2O2, and TGFβs have been found in aqueous humor, affecting the morphologic and physiologic alterations in TM cells. Izzotti [13] has found that outflow resistance in the anterior chamber increases in the presence of high levels of H2O2 [16]. Furthermore, tissues from the TM in glaucomatous patients contain high levels of metabolic products of lipid peroxidation, suggesting a role for oxidative stress in the pathophysiology of this disease [10, 11, 14, 17, 18, 19]. Several recent studies have shown that in vitro treatment of cultured human TM cells with TGFβ1/2 leads to phenotypic changes [20, 21] and to upregulation of genes encoding ECM proteins like α-SM-actin and βig-h3, which are implicated in several forms of pathogenesis. Zhao et al. [22] showed that treatment of human TM cells with either TGF-β1 or -β2 stimulates the expression of several ECM genes, including versican, elastin, collagens, fibrillin, laminin, and fibulin. In addition, TGFβ2-treated human TM cells altered the production of the enzymes promatrix metalloproteinase-2 (MMP-2) and plasminogen activator inhibitor (PAI)-1, each of which likely plays a role in ECM-remodeling [23]. We predicted that ROS-driven activation of TGF βs [3, 7] should be a factor responsible for overmodulation of ECM genes/proteins that in turn interrupt smooth flow of aqueous humor. TGFβ in the eye is present in a latent, inactive form and may be activated in the presence of excessive ROS [3]. That activation of TGFβs can be prevented by the presence of an antioxidant such as PRDX6.
PRDX6, a multifunctional or “moonlighting” protein, has GSH peroxidase as well as acidic Ca+2 -independent phospholipase (aiPLA2) activities [24]. The latter activity, present only in PRDX6 and not in other members of the peroxiredoxin family, can protect cells from damage to membrane, DNA, and protein mediated by lipid peroxidation [3, 7, 25]. PRDX6 is highly expressed in TM (current study), but its expression is significantly reduced in glaucomatous cells. We hypothesized that reduced expression of PRDX6 in glaucomatous TM cells may be a plausible cause of phenotypic abnormality and cell malfunction resulting in failure of outflow homeostasis. Using TM cells derived from glaucomatous and nonglaucomatous human subjects, we found that ROS induced the activation of TGFβs which are responsible for abnormal phenotypes of TM and overmodulation of genes that can lead to attenuation of TM cell function; these adverse changes were reversed by the delivery of PRDX6. Research in several laboratories has shown that TAT (transcription activator of transcription) linked PRDX6, when administered in vitro or in vivo, can be internalized into cells and protect them from stressors [3, 7]. The HIV-1 TAT that has 11 amino acids (aa; YGRKKRRQRRR), has been proven to have 100% potential for intracellular delivery of proteins [26]. In the present study, we demonstrated that glaucomatous TM cells display reduced expression of Prdx6, and that H2O2 and TGFβ1 and TGFβ2 are major culprits in phenotypic abnormality and overmodulation of ECM or non-ECM genes in these cells. Additionally, we have shown the ability of PRDX6 to abolish ROS- or TGFβ-driven stress-induced overstimulation of genes and resulting abnormalities in glaucomatous TM cells. The study documented the basis for using antioxidants such as PRDX6 for preventing and treating glaucoma or other ROS-induced disorders in general.
Materials and Methods
Cell culture
Primary TM cells from normal and glaucomatous subjects were obtained from Dr. Stamer, Department of Ophthalmology and Visual Sciences, University of Arizona maintained in Opti-MEM with 10%FBS and antibiotic-antimycotic solution containing 100 µg/ml streptomycin, 100 U/ml penicillin and 25 µg/ml fungizone as described elsewhere [27]. Methods for securing human cells/tissue were humane, included proper consent, and complied with Declaration of Helsinki. Cells were treated with either H2O2 or TGFβ1 or TGFβ2 at variable concentrations for variable times with or without TAT-HA-PRDX6 (4µg/ml) as optimized for experimental requirement. TM cells of passage 3 and onwards or cells showing senescence were used as needed for experiments.
Western analysis
Cell lysates of TM cells were prepared in RIPA buffer and extracted proteins were resolved on either 10% SDS-PAGE gel, 7.5% gel or 5–20% gradient gel, and blotted onto nitrocellulose membranes ( Immobilon-P Millipore). After blocking, membranes were incubated O/N with either PRDX6 monoclonal Ab (Lab Frontier) or other primary antibodies. TGFβ1 or 2, Fibulin-5, Thrombospondin −1, Tropomyosin-1and 2 , Transglutaminase-2, MMP-2, MMP-9, βig-h3, P16 and P21 (cyclin inhibitors) were purchased from SantaCruz Biotech and Fibronectin, α-sm-actin and β-actin antibodies were purchased from Sigma. All secondary antibodies obtained from SataCruz Biotech. The specific protein bands were visualized with luminol reagent. To detect TSP-1 and βig-h3, culture medium of normal and glaucomatous TM cells was collected, and aliquots (500 µL) were concentrated using centricon following the manufacturer’s instructions and protein contents were determined.
Real- time PCR analysis
Total RNA was isolated from normal and glaucomatous TM cells using Trizol Reagent according to manufacture’s protocol. After reverse transcription using the First-Strand cDNA kit (GE Healthcare), PCR was conducted in a 50-µl reaction mixture with 5 µl of 10x PCR buffer (Takara, Ohtsu, Japan), 1 µl of 10 mM dNTP mix, 1 µl of each specific 5' and 3' primer of PRDXs (10 pmol/µl), 0.25 µl of Ex-Taq DNA polymerase (5 U/µl; Takara), 2 µl of cDNA, and 37.5 µl of sterile distilled water. The DNA was amplified for 15–35 cycles at 94°C for 1 min, 55°C for 0.5 min, and 72°C for 3 min. Reaction mixtures (20 µl) were electrophoresed on 1% agarose gel. We performed relative quantification of Prdx 1–6 mRNA using the ABI PRISM 7000 sequence detection system (Applied Biosystems, Foster City, CA). PRDXs, Tropomyocin and GAPDH primers were purchased from Custom TaqMan Gene Expression Assays (Applied Biosystems). The comparative threshold cycle (Ct) method was used to calculate relative changes in expression levels using the 7000 SDS version 1.1 RQ software (Applied Biosystems). The Ct values of target genes were normalized to the levels of GAPDH as an endogenous control in each group [3, 8, 28].
Measurement of intracellular ROS
Intracellular redox state levels were measured using the fluorescent dye 2,7-dichlorofluorescein diacetate (H2-DCFH-DA) which is rapidly oxidized to the highly fluorescent 2',7'-dichloroflourescein in the presence of intracellular hydrogen peroxide and peroxidases [3] . Cells were cultured in 96 well plates overnight, washed with HBSS and incubated with dye for 30 min at 37°C. Intracellular flourescence was detected at 485nm excitation and 530nm emission using DTX880, Multimode Detector (Beckman Coulter) [5–7].
Measurement of bioactive TGFβ level
TGFβ is secreted by the cells in latent form and is activated in the presence of excessive ROS [3]. To know whether the expression and activation of TGFβ is increased in glaucomatous TM cells, we used TGFβ1 Emax ImmunoAssay system (Promega Corp., Madison, WI, USA) as described earlier [3]. Briefly, 96-well plates were coated with TGFβ Coat mAb, which binds to soluble TGFβ. TGFβ bound to a specific polyclonal antibody (pAb). After washing, the amount of specifically bound pAb was measured using a specific antibody conjugated to HRP. Readings were taken at 450 nm.
Construction of TAT-HA-PRDX6
PRDX6 cDNA isolated from the Lens Epithelium Cell library [25] was fused with a gene fragment encoding the 11-amino acid TAT protein transduction domain (RKKRRQRRR) of HIV-1 in a bacterial expression vector, pTAT-HA to generate a genetically engineered TAT-HA-PRDX6 fusion protein, and this recombinant protein linked to transduction domain was used to assess its ability in protecting cells/tissues against oxidative stress. Briefly, Prdx6 full length cDNA was cloned into a TA-cloning vector (Invitrogen) and transformed into a prokaryotic competent cell, and the plasmids of selected colonies were purified. This purified TA vector containing Prdx6 cDNA was subcloned into a pTAT-HA expression vector (a kind gift of Dr. S. F. Dowdy). The expressed recombinant protein TAT-HA-PRDX6 was purified using Ni2+-nitrilotriacetic acid Sepharose column (Invitrogen). The purified recombinant protein was either used for cell protection assay or aliquoted and stored frozen in 10% glycerol at –80°C for further use [7].
Transduction of TAT-HA-PRDX6 fusion protein into TM cells
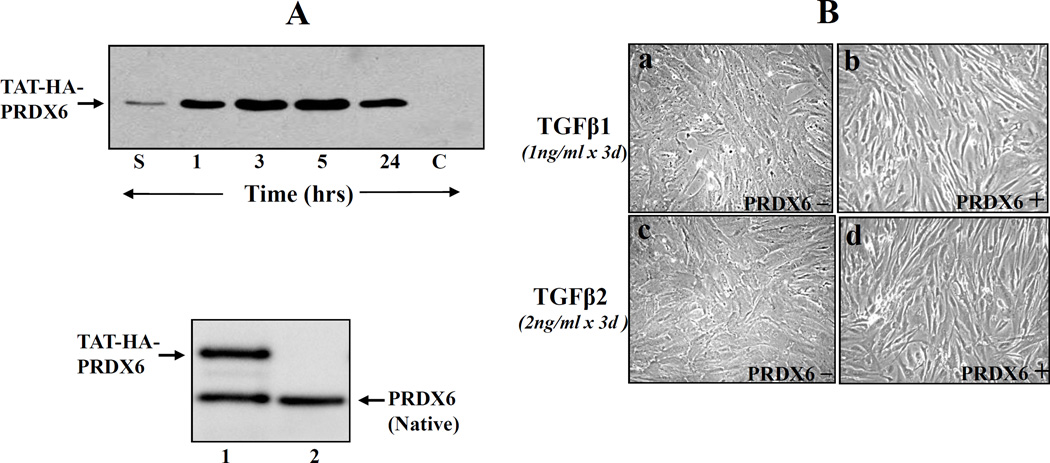
TM cells were grown overnight on a six-well plate, and then TAT-HA-PRDX6 fusion protein (4 µg/ml) was added to the culture media. After incubation periods of 1 h, 3 h, 5 h and 24 h, cells were washed and harvested for the preparation of cell extract. Western blot analysis was performed using anti-HisG HRP (Invitrogen). HA-PRDX6 was used as control. Similar experiments were conducted using PRDX6 monoclonal antibody (Lab Frontier, Seoul, Korea) to compare the levels of endogenous (native) and extrinsically supplied PRDX6.
Cell survival assay (MTS assay)
A colorimetric MTS assay (Promega) was performed as described earlier [3, 7, 8, 25]. This assay of cellular proliferation/viability uses 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2 to 4-sulfophenyl)-2H-tetrazolium salt (MTS; Promega, Madison, MI, USA). Upon addition to medium containing viable cells, MTS is reduced to a water-soluble formazan salt. The OD490 nm value was measured after 4 h with an ELISA reader.
Immuno-cytochemical analysis of 8-OhdG
TM cells, treated with or without TAT-HA-PRDX6 were fixed and immunostained using Alexa Fluor® 488 Signal-Amplification kit (Molecular Probes) following the company’s protocol. Briefly, after washing cells with PBS, all cell preparations were incubated for 30 minutes with the blocking solution supplied in the kit. Then they were exposed overnight at 4°C to the anti-8-OHdG monoclonal antibody. After washing, cells were exposed to Alexa Fluor second antibody solution and washed again. Visualization of antibody complex was carried out by fluorescent microscopy using absorption/emission maxima ~495/519 nm.
Statistical analysis
Data were analyzed by Student’s t-tests. A p-value less than <0.05 was considered significant.
Results
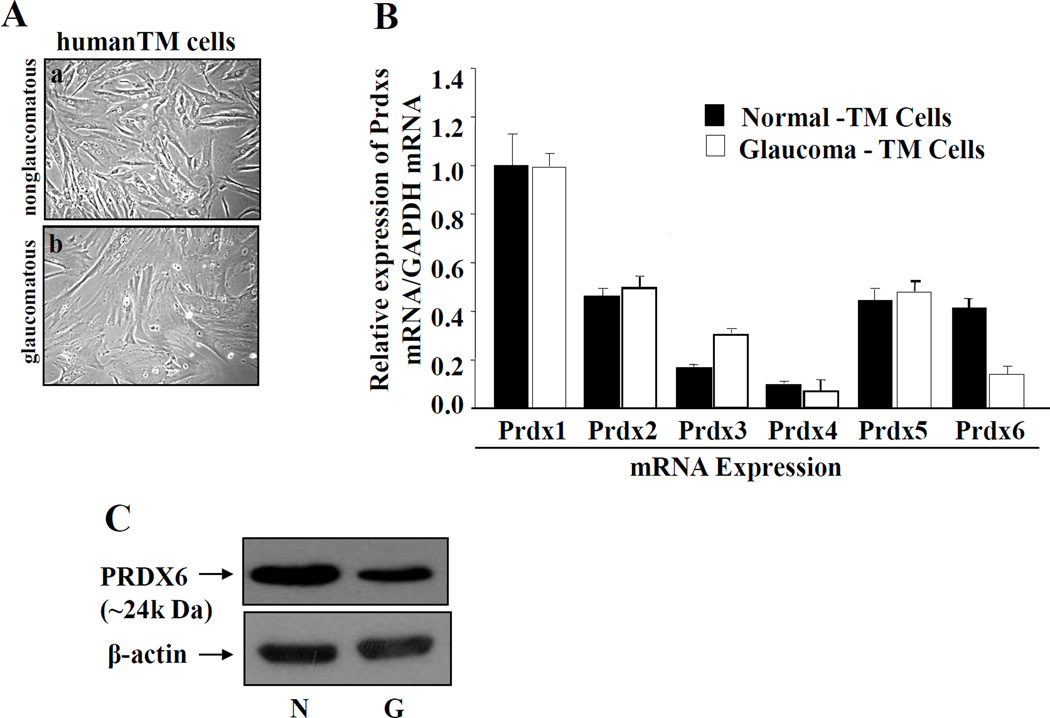
TM cells derived from glaucomatous subjects display abnormal phenotype with reduced expression of Prdx6 mRNA and protein
The expression levels of ROS generated by cells are tightly controlled by antioxidants, as excessive production of ROS in response to stressors leads to pathophysiology of cells/tissues. Reduced expression of PRDX6, an antioxidant, has been shown to cause impaired cell homeostasis and enhanced susceptibility to cellular abnormality as well as apoptosis against stressors [3, 5, 7, 24, 28, 29]. We believe this reduced expression of PRDX6 may be one cause of damage, abnormalities, and overmodulation of genes in TM cells. To test our hypothesis, we investigated the expression level of PRDX6 as well as other Prdxs, because comparative expression levels would reflect their abundance, which in turn would indicate their specific importance. To this end, we conducted quantitative real time PCR to measure levels of mRNA in glaucomatous and nonglaucomatous TM cells. Data analysis revealed that all six peroxiredoxins (Prdx 1–6) were present in both diseased and normal cells (Fig. 1), but importantly the level of PRDX6 mRNA was dramatically lower in glaucomatous TM cells (Prdx6, black bar) in comparison to other Prdxs. This reduced expression of PRDX6 may be a causative factor for the abnormal changes in glaucomatous TM cells, leaving the other PRDXs unable to maintain TM cell integrity (Fig. A, compare a and b). The difference in PRDX6 levels may be associated with its antioxidant and protective activity.
Figure 1. TM cells derived from glaucomatous human subjects display abnormal phenotypes and reduced expression of PRDX6 mRNA and protein.
A, Photomicrograph showing TM cell morphology, nonglaucomatous (a) and glaucomatous (b) cultured in vitro. Cells were maintained in Opti-MEM with 10% FBS. Glaucomatous TM cells appeared flattened in shape with larger nuclei (typical senescence-like morphology) compared to control. Figures are representative of experiments. B, Quantitative real-time PCR showing differential expression of Prdx1-6 mRNA in glaucomatous and nonglaucomatous TM cells using their specific primers. Total RNA was isolated and transcribed into cDNA. Real-time PCR was performed [3, 8, 28]. mRNA expression of each Prdx was adjusted to the mRNA copies of GAPDH. Results indicated that mRNA expression level of Prdx6 was significantly decreased in glaucomatous TM cells (B, open bar vs black bar) compared to other Prdxs. However, an abundant level of Prdx 3 mRNA was present but could not control cellular integrity of TM cells, suggesting that Prdx6 has a major role in maintaining cellular homeostasis in TM cells (B, open bar vs black bar). C, Western analysis showing significantly reduced expression of PRDX6 protein in glaucomatous TM cells (upper panel; right lane, G). β-actin bands represent equal loading (lower panel; N and G).
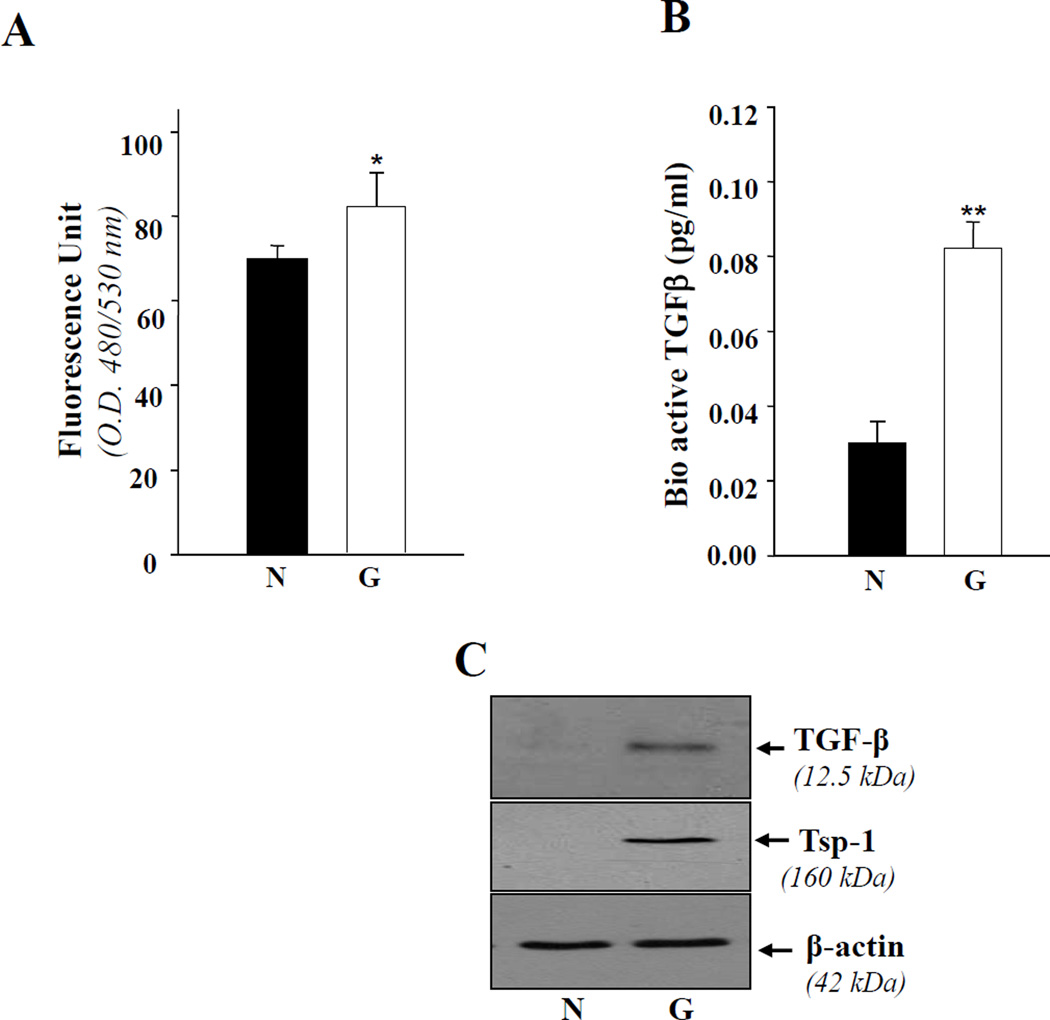
Glaucomatous TM cells bear increased ROS and bioactive TGFβ levels with elevated expression of TGF-β1 and TSP-1
TGFβs are implicated in the pathophysiology of glaucoma and act by overstimulation of ECM and/or other genes [22, 30, 3]. However, in view of the established relationship between ROS levels and PRDX6 expression [5, 24, 29, 32] and based on the above data as well as our earlier studies [3, 7], we predicted that glaucomatous TM cells may harbor higher levels of ROS that may activate the expression of Tsp-1 and TGFβ and cause release of bioactive TGFβ. We measured intracellular levels of ROS in glaucomatous TM cells by staining with H2DCF-DA [3]. Figure 2,A shows a accumulation of ROS in glaucomatous cells (open bar), demonstrating that PRDX6 diminution in glaucomatous TM cells is a cause of higher ROS levels that result in oxidative stress. We predicted that TGFβs would be activated and increasingly expressed in glaucomatous cells. TGFβ is normally secreted in the latent form (L-TGFβ), and must be cleaved from latency associated peptides (LAP) to generate the bioactive TGFβ form [3, 6, 7]. ROS and Tsp-1 are known to be involved in activation of TGFβ [3, 6, 33]. To demonstrate whether expression and release of bioactive TGFβ as well as Tsp-1 are increased, we performed Western analysis with specific antibody, and Emax ELISA assay (Promega) for active TGFβ. The culture supernatant of glaucomatous cells contained significantly higher levels of bioactive TGFβ (Figure 2, B; open bar). These cells also displayed increased expression of TGFβ (Figure 2C, upper panel; right lane) and Tsp-1 (Figure 2 C, lower panel; right lane) proteins. The results collectively imply that changes in glaucomatous cells are associated with ROS and TSP-1 dependent activation of TGFβ. ROS and Tsp-1 are potent activators of TGFβ both in vivo and in vitro [33, 34].
Figure 2. TM cells derived from glaucomatous subjects showing increased expression of ROS.
A, cells were cultured in 96 well plates containing Opti-MEM plus 10% FBS as described in Materials and Methods. The next day, the medium was replaced with HBSS containing 5–10µg of H2-DCFH-DA, and fluorescence intensity was measured using DTX 880, Multimode detector, adjusted at 485nm (excitation) and 535nm (emission) (Beckman Coulter). Histogram values are means ± SD of 3 independent experiments, each with triplicate wells, showing increased levels of ROS in glaucomatous TM cells (G, open bar, *p< 0.05). B, Glaucomatous TM cells secrete bioactive TGFβ. Cells were cultured in 96 well plate for 72 h, culture supernatant was collected, and bioactive TGFβ was assessed using Emax immunoassay system (Promega). Equal numbers of nonglaucomatous cells were also cultured and supernatant was used as control. A higher level of TGFβ1 was detected in glaucomatous cells (B, open bar, **p< 0.001) in comparison to nonglaucomatous TM cells. From another set of experiments, cells extract was prepared and used for Western analysis. The level of TGFβ1 protein was comparatively higher in glaucomatous TM cells (C, upper panel). To see the expression level of Tsp-1, a protein implicated in activation of TGFβ and glaucoma pathogenesis, the membrane stained earlier with PRDX6 or β-actin antibody, was stripped and reprobed with Tsp-1 antibody. The results revealed an increased level of Tsp-1 (C, right lane; G).
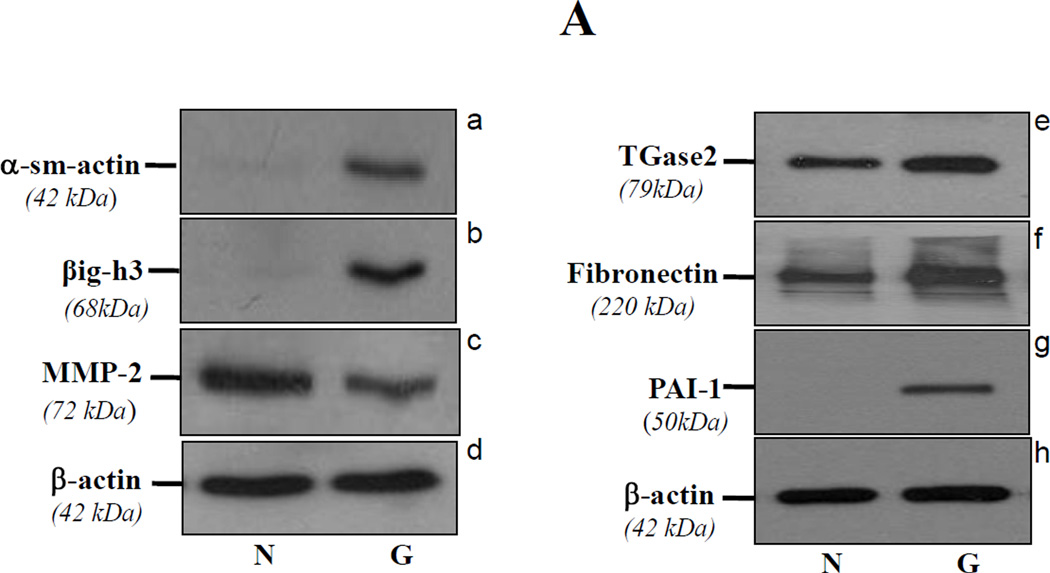
Glaucomatous TM cells display elevated expression of βig-h3, α-sm-actin, fibronectin, PAI-1, and TGase2 and reduced expression of MMP-2
TGFβ has been implicated to play a role in the pathophysiology of TM that leads to initiation and progression of glaucoma [9, 20, 22, 35]. To determine whether glaucomatous TM cells expressed elevated levels of TGFβ inducible genes, we conducted Western analysis of those genes known to be involved in TM cell insults. Protein levels of α-sm-actin, βig-h3, TGase2 (an enzyme cross-linking ECM protein) and fibronectin were significantly increased, suggesting a TGFβ- mediated effect (Figure 3, A). An elevated expression of TGase2 in glaucomatous TM cells was reported earlier by other group [36]. In addition, we assessed expression levels of MMP2, MMP9, and PAI-1 because ECM metabolism within the TM cells is regulated by MMPs [37, 38, 39] and PAI-1 [9, 23, 40, 41]. Western analysis with anti-MMP2, MMP9, and PAI-1 antibodies of glaucomatous cell extracts showed decreased expression of MMP2 (Figure 3, A; c) and increased expression of PAI-1(Figure 3, A; g), however, MMP9 could not be detected in either nonglaucomatous or glaucomatous cells. These results suggest that adverse changes as well as overmodulation of gene expression in glaucomatous cells are associated with oxidative stress or its induced activation of TGFβs.
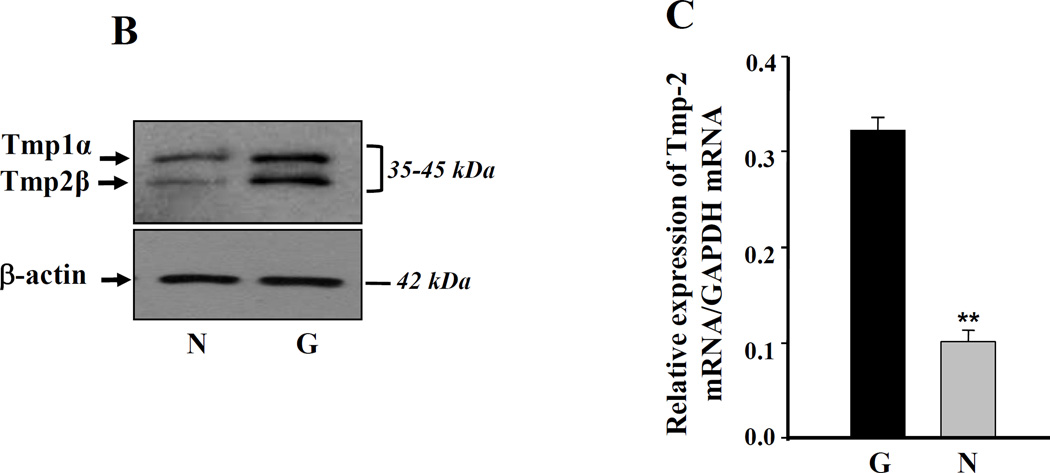
Figure 3. Western analysis of TM cells from glaucomatous (G) subjects showing increased expression of ECM proteins.
A, α-sm-actin (a, right lane), βig-h3 (b, right lane), TGase-2 (e, right lane), fibronectin (f, right lane) and PAI-1(g, right lane) expression was increased in glaucomatous cells, while these cells showed decreased expression of MMP2 (c, right lane). B and C, Western and real-time PCR analysis showing increased expression of tropomyosins (Tmp1α and Tmp2β) protein and Tmp 2β mRNA in glaucomatous TM cells. Cells were cultured, harvested, and processed for protein and RNA extraction and analyzed for protein and mRNA levels as described earlier [3, 7, 25]. Data disclosed elevated expression of Tmps protein (B, right lane) and mRNA (C, black bar, **p < 0.0001). No change in β-actin expression revealed equal loading of samples (B).
TM cells derived from glaucomatous subjects contain increased levels of Tmp1α and Tmp2β proteins
Tropomyosins (Tmps) are essential molecules for maintenance of cellular integrity. Changes in cellular microenvironment due to oxidative stress or TGFβ, and their effects on modulation of Tmps expression, have been shown to cause physiological abnormalities in cells. Because glaucomatous cells contain higher levels of both ROS and TGFβ, we investigated protein levels of Tmps using anti-Tmps specific antibodies. We found that Tmp1α and Tmp2β are overproduced in glaucomatous cells (Figure 3, B; right lane), further validating the belief that oxidative stress and TGFβ play pivotal roles in alterations of TM cell biology. To ascertain whether enhanced expression of Tmps are related to their enhanced transcription, we measured mRNA expression level of Tmp2β and carried out real time PCR analysis (Figure 3, black bar). The level of Tmp2β mRNA was significantly elevated in glaucomatous TM cells compared to nonglaucomatous cells. Tmps are known to be responsive to TGFβs [22, 42], and overstimulation of these genes has been documented in various types of cellular abnormality caused by over- or under-expression in cells. In this scenario, we think that overstimulation of Tmp2β may be a pathogenic marker for progression of TM cell damage.
Extrinsically supplied TAT-HA-PRDX6 internalizes into TM cells, attenuated TGF β-induced insults, and maintains cellular integrity
PRDX6 protects cells by removing H2O2 and by maintaining cell survival signaling [3, 5, 25, 24, 29]. Because glaucomatous TM cells bear low levels of PRDX6, we surmised that the reduced expression of PRDX6 by TGFβ [3, 7] could be a cause of abnormalities in glaucomatous TM cells. If so, an extrinsic supply of PRDX6 should restore normal physiology. To deliver PRDX6 to TM cells, we used TAT-linked PRDX6, which can internalize into cells in a concentration-dependent fashion [7, 8, 26, 43]. Using TM cells, we first confirmed cellular internalization of TAT-HA-PRDX6. We cultured cells in six-well plates containing medium supplemented with 5 µg/ml recombinant protein. Western analysis was conducted at 1, 3, 5, and 24h. TAT-HA-PRDX6 was efficiently transduced into the cells (Figure 4). Very small amounts of protein could be detected in supernatant collected at 1 h following addition (lane S), indicating that the protein was transduced into the cells. We could not detect any band when HA-PRDX6 was added to the culture medium (Figure 4, A; lane C). Similarly, Western analysis was conducted using PRDX6 monoclonal antibody. Results revealed the presence of two protein bands, TAT-HA-PRDX6 and native PRDX6 (Figure 4, A; lower panel, lane 1), and concentration of delivered TAT-HA-PRDX6 was almost equal to native PRDX6. Next, to determine whether adding PRDX6 attenuated TGFβs-induced abnormal changes, TM cells were exposed to TGFβ1 or TGFβ2 (Figure 4, B; a and c). In a parallel experiment, TM cells were supplied with recombinant PRDX6 (Figure 4, B; b and d). Changes in cellular morphology were analyzed and photomicrographed, and compared to the parallel culture of TM cells supplemented with PRDX6. Interestingly, TGFβs-induced changes in TM cells were similar to those of TM cells derived from glaucomatous subjects (Figure 1, A; b). More importantly, TM cells supplied with PRDX6 displayed normal phenotypes, indistinguishable from nonglaucomatous cells with limited damage (Figure 1, A; a), suggesting that PRDX6 has the ability to counteract TGF βs-induced damage of TM cells.
Figure 4. Western analysis showing internalization of TAT-HA-PRDX6 in TM cells.
A, Recombinant PRDX6 linked TAT were generated and purified as described earlier [7, 8]. The protein was supplied to the culture medium at a concentration of 4 µg/ml and transduction efficiency of TAT-HA-PRDX6 was evaluated at 1, 3, 5, and 24h. HA-PRDX6 was used as control (lane, c). 3 h later, cells were washed several times, cell extracts were prepared, and Western blot was carried out with anti-His antibody (Invitrogen). Lane S, culture medium after 1 h of addition; Protein band corresponding to lanes 1, 3, 5, 24 denotes time after TAT-HA-PRDX6 protein; lane C, control (HA-PRDX6 without TAT). Results showed that TAT-HA-PRDX6 internalized into cells while HA-PRDX6 did not. A, lower panel showed protein bands of TAT-HA-PRDX6 (lane1 upper band) and native PRDX6 (lane 1 and 2, lower band). To visualize delivered PRDX6 and native PRDX6, membrane was probed with PRDX6 monoclonal antibody and visualized as described in ‘Materials and Methods’ section. (B) Photomicrographs showing that a supply of PRDX6 attenuated TGFβ1 and TGFβ 2 induced adverse changes in TM cells (passage (P3), and restored normal phenotypes. TM cells derived from nonglaucomatous subjects (P3) were cultured in Opti-MEM plus 10% FBS. These cells were supplied with TAT-HA-PRDX6 or every third day up to P12. Photomicrographs were taken at P8 and are representative of three experiments.
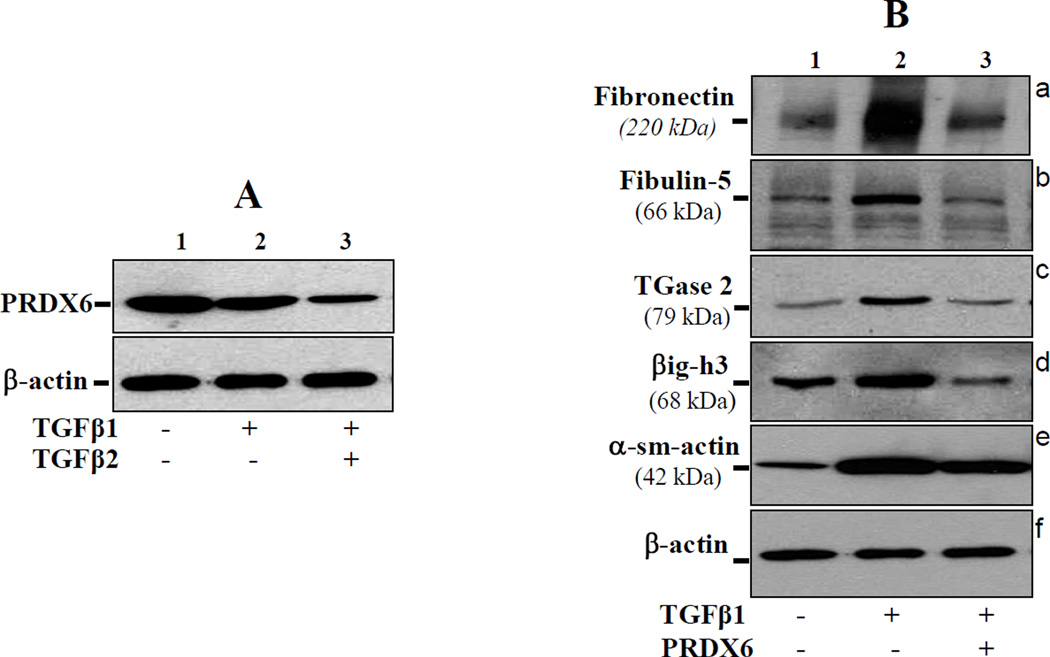
PRDX6 attenuate TGFβ1/β2 induced overstimulation or accumulation of ECM proteins and thereby optimized their expression
In glaucoma, TM cells lose their bona fide characteristics. The above experiments suggested the involvement of TGFβs in disturbance of TM cell homeostasis, and PRDX6 was able to attenuate their adverse effects. Figures 2 and 3 showed that glaucomatous cells expressed elevated levels of TGF-β and its inducible genes and overexpressed ECM proteins. Next, we evaluated whether TAT-HA-PRDX6 could prevent the overexpression of these genes induced by TGFβs. To test this, we treated normal TM cells with either TGFβ1 or TGFβ2. With both TGFβs, there was a substantial upregulation of genes, similar to findings in glaucomatous cells, and these results were consistent with pathological changes observed during the disease (Figure 3). Western analysis revealed that TM cells treated with TGFβs showed increased levels of fibronectin, Fibulin-5, TGase-2, α-sm-actin, and βig-h3 in comparison to untreated cells as expected, and that their expression was optimized (almost comparable to normal untreated cells) when the cells were treated with TAT-HA-PRDX6 (Figure 5). Importantly, as observed in glaucomatous cells, nonglaucomatous cells treated with TGFβs displayed reduced PRDX6 protein (Figure 5 A, lane 2 and 3), further suggesting that adverse changes in TM cells in glaucoma are related to reduced expression of PRDX6. From these results we concluded that abnormal modulation of genes and phenotypic changes in TM cells were associated with TGFβs, and could be attenuated by PRDX6 expression.
Figure 5. A, TM cells obtained from nonglaucomatous subjects treated with TGFβ1 or 2 showed reduced expression of PRDX6.
Cells (P3) were treated with TGFβs (1 or 2ng/ml) for 72h. Cell extract was prepared and Western blot was conducted using PRDX6 specific antibody. Results disclosed that TGFβ1/2 treatment of TM cells resulted in suppression of the PRDX6 expression, and the effect of TGFβ2 was more prominent than TGFβ1. B, Western blot showing that TGFβ-induced abnormal expression of ECM proteins in TM cells was reversed by PRDX6 delivery. Cells were treated with TGF-β1 at 1 ng/ml for 3 days. In parallel experiments, cells were supplied with TAT-HA-PRDX6 (4 µg/ml) 3 h prior to TGFβ treatment to evaluate its ability to prevent TGFβ-induced overexpression of ECM proteins. Cells supplied with PRDX6 showed reversal of the TGFβ-induced overmodulation of proteins (lane 3, panels; a-e).
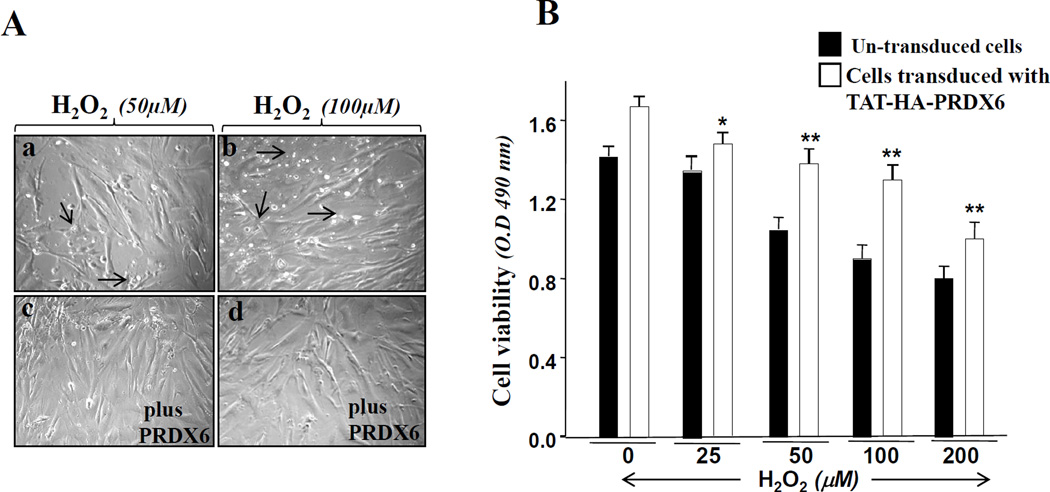
TM cells can be saved from H2O2-driven oxidative stress-induced damage
Oxygen-derived free radicals are known to play a role in the pathophysiology of ocular diseases including glaucoma. Aqueous humor, which is indirectly associated with TM, contains H2O2 and TGFβs. Importantly, H2O2 is an activator of TGFβ, and TGFβ enhances intracellular ROS [3]. Both are implicated in pathophysiology in the TM [44]. We envisaged a feed-forward process within the cellular microenvironment, a process that becomes deleterious to cells bearing lower levels of antioxidant such as PRDX6. A supply of PRDX6 should block the cycle initiated by locally high levels of ROS. To assess this possibility, we exposed TM cells to various concentrations of H2O2 (25, 50, 100 or 200 µM) for 2 h. In a parallel experiment, TAT-HA-PRDX6 (4µg/ml) was added to the cells prior to H2O2 exposure. Colorimetric MTS assay was performed after 24 h, and cell viability was monitored. Figure 6 represents experiments showing that TAT-HA-PRDX6 was able to save the TM cells from H2O2 induced damage (Figure 6, A; c and d, B, open bar).
Figure 6. Photomicrographs showing the ability of PRDX6 in protecting TM cells against oxidative stress.
Cells were cultured overnight in Opti-MEM +10% FBS. On the second day cells were exposed to H2O2 for 4h. After an overnight recovery period, cells were photomicrographed. Arrow heads indicate dead cells (a and b). A, supply of TAT-HA-PRDX6 prior to H2O2 treatment showed enhanced cellular survival (c and d). (B) MTS assay showing cell viability in the presence (Open bar) or absence (black bar) of PRDX6 (open bar vs black bar, *p <0.05; **p < 0.001), indicating that the protein had protected the cells from H2 O2- driven oxidative stress-induced cell death.
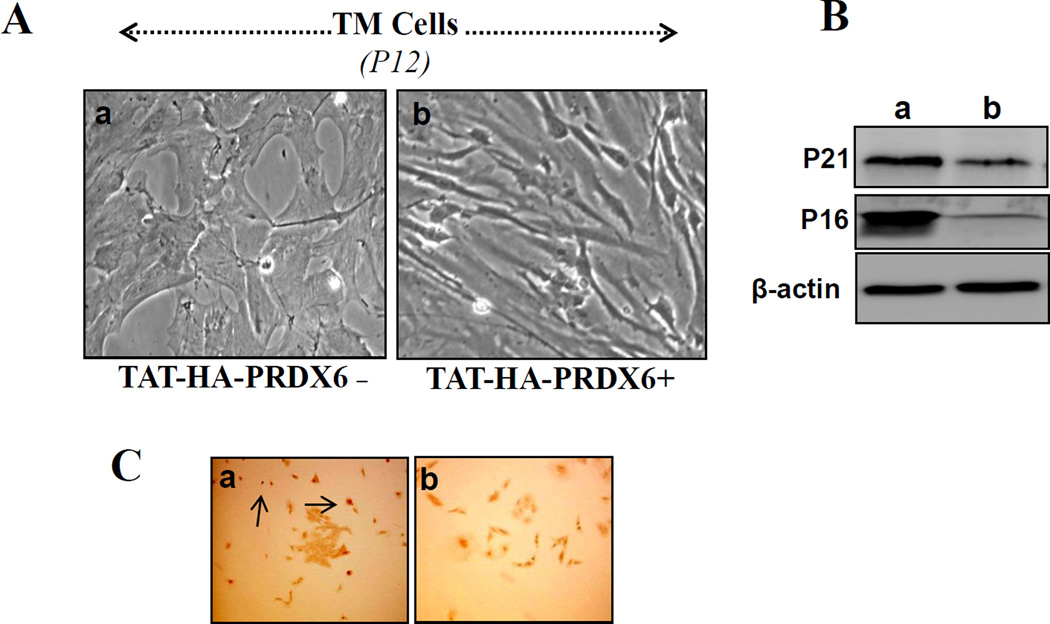
Delivery of PRDX6 attenuates TM cell senescence and DNA damage
Recently, TM cells in culture were shown to develop characteristics of senescence and to show changes after PDL 8 similar to those in glaucomatous cells [45]. Also, it has been demonstrated that oxidative damage to DNA is significantly increased in the TM of patients with glaucoma [10, 18]. These findings encouraged us to investigate whether adding PRDX6 to nonglaucomatous TM cells would interrupt the senescence process and protect DNA from damage. TM cells were cultured in the presence of TAT-HA-PRDX6 (4 µg/ml) or in its absence as described in Materials and Methods. Cells were observed routinely by microscope for morphological changes, if any, and photomicrographed. Figure 7 A is representative of experiment. Similar to the earlier findings by Yamazaki et al. [18], distinct morphological changes similar to senescence could be seen (Figure 7, A; a) and were comparable to changes in glaucomatous cells (Figure 1, A). Interestingly, delivery of PRDX6 maintained the typical morphology of TM cells. To validate whether PRDX6 could break the senescence process, we performed Western analysis using anti-P16 and P21 antibodies [46]. The levels of both proteins were dramatically decreased in TM cells that had received PRDX6 (Figure 7, B, right lane). Next we evaluated the ability of PRDX6 to block DNA damage. TM cells of passage 3 were cultured with or without PRDX6, and were stained at passage 12 (PDL 12) with 8-hydroxy-2’-deoxyguanosine [10, 18], following the protocols of the manufacturer (Molecular Probe). Results revealed that cells treated with PRDX6 (every 72 h) were significantly protected (Figure 7, b), suggesting that PRDX6 can break the senescence process of TM cells as well as DNA breakage. Based on these results, we conclude that TM cells entering senescence can be reversed by an extrinsic supply of PRDX6.
Figure 7. PRDX6 delivery attenuated the senescence process of TM cells.
TM cells cultured in vitro underwent senescence and showed morphology typical of glaucomatous TM cells. TM cells (P3) were cultured with or without PRDX6 (4µg/ml every third day) up to P12. Cells were photomicrographed (P12) and presented (A). To test whether PRDX6 reversed senescence markers, P21 and P16, Western analysis (B) was carried out using specific antibodies of the corresponding proteins. Results revealed the ability of PRDX6 in attenuating the senescence process in TM cells. Figure C demonstrates that PRDX6 protects TM cells against DNA damage occurring during the senescence process (C, a). TM cells, treated with or without TAT-HA-PRDX6 were fixed and immunostained using Alexa Fluor® 488 Signal-Amplification kit (Molecular Probes). Visualization of antibody complex was done by fluorescent microscopy using absorption/emission maxima ~495/519 nm.
Discussion
Recent evidence reveals that ROS, when overproduced in vivo, can cause significant damage to cells/tissues [3, 5, 19, 47, 48], leading to the initiation and progression of degenerative diseases including glaucoma. Oxidative stress-induced cellular insults become more severe when cells’ antioxidant defense has been weakened by aging or overstimulation of other physiological factors, such as growth factors, transcriptional proteins, and ROS, that directly or indirectly jeopardize the expression and functions of antioxidants. A particularly intriguing recent development in glaucoma research has been the realization that oxidative stress is pivotal in the decline of most measures of physiological performance, specifically TM cells, which are directly exposed to H2O2 and TGFβs present in aqueous humor [2, 23, 49]. The study described here was directed at understanding the role of oxidative stress and the underlying mechanisms involved in the induction of pathophysiology of glaucoma, by using TM cells derived from glaucomatous and nonglaucomatous subjects. Findings demonstrated that TM cells from glaucomatous subjects bear increased expression of ROS levels along with reduced expression of PRDX6 compared to other members of the Prdx family (Figures 1, B, C and 2, A), suggesting that PRDX6 may play an important role in maintaining TM cell integrity by controlling ROS-mediated adverse signaling. In addition, we found that reduced expression of PRDX6 in TM cells is related to phenotypic changes and overmodulation of many ECM or non ECM genes known to be involved in TM cell abnormalities. These changes in glaucomatous cells resemble those of TGFβ-induced alteration in nonglaucomatous TM cells (Figure 1, 3,4, 5). Interestingly, changes in glaucomatous cells involve higher ROS levels as assessed by H2DCFH-DA staining (Figure 2, A; open bar). We conclude that PRDX6 is a major protective molecule that is vitally important to maintaining cellular integrity, and that its expression is significantly reduced in glaucomatous TM cells exhibiting abnormal phenotypes (Figure 1). When extrinsically supplied with PRDX6, these cells show normal TM cell phenotypes, suggesting that the cellular changes are prevented by the delivery of PRDX6 (figure, 7). In addition, our finding indicates that the expression levels of PRDX1 are significantly higher than other PRDXs ( 2–6) in glaucomatous TM-cells, but could not counteract the changes occurred the abnormal changes in these cells, emphasizing a pivotal role of PRDX6 in maintaining cellular homeostasis of TM-cells. Furthermore, the presence of PRDX1 expression in TM-cells indicates that there is no balance between PRDX6 and PRDX1, since PRDX1 that exhibit cytosolic localization could not be able to attenuate the abnormalities of TM cells. This absence of balance can be explained by the fact that PRDX6 might have other cellular function(s) in addition to its protective antioxidant activity. Indeed, PRDX6 has been implicated in signal transduction in mammalian cells through control of ROS [3, 5, 7, 8]. Recently, using Prdx6−/− mouse, it has been shown that endogenously produced mouse PRDX6 functioned in vivo as an antioxidant enzyme, and its function was not redundant to other PRDXs and antioxidant enzyme [5]. Notably, the gene expression of Prdx 1-5, Gpx1-4, Cat, superoxide dismutase (Sod1-3), thioredoxin (Txn1-2), and glutaredoxin (Glrx1-2) was found similar between Prdx6−/− and Prdx6+/+ cells [5]. These studies emphasize that PRDX6 activities are independent of other enzymes. Moreover, several studies from others as well as from our laboratory have shown that PRDX6 plays a role in maintaining cellular homeostasis by optimizing cellular signaling, and it does so by controlling intracellular ROS levels [3, 5, 7, 24, 29]. Thus, we think that TM cells with reduced expression of PRDX6 due to aging or physiological stress are more susceptible to internal and external environmental stress, one cause of disturbances in TM cell biology that may lead to higher intraocular pressure (IOP) associated with the initiation and progression of glaucoma.
Glaucoma is a leading cause of blindness in the world. High IOP caused by reduction in normal aqueous outflow is a major casual risk factor for the disease. The TM, the specialized cells that control aqueous humor, plays an important role in the maintenance of IOP. Any damage or disturbance of TM may lead to the elevation of IOP [11]. Furthermore, the cells lining the TM synthesize various extracellular matrix (ECM) components that are believed to influence potency of the aqueous channels. An altered composition of ECM in TM may contribute to an increase in outflow resistance. Recently, several reports have indicated a major role for oxidative stress in the etiology and progression of glaucoma [2, 8, 11, 19, 43]. TM cells are continuously in contact with aqueous humor that contains H2O2 as well as TGFβs [16], and TGFβs are inducers of several matrix proteins. We believe that overproduction of ECM proteins in TM cells results from reduced expression of PRDX6 and higher expression and activation of TGFβs by H2O2 and Tsp-1. In the present study, we observed that TM cells exposed to TGFβs display abnormal phenotypes and increased expression of ECM proteins, abnormalities which are indistinguishable from those of glaucomatous TM cells (Figures 3, 4, 5). Zhao et al. [22] showed that treatment of human TM cells with either TGFß1 or ß2 stimulates the expression of several ECM genes, including versican, elastin, collagens, fibrillin, laminin, and fibulin. In addition, TGFß2-treated human TM cells alter the production of the enzymes promatrix metalloproteinase-2 and plasminogen activator inhibitor (PAI)-1, each of which likely plays a role in ECM-remodeling by the TM. Furthermore, treatment of human TM cell cultures with either TGFß1 or ß2 leads to significant increases in fibronectin and tissue transglutaminase, an enzyme that covalently cross-links ECM proteins, thereby conferring resistance to fibronectin degradation [36]. Fibronectin (FN) deposition in TM cells is known to occur with progression of glaucoma, and an excess FN deposition by TM cells may play a role in obstructing the passage of aqueous outflow [30, 50]. Fibulin 5 is essential for the polymerization of elastin. In comparing the expression of ECM among non glaucomatous, glaucomatous, and TGFβs-treated nonglaucomatous TM cells, we found that overmodulation of ECM proteins is associated with TGFβs-treated nonglaucomatous cells, suggesting that TGFβs are major culprits for TM cell disturbance. Furthermore, MMP2 expression is down-regulated in glaucomatous TM cells. MMPs are involved in ECM metabolism and have been shown to increase aqueous outflow facility [37, 38]. These proteins may play an important role in the maintenance and regulation of the trabecular extracellular matrix and, subsequently, of the aqueous humor outflow pathway. The reduced expression of these proteins may lead to interruption of smooth flow of aqueous humor. Recently, Clark and his coworkers [36] have shown an increase in the expression and enzymatic activity of Transglutaminase-2 in glaucomatous TM cells. We found higher expression of this protein in glaucomatous as well as nonglaucomatous cells treated with TGFβs (Figures, 3 and 5). However, we did not check the enzymatic activity. Moreover, the expression of Tropomyosin (Tmp1α and Tmp 2β) was elevated in glaucomatous TM (Figure 3). These proteins are also TGFβ- inducible [42]. An increase in TGFβ-inducible genes like βig-h3 and α-sm-actin in glaucomatous TM and other genes mentioned above (Figure 3 and 5) leads us to believe that ROS-driven activation of TGFβs may be a factor responsible for the overmodulation of ECM genes/proteins that in turn interrupts the smooth flow of aqueous humor.
Moreover, the concentration of ROS generated by cells in response to internal as well as external environment is tightly regulated by antioxidants, because excessive accumulation of ROS leads to cellular damage and results in cell death. We think that reduced expression of PRDX6 in TM cells may be associated with ROS-driven-activation of TGFβ-mediated overstimulation of genes in TM cells as well as DNA damage in these cells. Previously we have reported that ROS-driven oxidative stress could influence down-stream signaling [3], such as attenuation of transcriptional activity of LEDGF, an activator of PRDX6 transcription [7]. We found that repression of PRDX6 was due to activation and expression of TGFβ by overproduction of ROS [3]. Moreover, the concentration of ROS generated by cells in response to internal or external stimuli are tightly controlled by antioxidants, because excessive production usually results in failure cellular homeostasis or in cell death. We think that repression of PRDX6 in TM cells might be linked to ROS induced activation of TGFβ and its mediated attenuation of Prdx6 transcription. Furthermore, ROS, a source of oxidative stress, have received attention recently due to its role as a signaling molecule for various growth factors, including TGFβs. However, the adverse effect of TGFβ1 and TGFβ2 on anti-death factors has been reported [3, 7]. Moreover, TGFβs are normally present in the aqueous humor of the eye [16]. Increased IOP in human eye caused by TGFβs indicates that TGFβs are involved in pathogenesis of glaucoma; it causes increased ECM proteins and phenotypic changes in the TM cells [10]. TGFβ is known to be activated when ROS are produced, and that TGFβ subsequently induced ROS production. We believe that during glaucoma, due to down regulation of PRDX6, ROS production is increased; TGF β is activated that further enhances ROS production by down regulating PRDX6. We believe that by blocking ROS-mediated deleterious effects should reduce progression of glaucoma by interrupting the vicious cycle initiated by locally high levels of ROS and ROS-induced TGFβs activation and its overstimulated genes. However, it appears reduced expression of PRDX6 is associated with activation TGFβ by ROS. We and others have reported that cloned PRDX6 is a real savior of cells facing oxidative stress [3, 5, 7, 25, 28], in the present study we showed that TAT-HA-PRDX6 efficiently internalizes into TM cells, protecting them from TGFβs- or H2O2-induced abnormalities (Figures 1, 4, 5, 6). Most importantly, the present study demonstrated the ability of PRDX6 to attenuate the senescence process as well as DNA damage (Figure 7). Oxidative stress has been implicated in the aging process and in pathology of several age-related disorders [51, 52] including glaucoma [53]. Primary open glaucoma (POAG) is characterized by an accumulation of senescence cells [53, 54, 55]. In view of our findings, we believe that PRDX6 plays a pivotal role in maintaining TM cell biology, and its reduced expression in TM cells is a cause of TM cell abnormalities. However, a more complete understanding of the pathogenesis of glaucoma requires further attention to dissect out the roles of other cell types producing ECM and complex networks of growth factors involved in progression or initiation of the glaucoma associated pathological process. In summary, we presented evidence that ROS-mediated adverse signaling is a plausible cause of disturbances in TM cell biology that in turn lead to development of elevated IOP resulting in glaucoma. Because PRDX6 is able to reverse the ROS- or TGFβs-induced insult to TM cells as well as attenuate the senescence process, this protein may be a novel candidate to block or delay ROS-associated pathophysiology, including glaucoma.
Acknowledgement
Grant provided by the National Eye Institute (EY-13394 and EY017613) (to DPS), and Research for Preventing Blindness are gratefully acknowledged. Grant support by American Health Assistance Foundation (AHAF) (to NF) is gratefully acknowledged.
Footnotes
Declaration of interest
No conflicts of interest exist.
References
- 1.Chong ZZ, Li F, Maiese K. Oxidative stress in the brain: novel cellular targets that govern survival during neurodegenerative disease. Prog Neurobiol. 2005;75:207–246. doi: 10.1016/j.pneurobio.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 2.He Y, Leung KW, Zhang YH, Duan S, Zhong XF, Jiang RZ, Peng Z, Tombran-Tink J, Ge J. Mitochondrial complex I defect induces ROS release and degeneration in trabecular meshwork cells of POAG patients: protection by antioxidants. Invest Ophthalmol Vis Sci. 2008;49:1447–1458. doi: 10.1167/iovs.07-1361. [DOI] [PubMed] [Google Scholar]
- 3.Fatma N, Kubo E, Sharma P, Beier DR, Singh DP. Impaired homeostasis and phenotypic abnormalities in Prdx6−/−mice lens epithelial cells by reactive oxygen species: increased expression and activation of TGFbeta. Cell Death Differ. 2005;12:734–750. doi: 10.1038/sj.cdd.4401597. [DOI] [PubMed] [Google Scholar]
- 4.Azhar S, Cao L, Reaven E. Alteration of the adrenal antioxidant defense system during aging in rats. j Clin Invest. 1995;96:1414–1424. doi: 10.1172/JCI118177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang X, Phelan SA, Forsman-Semb K, Taylor EF, Petros C, Brown A, Lerner CP, Paigen B. Mice with targeted mutation of peroxiredoxin 6 develop normally but are susceptible to oxidative stress. J Biol Chem. 2003;278:25179–25190. doi: 10.1074/jbc.M302706200. [DOI] [PubMed] [Google Scholar]
- 6.Barcellos-Hoff MH, Dix TA. Redox-mediated activation of latent transforming growth factor-beta 1. Mol Endocrinol. 1996;10:1077–1083. doi: 10.1210/mend.10.9.8885242. [DOI] [PubMed] [Google Scholar]
- 7.Kubo E, Fatma N, Akagi Y, Beier DR, Singh SP, Singh DP. TAT-mediated PRDX6 protein transduction protects against eye lens epithelial cell death and delays lens opacity. Am J Physiol Cell Physiol. 2008;294:C842–C855. doi: 10.1152/ajpcell.00540.2007. [DOI] [PubMed] [Google Scholar]
- 8.Fatma N, Kubo E, Sen M, Agarwal N, Thoreson WB, Camras CB, Singh DP. Peroxiredoxin 6 delivery attenuates TNF-alpha-and glutamate-induced retinal ganglion cell death by limiting ROS levels and maintaining Ca(2+) homeostasis. Brain Res. 2008;1233:63–78. doi: 10.1016/j.brainres.2008.07.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fleenor DL, Shepard AR, Hellberg PE, Jacobson N, Pang IH, Clark AF. TGFbeta2-induced changes in human trabecular meshwork: implications for intraocular pressure. Invest Ophthalmol Vis Sci. 2006;47:226–234. doi: 10.1167/iovs.05-1060. [DOI] [PubMed] [Google Scholar]
- 10.Saccà SC, Pascotto A, Camicione P, Capris P, Izzotti A. Oxidative DNA damage in the human trabecular meshwork: clinical correlation in patients with primary open-angle glaucoma. Arch Ophthalmol. 2005;123:458–463. doi: 10.1001/archopht.123.4.458. [DOI] [PubMed] [Google Scholar]
- 11.Saccà SC, Izzotti A, Rossi P, Traverso C. Glaucomatous outflow pathway and oxidative stress. Exp Eye Res. 2007;84:389–399. doi: 10.1016/j.exer.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 12.Moreno MC, Campanelli J, Sande P, Sánez DA, Keller Sarmiento MI, Rosenstein RE. Retinal oxidative stress induced by high intraocular pressure. Free Radic Biol Med. 2004;37:803–812. doi: 10.1016/j.freeradbiomed.2004.06.001. [DOI] [PubMed] [Google Scholar]
- 13.Izzotti A, Bagnis A, Saccà SC. The role of oxidative stress in glaucoma. Mutat Res. 2006;612:105–114. doi: 10.1016/j.mrrev.2005.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Ohia SE, Opere CA, Leday AM. Pharmacological consequences of oxidative stress in ocular tissues. Mutat Res. 2005;579:22–36. doi: 10.1016/j.mrfmmm.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 15.Tamm ER, Fuchshofer R. What increases outflow resistance in primary open-angle glaucoma? Surv Ophthalmol. 2007;52:S101–S104. doi: 10.1016/j.survophthal.2007.08.002. [DOI] [PubMed] [Google Scholar]
- 16.Jampel HD, Roche N, Stark WJ, Roberts AB. Transforming growth factor-beta in human aqueous humor. Curr Eye Res. 1990;9:963–969. doi: 10.3109/02713689009069932. [DOI] [PubMed] [Google Scholar]
- 17.Baleriola J, García-Feijoo J, Martínez-de-la-Casa JM, Fernández-Cruz A, de la Rosa EJ, Fernández-Durango R. Apoptosis in the trabecular meshwork of glaucomatous patients. Mol Vis. 2008;14:1513–1516. [PMC free article] [PubMed] [Google Scholar]
- 18.Izzotti A, Saccà SC, Cartiglia C, De Flora S. Oxidative deoxyribonucleic acid damage in the eyes of glaucoma patients. Am J Med. 2003;114:638–646. doi: 10.1016/s0002-9343(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 19.Saccà SC, Izzotti A. Oxidative stress and glaucoma: injury in the anterior segment of the eye. Prog Brain Res. 2008;173:385–407. doi: 10.1016/S0079-6123(08)01127-8. [DOI] [PubMed] [Google Scholar]
- 20.Lütjen-Drecoll E. Morphological changes in glaucomatous eyes and the role of TGFbeta2 for the pathogenesis of the disease. Exp Eye Res. 2005;81:1–4. doi: 10.1016/j.exer.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 21.Wordinger RJ, Fleenor DL, Hellberg PE, Pang IH, Tovar TO, Zode GS, Fuller JA, Clark AF. Effects of TGF-beta2, BMP-4, and gremlin in the trabecular meshwork: implications for glaucoma. Invest Ophthalmol Vis Sci. 2007;48:1191–1200. doi: 10.1167/iovs.06-0296. [DOI] [PubMed] [Google Scholar]
- 22.Zhao X, Ramsey KE, Stephan DA, Russell P. Gene and protein expression changes in human trabecular meshwork cells treated with transforming growth factor-beta. Invest Ophthalmol Vis Sci. 2004;45:4023–4034. doi: 10.1167/iovs.04-0535. [DOI] [PubMed] [Google Scholar]
- 23.Fuchshofer R, Yu AH, Welge-Lüssen U, Tamm ER. Bone morphogenetic protein-7 is an antagonist of transforming growth factor-beta2 in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2007;48:715–726. doi: 10.1167/iovs.06-0226. [DOI] [PubMed] [Google Scholar]
- 24.Manevich Y, Fisher AB. Peroxiredoxin 6, a 1-Cys peroxiredoxin, functions in antioxidant defense and lung phospholipid metabolism. Free Radic Biol Med. 2005;238:1422–1432. doi: 10.1016/j.freeradbiomed.2005.02.011. 2. [DOI] [PubMed] [Google Scholar]
- 25.Fatma N, Singh DP, Shinohara T, Chylack LT., Jr Transcriptional regulation of the antioxidant protein 2 gene, a thiol-specific antioxidant, by lens epithelium-derived growth factor to protect cells from oxidative stress. J Biol Chem. 2001;276:48899–48907. doi: 10.1074/jbc.M100733200. [DOI] [PubMed] [Google Scholar]
- 26.Nagahara H, Vocero-Akbani AM, Snyder EL, Ho A, Latham DG, Lissy NA, Becker-Hapak M, Ezhevsky SA, Dowdy SF. Transduction of full-length TAT fusion proteins into mammalian cells: TAT-p27Kip1 induces cell migration. Nat Med. 1998;4:1449–1452. doi: 10.1038/4042. [DOI] [PubMed] [Google Scholar]
- 27.Stamer DW, Roberts BC, Epstein DL, Allingham RR. Isolation of primary open-angle glaucomatous trabecular meshwork cells from whole eye tissue. Curr Eye Res. 2000;20:347–350. [PubMed] [Google Scholar]
- 28.Kubo E, Miyazawa T, Fatma N, Akagi Y, Singh DP. Development- and age-associated expression pattern of peroxiredoxin 6, and its regulation in murine ocular lens. Mech Ageing Dev. 2006;127:249–256. doi: 10.1016/j.mad.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 29.Manevich Y, Sweitzer T, Pak JH, Feinstein SI, Muzykantov V, Fisher AB. 1-Cys peroxiredoxin overexpression protects cells against phospholipid peroxidation-mediated membrane damage. Proc Natl Acad Sci. 2002;99:11599–11604. doi: 10.1073/pnas.182384499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Acott TS, Kelley MJ. Extracellular matrix in the trabecular meshwork. Exp Eye Res. 2008;86:543–561. doi: 10.1016/j.exer.2008.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Keller KE, Aga M, Bradley JM, Kelley MJ, Acott TS. Extracellular matrix turnover and outflow resistance. Exp Eye Res. 2008 doi: 10.1016/j.exer.2008.11.023. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gallagher BM, Phelan SA. Investigating transcriptional regulation of Prdx6 in mouse liver cells. Free Radic Biol Med. 2007;42:1270–1277. doi: 10.1016/j.freeradbiomed.2007.01.023. [DOI] [PubMed] [Google Scholar]
- 33.Flügel-Koch C, Ohlmann A, Fuchshofer R, Welge-Lüssen U, Tamm ER. Thrombospondin-1 in the trabecular meshwork: localization in normal and glaucomatous eyes, and induction by TGF-beta1 and dexamethasone in vitro. Exp Eye Res. 2004;79:649–663. doi: 10.1016/j.exer.2004.07.005. [DOI] [PubMed] [Google Scholar]
- 34.Murphy-Ullrich JE, Poczatek M. Activation of latent TGF-beta by thrombospondin-1: mechanisms and physiology. Cytokine Growth Factor Rev. 2000;11:59–69. doi: 10.1016/s1359-6101(99)00029-5. [DOI] [PubMed] [Google Scholar]
- 35.Tane N, Dhar S, Roy S, Pinheiro A, Ohira A, Roy S. Effect of excess synthesis of extracellular matrix components by trabecular meshwork cells: possible consequence on aqueous outflow. Exp Eye Res. 2007;84:832–842. doi: 10.1016/j.exer.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 36.Tovar-Vidales T, Roque R, Clark AF, Wordinger RJ. Tissue transglutaminase expression and activity in normal and glaucomatous human trabecular meshwork cells and tissues. Invest Ophthalmo Vis Sci. 2008;49:622–628. doi: 10.1167/iovs.07-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Oh DJ, Martin JL, Williams AJ, Russell P, Birk DE, Rhee DJ. Effect of latanoprost on the expression of matrix metalloproteinases and their tissue inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2006;47:3887–3895. doi: 10.1167/iovs.06-0036. [DOI] [PubMed] [Google Scholar]
- 38.Pang IH, Hellberg PE, Fleenor DL, Jacobson N, Clark AF. Expression of matrix metalloproteinases and their inhibitors in human trabecular meshwork cells. Invest Ophthalmol Vis Sci. 2003;44:3485–3493. doi: 10.1167/iovs.02-0756. [DOI] [PubMed] [Google Scholar]
- 39.Rönkkö S, Rekonen P, Kaarniranta K, Puustjärvi T, Teräsvirta M, Uusitalo H. Matrix metalloproteinases and their inhibitors in the chamber angle of normal eyes and patients with primary open-angle glaucoma and exfoliation glaucoma. Graefes Arch Clin Exp Ophthalmol. 2007;245:697–704. doi: 10.1007/s00417-006-0440-1. [DOI] [PubMed] [Google Scholar]
- 40.Clark AF. New discoveries on the roles of matrix metalloproteinases in ocular cell biology and pathology. Invest Ophthalmol Vis Sci. 1998;39:2514–2516. [PubMed] [Google Scholar]
- 41.Eitzman DT, Ginsburg D. Of mice and men. The function of plasminogen activator inhibitors (PAIs) in vivo. Adv Exp Med Biol. 1997;425:131–141. [PubMed] [Google Scholar]
- 42.Bakin AV, Safina A, Rinehart C, Daroqui C, Darbary H, Helfman DM. A critical role of tropomyosins in TGF-beta regulation of the actin cytoskeleton and cell motility in epithelial cells. Mol Biol Cell. 2004;15:4682–4694. doi: 10.1091/mbc.E04-04-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sakai H, Park BC, Shen X, Yue BY. Transduction of TAT fusion proteins into the human and bovine trabecular meshwork. Invest Ophthalmol Vis Sci. 2006;47:4427–4434. doi: 10.1167/iovs.06-0047. [DOI] [PubMed] [Google Scholar]
- 44.Zhou L, Li Y, Yue BY. Oxidative stress affects cytoskeletal structure and cell-matrix interactions in cells from an ocular tissue: the trabecular meshwork. J Cell Physiol. 1999;180:182–189. doi: 10.1002/(SICI)1097-4652(199908)180:2<182::AID-JCP6>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 45.Yamazaki Y, Matsunaga H, Nishikawa M, Ando A, Kaneko S, Okuda K, Wada M, Ito S, Matsumura M. Senescence in cultured trabecular meshwork cells. Br J Ophthalmol. 2007;91:808–811. doi: 10.1136/bjo.2006.108423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caballero M, Liton PB, Challa P, Epstein DL, Gonzalez P. Effects of donor age on proteasome activity and senescence in trabecular meshwork cells. Biochem Biophys Res Commun. 2004;323:1048–1054. doi: 10.1016/j.bbrc.2004.08.195. [DOI] [PubMed] [Google Scholar]
- 47.Halliwell B. Free radicals, reactive oxygen species and human disease: a critical evaluation with special reference to atherosclerosis. Br J Exp Pathol. 1989;70:737–757. [PMC free article] [PubMed] [Google Scholar]
- 48.Fisher AB. Redox Signaling Across Cell Membranes. Antioxid Redox Signa. 2008 doi: 10.1089/ars.2008.2378. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Caballero M, Liton PB, Epstein DL, Gonzalez P. Proteasome inhibition by chronic oxidative stress in human trabecular meshwork cells. Biochem Biophys Res Commun. 2003;308:346–352. doi: 10.1016/s0006-291x(03)01385-8. [DOI] [PubMed] [Google Scholar]
- 50.Faralli JA, Schwinn MK, Gonzalez JM, Jr, Filla MS, Peters DM. Functional properties of fibronectin in the trabecular meshwork. Ex. Eye Res. 2008 doi: 10.1016/j.exer.2008.08.019. (In Press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Campisi J. Senescent cells, tumor suppression, and organismal aging: good citizens, bad neighbors. Cell. 2005;120:513–522. doi: 10.1016/j.cell.2005.02.003. [DOI] [PubMed] [Google Scholar]
- 52.Passos JF, Von Zglinicki T. Oxygen free radicals in cell senescence: are they signal transducers? Free Radic Res. 2006;40:1277–1283. doi: 10.1080/10715760600917151. [DOI] [PubMed] [Google Scholar]
- 53.Liton PB, Challa P, Stinnett S, Luna C, Epstein DL, Gonzalez P. Cellular senescence in the glaucomatous outflow pathway. Exp Gerontol. 2005;40:745–748. doi: 10.1016/j.exger.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Luna C, Li G, Liton PB, Qiu J, Epstein DL, Challa P, Gonzalez P. Resveratrol prevents the expression of glaucoma markers induced by chronic oxidative stress in trabecular meshwork cells. Food Chem Toxicol. 2009;47:198–204. doi: 10.1016/j.fct.2008.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Yu AL, Fuchshofer R, Kampik A, Welge-Lüssen U. Effects of oxidative stress in trabecular meshwork cells are reduced by prostaglandin analogues. Invest Ophthalmol Vis Sci. 2008;49:4872–4880. doi: 10.1167/iovs.07-0984. [DOI] [PubMed] [Google Scholar]