Abstract
Objectives
To evaluate the prevalence and prognostic impact of non-cardiac comorbidities in patients with heart failure (HF) with preserved ejection fraction (HFpEF) versus heart failure with reduced ejection fraction (HFrEF).
Background
There is paucity of information on the comparative prognostic significance of comorbidities between HFpEF and HFrEF patients.
Methods
In a national ambulatory cohort of Veterans with HF, we compared the comorbidity burden of 15 non-cardiac comorbidities and the impact of these comorbidities on hospitalization and mortality between HFpEF and HFrEF patients.
Results
The cohort consisted of 2,843 HFpEF and 6,599 HFrEF patients with 2 year follow-up. Compared to HFrEF, HFpEF patients were older and had higher prevalence of chronic obstructive pulmonary disease (COPD), diabetes, hypertension, psychiatric disorders, anemia, obesity, peptic ulcer disease and cancer, but lower prevalence of chronic kidney disease. HFpEF patients had lower HF hospitalization, higher non-HF hospitalization and similar overall hospitalization, compared with HFrEF patients (p<0.001, p<0.001, p=0.19, respectively). Increasing number of non-cardiac comorbidities was associated with higher risk of all-cause admissions (p<0.001). Comorbidities had similar impact on mortality in HFpEF vs. HFrEF patients except for COPD, which was associated with a higher hazard (1.62 [95% CI 1.36-1.92] vs. 1.23 [95% CI 1.11, 1.37], respectively; p=0.01 for interaction) in HFpEF patients.
Conclusions
There is a higher non-cardiac comorbidity burden associated with higher non-HF hospitalizations in HFpEF compared to HFrEF patients. However, individually, most comorbidities have similar impact on mortality in both groups. Aggressive management of comorbidities may have an overall greater prognostic impact in HFpEF compared to HFrEF.
Keywords: Heart failure with preserved ejection fraction, Heart failure with reduced ejection fraction, diastolic heart failure, comorbidities, prognosis
INTRODUCTION
Many patients with heart failure (HF) have a normal or nearly normal left ventricular ejection fraction (EF), referred to as diastolic HF or HF with preserved EF (HFpEF). Studies have reported the prevalence of HFpEF ranging from 30% to 70% (average ~ 50%) among HF patients (1-3). Furthermore, the prevalence of HFpEF among patients with a discharge diagnosis of HF has increased significantly over the last few decades (4). The prevalence of this condition is anticipated to keep increasing as the prevalence of the elderly with comorbid conditions such as hypertension, diabetes mellitus (DM) and obesity increases. Although the morbidity and mortality in patients with HFpEF in comparison with HF with reduced EF (HFrEF) has varied, there is consensus that HFpEF is associated with substantial morbidity and mortality (3-6).
Previously, the few analyses that examined the cause of death in HF patients, suggested that a higher proportion of deaths are due to non-cardiovascular causes in HFpEF compared to HFrEF patients (7-9). This is consistent with the belief that comorbidities may play a more significant role in outcomes in HFpEF compared with HFrEF. However, the relative impact of comorbidities on morbidity and mortality in HFpEF vs. HFrEF has not been well studied. Therefore, in a large national cohort of ambulatory patients with HF, we examined the prevalence and relative impact of a wide range of non-cardiac comorbidities on morbidity and mortality in HFpEF vs. HFrEF patients.
METHODS
Patient Cohort and Comorbidities
We performed a retrospective study of a national cohort of Veterans with HF treated in ambulatory clinics of Veterans Affairs (VA) Medical Centers between October 1, 2000 and September 30, 2002. We used the VA External Peer Review Program (EPRP) data. As described previously (10), the sampling pool of outpatients for EPRP included ambulatory patients with chronic diseases including HF, diabetes mellitus (DM), prior myocardial infarction, and chronic obstructive pulmonary disease (COPD), identified by ICD-9 codes. Abstractors reviewed electronic medical records for validation of inclusion criteria, including documentation by clinicians of the diagnosis of HF and other chronic diseases listed above (10). Patient-level data from the EPRP HF cohort was linked with five existing national VA databases to obtain demographic, comorbidity, laboratory, pharmacy and outcome data. The EF and date of its ascertainment were obtained from the EPRP database. Of patients with known EF (n=17456), only patients with the EF determination within one year prior to 3 months after the clinic visit (n=9451) were included in the current analyses. Patients were classified as HFpEF when the EF was ≥ 50% and as HFrEF when EF was < 50%.
Blood pressure, weight, height and comorbidities of prior myocardial infarction, DM, hypertension and COPD were obtained from the EPPRP database. Other comorbidities were ascertained using ICD-9 codes from VA Outpatient Clinic files (containing demographics, diagnoses, and outpatient services) and Patient Treatment files (containing abstracts for patients discharged from VA hospitals) over a time period of 2 years before, and at the index clinic visit (ICD-9 codes used to identify the comorbidities are listed in the online Appendix). Based on the Charlson comorbidity index (11) we included the following non-cardiac comorbidities: peripheral arterial disease, cerebrovascular disease (CVA), dementia, chronic pulmonary disease, rheumatological disorders, acquired immunodeficiency syndrome, peptic ulcer disease, DM, liver disease, malignancy and renal disease. Although not included in the Charlson comorbidity index, we included anemia, hypertension, psychiatric disorders and obesity because previous studies have identified them as significant prognostic variables in HF patients (12-14). For each patient we calculated the total number of non-cardiac comorbidities from these 15 comorbidities.
The most recent laboratory data within 1 year prior to 2 weeks after the index visit were used. Renal insufficiency was defined as eGFR < 60 ml/min/1.73m2, calculated by the four variable MDRD formula (15). Anemia was present if hemoglobin was <13gm/dL in males and <12gm/dL in females. Obesity was present if body mass index was ≥ 30 kg/m2. Four patients were excluded due to missing systolic blood pressure and 5 patients were excluded due to missing outcomes. For variables with < 20% missing values, imputation procedures were performed. Variables with missing values of > 20% were excluded. Missing values for serum sodium (6.1%), hemoglobin (15.9%) and creatinine (11.7%) were imputed. Missing values were imputed using linear regression with baseline variables as predictors and constraints applied based on observed minimum and maximum values. Analyses were repeated by excluding observations with imputed values and the results were found to be concordant. Thus, models using imputed data are shown.
Statistical Analyses
Univariate differences in baseline variables between HFpEF and HFrEF were evaluated using the Chi-square test for categorical and the two sample t-test for continuous variables. Covariates for multivariate models of mortality were selected based on backward stepwise Cox proportional hazard models with removal set at probability of 0.2. Based on these results, 19 variables were selected for the multivariate models. Additionally, history of hypertension, psychiatric disorder, peptic ulcer disease and rheumatological disorders were forced into the model in order to evaluate the effect of all non-cardiac comorbidities in this population. Multivariable Cox proportional hazards models were run separately for the HFpEF and HFrEF groups to calculate the hazard ratios for mortality in each EF group. Finally, 23 variables were used for the multivariate analyses: age, serum sodium, gender, systolic blood pressure, past hospitalization for HF, use of beta-blockers, angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) and statins, and the 15 comorbidities. In models of HFrEF, we also used a categorical variable for severity of depressed EF: mild (40% ≤ EF <50%), moderate (30% ≤ EF <40%) and severe (EF < 30%). To evaluate whether the prognostic impact of each comorbidity was different in the HFpEF vs. HFrEF group, we performed an interaction analysis. A backward elimination for the Cox proportional hazards analysis on the entire dataset was performed to examine the interaction of each of the 23 variables and the group variable representing HFpEF or HFrEF. In this analysis, we kept all 23 variables and the group in the model, allowing the interaction terms to be removed one by one for p value > 0.05.
Kaplan Meier survival curves were generated and the log-rank test was used to compare time to first HF and first non-HF hospitalization between HFpEF and HFrEF patients. Follow-up data were available for the first all-cause admission and HF admission. For survival analysis of non-HF admissions, only the first all-cause admission that was not documented as a HF admission was considered an event. In addition, occurrence of a HF admission was considered a censor for the observation of non-HF admission. All analyses were performed using SPSS v. 18. Data are presented as mean ± SD unless otherwise specified. P values < 0.05 are considered significant.
RESULTS
The cohort consisted of 9442 Veterans with HF, of which 2,843 patients (30%) had HFpEF and 6,599 patients (70%) had HFrEF. All patients had a 2 year follow-up. Patients had a mean age of 70 years and 95% were males. Of patients with HFrEF, 25% had mildly reduced, 31% had moderately reduced, and 44% had severely reduced EF.
As shown in Table 1, patients with HFpEF were older with a higher proportion of women and Caucasians. Compared to patients with HFrEF, patients with HFpEF had higher systolic blood pressure and serum sodium, higher prevalence of DM, hypertension, anemia, COPD, obesity, cancer, peptic ulcer disease and psychiatric disorders but lower prevalence of past MI and a mildly lower prevalence of renal insufficiency. In addition, patients with HFpEF had a lower frequency of HF hospitalization over the previous 2 years, and were less frequently prescribed beta-blockers, ACE inhibitors/ARBs and statins. Patients with HFpEF had a higher number of non-cardiac comorbidities per patient (mean 4.0 ± 1.7) compared to patients with HFrEF (3.5 ± 1.7; p<0.001), ranging from a minimum of 0 to a maximum of 11 comorbidities per patient. There was a significant increase in the proportion of patients with HFpEF with increasing number of non-cardiac comorbidities (Figure 1; p<0.001 by trend analysis).
Table 1.
Baseline Characteristics of patients with heart failure and preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF)
| HFpEF (n=2,843) | HFrEF (n=6,599) | p value | Age-Adjusted p value* | |
|---|---|---|---|---|
| Age (years) | 70.7 ± 10.1 | 69.5 ± 10.3 | <0.001 | NA |
| Male (%) | 91.1 | 96.4 | <0.001 | <0.001 |
| Race: | <0.001 | <0.001 | ||
| Caucasian (%) | 78.7 | 74.8 | ||
| African American (%) | 10.1 | 13.2 | ||
| Other / unknown | 11.2 | 11.9 | ||
| Diabetes mellitus (%) | 44.9 | 40.0 | <0.001 | <0.001 |
| Hypertension (%) | 70.5 | 62.2 | <0.001 | <0.001 |
| Peripheral arterial disease (%) | 27.5 | 27.8 | 0.76 | 0.42 |
| CVA (%) | 21.0 | 21.3 | 0.76 | 0.48 |
| Atrial Fibrillation (%) | 35.0 | 35.4 | 0.73 | 0.22 |
| Past MI | 27.1 | 40.4 | <0.001 | <0.001 |
| Renal insufficiency (%) | 48.8 | 51.9 | 0.005 | <0.001 |
| Anemia (%) | 33.2 | 28.4 | <0.001 | <0.001 |
| COPD (%) | 33.9 | 26.6 | <0.001 | <0.001 |
| Obesity (%) | 51.0 | 34.7 | <0.001 | <0.001 |
| Liver disease (%) | 1.7 | 1.7 | 1.0 | 0.81 |
| Cancer (%) | 21.6 | 18.6 | 0.001 | 0.01 |
| AIDS (%) | 0.3 | 0.3 | 1.0 | 0.84 |
| Dementia (%) | 3.0 | 2.6 | 0.31 | 0.56 |
| Psychiatric disoders (%) | 27.8 | 22.8 | <0.001 | <0.001 |
| Rheumatological disorders (%) | 4.4 | 3.8 | 0.20 | 0.22 |
| Peptic ulcer disease (%) | 8.1 | 6.0 | <0.001 | <0.001 |
| Systolic blood pressure (mmHg) | 132.2 ± 21.0 | 124.5 ± 20.9 | <0.001 | <0.001 |
| Serum sodium (meq/l) | 139.1 ± 3.4 | 139.0 ± 3.5 | 0.04 | 0.08 |
| HF hospitalizations within previous 2 years (%) | 17.2 | 22.9 | <0.001 | <0.001 |
| Medication use | ||||
| Beta-blockers (%) | 55.7 | 64.9 | <0.001 | <0.001 |
| ACE inhibitors/ARBs (%) | 72.8 | 85.5 | <0.001 | <0.001 |
| Statins (%) | 45.0 | 51.3 | <0.001 | <0.001 |
ACE inhibitors/ARBs: ACE inhibitors/Angiotensin receptor blockers; AIDS: Acquired immunodeficiency syndrome; COPD: Chronic obstructive pulmonary disease; CVA: cerebrovascular accident; HF: Heart failure; MI: Myocardial infarction.
Figure 1. Stacked bar chart showing relative composition of the HF population (HFpEF vs. HFrEF) stratified by the total number of non-cardiac comorbidities.
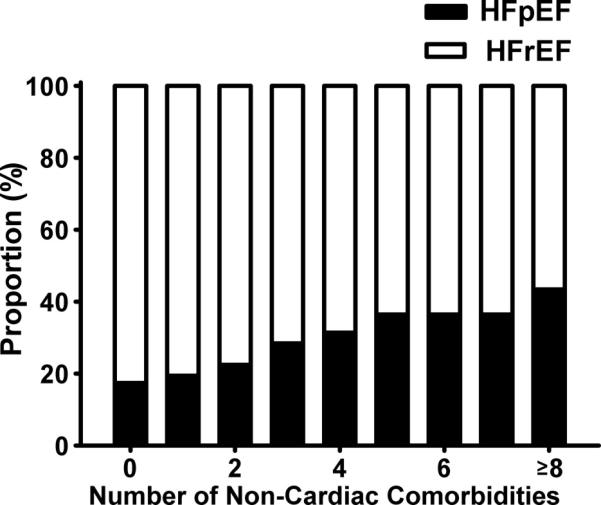
X-axis represents the total number of prevalent comorbidities, and the Y-axis demonstrates the relative proportion of patients with HFpEF and HFrEF for each category. As the number of prevalent comorbidities increases, there is a greater proportion of patients with HFpEF compared to patients with HFrEF (p<0.001 by trend analysis).
We then examined whether the higher prevalence of comorbidities in HFpEF patients was associated with more non-HF hospitalizations compared to HFrEF patients. Compared to HFrEF, a higher proportion of patients with HFpEF had at least one non-HF related admission (p<0.001), but a similar proportion of at least one any-cause admission (p=0.19) and lower proportion of at least one HF admission (p< 0.0001, Figure 2). Similar results were noted on time to event analysis demonstrated by a shorter time to first non-HF admission and longer time to first HF admission in HFpEF patients compared to HFrEF patients (Figure 3). There was no significant difference in time to any-cause hospitalization (p=0.6). Next, we examined whether the higher prevalence of non-cardiac comorbidities in HFpEF patients was a contributor to increased hospitalizations. On multivariate survival analysis, number of comorbidities (as a continuous variable) was significantly associated with time to all-cause admission (1.19, 95% CI: 1.17, 1.22, p<<0.001). When examined as a categorical variable, the increasing number of comorbidities also demonstrated an increasing hazard of hospitalization (p<<0.001, Figure 4).
Figure 2. Incidence of death and at least one all-cause admission, heart failure (HF) admission, and non-HF related admission, in HFpEF and HFrEF patients.
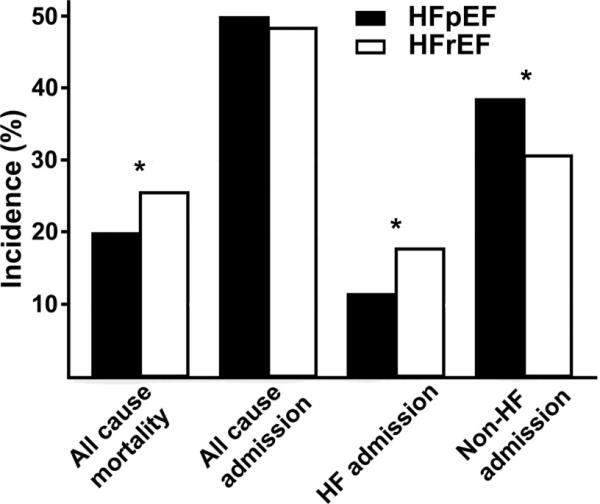
The median (inter quartile range) follow up for these end-points were 730 days (730 to 730 days), 518 days (138 to 730 days), 730 days (474 to 730 days) and 518 days (138 to 730 days), respectively. *p < 0.001
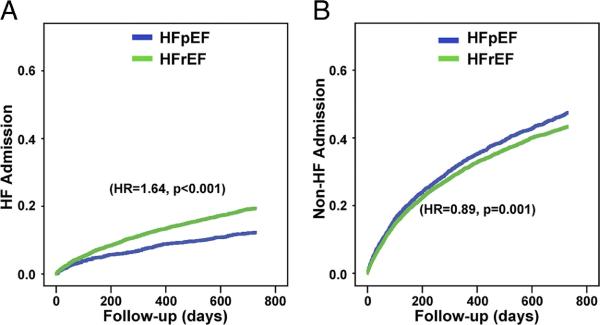
Figure 3. Kaplan Meier curves for hospitalization in patients with HFpEF and HFrEF.
A) Heart failure admissions, and B) Non-heart failure admissions. Hazard ratios (HR) shown are calculated using univariate Cox proportional hazards models.
Figure 4. Risk of all-cause hospitalization vs. number of non-cardiac comorbidities.
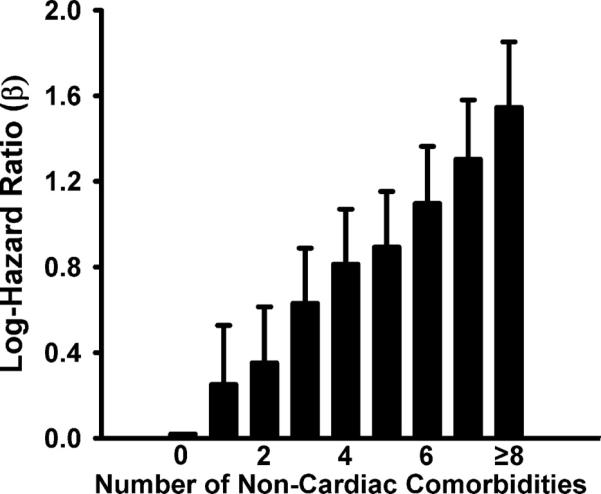
Log hazard ratios are based on Cox proportional hazard model (p<<0.001). Number of non-cardiac comorbidities used as a categorical variable. Error bars represent the upper limit of the 95% confidence interval.
During the two-year follow-up, there were 1,680 deaths among 6,599 HFrEF patients (25.5%), whereas there were 563 deaths among 2843 HFpEF patients (19.8%; p<0.001, Figure 2). In HFpEF, CVA, renal insufficiency, anemia, COPD, liver disease, cancer, dementia, rheumatological disorders and absence of obesity were independent predictors of all-cause mortality. In HFrEF patients, the association of baseline variables with mortality was found to be similar except for DM and peripheral arterial disease, which were significantly associated with mortality in addition to previously mentioned comorbidities; and history of cancer and rheumatological disorders which were not (Table 2). However, the interaction analyses revealed a significant interaction only between COPD and EF group (p=0.01). COPD contributed a higher hazard for mortality in HFpEF patients (1.61, 95% CI 1.36, 1.92) compared to HFrEF patients (1.23, 95% CI 1.11, 1.37). No other variables had a significant interaction with EF group indicating no significant differences in the prognostic impact of other comorbidities between the 2 EF groups.
Table 2.
Hazard ratios of Non-Cardiac Comorbidities for mortality in patients with heart failure with preserved ejection fraction (HFpEF) and heart failure with reduced ejection fraction (HFrEF)
| HFpEF Hazard Ratio (95% CI) | HFrEF Hazard Ratio (95% CI) | P value for interaction analyses | |
|---|---|---|---|
| Diabetes mellitus | 1.03 (0.86, 1.24) | 1.25‡ (1.13, 1.38) | 0.20 |
| Hypertension | 1.03 (0.85, 1.24) | 1.03 (0.93, 1.15) | 0.66 |
| Peripheral arterial disease | 1.17 (0.97, 1.40) | 1.38‡ (1.25, 1.53) | 0.11 |
| CVA | 1.25* (1.02, 1.52) | 1.18† (1.05, 1.32) | 0.53 |
| Renal insufficiency | 1.28† (1.07, 1.53) | 1.25‡ (1.12, 1.38) | 0.86 |
| Anemia | 1.35† (1.13, 1.61) | 1.42‡ (1.28, 1.57) | 0.84 |
| COPD | 1.61‡ (1.36, 1.91) | 1.23‡ (1.11, 1.37) | 0.01 |
| Obesity | 0.67‡ (0.56, 0.81) | 0.83† (0.74, 0.93) | 0.09 |
| Liver disease | 2.31‡ (1.48, 3.62) | 1.41* (1.05, 1.89) | 0.06 |
| Cancer | 1.24* (1.03, 1.49) | 1.10 (0.98, 1.24) | 0.33 |
| AIDS | 2.38 (0.76, 7.48) | 1.52 (0.72, 3.22) | 0.53 |
| Dementia | 1.75† (1.21, 2.51) | 1.48† (1.16, 1.90) | 0.70 |
| Psychiatric disorder | 1.05 (0.87, 1.27) | 1.02 (0.91, 1.15) | 0.57 |
| Rheumatological disorder | 1.52* (1.06, 2.17) | 0.96 (0.75, 1.23) | 0.054 |
| Peptic ulcer disease | 0.81 (0.60, 1.10) | 1.07 (0.89, 1.29) | 0.21 |
Other baseline covariates used in the model are age, gender, systolic blood pressure, serum sodium, past heart failure hospitalization and use of beta-blockers, ACE inhibitors/angiotensin receptor blockers and statins. In HFrEF group, reduced ejection fraction was used in the model as a categorical variable of mild/moderate/severely reduced ejection fraction.
AIDS: Acquired immunodeficiency syndrome; COPD: Chronic obstructive pulmonary disease; CVA: Cerebrovascular accident.
p < 0.05
p < 0.01
p < 0.001.
DISCUSSION
In a large national ambulatory HF cohort, we demonstrate that HFpEF patients have a significantly higher burden of non-cardiac comorbidities compared to HFrEF patients. Patients with HFpEF experienced significantly more non-HF hospitalizations compared to HFrEF patients, although overall hospitalizations were similar in both groups. The increasing number of comorbidities was associated with an increase in all-cause hospitalizations. Furthermore, individually, most of the non-cardiac comorbidities had a similar prognostic impact on mortality in HFpEF and HFrEF.
Our study adds to previous studies by demonstrating a higher burden of non-cardiac comorbidities in ambulatory HFpEF compared with HFrEF patients. Most of the large published studies evaluating comorbidities in HFpEF patients are based on hospitalized HF cohorts (1,2,4). In contrast, our study evaluated a large nationally representative cohort of 9,442 ambulatory HF patients, a setting wherein patients are more representative of the overall HF population rather than the sickest subgroup of hospitalized patients. In addition, most of the previous investigations examined only a limited number of comorbidities in each study and the specific comorbidities assessed varied across studies. In the current study, we included a comprehensive set of non-cardiac comorbidities. Our study confirmed findings from previous studies which demonstrated that HFpEF patients are typically older and have comorbidities including hypertension (55%–86%), DM (26%–45%), CVA (15-17%), obesity (41-62%), COPD (7-31%), and anemia (21%-53%), which were usually more prevalent than in HFrEF patients (1-4,6,16). Although the prevalence of diabetes has varied across studies, the majority of studies found a higher prevalence of diabetes in HFpEF, consistent with the findings in our study. In the OPTIMIZE-HF registry, lower serum creatinine was noted in HFpEF compared to HFrEF patients, while other studies demonstrated no significant difference in serum creatinine between the two groups (1,2,6). In our cohort, HFpEF patients had a slightly lower prevalence of renal insufficiency in comparison with HFrEF patients. For our analysis, renal insufficiency was defined by eGFR, which may be a more accurate measure of renal function than serum creatinine.
Although overall hospitalizations were similar between HFpEF and HFrEF, non-HF hospitalizations were significantly higher in the HFPEF group. These findings are supported by a previous study of 1077 HF patients from Olmstead County, Minnesota, which suggested a higher frequency of admissions for HF patients with preserved EF compared to reduced EF (40% vs. 34%, respectively) although in that study the difference was not statistically significant (p=0.069)(17). These findings are consistent with the greater burden of non-cardiac comorbidities that we found in patients with HFpEF, and underlines the importance of comorbidity management in reducing the overall morbidity in patients with HFpEF. Focusing predominantly on the reduction of HF admissions in these patients may result in a lower impact on their overall frequency of hospitalization. This has also been evident in recent large trials of in HFpEF targeting the renin-angiotensin system, some of which showed modest reduction in HF hospitalizations (18,19) but failed to reduce all-cause mortality and/or non-HF hospitalizations (18-20). In the Irbesartan in Heart Failure with Preserved Ejection Fraction (I-PRESERVE) trial, over 50% of patient hospitalizations were for non-cardiovascular causes. This higher rate of non-cardiovascular hospitalization occurred in spite of the trial having multiple exclusion criteria for significant comorbidities (20). Patients in clinical practice, such as our study cohort, would be expected to have an even greater contribution of co-morbidities to outcomes. Thus, it is possible that HF-specific treatment in HFpEF patients may not be able to reduce total hospitalization or total mortality that is largely driven by competing non-cardiac comorbidities.
The greater burden of comorbidities in HFpEF is also consistent with the prior finding of a higher proportion of non-cardiovascular deaths in patients with HFpEF compared to HFrEF (7-9). However, on examination of the relative prognostic impact of individual comorbidities on mortality in the preserved and reduced EF groups, we found that most comorbidites including renal disease, anemia, prior CVA, liver disease, cancer, dementia and obesity have a similar prognostic impact on mortality in the 2 EF groups. Although these comorbidities have been shown, in various combinations, to be associated with intermediate or long-term mortality in HFpEF or in HFrEF (4,6,21), most previous studies did not examine the differential prognostic impact of a comprehensive set of non-cardiac comorbidities in both these EF groups. We found that only COPD was associated with a significantly higher hazard of death in patients with HFpEF compared to HFrEF, although COPD was an independent predictor of mortality in both groups. While previous studies have found a higher prevalence of COPD in patients with HFpEF compared to HFrEF (2,6) and have demonstrated that COPD is associated with higher mortality in HF patients, few studies have addressed its comparative prognostic role in preserved and reduced EF groups (21,22). One small study of 528 hospitalized patients with HF demonstrated results similar to ours in that they found an increased risk of death associated with COPD in HFpEF compared with HFrEF (23). The complex relationship between COPD and HF including overlapping symptoms contribute to difficulties in making the diagnosis of one in the presence of the other and the role of each of the conditions in the progression and exacerbation of the other require further study (22).
Based on our findings a greater focus on the recognition and treatment of comorbidities in HFpEF appears warranted. Patients with HFpEF who are often older and have multiple chronic health conditions with complex health care needs may benefit from newer models of primary care in order to improve the fragmented and often ineffective care that such patients may receive in the current health-care system (24). In addition, studies have also shown that although the clinical signs and symptoms of HF are similar between HFpEF and HFrEF patients, HFpEF patients are less likely to receive diuretics for congestion, anticoagulation therapy for atrial fibrillation, smoking-cessation counseling, complete discharge instructions, have a cardiologist as a primary physician or get consultation with a cardiologist (1,6). Attempts to pursue case management strategies for HFpEF just as done for HFREF may help reduce the morbidity associated with this condition. Both conventional and novel strategies may be warranted to treat comorbidities. For example, as demonstrated by large randomized clinical trials, one of the most beneficial effects of better blood pressure control is the reduction of HF events (25,26). Smaller studies demonstrating benefits of treatment of anemia and sleep-disoreded breathing in HFpEF need further evaluation in larger clinical trials (27,28). In order to increase the applicability of clinical trial results to the general population, our findings support changes in clinical trial strategy as suggested recently by Kitzman et al (29). These include efforts to enroll a greater proportion of elderly patients in trials of HFpEF, to discourage exclusion of patients with multiple comorbidities as they are the driving force of outcomes in HFpEF and to include the primary evaluation of outcomes of functional ability rather than just mortality and HF hospitalizations.
Limitations
This study has limitations inherent to retrospective observational studies. Also, our database had missing data for some variables ranging from 6%-16%. This has potential to bias the study if the missing data were not completely random. To address this issue, we conducted the analyses both with imputed data and as well as by excluding patients with missing data and found concordant results. In addition, the study cohort is predominantly male (91%), representative of the VA population, and results may not be generalizable to females, who form a large proportion of patients with HFpEF. The male dominance may also explain the lower prevalence of HFpEF (30%) in our study cohort compared to other US databases. Furthermore, patients were initially identified by ICD-9 codes for HF. Thereafter, the data abstractors for EPRP confirmed physician documentation of HF in the electronic medical records. Relying on physician diagnosis of HF lends itself to the possibility of some misclassification, especially in patients with HFpEF, where coexistent obesity and/or COPD may confound the diagnosis of HF.
Conclusions
Although there is a higher prevalence of non-cardiac comorbidities in HFpEF compared to HFrEF patients, most individual comorbidities have comparable prognostic impact on mortality in both EF groups. The higher overall burden of comorbidities in HFPEF is associated with higher non-HF morbidity in patients with HFpEF compared to HFrEF. This underlines the importance of tharapeutic approaches with greater emphasis on management of comorbidites in HFpEF. Treatment strategies aimed mainly at reducing HF morbidity and mortality may have less overall impact on morbidity and mortality in patients with HFpEF.
ACKNOWLEDGEMENTS
The authors thank the Office of Quality and Performance of the Veterans Health Administration for providing EPRP data. The views expressed in this report are those of the authors and do not necessarily represent the views of the Department of Veterans Affairs.
Source(s) of funding: This study was supported in part by VA Health Services Research & Development Service grant # IIR 02-082-1 (to Dr. Deswal). S.A. is supported by American Heart Association predoctoral fellowship (2010-2012) and Alkek Foundation fellowship (2009-2012)
ABBREVIATIONS
- CKD
Chronic Kidney Disease
- COPD
Chronic Obstructive Pulmonary Disease
- DM
Diabetes mellitus
- EF
Ejection fraction
- EPRP
External Peer Review Program
- HF
Heart failure
- HFpEF
Heart failure with preserved ejection fraction
- HFrEF
Heart failure with reduced ejection fraction
- HR
Hazard ratio
- VA
Veterans Affairs
Appendix
Appendix.
ICD-9 codes used to ascertain selected comorbidities
| Comorbidity Class | ICD 9-CM codes |
|---|---|
| Peripheral arterial disease | 440, 441, 443, 444, 442.0, 442.1, 442.2, 442.3, 442.8 |
| Cerebrovascular disease & h/o CVA | 430, 431, 432, 433, 434, 435, 436, 437, 438 |
| PUD | 531.xx-534.xx |
| Liver disease | 070,456.0, 456.1, 456.2, 571.1-571.9, 572.2-572.8, V42.7 |
| Non skin malignancy | 140, 141, 142, 143-172, 174, 175, 179-195, 196-199, V10, 200-208 |
| AIDS | 042 |
| Dementing disorders (incl. Alzheimer's) | 290.0, 290.1, 290.2, 290.3, 290.4, 290.9, 331.0 |
| Other psychiatric disorders | 295, 296, 297, 298, 309.81, 311 |
| Rheumatologic disease | 710.0, 710.1, 710.4, 714.0-714.2, 714.81, 725 |
| Atrial fibrillation | 427.31 |
Footnotes
Conflict(s) Of Interest/Disclosure(s): None of the authors have any financial or other relations that could lead to a conflict of interest.
References
- 1.Fonarow GC, Stough WG, Abraham WT, et al. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: a report from the OPTIMIZE-HF Registry. J Am Coll Cardiol. 2007;50:768–77. doi: 10.1016/j.jacc.2007.04.064. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Lopatin M, Stevenson LW, De MT, Fonarow GC. Clinical presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. doi: 10.1016/j.jacc.2005.09.022. [DOI] [PubMed] [Google Scholar]
- 3.Hogg K, Swedberg K, McMurray J. Heart failure with preserved left ventricular systolic function; epidemiology, clinical characteristics, and prognosis. J Am Coll Cardiol. 2004;43:317–27. doi: 10.1016/j.jacc.2003.07.046. [DOI] [PubMed] [Google Scholar]
- 4.Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355:251–9. doi: 10.1056/NEJMoa052256. [DOI] [PubMed] [Google Scholar]
- 5.Senni M, Redfield MM. Heart failure with preserved systolic function. A different natural history? J Am Coll Cardiol. 2001;38:1277–82. doi: 10.1016/s0735-1097(01)01567-4. [DOI] [PubMed] [Google Scholar]
- 6.Bhatia RS, Tu JV, Lee DS, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355:260–9. doi: 10.1056/NEJMoa051530. [DOI] [PubMed] [Google Scholar]
- 7.Curtis JP, Sokol SI, Wang Y, et al. The association of left ventricular ejection fraction, mortality, and cause of death in stable outpatients with heart failure. J Am Coll Cardiol. 2003;42:736–42. doi: 10.1016/s0735-1097(03)00789-7. [DOI] [PubMed] [Google Scholar]
- 8.Lee DS, Gona P, Albano I, et al. A systematic assessment of causes of death after heart failure onset in the community: impact of age at death, time period, and left ventricular systolic dysfunction. Circ Heart Fail. 2011;4:36–43. doi: 10.1161/CIRCHEARTFAILURE.110.957480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Henkel DM, Redfield MM, Weston SA, Gerber Y, Roger VL. Death in heart failure: a community perspective. Circ Heart Fail. 2008;1:91–7. doi: 10.1161/CIRCHEARTFAILURE.107.743146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Deswal A, Petersen NJ, Urbauer DL, Wright SM, Beyth RJ. Racial variations in quality of care and outcomes in an ambulatory heart failure cohort. Am Heart J. 2006;152:348–54. doi: 10.1016/j.ahj.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–83. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 12.Braunstein JB, Anderson GF, Gerstenblith G, et al. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J Am Coll Cardiol. 2003;42:1226–33. doi: 10.1016/s0735-1097(03)00947-1. [DOI] [PubMed] [Google Scholar]
- 13.Ezekowitz JA, McAlister FA, Armstrong PW. Anemia is common in heart failure and is associated with poor outcomes: insights from a cohort of 12 065 patients with new-onset heart failure. Circulation. 2003;107:223–5. doi: 10.1161/01.cir.0000052622.51963.fc. [DOI] [PubMed] [Google Scholar]
- 14.Bozkurt B, Deswal A. Obesity as a prognostic factor in chronic symptomatic heart failure. Am Heart J. 2005;150:1233–9. doi: 10.1016/j.ahj.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Coresh J, Greene T, et al. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med. 2006;145:247–54. doi: 10.7326/0003-4819-145-4-200608150-00004. [DOI] [PubMed] [Google Scholar]
- 16.Smith GL, Masoudi FA, Vaccarino V, Radford MJ, Krumholz HM. Outcomes in heart failure patients with preserved ejection fraction. Mortality, readmission, and functional decline. J Am Coll Cardiol. 2003;41:1510–8. doi: 10.1016/s0735-1097(03)00185-2. [DOI] [PubMed] [Google Scholar]
- 17.Dunlay SM, Redfield MM, Weston SA, et al. Hospitalizations after heart failure diagnosis a community perspective. J Am Coll Cardiol. 2009;54:1695–702. doi: 10.1016/j.jacc.2009.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yusuf S, Pfeffer MA, Swedberg K, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet. 2003;362:777–81. doi: 10.1016/S0140-6736(03)14285-7. [DOI] [PubMed] [Google Scholar]
- 19.Cleland JGF, Tendera M, Adamus J, et al. The perindopril in elderly people with chronic heart failure (PEP-CHF) study. Eur Heart J. 2006;27:2338–45. doi: 10.1093/eurheartj/ehl250. [DOI] [PubMed] [Google Scholar]
- 20.Massie BM, Carson PE, McMurray JJ, et al. Irbesartan in Patients with Heart Failure and Preserved Ejection Fraction. N Engl J Med. 2008;359:2456–67. doi: 10.1056/NEJMoa0805450. [DOI] [PubMed] [Google Scholar]
- 21.Tribouilloy C, Rusinaru D, Mahjoub H, et al. Prognosis of heart failure with preserved ejection fraction: a 5 year prospective population-based study. Eur Heart J. 2008;29:339–47. doi: 10.1093/eurheartj/ehm554. [DOI] [PubMed] [Google Scholar]
- 22.Hawkins NM, Petrie MC, Jhund PS, Chalmers GW, Dunn FG, McMurray JJ. Heart failure and chronic obstructive pulmonary disease: diagnostic pitfalls and epidemiology. Eur J Heart Fail. 2009;11:130–9. doi: 10.1093/eurjhf/hfn013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Berry C, Hogg K, Norrie J, Stevenson K, Brett M, McMurray J. Heart failure with preserved left ventricular systolic function: a hospital cohort study. Heart. 2005;91:907–13. doi: 10.1136/hrt.2004.041996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boult C, Wieland GD. Comprehensive primary care for older patients with multiple chronic conditions: “Nobody rushes you through”. JAMA. 2010;304:1936–43. doi: 10.1001/jama.2010.1623. [DOI] [PubMed] [Google Scholar]
- 25.Major outcomes in high-risk hypertensive patients randomized to angiotensin-converting enzyme inhibitor or calcium channel blocker vs diuretic: The Antihypertensive and Lipid-Lowering Treatment to Prevent Heart Attack Trial (ALLHAT). JAMA. 2002;288:2981–97. doi: 10.1001/jama.288.23.2981. [DOI] [PubMed] [Google Scholar]
- 26.Beckett NS, Peters R, Fletcher AE, et al. Treatment of hypertension in patients 80 years of age or older. N Engl J Med. 2008;358:1887–98. doi: 10.1056/NEJMoa0801369. [DOI] [PubMed] [Google Scholar]
- 27.Cohen RS, Karlin P, Yushak M, Mancini D, Maurer MS. The effect of erythropoietin on exercise capacity, left ventricular remodeling, pressure-volume relationships, and quality of life in older patients with anemia and heart failure with preserved ejection fraction. Congest Heart Fail. 2010;16:96–103. doi: 10.1111/j.1751-7133.2009.00135.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bitter T, Westerheide N, Faber L, et al. Adaptive servoventilation in diastolic heart failure and Cheyne-Stokes respiration. Eur Respir J. 2010;36:385–92. doi: 10.1183/09031936.00045609. [DOI] [PubMed] [Google Scholar]
- 29.Kitzman DW, Rich MW. Age disparities in heart failure research. JAMA. 2010;304:1950–1. doi: 10.1001/jama.2010.1592. [DOI] [PMC free article] [PubMed] [Google Scholar]