Abstract
With the increase in life expectancy, there is also growing interest in anti-aging treatments and technologies. The development of anti-aging functional drugs for the skin, and foods from natural sources, may offer solutions to this global matter. Aging involves structural, functional and biochemical changes that occur throughout cells and bodily tissues; the amount of hormones secreted from of all human organs, including the skin, decreases over time. Matrix metalloproteinase (MMP) genes (MMP-1 and -8) play an important role in the aging of skin fibroblasts. For example, an increased MMP expression causes accelerated aging and the degradation of skin elasticity-related genes. In the present study, we examined the anti-wrinkle effects of tuna heart extract which are mediated through the inhibition of MMPs in skin cells. Generally, tuna contains high concentrations of selenium and antioxidants, which serve to remove free radicals, and is known to delay skin and body aging. In addition, unsaturated fatty acids in tuna help to maintain the natural glossy look of skin, and increase skin elasticity, providing moisture for dry skin. A recent study confirmed the various bio-effects of boiled tuna extract and muscle. However, bioactivity studies using tuna heart are limited. Thus, in the present study, we obtained extracts and fractions of tuna heart, and examined their effects on Hs27 human fibroblast proliferation using an MTS assay. In addition, we measured procollagen type 1 levels and elastase activity, and performed β-galactosidase staining. We then measured the expression levels of phosphatidylinositol 3-kinase/Akt and MMP-related genes by western blot analysis and RT-PCR. Our results revealed that tuna heart extract decreased MMP expression by upregulating tissue inhibitors of metallopro-teinase-1 (TIMP-1) and decreasing elastase activity, thus exerting anti-aging and anti-wrinkle effects by increasing collagen synthesis and promoting skin fibroblast proliferation. Thus, our data suggest that tuna heart (TH)-H2O fractions exert anti-wrinkle effects on Hs27 cells.
Keywords: Hs27 cells, tuna heart, matrix metalloproteinase, procollagen type 1
Introduction
Over the years, the average global lifespan has increased due to economic development and advancements in medicine, and there is great interest in skin health and anti-aging technologies in order to delay the aging process and maintain youth (1). Studies on anti-wrinkle agents have been conducted with the aim of maintaining skin health (2). As a result, various cosmetics and foods which assist in delaying the aging process have been developed (3). Aging involves structural, functional and biochemical changes that occur throughout cells and bodily tissues; the amount of hormones secreted from of all human organs, including the skin, decreases over time (4). Skin aging is associated with environmental and genetic factors, and is divided into extrinsic aging caused by various environmental factors, such as UV rays, polluted air and gravity (5); by contrast, endogenous aging (which is associated with genetic factors) can also lead to skin wrinkling (6,7). Wrinkles are a typical symptom of skin aging caused by the loss of elasticity, which is caused by a reduction in the amount of collagen associated with the elasticity of the skin dermal tissue. Collagen (approximately 90% of which is found in dermal cells) has a close association with skin elasticity, as it protects the skin against external stimuli and provides it with tension and strength (3). Procollagen type 1 and the extracellular matrix are decomposed and the expression of elastin (related to skin elasticity) is inhibited by matrix metalloproteinases (MMPs) induced by the transcription factor, activating protein-1 (AP-1) and MMP-1 and -8, which are known to degrade collagen. Tissue inhibitors of metalloproteinase-1 (TIMP-1) exert the opposite effect by inhibiting the activity of MMPs (8,9). In addition, elastase derived from fibroblasts plays an important role in the three-dimensional distortion of skin elasticity fibers, and increased elastase activity contributes to the acceleration of wrinkle formation by reducing the number of elastic fibers in the skin (10). UV rays and the production of reactive oxygen species cause skin aging and damage cells by attacking the plasma membrane, resulting in loss of function. This creates a harsh environment and causes skin wrinkles by reducing the glossy appearance of skin (11,12). Of the factors involved in cell proliferation, phosphatidylinositol 3-kinase (PI3K) is an important protein that performs regulatory functions in a number of signaling pathways and consists of two subunits (p110 and p85) and increases the activity of Akt, a serine/threonine kinase (13). Serine 473 autophosphorylation promotes the proliferation and differentiation of cells by activating sub-molecules and transcription factors (14).
The Akt/PI3K signaling pathway is a signal transduction pathway that promotes cell growth and survival in response to extracellular signals. In order to maintain the elasticity of skin cells, it is important to increase procollagen type 1 c-peptide (PIP) and elastin activity by increasing TIMP-1 expression and inhibiting that of MMP, and it is also necessary to activate the PI3K/Akt pathway to directly induce cell growth. In this study, we confirmed the anti-wrinkle effects of tuna heart extract, which were mediated through the inhibition of the expression of MMPs and the increase in PI3K/Akt levels. By-products of global seafood processing amount to 75% of the total catch, and these by-products are disposed of together with a considerable amount of useable ingredients. Omega-3 fatty acid separated through protein hydrolysate, and concentrates of its by-products, has previously been used in the food and pharmaceutical industries (15–17). Studies on immune enhancement and on unsaturated fats in tuna by-products have previously been performed (18–20). In this study, we examined the anti-wrinkle effects of a physiologically active substance isolated from tuna heart extract on skin by measuring the expression levels MMPs in skin fibroblasts and determining whether they are directly involved in the anti-wrinkle effects.
Materials and methods
Extraction and fractionation of tuna heart
Lyophilized powder (200 g) of tuna heart was refluxed with 70% ethanol (EtOH; 1.2 liters for 3 times) for 3 h, and each filtrate was concentrated to dryness in vacuo at 40°C to obtain the 70% EtOH extract (54 g). The 70% EtOH extract was then suspended in distilled water (H2O) and partitioned in sequence with dichloromethane (CH2Cl2), ethyl acetate (EtOAc), n-butanol (n-BuOH) and H2O to yield 4 fractions. The respective yields of the CH2Cl2 (4.05 g), EtOAc (3.91 g), n-BuOH (9.06 g) and H2O (36.98 g) fractions were 2.03, 1.96, 4.53 and 18.49%, respectively (Fig. 1). The CH2Cl2, EtOAc and n-BuOH fraction had no effect on the viability of Hs27 cells (data not shown). In this study, we confirm the effects of the H2O fraction on the viability of Hs27 cells. Thus, we used various concentrations of the H2O fraction (25, 50 and 100 µg/ml).
Figure 1.
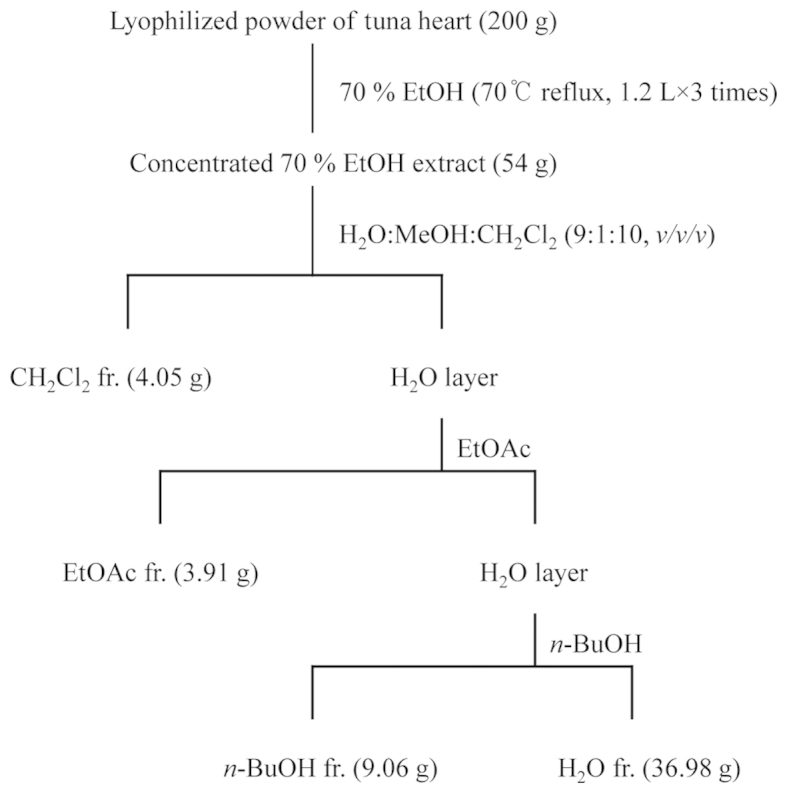
Procedure for the extraction and fractionation of the tuna heart (TH)-H2O fraction.
Cell culture
Hs27 human skin fibroblasts (American Type Culture Collection, Manassas, VA, USA) were maintained at 37°C in a humidified atmosphere with 5% CO2. The cells were cultured in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS; HyClone, Logan, UT, USA) and 100 U/ml penicillin/100 mg/ml streptomycin. The Hs27 cells were cultured to ~60–80% confluence in a 100-mm diameter plate. The medium was replaced daily.
Cell proliferation assays
Hs27 cell proliferation was measured using a CellTiter 96® aqueous non-radioactive cell proliferation assay (Promega, Madison, WI, USA), which is based on the cleavage of 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxy-methoxyphenyl)-2-(4-sulfonyl)-2H-tetrazolium (MTS) into a formazan product which is soluble in tissue culture medium. The cells were seeded onto 96-well plates at 2×104 cells/well, and the medium was replaced with serum-free medium (SFM) following culture for 24 h. After a further 24 h, the medium was replaced with SFM containing tuna heart (TH)-H2O fraction (25, 50 and 100 µg/ml), followed by incubation for 24 h. For the assay, MTS solution was added to the cells in each well and was allowed to react for 30 min at 37°C. The absorbance at 490 nm of the solution in each well was measured using a microplate reader (Benchmark microplate reader; Bio-Rad Laboratories, Hercules, CA, USA).
Determination of elastase activity
To determine the activity of elastase and its effects on elastic fibers, the decomposition of elastase was measured. Elastic fibers are bundles of elastin found in the extracellular matrix of connective tissue and produced by skin fibroblasts (21). Elastic fiber includes elastin. The upregulation of elastin is important in order to prevent skin cell aging (22). The cells were seeded into 6-well plates at 2×104 cells/well and the medium was replaced with SFM containing the TH-H2O fraction (25, 50 and 100 µg/ml) for 24 h. The cells were collected in 100 mM Tris-HCl buffer (pH 7.6) with 0.1% Triton X-100 buffer. The supernatant was then transferred to the 96-well plate. Subsequently, 2 mM N-Succinyl-Ala-Ala-Ala-p-nitroanilide was added to each well (Sigma-Aldrich, St. Louis, MO, USA), adjusted to a 100-µl volume using elastase enzyme solution, and incubated at 37°C for 30 min. The absorbance at 410 nm was measured using a microplate reader (Benchmark microplate reader; Bio-Rad Laboratories).
β-galactosidase staining
β-galactosidase staining was performed using the Takara V2600 kit (Takara Bio Inc., Tokyo, Japan). The cells were seeded into 6-well plates at 2×104 cells/well and the medium was replaced with SFM containing the TH-H2O fraction (25, 50 and 100 µg/ml) for 24 h. Cell fixative working solution (0.5 ml) was added to each well and incubated at room temperature for 5 min, after which the working solution was removed. Each well was then washed twice with 1 ml phosphate-buffered saline (PBS), and 0.5 ml of cell staining working solution was added. Cell morphology and staining were observed using a light microscope (ECLIPSE TS100-F; Nikon, Tokyo, Japan).
PIP EIA assay
PIP EIA assay was performed using the Takara MK101 kit (Takara Bio Inc.). The cells were seeded into 6-well plates at 2×104 cells/well and the medium was replaced with SFM containing the TH-H2O fraction (25, 50 and 100 µg/ml) for 24 h. The cell supernatant was then collected by centrifugation (12,000 rpm, 4°C, 10 min). Antibody-POD conjugate solution (100 µl) and 20 µl of cell supernatant were added to each well, followed by incubation at 37°C for 3 h. Each well was washed 4 times with 400 µl PBS, after which 100 µl of substrate solution (TMBZ, 3,3′,5,5′-tetramethylbenzidine) was added, followed by incubation at room temperature for 15 min. Finally, 100 µl of stop solution (1 N H2SO4) were added and the absorbance at 450 nm was measured using a microplate reader (Benchmark microplate reader; Bio-Rad Laboratories).
Western blot analysis
The Hs27 cells in 100-mm diameter plates were cultured to 60% confluence and incubated in SFM containing the TH-H2O fraction (25, 50 and 100 µg/ml) for 24 h. The collected cells were washed with PBS, after which extraction lysis buffer (20 mM Tris-base, pH 8, 150 mM NaCl, 100 µM sodium vanadate, 100 µM ammonium molybdate, 10% glycerol, 0.1% Nonidet P-40, 0.1% SDS, 1 mM glycerophosphate, 1 µg/ml aprotinin, 1 µg/ml leupeptin, 1 µg/ml pepstatin A and 1 mM PMSF) was added. The proteins were separated on 7–15% SDS-PAGE gels and transferred onto polyvinylidene fluoride membranes (Millipore, Billerica, MA, USA). The membranes were blocked at room temperature with 1% bovine serum albumin in TBS-T (10 mM Tris-HCl, pH 7.5, 150 mM NaCl, 0.1% Tween-20) and then incubated with the following primary antibodies: anti-MMP-1 (sc-21731, anti-mouse, 1:1,000), anti-MMP-8 (sc-8848, anti-goat, 1:1,000), anti-elastin (sc-166543, anti-mouse, 1:1,000), anti-col1A (sc-59772, anti-mouse, 1:1,000), anti-PI3K (sc-7248, anti-goat, 1:1,000), anti-Akt (sc-8312, anti-rabbit, 1:1,000), anti-TIMP1 (sc-5538, anti-rabbit, 1:1,000), and anti-GAPDH (sc-25778, anti-rabbit, 1:1,000) (Santa Cruz Biotechnology, Inc., Santa Cruz, CA, USA). The secondary antibody was a peroxidase-conjugated goat (sc-2741), mouse (sc-2032), or rabbit (sc-2031) antibody (1:10,000; Santa Cruz Biotechnology, Inc.). The signal was developed using SuperSignal West Pico Stable Peroxide Solution and the SuperSignal West Pico Luminol/Enhancer solution (Thermo Fisher Scientific, Rockford, IL, USA) before exposure to Kodak X-ray film.
Reverse transcription-polymerase chain reaction (RT-PCR) for analysis of mRNA expression
The Hs27 cells in 100-mm diameter plates were cultured to 60% confluence and incubated in SFM containing the TH-H2O fraction (25, 50 and 100 µg/ml) for 24 h. The cells were then treated with TRIzol reagent (Invitrogen, Carlsbad, CA, USA), after which the extracted RNA was used as a template for cDNA synthesis using an oligo(dT) primer (Intron Co., Gyeonggi, Korea). The synthe sized cDNA was mixed with 2X TOPsimple DyeMIX-nTaq (Enzynomics Inc., Daejeon, Korea) and primers (MMP-1 forward, AAAGGG AATAAGTACTGGGC and reverse, AATTCCAGGAAAG TCATGTG; MMP-8 forward, GCTCATTTTGATGCCGAAG and reverse, AGTAGTTGCTGGTTTCCCT; col1A forward, CTCCAACGAGATCGAGATC and reverse, GTTACAGGAA GCAGACAGG; TIMP-1 forward, TGGGGACACCAG AAGTCAAC and reverse, TTTTCAGAGCCTTGGAGGAG; PI3K forward, AGGAGCGGTACAGCAAAGAA and reverse, GCCGAACACCTTTTTGAGTC; Akt forward, CAACTTCT CTGTGGCGCAGTG and reverse, GACAGGTGGAAGAA CAGCTCG; GAPDH forward, AAGGGCTCATGACCAC AGTC and reverse, GGATGCAGGGATGTTCT) in 0.1% diethylpyrocarbonate (DEPC)-treated water for PCR. Using a 1% agarose gel, the PCR products were separated and stained with Red Safe nucleic acid staining solution (Intron Co.).
Statistical analysis
The results are expressed as the means ± SDs. SPSS software (v. 10.0; SPSS, Inc., Chicago, IL, USA) was used. Comparisons were made using ANOVA and Duncan's multiple range test. A p-value <0.05 was deemed to indicate a statistically significant difference.
Results
Effects of TH-H2O fraction on Hs27 cell proliferation
The effect of the TH-H2O fraction on Hs27 cell proliferation was examined by MTS assay. The Hs27 cells treated with the TH-H2O fraction at 25, 50 and 100 µg/ml exhibited a dose-dependent increase in proliferation, with a maximum increase of 47% compared to the controls (untreated cells) being observed in the cells treated with 100 µg/ml of the TH-H2O fraction. In other words, treatment of Hs27 skin fibroblasts with the TH-H2O fraction did not result in toxicity and may have affected differentiation and reproduction (Fig. 2).
Figure 2.
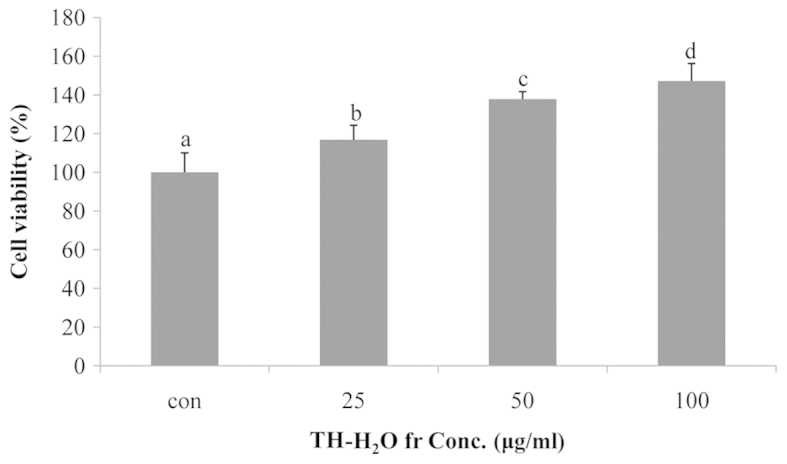
Tuna heart (TH)-H2O fraction affects the proliferation of Hs27 human skin fibroblasts. Cells were treated with the TH-H2O fraction (25, 50 and 100 µg/ml) for 24 h. Cell proliferation was determined by MTS assay. Values are represented as the means ± SD. p<0.05 as shown by ANOVA. Bars labeled with different letters indicate a significant difference compared to the untreated controls, according to Duncan's multiple range test.
Inhibitory effects of the TH-H2O fraction on elastase activity
Elastin is a substrate protein that accounts for 4% of the dermis and plays a role in maintaining skin elasticity (23). Elastin is degraded by elastase in neutrophil granulocytes; elastase is a non-specific hydrolytic enzyme capable of degrading the matrix protein collagen. Elastase degrades matrix proteins in the network structure of the basal cells of the skin and causes wrinkles and/or destroys tissue (1). In addition, as previously demonsrated in a study on skin wrinkles, increased elastase activity accelerates wrinkle formation by reducing the amount of elastic fibers in the skin (10). In this study, we noted that treatment of the Hs27 cells with the TH-H2O fraction inhibited elastase activity. The level of elastase was reduced in a dose-dependent manner, with a maximum decrease of 35% compared to the controls (untreated cells) being observed in the cells treated with 100 µg/ml of the TH-H2O fraction (Fig. 3).
Figure 3.
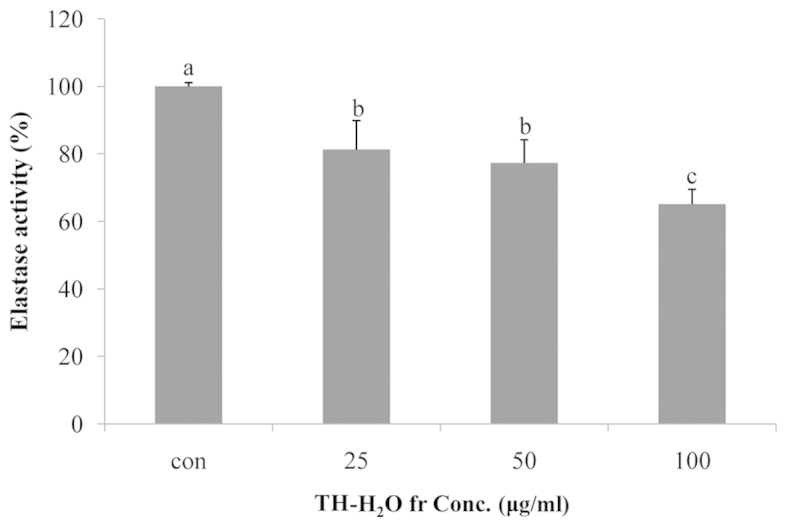
Inhibitory effects of tuna heart (TH)-H2O fraction on elastase activity. Hs27 cells were treated with the TH-H2O fraction at 25, 50 and 100 µg/ml for 24 h. Elastase activity was measured by enzyme-linked immunosorbent assay (ELISA). Values are represented as the means ± SD; p<0.05 as shown by ANOVA. Bars labeled with different letters indicate a significant difference compared to the untreated controls, according to Duncan's multiple range test.
Morphological changes and β-galactosidase staining
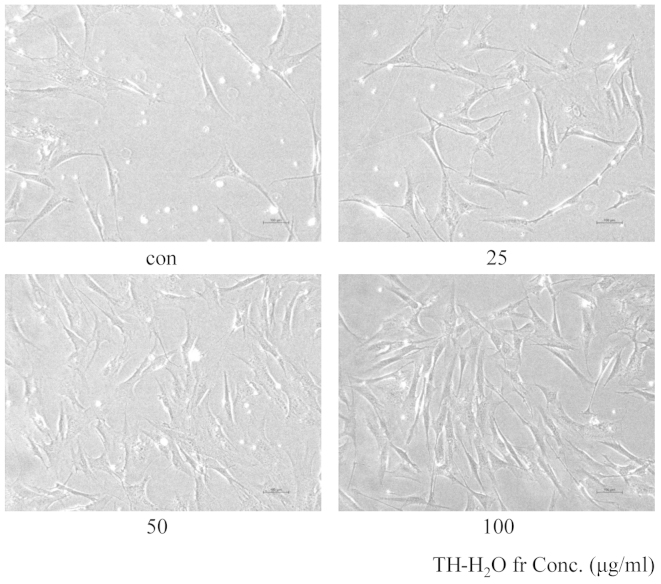
The aging of skin fibroblasts is evidenced by blue color upon β-galactosidase staining. The decomposition of X-gal by β-galactosidase in transfected cells leads to the generation of the blue color. Treatment with the TH-H2O fraction at 25, 50 and 100 µg/ml resulted in a dose-dependent increase in the number of Hs27 cells and in no β-galactosidase staining (Fig. 4). This suggests that cell aging did not occur and that the cells were proliferating.
Figure 4.
Effects of tuna heart (TH)-H2O fraction on Hs27 human skin fibroblast morphology and viability. Aging was determined by senescence-associated β-galactosidase (SA-β-gal). Hs27 cells were treated with the TH-H2O fractoin at 25, 50 and 100 µg/ml for 24 h. Cells not stained blue by SA-β-gal did not experience aging, which is suggestive of increased cell growth. No blue staining was observed in the cells in our study, thus the cells did not age.
Effects of the TH-H2O fraction on PIP levels
Collagen is the main structural protein in extracellular space in connective tissues in mammals. Collagen is present at high concentrations in the skin, bones, ligaments, cartilage and teeth, and is a particularlyimportant component of the extracellular matrix in fibroblasts (24). In addition, collagen is associated with the mechanical robustness of skin, resistance of the skin connective tissue, tissue bonding, support of cell adhesion, differentiated function and the induction of cell division (23). Collagen is synthesized from the precursor, procollagen, which has a propeptide sequence at the amino and carboxyl termini (25). The propeptide plays a role in the folding of procollagen, and upon polymerization it is separated from collagen molecules. This suggests that increased PIP activation is associated with cell proliferation and anti-wrinkle effects, as it promotes collagen synthesis (26). In this study, we measured the PIP levels using the Takara MK101 kit. We noted a dose-dependent increase in PIP levels, with a maximum increase of 43% compared to the controls being observed in the cells treted with 100 µg/ml of the TH-H2O fraction (Fig. 5).
Figure 5.
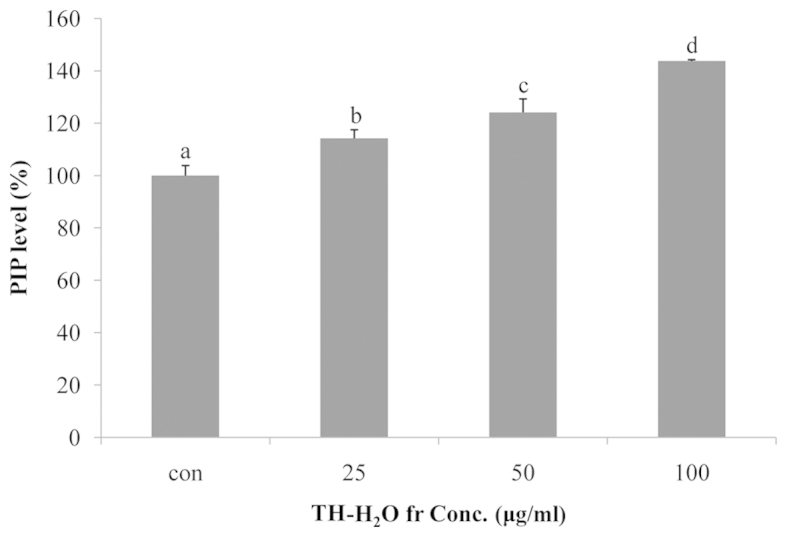
Effects of tuna heart (TH)-H2O fraction on collagen type I synthesis in Hs27 cells. Cells were cultured with the TH-H2O fraction at 25, 50 and 100 µg/ml for 24 h. The supernatant and pellet were collected from each well and procollagen type 1 c-peptide (PIP) was determined using the EIA kit. Values are represented as the means ± SD; p<0.05 by ANOVA. Bars labeled with different letters indicate a significant difference compared to the untreated controls, according to Duncan's multiple range test.
Effect of the TH-H2O fraction on the mRNA expression levels of PI3K/Akt and MMP in Hs27 cells
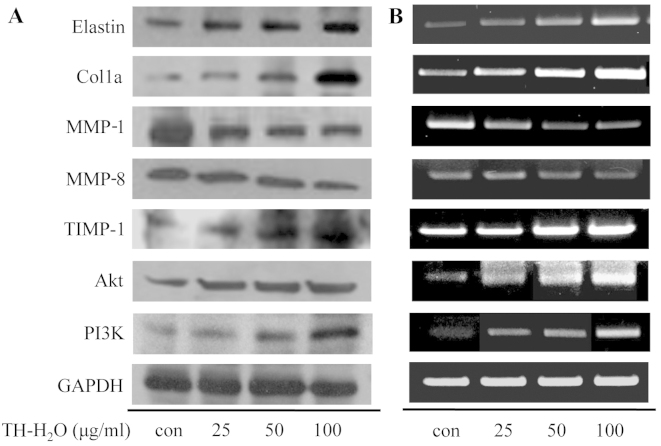
MMP-1 and -8 are enzymes that degrade collagen and inhibit collagen synthesis by specifically decomposing procollagen. In addition, they degrade the extracellular matrix (27) and inhibit the expression of elasticity-related genes [elastin and collagen, type I, alpha 1 (Col1a)]. The MMPs are inhibited by specific endogenous TIMPs, and TIMP-1 regulates MMP activity and protects against collagen and elastin degradation (28). Thus, upregulation of TIMP-1 plays an important anti-wrinkle role. One of the genes involved in cell growth, PI3K, is involved in the lipid components of phophoinisitide and components of the cell membrane, and functions as a lipid kinase that phosphorylates and activates Akt by generating a secondary transfer (29,30). We confirmed increased cell proliferation and PIP levels. Thus, we measured the expression levels of MMP-encoding genes and PI3K/Akt by western blot analysis and RT-PCR. The MMP-1 and -8 levels decreased upon the activation of TIMP-1 in the cells treated with the TH-H2O fraction, and the Col1a and elastin levels increased. In addition, the TH-H2O fraction upregulated the PI3K/Akt levels in a dose-dependent manner (Fig. 6).
Figure 6.
Effects of tuna heart (TH)-H2O fraction on phosphatidylinositol 3-kinase (PI3K)/Akt and matrix metalloproteinase (MMP) gene expression. Hs27 cells were cultured in 100-mm dishes with TH-H2O fraction (25, 50 and 100 µg/ml) for 24 h, after which time cellular protein and total RNA were isolated. (A) Protein levels were assessed by western blot analysis. (B) RT-PCR analysis was achieved by electrophoresis in a 1% agarose gel stained with Red Safe nucleic acid staining solution.
Discussion
Interest in a healthy lifestyle has increased worldwide and living standards have also increased; thus it is important to also improve the treatments associated with the bioactive effect of various marine resources (seaweed and fish), in order to further protect against various diseases (31). Interest in marine resources and fishery byproducts to replace depleted land resources has thus increased. Tuna is mainly used in canning, and there is a growing demand for tuna to be used in simple and highly functional foods. However, the processing of marine products, such as tuna generates 30–35% byproducts of total materials (32). Recently, certain studies have been conducted in order to take advantage of fishery by-products since they contain a variety of functional proteins (20). Typically, tuna is a high-protein food which exerts anti-atherosclerotic and anticancer effects, and is known to protect against high cholesterol levels in the blood (18). Thus, the present study was conducted on tuna waste, and previous studies reported on the anti-obesity effects of boiled tuna extract on 3T3-L1 cells (18,19) and the anti-atopic effect of an ethanol extract of tuna heart (20). However, bioactivity studies using tuna heart are not common. In this study we confirmed the anti-wrinkle effect of the TH-H2O fraction on Hs27 human fibroblasts. First, we evaluated cell proliferation following treatment with 25, 50 and 100 µg/ml of the TH-H2O fraction by MTS assay. As shown in Fig. 2, we noted a dose-dependent increase in cell proliferation, with a maximum increase of 47% compared to the controls in the cells treated with 100 µg/ml of the TH-H2O fraction. Elastase activity induces skin wrinkling by destroying the network structure of the basal cells of the skin through the degradation of matrix proteins (1).
The upregulation of elastase activity leads to tissue destruction and loss of elasticity (33,34). The data in Fig. 3 show a dose-dependent decrease in elastase activity upon treatment with the TH-H2O fraction, with a maximum inhibition of 35% compared to the controls in the cells treated with 100 µg/ml of the TH-H2O fraction. In addition, we noted an increase in the number of cells, but not in blue β-galactosidase staining. This suggests that the TH-H2O fraction is not induced in aging cells (Fig. 4). To explore the mechanism underlying this induction of cell proliferation, we examined the PIP levels by PIP EIA assay. Collagen is present at the highest concentration in the dermal layer of the skin, accounting for 70–80% of the total dry weight, and plays a role in supporting the skin (35). However, stress caused by genetic factors and the environment induces wrinkles by increasing collagenase activity and reactive oxygen species (36,37). In other words, collagen is an important factor for anti-wrinkle effects and cell growth. The level of collagen biosynthesis can be determined based on the biosynthetic effects by measuring the amount of propeptide (26). As shown in Fig. 5, we noted a dose-dependent increase in PIP levels upon treatment with the TH-H2O fraction, with a maximum increase of 43% compared to the controls being observed in the cells treated with 100 µg/ml of the TH-H2O fraction. This indicates increased collagen synthesis due to elevated PIP levels. MMP-1 and -8 can degrade collagen, and TIMP-1 exerts the opposite effect by inhibiting MMP activity. In addition, tissue-specific inhibitors of TIMP-1 are known to maintain the biological equilibrium by inactivating MMPs (38). In the present study we examined the changes in protein and mRNA expression by western blot analysis and RT-PCR. The MMP-1 and -8 levels were decreased by the upregulation of TIMP-1. This suggests that collagen biosynthesis is promoted by decreased MMP expression. In addition, the expression levels of elastin and Col1a were increased by the inhibition of elastase. Moreover, treatment with the TH-H2O fraction induced the upregulation of PI3K/Akt (Fig. 6). TH-H2O induced the upregulation of TIMP-1 and inhibited elastase expression. Thus, the increased expression of the genes encoding elastin, Col1a and PI3K/Akt is a factor involved in skin elasticity, anti-wrinkling and cell growth. In conclusion, the TH-H2O fraction significantly decreased MMP levels and upregulated the expression of genes associated with cell growth. Therefore, we posit that tuna heart extract modulates cell growth and induces an anti-wrinkle effect.
Acknowledgments
This study was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education (2014R1A6A3A01058410). This study was a part of the project entitled 'Functional materials and foods using fisheries by-products', funded by the Ministry of Oceans and Fisheries, Korea (20130279).
References
- 1.Kim SH, Yong HJ, Shin C, Ko SG. Research of traditional herbal medicines for anti-aging, inhibition effect of wrinkle and whitening effect in the skin. Korean J Ori Physiol Pathol. 2008;22:691–698. In Korean. [Google Scholar]
- 2.Gancevicine R, Liakou AI, Theodoridis A, Makrantonaki E, Zouboulis CC. Skin anti-aging strategies. Dermato-Endocrinol. 2012;4:308–319. doi: 10.4161/derm.22804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Park KJ, Park SH, Kim JK. Anti-wrinkle activity of Acanthopanax senticosus extract in ultraviolet B (UBV)-induced photoaging. J Korean Soc Food Sci Nutr. 2010;39:42–46. doi: 10.3746/jkfn.2010.39.1.042. In Korean. [DOI] [Google Scholar]
- 4.Gilchrest BA. Skin aging and photoaging. Dermatol Nurs. 1990;2:79–82. [PubMed] [Google Scholar]
- 5.Ha TY. Development of functional food materials for healthy life. Korean J Crop Sci. 2006;51:26–39. In Korean. [Google Scholar]
- 6.Makrantonaki E, Zouboulis CC. Molecular mechanisms of skin aging: state of the art. Ann NY Acad Sci. 2007;1119:40–50. doi: 10.1196/annals.1404.027. [DOI] [PubMed] [Google Scholar]
- 7.Seo MY, Chung SY, Choi WK, Seo YK, Jung SH, Park JM, Seo MJ, Park JK, Kim JW, Park CS. Anti-aging effect of rice wine in cultured human fibroblasts and keratinocytes. J Biosci Bioeng. 2009;107:266–271. doi: 10.1016/j.jbiosc.2008.11.016. [DOI] [PubMed] [Google Scholar]
- 8.Seo JE, Kim S, Shin MH, Kim MS, Eun HC, Park CH, Chung JH. Ultraviolet irradiation induces thrombospondin-1 which attenuates type I procollagen downregulation in human dermal fibroblasts. J Dermatol Sci. 2010;59:16–24. doi: 10.1016/j.jdermsci.2010.04.010. [DOI] [PubMed] [Google Scholar]
- 9.Kang TH, Park HM, Kim YB, Kim H, Kim N, Do JH, Kang C, Cho Y, Kim SY. Effects of red ginseng extract on UVB irradiation-induced skin aging in hairless mice. J Ethnopharmacol. 2009;123:446–451. doi: 10.1016/j.jep.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 10.Tsukahara K, Nakagawa H, Moriwaki S, Takema Y, Fujimura T, Imokawa G. Inhibition of ultraviolet-B-induced wrinkle formation by an elastase-inhibiting herbal extract: implication for the mechanism underlying elastase-associated wrinkles. Int J Dermatol. 2006;45:460–468. doi: 10.1111/j.1365-4632.2006.02557.x. [DOI] [PubMed] [Google Scholar]
- 11.Kim MJ, Kim JY, Jung TK, Choi SW, Yoon KS. Skin anti-aging effect of Forsythia viridissima L. extract. Korean J Biotechnol Bioeng. 2006;21:444–450. [Google Scholar]
- 12.Yeom MH, Lee JY, Kim JS, Park CW, Kim DH, Kim HK. The anti-aging effects of Korean ginseng berry in the skin. Korean J Pharmacogn. 2010;41:26–30. [Google Scholar]
- 13.Klippel A, Reinhard C, Kavanaugh WM, Apell G, Escobedo MA, Williams LT. Membrane localization of phosphatidylinositol 3-kinase is sufficient to activate multiple signal-transducing kinase pathways. Mol Cell Biol. 1996;16:4117–4127. doi: 10.1128/MCB.16.8.4117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bellacosa A, Kumar CC, Di Cristofano A, Testa JR. Activation of AKT kinases in cancer: implications for therapeutic targeting. Adv Cancer Res. 2005;94:29–86. doi: 10.1016/S0065-230X(05)94002-5. [DOI] [PubMed] [Google Scholar]
- 15.Long AM, Haard NF. The effect of carotenoid-protein association on pigmentation and growth rates of rainbow trout (Salmo gairdneri) Bull Aquacult Assoc Can. 1988;2:98–100. [Google Scholar]
- 16.Shahidi F, Synowiecki J. Isolation and characterization of nutrients and value-added products from snow crab (Chinoecetes opilio) and shrimp (Pandalus borealis) processing discards. J Agric Food Chem. 1991;39:1527–1532. doi: 10.1021/jf00008a032. [DOI] [Google Scholar]
- 17.Shahidi F, Naczk M, Pegg R, Synowiecki J. Chemical composition and nutritional value of processing discards of cod (Gadus morhua) Food Chem. 1991;42:145–151. doi: 10.1016/0308-8146(91)90030-R. [DOI] [Google Scholar]
- 18.Kim YM, Kim EY, Kim IH, Nam TJ. Peptide derived from desalinated boiled tuna extract inhibits adipogenesis through the downregulation of C/EBP-α and PPAR-γ in 3T3-L1 adipocytes. Int J Mol Med. 2015;35:1362–1368. doi: 10.3892/ijmm.2015.2127. [DOI] [PubMed] [Google Scholar]
- 19.Kim YM, Kim EH, Choi JW, Lee MK, Nam TJ. The anti-obesity effects of a tuna peptide on 3T3-L1 adipocytes are mediated by the inhibition of the expression of lipogenic and adipogenic genes and by the activation of the Wnt/β-catenin signaling pathway. Int J Mol Med. 2015;36:327–334. doi: 10.3892/ijmm.2015.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kang BK, Kim KBWR, Kim MJ, Bark SW, Park WM, Kim BR, Ahn NK, Choi YU, Bae NY, Park JH, et al. Anti-atopic activity of tuna heart ethanol extract. J Korean Soc Food Sci Nutr. 2015;44:1–6. doi: 10.3746/jkfn.2015.44.1.001. In Korean. [DOI] [Google Scholar]
- 21.Liu X, Zhao Y, Gao J, Pawlyk B, Starcher B, Spencer JA, Yanagisawa H, Zuo J, Li T. Elastic fiber homeostasis requires lysyl oxidase-like 1 protein. Nat Genet. 2004;36:178–182. doi: 10.1038/ng1297. [DOI] [PubMed] [Google Scholar]
- 22.Patel A, Fine B, Sandig M, Mequanint K. Elastin biosynthesis: The missing link in tissue-engineered blood vessels. Cardiovasc Res. 2006;71:40–49. doi: 10.1016/j.cardiores.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 23.Suganuma K, Nakajima H, Ohtsuki M, Imokawa G. Astaxanthin attenuates the UVA-induced up-regulation of matrix-metalloproteinase-1 and skin fibroblast elastase in human dermal fibroblasts. J Dermatol Sci. 2010;58:136–142. doi: 10.1016/j.jdermsci.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 24.Sandhu SV, Gupta S, Bansal H, Singla K. Collagen in health and disease. J Orofac Res. 2012;2:153–159. doi: 10.5005/jp-journals-10026-1032. [DOI] [Google Scholar]
- 25.Kim YH, Chung CB, Kim JG, Ko KI, Park SH, Kim JH, Eom SY, Kim YS, Hwang YI, Kim KH. Anti-wrinkle activity of ziyuglycoside I isolated from a Sanguisorba officinalis root extract and its application as a cosmeceutical ingredient. Biosci Biotechnol Biochem. 2008;72:303–311. doi: 10.1271/bbb.70268. [DOI] [PubMed] [Google Scholar]
- 26.Tsuji-Naito K, Ishikura S, Akagawa M, Saeki H. Alpha-Lipoic acid induces collagen biosynthesis involving prolyl hydroxylase expression via activation of TGF-beta-Smad signaling in human dermal fibroblasts. Connect Tissue Re. 2010:1–10. doi: 10.3109/03008200903486188. [DOI] [PubMed] [Google Scholar]
- 27.Kim HH, Cho S, Lee S, Kim KH, Cho KH, Eun HC, Chung JH. Photoprotective and anti-skin-aging effects of eicosapentaenoic acid in human skin in vivo. J Lipid Res. 2006;47:921–930. doi: 10.1194/jlr.M500420-JLR200. [DOI] [PubMed] [Google Scholar]
- 28.Rabe JH, Mamelak AJ, McElgunn PJ, Morison WL, Sauder DN. Photoaging: mechanisms and repair. J Am Acad Dermatol. 2006;55:1–19. doi: 10.1016/j.jaad.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 29.Kivirikko KI, Myllyharju J. Prolyl 4-hydroxylases and their protein disulfide isomerase subunit. Matrix Biol. 1998;16:357–368. doi: 10.1016/S0945-053X(98)90009-9. [DOI] [PubMed] [Google Scholar]
- 30.Lee JH, Park SM, Jung HJ, Kim HS, Yu TS. Characteristics of Ju-back and effect of Ju-back fertilizer on growth of crop plants. J Life Sci. 2007;17:1562–1570. doi: 10.5352/JLS.2007.17.11.1562. [DOI] [Google Scholar]
- 31.Tsuji N, Moriwaki S, Suzuki Y, Takema Y, Imokawa G. The role of elastases secreted by fibroblasts in wrinkle formation: implication through selective inhibition of elastase activity. Photochem Photobiol. 2001;74:283–290. doi: 10.1562/0031-8655(2001)074<0283:TROESB>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Shin MO, Ku MJ, Bae SJ. Cytotoxicity and quinine reductase activity stimulating effects of fin of Thunnus thynnus extracts in various cancer cells. Korean J Nutr. 2007;40:147–153. [Google Scholar]
- 33.DeWitt DL, Rollins TE, Day JS, Gauger JA, Smith WL. Orientation of the active site and antigenic determinants of prostaglandin endoperoxide synthase in the endoplasmic reticulum. J Biol Chem. 1981;256:10375–10382. [PubMed] [Google Scholar]
- 34.Roth GJ, Siok CJ, Ozols J. Structural characteristics of prostaglandin synthetase from sheep vesicular gland. J Biol Chem. 1980;255:1301–1304. [PubMed] [Google Scholar]
- 35.Makrantonaki E, Zouboulis CC, German National Genome Research Network 2 The skin as a mirror of the aging process in the human organism - state of the art and results of the aging research in the German National Genome Research Network 2(NGFN-2) Exp Gerontol. 2007;42:879–886. doi: 10.1016/j.exger.2007.07.002. [DOI] [PubMed] [Google Scholar]
- 36.Kim SN, Lee SH, Lee BK, Jang IS. Acompositions containing Anthriscus sylvestris Hoffmann extract or Petroselinum sativum Miller extract for external application having effects of improving skin wrinkle. 10-2002-0079594. Korea Patent. 2002
- 37.El-Domyati M, Attia S, Saleh F, Brown D, Birk DE, Gasparro F, Ahmad H, Uitto J. Intrinsic aging vs. photoaging: a comparative histopathological, immunohistochemical, and ultrastructural study of skin. Exp Dermatol. 2002;11:398–405. doi: 10.1034/j.1600-0625.2002.110502.x. [DOI] [PubMed] [Google Scholar]
- 38.Berneburg M, Plettenberg H, Krutmann J. Photoaging of human skin. Photodermatol Photoimmunol Photomed. 2000;16:239–244. doi: 10.1034/j.1600-0781.2000.160601.x. [DOI] [PubMed] [Google Scholar]