Abstract
Purpose of review
The aims of this review are to 1) discuss the impact of chronic high dose allergen exposure on allergen-specific CD4(+) T cell subset during Allergen-Specific Immunotherapy (AIT) and 2) discuss recent advances supporting novel mechanisms for desensitization and tolerance induction during AIT.
Recent findings
New technologies for direct molecular and cellular analysis have now provided an unprecedented opportunity to compare the functions and phenotypes of allergen-specific T cells at a single cell level, both in the context of disease and clinical intervention. Recent studies have demonstrated that AIT may restore tolerance by transiently inducing IL-10 producing T cells followed by selective deletion of allergen-specific TH2 cell subset.
Summary
With antigen-specific TH2 cells at the core of the allergic process in atopic individuals, the duration and dose of antigen exposure can be the driving force behind current AIT protocol. Mechanisms modulating allergen-specific CD4(+) T cell responses may include 1) autocrine IL-10 production to limit excessive TH2 cell effector responses, 2) T cell exhaustion and 3) preferential pro-allergic TH2 cell deletion allowing concurrent down-regulating T cell responses to emerge. Administration of AIT in the context of immune modulating strategies able to induce counter-regulatory immune response may lead to improved AIT with durable clinical benefit.
Keywords: Allergy, IL-10, CD4+ T cells, tolerance, desensitization, allergen immunotherapy, apoptosis
Introduction
For more than 100 years, allergen-specific immunotherapy (AIT) has been used to treat allergic individuals and continues to be the only clinical therapy available for IgE-mediated allergy. The goal of AIT is to reduce symptoms caused by allergy (desensitization) and ultimately restore a durable harmless response to natural allergen exposure (tolerance). To date, long term clinical benefit following AIT can typically be achieved by the administration (subcutaneous, sublingual, oral, or epicutaneous) of escalating doses of the sensitizing allergen, until a high enough dose is reached and maintained. While clinical benefits associated with AIT are well documented, it faces several problems related to side effects, high costs of lengthy protocols and efficacy that has not yet been proven to be permanent. Part of the reason is that the underlying mechanisms leading to natural tolerance to allergen are not yet understood. It also remains unclear whether restoration of harmless response to allergen is a function of the dose or duration of AIT and can be achieved in any allergic individuals. Whether the immunologic response to allergens absorbed through the oral mucosa is different from that to allergens administrated subcutaneously is also an area of ongoing investigation, although similar immunologic mechanisms appear to be involved (1). In contrast to symptomatic treatment, accumulating evidence now suggests that current allergy vaccine intervenes in the disease at the level of T helper (TH) cells, redirecting inappropriate allergen-specific T cell responses that trigger downstream pro-inflammatory responses (2-5). Therefore, a good knowledge of the targeted CD4(+) T cell population is likely to be key to the design of better immunotherapy. In the case of type 1 allergic disease, current dogma holds that TH2 cells cause pathology, and induction of concurrent immuno-regulating T cells responses is considered a highly desirable therapeutic goal(6). AIT can alter the responses of allergen-specific T cells via various non-exclusive mechanisms involving either i) a switch from a TH2 to a TH1 cell-dominated immune reaction (immune-deviation), ii) the induction of regulatory T cells (immune-regulation) or iii) deletion/anergy of pathogenic allergen-reactive T cells (immune disease induction model). For many years, mechanistic studies investigating the effect of AIT on CD4(+) T cells have shown contradictory results about the mechanism involved, both during desensitization phase and tolerance induction. This discrepancy between studies may arise from the absence of adequately sensitive approaches to directly assess immunological changes within rare allergen-specific CD4(+) T cells. Although still technically challenging, it is now possible to interrogate ex vivo antigen-specific CD4(+) T cell responses in the peripheral blood of patients during the course of immunotherapies, revealing new insights into disease pathogenesis(7, 8). This review will detail the changes in peripheral CD4(+) T cell responses during current immunotherapy and discuss a new paradigm for how allergen-specific CD4(+) T responses may be regulated during AIT.
Allergen-specific regulatory T cells and immunotherapy
Understanding the nature of CD4(+) T cells responses in healthy individuals is critical to improving current allergy vaccines. This is with the assumption that these cells are protective and that allergen-specific immunotherapy should restore and maintain such immuno-regulating T cells responses. Regulatory T (Treg) cells are important mediators of immune tolerance, preventing inappropriate or overwhelming immune response. Interestingly, the high-dose models of beekeepers and cat allergen-exposed individuals suggest that Treg cells can be induced by chronic high dose allergen challenges(9).
Among the known subpopulation of Treg cells, both forkhead box P3(+) (Foxp3) Treg cells (10-12) and inducible Type 1 regulatory T (Tr1) cells (2, 6, 13) have been linked to clinical benefit induced by AIT. However, for many years there has been heated debate about the definition of such Treg cells (14) and their precise monitoring in humans (Table 1). Nevertheless, induction of CD4(+) T cells able to produce the anti-inflammatory Interleukin-10 (IL-10), has emerged as a consistent finding, either in the context of natural responses in non-allergic individuals or as a consequence of successful AIT protocols(15-17). As previously reviewed(18), IL-10 is a pleiotropic cytokine that plays a key role both in suppressing allergic inflammatory pathways and in promoting the induction of IL-10-secreting regulatory T cells. However, under chronic stimulation, IL-10 can be produced by many cell-types of the immune system, including effector TH1 and TH2 cells, increasing uncertainty over the mechanism of AIT. As recently highlighted, AIT appears to be dependent on the interplay between IL-10 and regulatory T cells (16). Yet, recent studies suggest that mainly IL-10-producing Tr1 cells, but not Foxp3(+) Treg cells, are involved in the effectiveness of AIT(2, 15). This is consistent with studies showing that AIT-mediated suppression could be abrogated by the use of neutralizing antibodies against IL-10 signaling (16), whereas depletion of naturally occurring Foxp3(+) Treg cells only partly abrogates the suppressive effects induced by AIT(19).
Table 1. Current known type of regulatory CD4+ T cells and their characteristics.
| Subset | Specific marker | Distinctive cytokine | Origin |
|---|---|---|---|
| Thymus-derived Treg (tTreg) | CD25+ CD127- Helios+ CD39+ CD73+ CTLA4+ Foxp3+ Nrp1+ CD45RA+ | IL-10, TGF-b, IL-35 | Thymus |
| Peripherally-derived Treg (pTreg) | CD25+ CD127- CD39+ CD73+ CTLA4+ Foxp3+ Eos+ CD45RO+ | IL-10, TGF-b, IL-35 | Periphery (CD25-Foxp3- T cells) |
| IL-10 producing Tr1 cells (Tr1) | CD25+ LAG3+ CD49b+ CD45RO+ | IL-10 | Periphery (T helper cells) |
It remains unclear whether AIT directly enhances Treg cell activity or simply increases their absolute number to correct the imbalance between allergen-specific T cell subsets. For instance, the number of Tr1 cells has been found to be reduced in peripheral blood from atopic subjects(20), whereas it was elevated in AIT-treated patients (2, 15). Conversely, CD4(+)CD25(+) Treg suppressive activity has been shown to be defective in birch allergic individuals (21), while recent works by Nadeau et al showed restoration of the suppressive capacity of Foxp3(+) Treg cells following therapy.(22, 23) In this context, regulatory T cells have been regarded for many years as an obvious therapeutic target during AIT to improve its safety and long-term benefit.
Allergen-specific TH2 cells and immunotherapy
While numerous studies support the central role of allergen-specific TH2 cells in pathophysiological responses, there is now compelling evidence that they may also act to re-enforce their own induction. As an example, TH2 functional activities have been shown to antagonize both the induction of IL-10–secreting Tr1 cells (24, 25) and the post-thymic development of Foxp3(+) Treg cells in response to antigen stimulation (26). In parallel, production of IL-4 may amplify immunogenic allergen presentation to other T cells (27) and cause TH2 cells to become resistant to regulatory T cell-mediated suppression (28). Thus, one could argue that inhibition of pro-allergic TH2 cell responses may represent a first critical step to restore tolerance during AIT. To illustrate this concept, we recently showed that pro-allergic TH2 cells, compared to other sub-dominant allergen-specific T cell subsets, were terminally differentiated (CD27-) memory T cells with low expression of Bcl-2, a key inhibitor of apoptosis (29, 30). This shared phenotype expressed among all pro-allergic TH2 cells is consistent with cells highly sensitive to activation-induced cell death (31). Interestingly, while all groups tested (i.e. non-allergic individuals, patient post-AIT and allergic subject) had low frequencies of the TH1/Tr1-like CD27(+) counterpart, pro-allergic TH2 cells (CD27-) were confined to allergic individuals (29, 30), suggesting that their presence might be sufficient for the pathogenesis of allergic diseases, regardless of the balance of TH subset. Likewise, a recent work by Ciepiela et al, revealed that allergen-specific TH1 cells have a survival benefit during SLIT compared to TH2 cells (32) Together, these results indicate that high cumulative dose of allergen during AIT may induce selective functional deletion of pathogenic TH2 response, allowing other T cell responses to emerge.
Chronic high dose allergen stimulation may have other direct consequences on pro-allergic TH2 cell function. Under this stimulation, a negative feedback mechanism to limit effector T cell responses is commonly observed and characterized by autocrine IL-10 production of the responding T cells (33). Remarkably, it has also been shown that antigen-specific TH2 memory cells can be converted into Foxp3(+) regulatory T cells, suppressing TH2-mediated allergic asthma (34). As recently underlined, chronic high dose allergen stimulation of TH2 cells during AIT may also directly enhance epigenetic mechanisms on TH2-related genes such as GATA-3, IL-5 and IL-4 (35). For instance, using milk epicutaneous immunotherapy (EPIT) treated mice, Mondoulet et al. demonstrated that EPIT increased the methylation of the GATA-3 promoter in correlation with decreased TH2 cell activity (36). In this context, pro-allergic TH2 cells can also be regarded as an obvious therapeutic target during AIT.
Sequential effect of AIT on CD4(+) T cell subset
1) Desensitization phase
Desensitization is the first change noted with the initiation of AIT and generally occurs early in the protocol when the threshold of clinical reactivity to natural allergen exposure is temporary increased. In a recent study using pMHCII-tetramer, Bonvalet et al, demonstrated that initial clinical improvement during sublingual AIT was not associated with dramatic alterations in T lymphocyte responses (37). Nevertheless, growing evidence suggests a direct, but transient, role for IL-10 in this process (15-17), which in turn induces production of allergen-specific IgG4 antibody that attenuates IgE mediating allergic symptoms (38). There is still debate as to what extent the dose and duration of AIT enhances activity of IL-10-secreting T cells. Since differentiation and induction of Tr1 cells required a previously established tolerogenic (IL-10) environment, in absence of IL-4 (18), it is also unclear whether these IL-10 producing CD4(+) T cells are a regulatory or an effector T cell subset. In this regard, early induction of allergen-specific Tr1 cells is commonly observed during AIT (2, 15). However, with TH2 cell subset at the core of the allergic process, initiation phase of AIT may likely create an inhibitory milieu that hampers establishment of such regulatory Tr1 cells. Hence, one can argue that administration of escalating doses of allergen may rapidly establish a negative auto-regulatory feedback loop to prevent excessive pro-allergic TH2 responses. Accordingly, these cells may directly represent the initial and principal source of IL-10 during early desensitization phase. Such an activation-induced feedback inhibition mechanism is powerfully evidenced by the work of Altin et al (39). In their study of discrete subsets of TH2 cells during chronic inflammation, they established that IL-10-producing TH2 cells, fulfilling the criteria of inducible type 1 regulatory T (Tr1) cells, can arise directly from non-suppressive TH2 cells once a specific threshold of activation is achieved. In further proof of this mechanism, our group recently observed, during initiation phase of AIT, selective increased mRNA expression of IL-10 and FOXP-3 within the pathogenic CD27(-) allergen-specific TH2 (IL5+, IL4+, IL9+) cell subset, but not in the TH1/Tr1-like CD27(+) counterpart. Consistent with a study showing that IL-10 increases IL-4-dependant IgG4 class switching(40), we further highlighted the direct role of TH2 cells in early reinforcement of IgG4 production during chronic cat-allergen exposure, which in turn drives desensitization without induction of T cell tolerance (41). Consistent with transient clinical benefits observed during desensitization phase, induction of such “regulatory” TH2 cells may persist as long as the allergen is administrated. Thereafter, if treatment is not continued long enough to further trigger selective TH2 cell exhaustion, the initial pathogenic feature of these TH2 cells may gradually recover after discontinuation (Figure 1).
Figure 1. Schematic representation of sequential immune mechanisms leading to desensitization state during AIT.
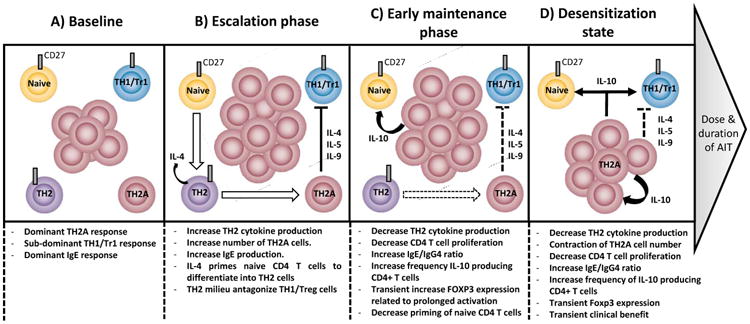
At baseline (A), CD27 expression distinguishes pro-allergic TH2 (TH2A) cell subset from non-pathogenic (CD27+) allergen-specific T cell subset in allergic subjects. While low frequency of CD27(+) allergen-specific TH1/Tr1 cell subset can be observed both in allergic and non-allergic individuals, allergen-specific TH2A cells (CD27-) are confined to allergic individuals and dominate the allergen-specific T cell response. Some CD27(+) allergen-specific TH2 cells (IL-4+) may differentiate from the naïve T cell pool to serve as reservoir to replenished TH2A cells (IL-4+, IL-5+, IL-9+) following chronic allergen challenge. Similar to natural allergen exposure, first low dose exposure to the allergen during escalation phase (B) leads to an increase of allergen-specific TH2A cell response and production of related specific-IgE. This dominant pro-allergic TH2 functional activity antagonizes the induction of regulatory T cells. Early maintenance phase (C) rapidly establishes a negative auto-regulatory feedback loop to prevent excessive pro-allergic TH2 responses.;these in turn drive a desensitization state (D) via decreased TH2 cell activity, IL-10 production and change in the IgE/IgG4 ratio. At this stage, if treatment is not continued long enough, the initial pathogenic feature of CD27(-) allergen-specific T cells may gradually recover after treatment discontinuation.
Altogether, these data suggest a model in which chronic stimulation of allergen-specific TH2 cells, during initial phase of AIT, culminates in a counter-regulatory immune response consisting of pathogenic TH2 cells driven to an anergic, regulatory-like phenotype transiently preventing allergic symptoms through the production of IL-10. Lastly, they also explain the discrepancy between studies that showed decreased TH2-cytokine production in response to allergen stimulation during the early phase of AIT (42) versus others that failed to document significant decrease in the absolute number of allergen-specific TH2 cells during the first 3 to 12 months of treatment (15, 37).
2) Tolerance induction phase
Tolerance refers to the induction of permanent clinical non-responsiveness to allergen, even after discontinuation of treatment. In contrast to desensitization phase, skewing of allergen-specific effector T cells away from the pro-allergic TH2 response appears to be a key event in the development of long-lasting peripheral tolerance to allergen. Given that regulatory T cells are crucial for maintaining T-cell tolerance, they are thought to be the ‘holy grail’ in successful AIT. However, solid evidence for induction of allergen-specific Tregs mediating T cell-tolerance during current AIT protocols remains elusive.
Emerging results substantiate the notion that selective deletion of pro-allergic TH2 cells can be a causative mechanism for the pronounced clinical benefit that is seen in many patients receiving more than 2 years of treatment (29, 30, 32, 43). Indeed, given that IL-10 is an important mediator of T cell exhaustion [47] and functional deletion of highly mature antigen-specific CD4(+) T cells can occur when the antigen dose is both extremely high and persistent, it is possible that induction of peripheral tolerance to allergen acts through this mechanism. Accordingly if AIT protocol is continued long enough following the desensitization phase, T cell exhaustion may become established on terminally differentiated (CD27-) TH2 cells, and in this state, the initial pathogenic feature could not recover after discontinuation. (44). Thereafter, this selective deletion of pro-allergic TH2 cells may allow previously sub-dominant response with survival benefit (Bcl2+, CD27+) to emerge (Figure 2). Change in the ratio CD27(-)/CD27(+) within allergen-specific memory T cells may thus represent a serious biomarker of good prognosis in patients receiving AIT. As a result of a change in the cytokine environment (decrease IL-4, increase IL-10), antigen-triggered de novo generation of IL-10–secreting Tr1 and of Foxp3(+) Tregs in the periphery may also induce a more permanent state of tolerance to allergen along with IL-10-producing dendritic cells favoring further suppressive mechanisms (45). However, the extremely low frequency of the CD27(+) allergen-specific T cells subset, observed both in the development of healthy immune response to allergens and in successful outcome in AIT, suggests that long-term allergen tolerance might not be attributed to active T cell contribution, but rather to diminished pro-allergic TH2 cells in general.
Figure 2. Schematic representation of sequential immune mechanisms restoring peripheral tolerance state during AIT.
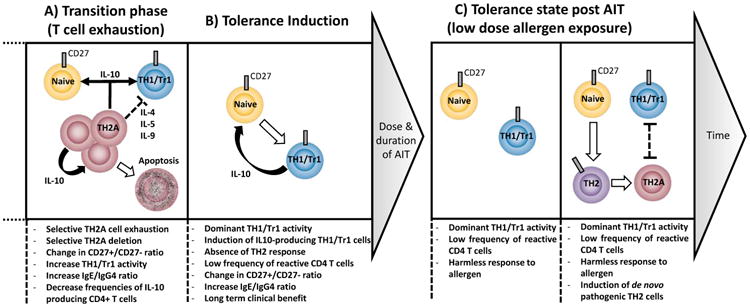
Following the desensitization state, persistent high dose allergen stimulation of CD27(-) allergen-specific T cells triggers selective T cells exhaustion followed by T cells deletion of them; this allows survival sub-dominant CD27(+) allergen-specific T cells to emerge (A). AIT restores a state of natural tolerance to allergen similar to non-allergic individuals. Further chronic stimulation in this tolerogenic environment can trigger de novo generation of regulatory T cells and induce IL-10 production in remaining CD27(+) TH1 cells (B). Following treatment discontinuation, hypo-responsive state is maintained during natural allergen challenge (C). With time, the effector memory pro-allergic TH2 cell pool may be replenished by allergen-specific cells differentiated from the naïve T cell pool.
Selective apoptosis of pathogenic Th2 cell population represents an alternative model of the classical pathogenic/protective T cell response balance model used to explain allergic disease and the effect of current AIT on CD4(+) T cells. In this model recently reviewed by Endo et al (46), allergic diseases are considered to be the result of the induction of a distinct TH2 cell subset, unique to allergic individuals and regardless of the balance of other TH subsets. Selective apoptosis of such a pathogenic TH population would therefore restore a hypo-responsive state without counter immuno-regulating T cell responses. In support of this concept, our group recently discovered a subset of human memory TH2 cells that includes all allergen-specific TH2 cells and confined to atopic individuals (47) (and manuscript submitted). Interestingly, this pro-allergic TH2 cell subset (denoted TH2A cell subset) exhibits numerous phenotypic and functional attributes distinctive of conventional TH2 cells and was preferentially deleted during AIT.
Conclusion
In this review, we have summarized the mechanisms by which current AIT protocol can modulate allergen-specific CD4(+) T cell responses in allergic individuals. It is likely that the dose and duration of allergen exposure during current AIT protocol sequentially target dominant pro-allergic TH2 cells including T cell exhaustion followed by T cell deletion. Determining whether development of new therapeutic approaches that selectively target pro-allergic TH2 cells without concurrent down-regulating T cell responses will be sufficient for long-term tolerance induction to allergen, is an important but unresolved question. Administration of AIT in the context of immune modulating strategies able to either induce counter-regulatory immune response or to block de novo generation of pro-allergic TH2 cells may lead to improved AIT with durable clinical benefit.
Key Points.
- Direct assessment of allergen-specific T cells is essential to determine critical node in both disease pathogenesis and tolerance induction.
- Administration of escalating doses of allergen rapidly establish a negative auto-regulatory feedback loop to prevent excessive pro-allergic TH2 responses and induce transient desensitization state.
- Preferential allergen-specific TH2 cell deletion during AIT can be another independent mechanism to restore tolerance during immunotherapy, allowing other sub-dominant responses to emerge.
Acknowledgments
We would like to thank collaborators at Benaroya Research Institute for assistance in the study and Cynthia Cousens-Jacobs, for excellent administrative assistance
Financial support and sponsorship: This work was supported by NIH/NIAID grants R01 AI108839 and AI095074.
Footnotes
Conflict of interest: None
References
- 1.Moingeon P. Update on immune mechanisms associated with sublingual immunotherapy: practical implications for the clinician. J Allergy Clin Immunol Pract. 2013;1(3):228–41. doi: 10.1016/j.jaip.2013.03.013. [DOI] [PubMed] [Google Scholar]
- 2.Mobs C, Slotosch C, Loffler H, Jakob T, Hertl M, Pfutzner W. Birch pollen immunotherapy leads to differential induction of regulatory T cells and delayed helper T cell immune deviation. Journal of immunology. 2010;184(4):2194–203. doi: 10.4049/jimmunol.0901379. (Of outstanding interest); A very interesting longitudinal study that directly focuses on the frequencies of distinct T cell population in the pivotal phases of AIT. [DOI] [PubMed] [Google Scholar]
- 3.Burton BR, Britton GJ, Fang H, Verhagen J, Smithers B, Sabatos-Peyton CA, et al. Sequential transcriptional changes dictate safe and effective antigen-specific immunotherapy. Nature communications. 2014;5:4741. doi: 10.1038/ncomms5741. (Of specific interest); A good example for addressing how analysis of the CD4+ T cell transcript me helps to elucidate sequential mechanism involved during AIT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Suarez-Fueyo A, Ramos T, Galan A, Jimeno L, Wurtzen PA, Marin A, et al. Grass tablet sublingual immunotherapy downregulates the TH2 cytokine response followed by regulatory T-cell generation. The Journal of allergy and clinical immunology. 2014;133(1):130–8.e1. 2. doi: 10.1016/j.jaci.2013.09.043. [DOI] [PubMed] [Google Scholar]
- 5.Maggi E. T-cell responses induced by allergen-specific immunotherapy. Clinical and experimental immunology. 2010;161(1):10–8. doi: 10.1111/j.1365-2249.2010.04148.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akdis CA, Akdis M. Mechanisms of allergen-specific immunotherapy and immune tolerance to allergens. The World Allergy Organization journal. 2015;8(1):17. doi: 10.1186/s40413-015-0063-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wambre E, Van Overtvelt L, Maillere B, Humphreys R, von Hofe E, Ferhat L, et al. Single cell assessment of allergen-specific T cell responses with MHC class II peptide tetramers: methodological aspects. International archives of allergy and immunology. 2008;146(2):99–112. doi: 10.1159/000113513. [DOI] [PubMed] [Google Scholar]
- 8.Kwok WW, Roti M, Delong JH, Tan V, Wambre E, James EA, et al. Direct ex vivo analysis of allergen-specific CD4+ T cells. The Journal of allergy and clinical immunology. 2010;125(6):1407–9.e1. doi: 10.1016/j.jaci.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Meiler F, Zumkehr J, Klunker S, Ruckert B, Akdis CA, Akdis M. In vivo switch to IL-10-secreting T regulatory cells in high dose allergen exposure. The Journal of experimental medicine. 2008;205(12):2887–98. doi: 10.1084/jem.20080193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Radulovic S, Jacobson MR, Durham SR, Nouri-Aria KT. Grass pollen immunotherapy induces Foxp3-expressing CD4+ CD25+ cells in the nasal mucosa. The Journal of allergy and clinical immunology. 2008;121(6):1467–72. 72.e1. doi: 10.1016/j.jaci.2008.03.013. [DOI] [PubMed] [Google Scholar]
- 11.Rolland JM, Gardner LM, O'Hehir RE. Functional regulatory T cells and allergen immunotherapy. Curr Opin Allergy Clin Immunol. 2010;10(6):559–66. doi: 10.1097/ACI.0b013e32833ff2b2. [DOI] [PubMed] [Google Scholar]
- 12.Ling EM, Smith T, Nguyen XD, Pridgeon C, Dallman M, Arbery J, et al. Relation of CD4+CD25+ regulatory T-cell suppression of allergen-driven T-cell activation to atopic status and expression of allergic disease. Lancet (London, England) 2004;363(9409):608–15. doi: 10.1016/S0140-6736(04)15592-X. [DOI] [PubMed] [Google Scholar]
- 13.Pellerin L, Jenks JA, Begin P, Bacchetta R, Nadeau KC. Regulatory T cells and their roles in immune dysregulation and allergy. Immunologic research. 2014;58(2-3):358–68. doi: 10.1007/s12026-014-8512-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abbas AK, Benoist C, Bluestone JA, Campbell DJ, Ghosh S, Hori S, et al. Regulatory T cells: recommendations to simplify the nomenclature. Nature immunology. 2013;14(4):307–8. doi: 10.1038/ni.2554. [DOI] [PubMed] [Google Scholar]
- 15.Mobs C, Ipsen H, Mayer L, Slotosch C, Petersen A, Wurtzen PA, et al. Birch pollen immunotherapy results in long-term loss of Bet v 1-specific TH2 responses, transient TR1 activation, and synthesis of IgE-blocking antibodies. The Journal of allergy and clinical immunology. 2012;130(5):1108–16.e6. doi: 10.1016/j.jaci.2012.07.056. [DOI] [PubMed] [Google Scholar]
- 16.Bohm L, Maxeiner J, Meyer-Martin H, Reuter S, Finotto S, Klein M, et al. IL-10 and regulatory T cells cooperate in allergen-specific immunotherapy to ameliorate allergic asthma. Journal of immunology. 2015;194(3):887–97. doi: 10.4049/jimmunol.1401612. [DOI] [PubMed] [Google Scholar]
- 17.Akdis CA, Akdis M. Mechanisms of immune tolerance to allergens: role of IL-10 and Tregs. J Clin Invest. 2014;124(11):4678–80. doi: 10.1172/JCI78891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hawrylowicz CM, O'Garra A. Potential role of interleukin-10-secreting regulatory T cells in allergy and asthma. Nature reviews Immunology. 2005;5(4):271–83. doi: 10.1038/nri1589. [DOI] [PubMed] [Google Scholar]
- 19.Maazi H, Shirinbak S, Willart M, Hammad HM, Cabanski M, Boon L, et al. Contribution of regulatory T cells to alleviation of experimental allergic asthma after specific immunotherapy. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2012;42(10):1519–28. doi: 10.1111/j.1365-2222.2012.04064.x. [DOI] [PubMed] [Google Scholar]
- 20.Akdis M, Verhagen J, Taylor A, Karamloo F, Karagiannidis C, Crameri R, et al. Immune responses in healthy and allergic individuals are characterized by a fine balance between allergen-specific T regulatory 1 and T helper 2 cells. The Journal of experimental medicine. 2004;199(11):1567–75. doi: 10.1084/jem.20032058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grindebacke H, Wing K, Andersson AC, Suri-Payer E, Rak S, Rudin A. Defective suppression of Th2 cytokines by CD4CD25 regulatory T cells in birch allergics during birch pollen season. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2004;34(9):1364–72. doi: 10.1111/j.1365-2222.2004.02067.x. [DOI] [PubMed] [Google Scholar]
- 22.Syed A, Garcia MA, Lyu SC, Bucayu R, Kohli A, Ishida S, et al. Peanut oral immunotherapy results in increased antigen-induced regulatory T-cell function and hypomethylation of forkhead box protein 3 (FOXP3) The Journal of allergy and clinical immunology. 2014;133(2):500–10. doi: 10.1016/j.jaci.2013.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Swamy RS, Reshamwala N, Hunter T, Vissamsetti S, Santos CB, Baroody FM, et al. Epigenetic modifications and improved regulatory T-cell function in subjects undergoing dual sublingual immunotherapy. The Journal of allergy and clinical immunology. 2012;130(1):215–24.e7. doi: 10.1016/j.jaci.2012.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mantel PY, Kuipers H, Boyman O, Rhyner C, Ouaked N, Ruckert B, et al. GATA3-driven Th2 responses inhibit TGF-beta1-induced FOXP3 expression and the formation of regulatory T cells. PLoS biology. 2007;5(12):e329. doi: 10.1371/journal.pbio.0050329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hadjur S, Bruno L, Hertweck A, Cobb BS, Taylor B, Fisher AG, et al. IL4 blockade of inducible regulatory T cell differentiation: the role of Th2 cells, Gata3 and PU.1. Immunology letters. 2009;122(1):37–43. doi: 10.1016/j.imlet.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 26.Wei J, Duramad O, Perng OA, Reiner SL, Liu YJ, Qin FX. Antagonistic nature of T helper 1/2 developmental programs in opposing peripheral induction of Foxp3+ regulatory T cells. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(46):18169–74. doi: 10.1073/pnas.0703642104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Eisenbarth SC, Zhadkevich A, Ranney P, Herrick CA, Bottomly K. IL-4-dependent Th2 collateral priming to inhaled antigens independent of Toll-like receptor 4 and myeloid differentiation factor 88. Journal of immunology. 2004;172(7):4527–34. doi: 10.4049/jimmunol.172.7.4527. [DOI] [PubMed] [Google Scholar]
- 28.Pillemer BB, Qi Z, Melgert B, Oriss TB, Ray P, Ray A. STAT6 activation confers upon T helper cells resistance to suppression by regulatory T cells. Journal of immunology. 2009;183(1):155–63. doi: 10.4049/jimmunol.0803733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wambre E, DeLong JH, James EA, LaFond RE, Robinson D, Kwok WW. Differentiation stage determines pathologic and protective allergen-specific CD4+ T-cell outcomes during specific immunotherapy. The Journal of allergy and clinical immunology. 2012;129(2):544–51. 51.e1–7. doi: 10.1016/j.jaci.2011.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wambre E, DeLong JH, James EA, Torres-Chinn N, Pfutzner W, Mobs C, et al. Specific immunotherapy modifies allergen-specific CD4(+) T-cell responses in an epitope-dependent manner. The Journal of allergy and clinical immunology. 2014;133(3):872–9.e7. doi: 10.1016/j.jaci.2013.10.054. (Of specific interest); In this study we used an ex vivo tetramer approach to characterize grass pollen-specific CD4+ T cell responses at epitope level and showed that the differentiation stage divides allergen-specific CD4(+) T cells into 2 distinct subpopulations with unique functional properties and different fates during SIT. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pepper M, Linehan JL, Pagan AJ, Zell T, Dileepan T, Cleary PP, et al. Different routes of bacterial infection induce long-lived TH1 memory cells and short-lived TH17 cells. Nature immunology. 2010;11(1):83–9. doi: 10.1038/ni.1826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ciepiela O, Zawadzka-Krajewska A, Kotula I, van Overveld F, Kulus M, Demkow U. Sublingual Immunotherapy for Asthma: Affects T-Cells but Does not Impact Basophil Activation. Pediatric allergy, immunology, and pulmonology. 2014;27(1):17–23. doi: 10.1089/ped.2014.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Saraiva M, Christensen JR, Veldhoen M, Murphy TL, Murphy KM, O'Garra A. Interleukin-10 production by Th1 cells requires interleukin-12-induced STAT4 transcription factor and ERK MAP kinase activation by high antigen dose. Immunity. 2009;31(2):209–19. doi: 10.1016/j.immuni.2009.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kim BS, Kim IK, Park YJ, Kim YS, Kim YJ, Chang WS, et al. Conversion of Th2 memory cells into Foxp3+ regulatory T cells suppressing Th2-mediated allergic asthma. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(19):8742–7. doi: 10.1073/pnas.0911756107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Begin P, Nadeau KC. Epigenetic regulation of asthma and allergic disease. Allergy, asthma, and clinical immunology : official journal of the Canadian Society of Allergy and Clinical Immunology. 2014;10(1):27. doi: 10.1186/1710-1492-10-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mondoulet L, Dioszeghy V, Puteaux E, Ligouis M, Dhelft V, Plaquet C, et al. Specific epicutaneous immunotherapy prevents sensitization to new allergens in a murine model. The Journal of allergy and clinical immunology. 2015;135(6):1546–57.e4. doi: 10.1016/j.jaci.2014.11.028. [DOI] [PubMed] [Google Scholar]
- 37.Bonvalet M, Moussu H, Wambre E, Ricarte C, Horiot S, Rimaniol AC, et al. Allergen-specific CD4+ T cell responses in peripheral blood do not predict the early onset of clinical efficacy during grass pollen sublingual immunotherapy. Clinical and experimental allergy : journal of the British Society for Allergy and Clinical Immunology. 2012;42(12):1745–55. doi: 10.1111/cea.12015. [DOI] [PubMed] [Google Scholar]
- 38.James LK, Shamji MH, Walker SM, Wilson DR, Wachholz PA, Francis JN, et al. Long-term tolerance after allergen immunotherapy is accompanied by selective persistence of blocking antibodies. The Journal of allergy and clinical immunology. 2011;127(2):509–16.e1. 5. doi: 10.1016/j.jaci.2010.12.1080. [DOI] [PubMed] [Google Scholar]
- 39.Altin JA, Goodnow CC, Cook MC. IL-10+ CTLA-4+ Th2 inhibitory cells form in a Foxp3-independent, IL-2-dependent manner from Th2 effectors during chronic inflammation. Journal of immunology. 2012;188(11):5478–88. doi: 10.4049/jimmunol.1102994. (Of outstanding interest); This study provides evidence that TH2 responses can also initiate a counterbalancing negative feedback to prevent excessive effector response during chronic inflammation. [DOI] [PubMed] [Google Scholar]
- 40.Tanaka A, Moriyama M, Nakashima H, Miyake K, Hayashida JN, Maehara T, et al. Th2 and regulatory immune reactions contribute to IgG4 production and the initiation of Mikulicz disease. Arthritis Rheum. 2012;64(1):254–63. doi: 10.1002/art.33320. [DOI] [PubMed] [Google Scholar]
- 41.Renand A, Archila LD, McGinty J, Wambre E, Robinson D, Hales B, et al. Chronic cat-allergen exposure induces a TH2 cell-dependent IgG4 response related to low-sensitization. The Journal of allergy and clinical immunology. 2015 doi: 10.1016/j.jaci.2015.07.031. In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moverare R, Elfman L, Bjornsson E, Stalenheim G. Changes in cytokine production in vitro during the early phase of birch-pollen immunotherapy. Scandinavian journal of immunology. 2000;52(2):200–6. doi: 10.1046/j.1365-3083.2000.00764.x. [DOI] [PubMed] [Google Scholar]
- 43.Gardner LM, O'Hehir RE, Rolland JM. High dose allergen stimulation of T cells from house dust mite-allergic subjects induces expansion of IFN-gamma+ T Cells, apoptosis of CD4+IL-4+ T cells and T cell anergy. International archives of allergy and immunology. 2004;133(1):1–13. doi: 10.1159/000075248. [DOI] [PubMed] [Google Scholar]
- 44.Brooks DG, Trifilo MJ, Edelmann KH, Teyton L, McGavern DB, Oldstone MB. Interleukin-10 determines viral clearance or persistence in vivo. Nature medicine. 2006;12(11):1301–9. doi: 10.1038/nm1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mittal SK, Roche PA. Suppression of antigen presentation by IL-10. Current opinion in immunology. 2015;34:22–7. doi: 10.1016/j.coi.2014.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Endo Y, Hirahara K, Yagi R, Tumes DJ, Nakayama T. Pathogenic memory type Th2 cells in allergic inflammation. Trends in immunology. 2014;35(2):69–78. doi: 10.1016/j.it.2013.11.003. (Of specific interest); This review proposes an alternative model to the classical pathogenic/protective T cell response balance model used to explain immune-mediated disease. [DOI] [PubMed] [Google Scholar]
- 47.Wambre E, Delong EA, James EA, Robinson D, Kwok WW. TH2A Cells As a Unique TH2 Cell Subset in Allergic Individuals: Steps Toward a T Cell Biomarker For Allergy. The Journal of allergy and clinical immunology. 2012;129(2 (Supplemental)):AB129. [Google Scholar]


