Abstract
Objective
To review recent advances in understanding the cellular mechanisms that regulate fat distribution.
Methods
We highlight new insights into depot- and sex-differences in the developmental origins and growth of adipose tissues as revealed by studies that use new methods, including lineage tracing.
Results
Variations in fat distribution during normal growth and in response to alterations in nutritional or hormonal status are driven by intrinsic differences in cells found in each adipose depot. Adipose progenitor cells and preadipocytes in different anatomical adipose tissues derive from cell lineages that determine their capacity for proliferation and differentiation. As a result, rates of hypertrophy and hyperplasia during growth and remodeling vary among depots. The capacities of adipose cells are also determined by variations in the expression of key transcription factors and non-coding RNAs. These developmental events are influenced by sex chromosomes, hormonal and nutrient signals that determine the adipogenic, metabolic, and functional properties of each depot.
Conclusions
These new developments in our understanding of fat distribution provide a sound basis for understanding the association of body shape and health in non-obese and obese men and women.
Introduction
Both total adiposity and fat distribution influence systemic metabolism and variations in both parameters are associated with risk for metabolic diseases. The concept that anatomically distinct adipose depots have specialized functions has gained widespread acceptance in the obesity field. Substantial research effort has been directed at understanding depot-differences in adipose tissue growth and function. In this review we will focus on findings in human adipose tissues, and discuss how recent studies shed light on understanding the mechanisms involved.
10 to 100 billion so-called white adipocytes are found in distinct anatomical depots of humans. Individual adipocytes within each depot differ greatly in size, from 10 to over 200 microns in diameter, due to the amount of triglyceride they are storing at a given point in time (1, 2). Under the microscope, all ‘white’ adipocytes generally look alike, i.e. they appear to have a single lipid droplet, a thin rim of cytoplasm, and a nucleus which is forced to the side. Appearances can be deceiving, however, as adipocytes from different anatomical depots exhibit different morphology (i.e. adipocyte size and distribution) and functional capacities (3).
The importance of understanding the biology of different adipose depots is that they have differential effects on health. In general, the size of lower body subcutaneous depots (gluteal and femoral) are associated with metabolic health and lower risk for abnormalities of glucose and lipid homeostasis, while upper body, especially central depots, both visceral and abdominal subcutaneous, are associated with higher risk for type 2 diabetes and cardiovascular disease (3, 4). Variations in the distribution of subcutaneous adipose tissues of the trunk, i.e. in subscapular depot as well as the more commonly measured abdominal subcutaneous depot, also contribute to metabolic risk. Visceral depots may have specific functions, for example in immunity, over and beyond their roles in energy homeostasis (5, 6).
Identification of genes that regulate body shape and fat distribution
Body circumferences and imaging methods allow increasingly detailed descriptions of regional fatness in epidemiological and clinical studies. Recent genome-wide association studies point to strong genetic effects on body shape in both men and women, with stronger associations in women (7, 8). The role of these newly-identified genetic loci in the regulation of fat distribution remains poorly understood (7). Only a few genes identified in human genetic studies have been studied from a functional perspective.
Metabolic differences among adipocytes from different adipose tissues
A number of recent reviews focus on recent advances in the metabolic and endocrine functions of ‘white’ adipocytes from different depots (3, 9), so we will just briefly describe these here. From a functional point of view, white adipocytes have the capacity to store triglyceride and to mobilize it in the form of fatty acids in response to hormonal, neural or local signals (e.g. cytokines or chemokines). White adipocytes are also endocrine cells that secrete numerous hormones (e.g. leptin, adiponectin) that regulate virtually every physiological system. All white adipocytes appear to share these functions, but there are depot-dependent biologically important quantitative and qualitative differences. For example, the distribution of adrenergic receptors subtypes on adipocytes varies between abdominal and gluteal adipocytes and this determines the lipolytic response to epinephrine and norepinephrine – e.g. lower in gluteal and femoral than abdominal subcutaneous adipose tissues of humans (10). There are also differences in the expression of proteins and enzymes that direct net fatty acid uptake and esterification. For example, CD36, ACS and DGAT expression and activity are higher in lower body subcutaneous depots (11).
Results
Depot-Dependent Adipose Tissue Growth through Hyperplasia and/or Hypertrophy
Depots vary in their capacity to expand by hyperplasia and hypertrophy
Normal growth is associated with an increase in both the size and number of adipocytes. A variety of experimental approaches, including the classic cell sizing and counting, document increases in the number of adipocytes with age as animals grow fatter (spontaneous obesity) (12). Time course studies of the size and number of adipocytes lead to the idea that a ‘critical’ adipocytes size exists, which could differ in different depots and somehow trigger the recruitment of preadipocytes that eventually differentiated into mature adipocytes (12). Studies in mice showed that in the epididymal fat pad cell number increased up to a certain age and then grew mainly by hypertrophy (13). However, in other depots (subcutaneous and retroperitoneal) prolonged high fat feeding was accounted for in part by hyperplasia (13). This result seems in contrast to results of greater differentiation of subcutaneous than gonadal/visceral preadipocytes in vitro in mice and humans, but may be explained by variations in local factors in the in vivo situation (as discussed below).
The gonadal/visceral depots, especially of males, are more susceptible to high fat induced adipocyte death yet can completely remodel (14). In addition to recruitment of preadipocytes, adipose tissues undergo continual remodeling in which older, dysfunctional adipocytes are replaced with newly differentiated ones (14). It is estimated that the lifespan of a subcutaneous adipocyte in humans is 10 years (2). Studies of net changes in cellularity cannot address the dynamic aspects of adipose tissue growth. A careful time course of measurements in adipocyte turnover (recruitment of adipocyte precursor cells (APCs) and death of older, mature adipocytes) is therefore critical for understanding the ability of the tissue to recover after the initial stress of a very high fat diet.
Two sophisticated tracking studies allow marking of adipocytes at specific stages of development (reviewed in (15)). The adipochaser mouse (16) is an inducible system that permanently labels mature adipocytes and hence allows analysis of the dilution of that label by new adipocytes. On the other hand, the PdgfRα-H2B-GFP mouse labels APCs (17). Both approaches find evidence for depot-dependent variations in both hypertrophy and hyperplasia, and a surprising result based on prior descriptive studies: the gonadal/epididymal fat pad of the male, of relatively young mouse exhibits a higher proliferative rate. In the rodent, subcutaneous adipocytes (inguinal and dorsal) are on average smaller than gonadal.
Mice as a model for human adipose development
When translating results from mice to humans, it is important to recall that in humans, in contrast to young rodents, lower body subcutaneous adipocytes are larger than abdominal subcutaneous or omental ones in both males and females (18), while in women, abdominal subcutaneous are larger than omental adipocytes and in men they are about the same size (19, 20). Thus, if adipocyte size is a variable in mediating adipocyte turnover and growth, results from mice/rats should be extrapolated to humans with caution.
Adipocyte hypertrophy versus hyperplasia and metabolic health
Studies of the relationship of depot differences in adipocyte size and the ‘expandability’ of specific depots by hyperplasia to metabolic health are mixed. An increase in the average size of abdominal subcutaneous adipocyte is associated with greater insulin resistance and metabolic dysfunction (21). Recent studies suggest that ‘unhealthy obesity’, is associated with a smaller pool of adipose precursors that restricts this hyperplastic expansion remodeling, leading to adipocyte hypertrophy, adipose tissue and systemic metabolic dysfunction (22). Conversely, a higher capacity for hyperplastic expansion in abdominal subcutaneous depots is associated with the metabolic health of the tissue independent of the level of obesity (23). Most studies have examined the abdominal subcutaneous depots, with a few notable exceptions (24) and do not address sex differences and influence of apple vs pear shape or more direct measures of body fat distribution.
The expandability hypothesis has been tested in overfeeding experiments in humans. Studying women and men, Jensen’s group (24) found evidence that the femoral but not abdominal depot tended to increase in mass due to hyperplasia. Overfeeding young men leads to increases in abdominal subcutaneous adipocyte size which was mainly accounted for by an increase in size of smaller adipocytes within the depot, and the increase in adipocyte number was greater in those with initially larger adipocytes (23). There are numerous technical challenges with determining changes in adipocyte number and turnover in humans which may contribute to these discrepancies, as well as likely high variability among men and women, and initial body fat distribution and genetic factors. Thus, the basic biology can be best dissected with animal models, although subsequent validation in humans is always essential.
Are Depot Differences in Adipogenesis Cell Autonomous?
Developmental influences on depot dependent APC and adipocyte phenotypes may be cell autonomous
Recent evidence supports the notion that there are different ‘types’ of white adipocytes, and that they may derive from different ‘types’ of APCs and possess variable capacities for growth and functional characteristics. To make it even more complicated, APCs are plastic, such that their fate, whether or not they differentiate into functional adipocytes, is influenced by biological sex, hormones, cytokines, growth factors, and other factors in the local environment within a specific, anatomically distinct depot (Figure 1). To address the question of whether there are different ‘types’ of white adipocytes, it is important to understand the developmental origins and functional capacities of a ‘white adipocyte’ from a given depot. Thus, it is necessary to ask (experimentally): “When and where were you born? Where did you grow up? Has your neighborhood changed over the years? Where do you live now? How old are you?” In other words, new studies are dissecting the molecular mechanisms that shape the phenotype of ‘white’ adipocytes and lead to their specialized functions at different steps of life cycle. Similarly, the capacity for an adipose depot to grow depends on the size of the pool of adipose progenitors (preadipocytes) and their capacity for self-renewal and differentiation under specific conditions.
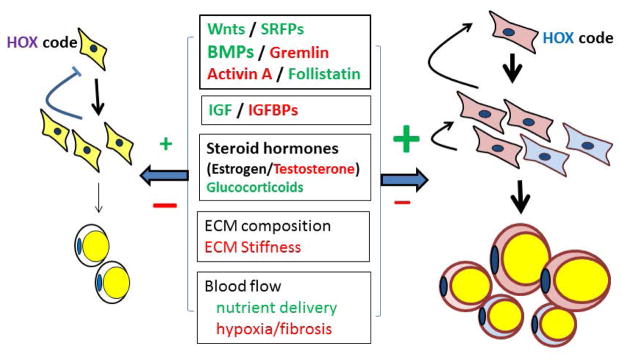
Figure 1. Simplified model of the depot-dependent factors that influence the proliferation and differentiation of adipocytes.
The development of two theoretical depots is illustrated. The color of the adipose progenitor cell signifies its lineage. Within a depot, paracrine interactions create an environment that promotes or inhibits the differentiation and progression of these cells through the adipogenic program (as reviewed well by Macdougal (78)). These local factors are produced by the adipose progenitors and preadipocytes as well as other cell types present within a depot (e.g. mesothelial cells in visceral only, immune cells, endothelial) as well as the mature adipocyte itself. Factors for which there is strongest evidence as local effectors of adipogenesis and tissue remodeling include members of the TGFβ superfamily (BMPs, activins, their receptors and antagonists (23, 79) and well as insulin, IGFs and steroids (locally or systemically produced (not illustrated)). All of the cells within the tissue secrete components of the extracellular matrix which modulates the activity of growth factors and cytokines/chemokines. Physiological effectors such as blood flow and innervation also modulate the activity of all of these processes in a depot-dependent manner. Red font indicates inhibitory effect. Green font indicates stimulatory effect.
Despite rapid advances in technology, we still cannot definitively identify APCs. Available evidence shows that there are likely multiple stem cell lineages that can differentiate to an adipocyte phenotype. As reviewed extensively in (25–27), it is possible to use various combinations of cell surface markers and flow activated cell sorting to isolate cells from the stromal vascular cell fraction of adipose tissues that can be differentiated into adipocytes in vitro and in vivo. Intriguingly, the results of some lineage tracing studies have suggested that white adipocytes can derive from a mural cell/pericyte niche (28), while others support a sole origin from very early Pref-1-positive mesenchymal cells (29). It has also been suggested that visceral adipocytes can derive from mesothelial cells, which themselves derive from a mesenchymal lineage (30).
The molecular mechanisms that link developmental origin to depot- and sex-dependent functional characteristics of mature adipocytes in specific depots remain far from clear. Interesting hints come from recent studies from the Guertin lab (31, 32). They discovered that Myf5-Cre, previously thought to only label brown adipocytes, also labels some white adipocytes (31), and sex dependent differences were documented (32). Using a knockin approach, they found that ~31% of APCs in all depots except the male gonadal were Myf5-lin positive, which were instead marked by a Pax3 lineage, Pax3 being another myogenic transcription factor, upstream of Myf5. In contrast, less than 1% of the female gonadal APCs were Pax3-lin positive, demonstrating clear depot- and sex-dependent effects. Intriguingly, although APCs isolated from the anterior (dorsal) subcutaneous depot were a mix of Pax3- and Myf5-lin positive cells, all adipocytes from these depots had a Myf5-positive lineage, except at the ventral tip of this depot. These data implicate local factors in the adipogenic differentiation. This comprehensive survey of all of the major subcutaneous and intra-abdominal depots are consistent with other data in the field that examined fewer depots (16, 17, 33), and demonstrate clear heterogeneity in the origin of both APCs and adipocytes, and the sex-dependency of these effects.
Modulation of depot-dependent and lineage-specific adipose growth
Age and short-term high fat feeding influence the relative proportional of the two cell lineages (32). Myf5 positive-lin cells increased whereas the numbers of Pax3-lin is more stable. These studies suggest that changes in fat distribution reflect plasticity in the ‘types’ of adipose cells within a depot, and likely determine their functional properties e.g. metabolic, endocrine. They found that Myf5 positive-lin cells increased whereas the numbers of Pax3-lin is more stable. These studies suggest that changes in fat distribution reflect plasticity in the ‘types’ of adipose cells within a depot, and likely determine their functional properties.
With regard to the signaling mechanisms, deleting PTEN in Myf5-lin positive increases the adipocytes arising from Myf5-positive lineage and redistributes body fat: the brown (inter- and sub-scapular), anterior-subcutaneous and retroperitoneal depots expand while the posterior-subcutaneous, mesenteric and perigonadal depots disappear (34). Deleting IRβ in Myf5-lin results in the opposite phenotype (32). Jeffery et al found that modulating insulin signaling by deletion of AKT2 impaired hyperplasia of APCs in gonadal adipose tissue of male mice on high fat diet (17).
Are there cell autonomous differences in abdominal subcutaneous and visceral fat?
Studies from several laboratories suggest that APCs isolated from the omental differentiate less well than APCs from the abdominal subcutaneous depot (22, 35, 36). In mice, Macotela et al. found clear, cell autonomous differences in the differentiation potential of mouse subcutaneous (inguinal, lower body, mainly anterior subcutaneous) as compared to visceral (in the case of the mouse, intraabdominal gonadal) adipose tissue of males (37). They found that addition of bone morphogenic protein 4 (BMP4) could overcome the block in differentiation present in epididymal APCs cultures. As most groups find that human omental adipocytes, which are developmentally similar to epididymal/gonadal in the male mice (38), differentiate more poorly than abdominal subcutaneous ones, it is tempting to speculate that human omental/visceral APCs are similarly stalled. Although BMP2 and 4 are proadipogenic in human preadipocytes, few studies of depot differences in the expression and sensitivity to these important developmental regulators are available.
Remodeling of adipose tissues is critical for the maintenance of ‘healthy, insulin sensitive adipose tissue’
As mentioned, adipocytes like all other cell types do turnover, albeit slowly, and this allows the tissue to recover function that was disrupted as the result of the accumulation of older dysfunctional preadipocytes as well as adipocytes (10, 11). Identification, quantification and analysis of the pool of APCs in adult tissues that is responsible for normal remodeling will be critical for understanding the dynamics of cell turnover in human adipose tissues. A recent paper from Graff’s lab suggested that the progenitors responsible for remodeling differ from the pool that determined the early development of adipose tissues (39). How this pool is regulated with aging, obesity and fat distribution will be an important line of inquiry. It will allow understanding the factors that facilitate recruitment of new, insulin sensitive adipocytes to replace older, inflamed and insulin-resistant adipocytes.
Local factors may be the key to understanding APC self-renewal and recruitment
An important question to be resolved is whether depot-dependent variations in the proliferation and adipogenic potential of APCs are related to ‘intrinsic/genetic differences’ at the level of the adipose progenitor itself, or whether the variations in function are driven by differences in the extracellular matrix composition, differences in cell composition and hence paracrine and cell-cell interactions or physiologic factors such as innervation and blood flow (Figure 1). With regard to paracrine factors, it will be important for future studies to analyze depot-dependent differences in the expression of BMPs and their antagonists, including gremlin1, in APC populations of visceral and subcutaneous human adipose tissues (see review by Smith (46)).
Similarly, inflammatory cytokines such as TNFα have well-established inhibitory effects on adipogenesis, yet at low concentrations can act as growth factors (40). Consistent with this idea, a recent study from Scherer’s group showed that some inflammation is required to maintain healthy adipose tissue and prevent inflammation (5).
It seems likely that all multiple local factors interact and may vary over developmental stages, aging, hormone, nutrient or environmental exposures. Potentially, these exposures could have epigenetic effects that can be transmitted across generations to limit the capacity of adipose depots to undergo healthy remodeling and/or expansion.
Sex difference in the regulation of fat distribution: importance of sex chromosomes and hormones
Women have higher total body fat, most of which (80–90%) is stored in subcutaneous depots, and a more ‘pear-shaped’ fat distribution due to the expansion of lower body, gluteal-femoral fat depots. In contrast, men tend to have a more central obesity with preferential expansion of visceral fat depots (4, 12). Expansion of lower body subcutaneous depots in women is associated with protection from impairments in glucose-insulin homeostasis and hypertriglyceridemia (41, 42). In both sexes, elevated waist circumference is associated with higher risk of metabolic diseases, including type 2 diabetes (T2D), whereas high hip circumference has a protective effect, independent of total body fat (12). Additionally, abdominal subcutaneous fat also has negative consequences for health (43, 44). Elegant recent studies of a novel mouse model in which chromosomal sex is dissociated from gonadal sex (i.e. XX or XY mice with either male or female gonads) indicate that the presence of two X chromosomes is associated with two-fold higher body fat without a change in fat distribution, and independent of gonadal steroids (45, 46). These data suggest that the cellular mechanisms that modulate the growth and function of male and female adipocytes are driven by cell autonomous properties linked to chromosomal complement and its architecture (i.e. X-inactivation, genes on the Y chromosome, and epigenetic imprinting). Recent studies from our laboratory (41, 47) and others (48) are consistent with the hypothesis that adipocytes from different subcutaneous depots (abdominal vs. gluteal) are developmentally distinct in a partially sex-dependent manner, and that the depot differences are cell autonomous. However, no study to date has been powered to address the question of cell autonomous, sex-linked differences in adipose tissue biology.
Sex differences in subcutaneous adipose tissue metabolism in vivo and in vitro
As summarized in recent reviews, accumulating evidence documents differences in the metabolic and endocrine properties of male and female adipose tissues (12, 45). Studies from the Jensen lab indicate that compared to males, female lower body adipose tissues are more efficient at taking up triglyceride-fatty acids from meals via LPL, and free fatty acids directly from the circulation (49). As a consequence, lower body adipocytes of premenopausal women are larger, yet remain insulin sensitive (50). Thus, lower body fat of females is considered a ‘safe depot’ that can expand through the recruitment of APCs/preadipocytes to prevent ectopic fat deposition in muscle and liver as well as excess visceral adiposity. Furthermore, APCs are recruited to replace dysfunctional enlarged or old adipocytes (2, 51). It is also possible, as suggested by the transplant and lipectomy studies (52, 53) that lower body subcutaneous adipose tissue may secrete adipokines or other proteins that facilitate the usage of fuels systemically and such inter-organ communication could mediate effects of fat distribution on metabolic risk.
Differences in developmental gene expression were initially shown between subcutaneous and intraabdominal depots of both mice and humans. Differences were present in both isolated adipocyte and stromal vascular fractions (54). HOXA5, HOXC8, NR2F1, and thrombomodulin were higher in visceral fat, while SHOX2, EN1, GPC4 were higher in abdominal subcutaneous fat in both males and females. HOXC9 was higher in subcutaneous fat, but only in females (54). Furthermore, at least in mice, Shox2 is a regulator of lipolysis and of fat deposition specifically in the subcutaneous depots (55).
In a cohort of young, healthy men and women, we found that mRNA expression of 284 genes (2.6% of total) differed significantly between the abdominal and gluteal depots. Significantly more mRNAs were depot different in women (n = 159 genes or 56%) than men (n = 66 genes or 23%), and 9 (21%) were different in both sexes. The 59 genes that were depot specific in both sexes showed very highly significant associations with Gene Ontology terms related to embryonic development and pattern specification and included multiple homeobox (HOX) genes: HOXA2, HOXA3, HOXA4, HOXA5, HOXA9, HOXA10, HOXB7, HOXB8, HOXC8, HOXD4, and IRX2, transcription factors that drive embryonic development. HOXC13 was exclusively expressed in the gluteal depot. In addition, HOXA2, HOXB8 and HOXC13 were also sex-different (41). Searching for other depot-specific genes, we identified the long, non-coding HOTAIR as a gluteal-exclusive transcript (47). Similarly, Pinnick et al. (48) identified 887 genes that exhibited a depot and sex interaction using a cohort of 31 men and women with an average age of 48–50 and a wide range of BMI. These also included HOTAIR and multiple HOX genes and clustered in the GO term “sequence-specific DNA binding”. In addition, the depot-difference in HOTAIR persisted in primary cultures of preadipocytes and adipocytes and was a result of differential DNA methylation of the HOTAIR promoter (48). Although both studies showed that the depot differences in expression of HOX genes and HOTAIR were cell autonomous (47), they did not investigate whether sex differences were also cell autonomous. A global methylation analysis study identified 223 genes that were significantly differentially methylated between abdominal subcutaneous and gluteal fat. Of these, 25 also showed differential expression and involved multiple HOX genes (56). Taken together, as illustrated in Figure 2, these studies show that upper- and lower-body locations within the subcutaneous compartment are developmentally distinct, and provide a strong rationale for studies of the genetic and epigenetic control of adipose depot-dependent variations in gene expression.
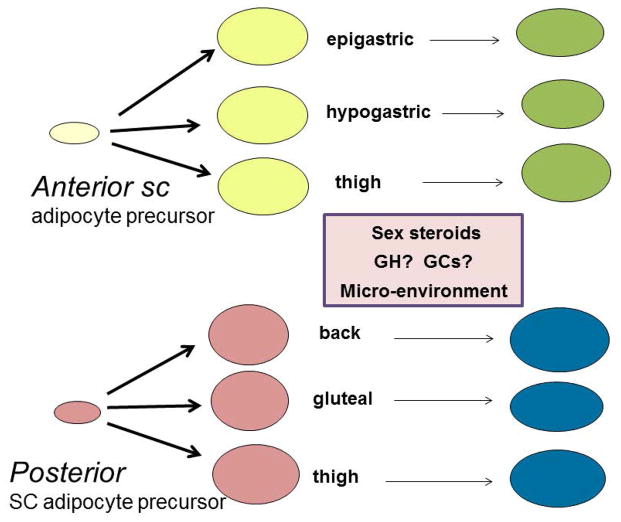
Figure 2. Different types of ‘white’ subcutaneous adipocytes.
The scheme illustrates that adipose progenitors isolated from different subcutaneous adipose depots retain their properties, likely dictated by the expression of different HOX genes and other developmental transcription factors that reflect the anterior or posterior anatomical origins. As they differentiate, their phenotype is influenced by the local microenvironment of the issue as well as exposure to paracrine and endocrine factors.
Limited data indicate that sex and depot differences are cell autonomous
A few studies used rodent fat transplantation approach to interrogate the potential cell autonomous effects of visceral and subcutaneous adipose tissue on systemic metabolism, with both agreements and controversies. Most studies agree that when subcutaneous fat was transplanted into intra-abdominal cavities, insulin sensitivity and metabolic phenotype improves, supporting the cell autonomous mechanism. But some studies found that when gonadal fat was transplanted into the intra-abdominal cavity, insulin sensitivity was improved (57, 58), while another found a slight trend in deteriorating insulin sensitivity (52). In a recent study, male gonadal fat was transplanted into portally-drained locations and recipient mice became insulin resistant, supporting the “portal theory” (59). Thus, both the anatomical locations as well as cell autonomous (intrinsic) properties have been found involved in the outcome of the transplantation and it is very context specific considering the potential relation between anatomical location and cell autonomous effects. Studies of female adipose tissue transplants are currently lacking, but have the potential to test the hypothesis that female and male subcutaneous adipose tissues have distinct phenotypes even when placed in a ‘neutral’ environment. Our preliminary studies (Wu, Lee, and Fried) suggest that when adipose tissue from the gonadal adipose tissues of males or females, or subcutaneous adipose tissue of males was transplanted into the subcutaneous compartment of males, adipocyte size is maintained, but female subcutaneous adipocytes become smaller.
Available studies on sex differences in adipogenesis are limited and underpowered
A study from Tchoukalova et al of preadipocytes isolated from non-obese men and women suggested that female adipose tissue contains 10% more of early stage preadipocytes in the stromal vascular fraction of abdominal subcutaneous and 35% more in the femoral depot (aP2+/CD68− population) (60, 61). Furthermore, they observed a poorer differentiation capacity of femoral but not gluteal preadipocytes in men as indicated by a significant depot x sex interaction for PPARγ expression after differentiation. Paradoxically, they noted that in women femoral adipocytes differentiate less well than abdominal subcutaneous (62), similar to our results comparing gluteal and abdominal ASC cultures (Lee, Karastergiou and Fried, unpublished observation).
A recent study implicated WNT signaling as a molecular pathway mediating depot-differences in adipogenesis. Subjects with rare, gain of function mutations in low-density-lipoprotein receptor-related protein 5 (LRP5) which serves as a WNT co-receptor show high bone mass, increased lower-body fat accumulation and improved insulin sensitivity. In vitro, LRP5 knockdown inhibits WNT signaling in human preadipocytes; in addition, LRP5 knockdown suppressed proliferation of both abdominal and gluteal preadipocytes, and differentiation preferentially in gluteal cells (63).
Integrin and glucocorticoid signaling pathways have been implicated in the regulation of adipogenesis specifically in visceral preadipocytes. IGFBP2 is highly secreted by visceral adipocytes in vitro and suppresses adipogenesis preferentially in visceral compared to subcutaneous preadipocytes, not through IGF-I but via integrin pathways (64). Glucocorticoids increase the expression of the proadipogenic factor, LIM domain only 3 (LMO3) in human but not murine adipocytes; LMO3 is in turn higher in visceral fat and may thus be implicated in the development of central obesity (65).
Influence of sex steroids on adipose tissue development and remodeling
As reviewed by Clegg and colleagues (66), in vivo evidence indicates that estrogen inhibits central fat deposition and may thereby contribute to alterations in fat distribution after menopause. Evidence for direct effects of estrogen on human preadipocytes or adipocytes is limited. Whereas in male mice, high fat induced obesity leads to adipose tissue inflammation, fibrosis and dysfunction, female mice are protected from this effect. Deletion of the estrogen receptor (ER)-α selectively in adipose tissues shows that estrogen plays a critical role in this protection. Most interestingly, adipose specific deletion of ERα increases total body fat due to an increase in the mass of the gonadal adipose tissue in female mice, due to adipocyte hypertrophy, but does not affect adiposity in males. A recent study showed that ERα is necessary for promoting the identity and differentiation of white adipose progenitor cells (33).
Sex differences in browning/britening of white adipocytes?
Limited data indicate that women have a greater quantity of brown-like cells (PET positive in vivo) (67) and a greater ‘britening’ response in white adipose (68). Although it is possible to convert APCs to a brite/beige phenotype (69), no studies have directly addressed the question of whether the number of beige precursors or ‘brite’ cells differs by sex in humans or even in mice.
Functional consequences of the developmental origin of adipocytes
An important question is whether there are functional differences in adipocytes within a given adipose depot are associated with different lineages. Remarkably, the Pax3-lin positive cells mature within the male perigonadal depot (epididymal) were smaller than the Myf5-lin positive ones (32). The authors speculated that the lineage of these cells determines their size, but mechanisms involved are yet to be elucidated. The data on retroperitoneal and mesenteric depots also showed discrepancies in the lineages, suggesting that heterogeneity of the size of adipocytes within a depot is related to developmental origins, and could be considered different ‘types’ of adipocytes.
Are lessons from mouse models applicable to humans?
Whereas most of total body fat in humans is located in subcutaneous compartments, in mice, most is intra-abdominal and a subset in visceral depots. Analysis of the transcriptome of human omental and mouse epididymal adipose tissues show similarities (38), suggesting the latter is an acceptable model. Both depots are found overlaying the gut, suggesting common functions as an immune barrier to ‘leaky gut’ (5). On the other hand, human males do not have a fat depot associated with the epididymis/testes and human females do not have a ‘parametrial’ fat depot in close proximity to the uterus and ovaries. With regard to subcutaneous depots, Cinti has recently suggested that female anterior and posterior adipose tissues of the mouse should be defined as ‘pink’, i.e they respond to pregnancy (70). His data suggest that epithelial cells are derived from ‘white’ adipocytes. The importance of this observation for the subcutaneous adipose tissue of the breast remains to be determined. In addition, the femoral adipose tissue of women may have a special role in supplying fatty acids for energy during lactation (71). Thus, defining the homologous fat depots of mice and human will be important for a full understanding to the diversity and specialization of depots within the adipose organ.
The role of the depot microenvironment
The cellular composition and extracellular matrix of different depots varies substantially and is likely a source for depot-dependent variations in growth, remodeling, metabolic and endocrine function. For example, the visceral (omental) depot as well as the epicardial adipose tissues of humans (but not mice) express the depot-specific adipokine omentin which has insulin-sensitizing properties (72). Well-documented differences in immune cell populations, including macrophages with varying levels of activation, B cells, T cells in visceral compared to subcutaneous tissue, undoubtedly influence adipogenesis and adipocyte function. Also, differences in the expression of inflammatory cytokines and growth factors (and their binding proteins) vary between abdominal and gluteal, and gluteal and thigh adipocytes likely reflect variations in cell composition, although this is less studied in the literature. Depot differences in expression of collagens and other extracellular matrix proteins are evident from analyses of the transcriptome of each depot, and in proteomic studies (73, 74). These differences are important for determining the activation and locally available concentrations of growth factors. Clear differences in the stiffness of the extracellular matrix of visceral and subcutaneous adipose tissue can be detected (75). As reviewed recently (76), the level of angiogenesis and hence blood flow, to different adipose tissues determine susceptibility to hypoxia within different depots and in response to overnutrition. The topic of how innervation of adipose tissue influences the proliferation and differentiation of adipose progenitors as well as having direct effects on lipolysis and blood flow is also critical to understanding the depot-specific biology of adipose tissue (3).
Conclusion
Rapid advances in our knowledge of the development of different anatomical depots point to the conclusion that they derive from different lineages. In addition, the HOX code of different depots ensures that adipose depots from anterior, posterior, and head to toe have a system to ‘remember’ where they were born, so that the remodeled tissue will retain the same functional properties. How HOX transcription factors interact with other transcription factors to determine the functional capacities (growth, metabolism) of specific adipose tissues is a key challenge for future research.
It is clear that we should be discussing ‘shades of white’ just as we have been discussing ‘shades of brown’ (77). In addition, analyses of multiple major and minor depots are essential for understanding the biology of the adipose tissues. Global analyses of depot differences in the transcriptome, miRNAs and lncRNA and proteome are pointing to the presence of multiple cell types within different adipose depots. The cellular composition within specific depots undoubtedly has the potential to switch the balance of pro- and anti-adipogenic factors during normal growth and development, and in states of energy balance and imbalance.
What major reviews have already been published on this subject?
A number of excellent recent reviews focus on depot differences in adipose tissue function.
Several reviews address sex differences in adipose biology
Several recent reviews focus on the development of white, beige, and brown adipocytes.
What does your review add?
This review integrates recent advances in knowledge of the developmental origins of rodent and human adipose tissues.
We highlight new advances and gaps in knowledge of depot- and sex-differences in human adipose tissues.
We highlight the question of whether depot- and sex-differences are cell-autonomous.
Acknowledgments
We would like to thank Steven R. Smith for many discussions of sex and depot differences in adipose biology and being a superb collaborator. This work was supported by NIH grants P30DK-46200, RO1-080448 and a grant from the Society for Women’s Health Research. We apologize for omitting many excellent primary references due to limitations on the reference numbers in this review..
Footnotes
The authors declare no conflict of interest.
References
- 1.Knittle JL, Timmers K, Ginsberg-Fellner F, Brown RE, Katz DP. The growth of adipose tissue in children and adolescents. Cross-sectional and longitudinal studies of adipose cell number and size. J Clin Invest. 1979;63(2):239–46. doi: 10.1172/JCI109295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Spalding KL, Arner E, Westermark PO, et al. Dynamics of fat cell turnover in humans. Nature. 2008;453(7196):783–7. doi: 10.1038/nature06902. [DOI] [PubMed] [Google Scholar]
- 3.Lee MJ, Wu Y, Fried SK. Adipose tissue heterogeneity: implication of depot differences in adipose tissue for obesity complications. Mol Aspects Med. 2013;34(1):1–11. doi: 10.1016/j.mam.2012.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Karpe F, Pinnick KE. Biology of upper-body and lower-body adipose tissue--link to whole-body phenotypes. Nat Rev Endocrinol. 2015;11(2):90–100. doi: 10.1038/nrendo.2014.185. [DOI] [PubMed] [Google Scholar]
- 5.Wernstedt AI, Tao C, Morley TS, et al. Adipocyte inflammation is essential for healthy adipose tissue expansion and remodeling. Cell Metab. 2014;20(1):103–18. doi: 10.1016/j.cmet.2014.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grant RW, Dixit VD. Adipose tissue as an immunological organ. Obesity (Silver Spring) 2015;23(3):512–8. doi: 10.1002/oby.21003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shungin D, Winkler TW, Croteau-Chonka DC, et al. New genetic loci link adipose and insulin biology to body fat distribution. Nature. 2015;518(7538):187–96. doi: 10.1038/nature14132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heid IM, Jackson AU, Randall JC, et al. Meta-analysis identifies 13 new loci associated with waist-hip ratio and reveals sexual dimorphism in the genetic basis of fat distribution. Nat Genet. 2010;42(11):949–60. doi: 10.1038/ng.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hilton C, Karpe F, Pinnick KE. Role of developmental transcription factors in white, brown and beige adipose tissues. Biochim Biophys Acta. 2015 doi: 10.1016/j.bbalip.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Leibel RL, Edens NK, Fried SK. Physiologic basis for the control of body fat distribution in humans. Annu Rev Nutr. 1989;9:417–43. doi: 10.1146/annurev.nu.09.070189.002221. [DOI] [PubMed] [Google Scholar]
- 11.Morgan-Bathke M, Chen L, Oberschneider E, Harteneck D, Jensen MD. Sex and Depot Differences in ex vivo Adipose Tissue Fatty Acid Storage and Glycerol-3-phosphate acyltransferase Activity. Am J Physiol Endocrinol Metab. 2015 doi: 10.1152/ajpendo.00424.2014. ajpendo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karastergiou K, Smith SR, Greenberg AS, Fried SK. Sex differences in human adipose tissues - the biology of pear shape. Biol Sex Differ. 2012;3(1):13. doi: 10.1186/2042-6410-3-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Faust IM, Johnson PR, Stern JS, Hirsch J. Diet-induced adipocyte number increase in adult rats: a new model of obesity. Am J Physiol. 1978;235(3):E279–E286. doi: 10.1152/ajpendo.1978.235.3.E279. [DOI] [PubMed] [Google Scholar]
- 14.Strissel KJ, Stancheva Z, Miyoshi H, et al. Adipocyte death, adipose tissue remodeling, and obesity complications. Diabetes. 2007;56(12):2910–8. doi: 10.2337/db07-0767. [DOI] [PubMed] [Google Scholar]
- 15.Wang QA, Scherer PE, Gupta RK. Improved methodologies for the study of adipose biology: insights gained and opportunities ahead. J Lipid Res. 2014;55(4):605–24. doi: 10.1194/jlr.R046441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang QA, Tao C, Gupta RK, Scherer PE. Tracking adipogenesis during white adipose tissue development, expansion and regeneration. Nat Med. 2013;19(10):1338–44. doi: 10.1038/nm.3324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jeffery E, Church CD, Holtrup B, Colman L, Rodeheffer MS. Rapid depot-specific activation of adipocyte precursor cells at the onset of obesity. Nat Cell Biol. 2015 doi: 10.1038/ncb3122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grove KL, Fried SK, Greenberg AS, Xiao XQ, Clegg DJ. A microarray analysis of sexual dimorphism of adipose tissues in high-fat-diet-induced obese mice. Int J Obes (Lond) 2010 doi: 10.1038/ijo.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fried SK, Kral JG. Sex differences in regional distribution of fat cell size and lipoprotein lipase activity in morbidly obese patients. Int J Obes. 1987;11(2):129–40. [PubMed] [Google Scholar]
- 20.Tchernof A, Belanger C, Morisset AS, et al. Regional differences in adipose tissue metabolism in women: minor effect of obesity and body fat distribution. Diabetes. 2006;55(5):1353–60. doi: 10.2337/db05-1439. [DOI] [PubMed] [Google Scholar]
- 21.McLaughlin T, Lamendola C, Coghlan N, et al. Subcutaneous adipose cell size and distribution: relationship to insulin resistance and body fat. Obesity (Silver Spring) 2014;22(3):673–80. doi: 10.1002/oby.20209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lessard J, Laforest S, Pelletier M, Leboeuf M, Blackburn L, Tchernof A. Low abdominal subcutaneous preadipocyte adipogenesis is associated with visceral obesity, visceral adipocyte hypertrophy, and a dysmetabolic state. Adipocyte. 2014;3(3):197–205. doi: 10.4161/adip.29385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gustafson B, Hedjazifar S, Gogg S, Hammarstedt A, Smith U. Insulin resistance and impaired adipogenesis. Trends Endocrinol Metab. 2015 doi: 10.1016/j.tem.2015.01.006. [DOI] [PubMed] [Google Scholar]
- 24.Tchoukalova YD, Votruba SB, Tchkonia T, Giorgadze N, Kirkland JL, Jensen MD. Regional differences in cellular mechanisms of adipose tissue gain with overfeeding. Proc Natl Acad Sci U S A. 2010;107(42):18226–31. doi: 10.1073/pnas.1005259107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Berry R, Jeffery E, Rodeheffer MS. Weighing in on adipocyte precursors. Cell Metab. 2014;19(1):8–20. doi: 10.1016/j.cmet.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berry DC, Stenesen D, Zeve D, Graff JM. The developmental origins of adipose tissue. Development. 2013;140(19):3939–49. doi: 10.1242/dev.080549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee YH, Mottillo EP, Granneman JG. Adipose tissue plasticity from WAT to BAT and in between. Biochim Biophys Acta. 2014;1842(3):358–69. doi: 10.1016/j.bbadis.2013.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tang W, Zeve D, Suh JM, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322(5901):583–6. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hudak CS, Gulyaeva O, Wang Y, et al. Pref-1 marks very early mesenchymal precursors required for adipose tissue development and expansion. Cell Rep. 2014;8(3):678–87. doi: 10.1016/j.celrep.2014.06.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chau YY, Bandiera R, Serrels A, et al. Visceral and subcutaneous fat have different origins and evidence supports a mesothelial source. Nat Cell Biol. 2014;16(4):367–75. doi: 10.1038/ncb2922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sanchez-Gurmaches J, Hung CM, Sparks CA, Tang Y, Li H, Guertin DA. PTEN loss in the Myf5 lineage redistributes body fat and reveals subsets of white adipocytes that arise from Myf5 precursors. Cell Metab. 2012;16(3):348–62. doi: 10.1016/j.cmet.2012.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sanchez-Gurmaches J, Guertin DA. Adipocytes arise from multiple lineages that are heterogeneously and dynamically distributed. Nat Commun. 2014;5:4099. doi: 10.1038/ncomms5099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lapid K, Lim A, Clegg DJ, Zeve D, Graff JM. Oestrogen signalling in white adipose progenitor cells inhibits differentiation into brown adipose and smooth muscle cells. Nat Commun. 2014;5:5196. doi: 10.1038/ncomms6196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Seale P, Bjork B, Yang W, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454(7207):961–7. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tchkonia T, Giorgadze N, Pirtskhalava T, et al. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006;55(9):2571–8. doi: 10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- 36.Isakson P, Hammarstedt A, Gustafson B, Smith U. Impaired preadipocyte differentiation in human abdominal obesity: role of Wnt, tumor necrosis factor-alpha, and inflammation. Diabetes. 2009;58(7):1550–7. doi: 10.2337/db08-1770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Macotela Y, Emanuelli B, Mori MA, et al. Intrinsic differences in adipocyte precursor cells from different white fat depots. Diabetes. 2012;61(7):1691–9. doi: 10.2337/db11-1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gesta S, Bluher M, Yamamoto Y, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A. 2006;103(17):6676–81. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang Y, Berry DC, Tang W, Graff JM. Independent stem cell lineages regulate adipose organogenesis and adipose homeostasis. Cell Rep. 2014;9(3):1007–22. doi: 10.1016/j.celrep.2014.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kras KM, Hausman DB, Martin RJ. Tumor necrosis factor-alpha stimulates cell proliferation in adipose tissue-derived stromal-vascular cell culture: promotion of adipose tissue expansion by paracrine growth factors. Obes Res. 2000;8(2):186–93. doi: 10.1038/oby.2000.20. [DOI] [PubMed] [Google Scholar]
- 41.Karastergiou K, Fried SK, Xie H, et al. Distinct developmental signatures of human abdominal and gluteal subcutaneous adipose tissue depots. J Clin Endocrinol Metab. 2013;98(1):362–71. doi: 10.1210/jc.2012-2953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Amati F, Pennant M, Azuma K, et al. Lower thigh subcutaneous and higher visceral abdominal adipose tissue content both contribute to insulin resistance. Obesity (Silver Spring) 2012;20(5):1115–7. doi: 10.1038/oby.2011.401. [DOI] [PubMed] [Google Scholar]
- 43.Nielsen S, Guo Z, Johnson CM, Hensrud DD, Jensen MD. Splanchnic lipolysis in human obesity. J Clin Invest. 2004;113(11):1582–8. doi: 10.1172/JCI21047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Jensen MD. Role of body fat distribution and the metabolic complications of obesity. J Clin Endocrinol Metab. 2008;93(11 Suppl 1):S57–S63. doi: 10.1210/jc.2008-1585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Link JC, Chen X, Arnold AP, Reue K. Metabolic impact of sex chromosomes. Adipocyte. 2013;2(2):74–9. doi: 10.4161/adip.23320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Chen X, McClusky R, Chen J, et al. The number of x chromosomes causes sex differences in adiposity in mice. PLoS Genet. 2012;8(5):e1002709. doi: 10.1371/journal.pgen.1002709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Divoux A, Karastergiou K, Xie H, et al. Identification of a novel lncRNA in gluteal adipose tissue and evidence for its positive effect on preadipocyte differentiation. Obesity (Silver Spring) 2014;22(8):1781–5. doi: 10.1002/oby.20793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pinnick KE, Nicholson G, Manolopoulos KN, et al. Distinct developmental profile of lower-body adipose tissue defines resistance against obesity-associated metabolic complications. Diabetes. 2014;63(11):3785–97. doi: 10.2337/db14-0385. [DOI] [PubMed] [Google Scholar]
- 49.Shadid S, Koutsari C, Jensen MD. Direct free fatty acid uptake into human adipocytes in vivo: relation to body fat distribution. Diabetes. 2007;56(5):1369–75. doi: 10.2337/db06-1680. [DOI] [PubMed] [Google Scholar]
- 50.Johnson JA, Fried SK, Pi-Sunyer FX, Albu JB. Impaired insulin action in subcutaneous adipocytes from women with visceral obesity. Am J Physiol Endocrinol Metab. 2001;280(1):E40–E49. doi: 10.1152/ajpendo.2001.280.1.E40. [DOI] [PubMed] [Google Scholar]
- 51.Tchkonia T, Thomou T, Zhu Y, et al. Mechanisms and metabolic implications of regional differences among fat depots. Cell Metab. 2013;17(5):644–56. doi: 10.1016/j.cmet.2013.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tran TT, Yamamoto Y, Gesta S, Kahn CR. Beneficial effects of subcutaneous fat transplantation on metabolism. Cell Metab. 2008;7(5):410–20. doi: 10.1016/j.cmet.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Shi H, Strader A, Woods SC, Seeley RJ. The Effect of Fat Removal on Glucose Tolerance is Depot Specific in Male and Female Mice. Am J Physiol Endocrinol Metab. 2007 doi: 10.1152/ajpendo.00649.2006. [DOI] [PubMed] [Google Scholar]
- 54.Gesta S, Bluher M, Yamamoto Y, et al. Evidence for a role of developmental genes in the origin of obesity and body fat distribution. Proc Natl Acad Sci U S A. 2006;103(17):6676–81. doi: 10.1073/pnas.0601752103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee KY, Yamamoto Y, Boucher J, et al. Shox2 is a molecular determinant of depot-specific adipocyte function. Proc Natl Acad Sci U S A. 2013;110(28):11409–14. doi: 10.1073/pnas.1310331110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gehrke S, Brueckner B, Schepky A, et al. Epigenetic regulation of depot-specific gene expression in adipose tissue. PLoS One. 2013;8(12):e82516. doi: 10.1371/journal.pone.0082516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Konrad D, Rudich A, Schoenle EJ. Improved glucose tolerance in mice receiving intraperitoneal transplantation of normal fat tissue. Diabetologia. 2007;50(4):833–9. doi: 10.1007/s00125-007-0596-1. [DOI] [PubMed] [Google Scholar]
- 58.Foster MT, Shi H, Softic S, Kohli R, Seeley RJ, Woods SC. Transplantation of non-visceral fat to the visceral cavity improves glucose tolerance in mice: investigation of hepatic lipids and insulin sensitivity. Diabetologia. 2011 doi: 10.1007/s00125-011-2259-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Rytka JM, Wueest S, Schoenle EJ, Konrad D. The portal theory supported by venous drainage-selective fat transplantation. Diabetes. 2011;60(1):56–63. doi: 10.2337/db10-0697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tchoukalova YD, Koutsari C, Votruba SB, et al. Sex- and depot-dependent differences in adipogenesis in normal-weight humans. Obesity (Silver Spring) 2010;18(10):1875–80. doi: 10.1038/oby.2010.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.White UA, Tchoukalova YD. Sex dimorphism and depot differences in adipose tissue function. Biochim Biophys Acta. 2014;1842(3):377–92. doi: 10.1016/j.bbadis.2013.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tchoukalova Y, Koutsari C, Jensen M. Committed subcutaneous preadipocytes are reduced in human obesity. Diabetologia. 2007;50(1):151–7. doi: 10.1007/s00125-006-0496-9. [DOI] [PubMed] [Google Scholar]
- 63.Loh NY, Neville MJ, Marinou K, et al. LRP5 Regulates Human Body Fat Distribution by Modulating Adipose Progenitor Biology in a Dose- and Depot-Specific Fashion. Cell Metab. 2015;21(2):262–72. doi: 10.1016/j.cmet.2015.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Yau SW, Russo VC, Clarke IJ, Dunshea FR, Werther GA, Sabin MA. IGFBP-2 inhibits adipogenesis and lipogenesis in human visceral, but not subcutaneous, adipocytes. Int J Obes (Lond) 2014 doi: 10.1038/ijo.2014.192. [DOI] [PubMed] [Google Scholar]
- 65.Lindroos J, Husa J, Mitterer G, et al. Human but not mouse adipogenesis is critically dependent on LMO3. Cell Metab. 2013;18(1):62–74. doi: 10.1016/j.cmet.2013.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Palmer BF, Clegg DJ. The sexual dimorphism of obesity. Mol Cell Endocrinol. 2015;402C:113–9. doi: 10.1016/j.mce.2014.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cypess AM, Lehman S, Williams G, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360(15):1509–17. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Scalzo RL, Peltonen GL, Giordano GR, et al. Regulators of human white adipose browning: evidence for sympathetic control and sexual dimorphic responses to sprint interval training. PLoS One. 2014;9(6):e90696. doi: 10.1371/journal.pone.0090696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wu J, Cohen P, Spiegelman BM. Adaptive thermogenesis in adipocytes: is beige the new brown? Genes Dev. 2013;27(3):234–50. doi: 10.1101/gad.211649.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giordano A, Smorlesi A, Frontini A, Barbatelli G, Cinti S. White, brown and pink adipocytes: the extraordinary plasticity of the adipose organ. Eur J Endocrinol. 2014;170(5):R159–R171. doi: 10.1530/EJE-13-0945. [DOI] [PubMed] [Google Scholar]
- 71.Rebuffe-Scrive M, Enk L, Crona N, et al. Fat cell metabolism in different regions in women. Effect of menstrual cycle, pregnancy, and lactation. J Clin Invest. 1985;75(6):1973–6. doi: 10.1172/JCI111914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Yang RZ, Xu AH, Pray J, et al. Cloning of omentin, a new adipocytokine from omental fat tissue in humans. Diabetes. 2003;52:A1. [Google Scholar]
- 73.Liu M, Guo L, Liu Y, et al. Adipose stromal-vascular fraction-derived paracrine factors regulate adipogenesis. Mol Cell Biochem. 2014;385(1–2):115–23. doi: 10.1007/s11010-013-1820-6. [DOI] [PubMed] [Google Scholar]
- 74.Hoggard N, Cruickshank M, Moar KM, Bashir S, Mayer CD. Using gene expression to predict differences in the secretome of human omental vs. subcutaneous adipose tissue. Obesity (Silver Spring) 2012;20(6):1158–67. doi: 10.1038/oby.2012.14. [DOI] [PubMed] [Google Scholar]
- 75.Lackey DE, Burk DH, Ali MR, et al. Contributions of adipose tissue architectural and tensile properties toward defining healthy and unhealthy obesity. Am J Physiol Endocrinol Metab. 2014;306(3):E233–E246. doi: 10.1152/ajpendo.00476.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Sun K, Tordjman J, Clement K, Scherer PE. Fibrosis and adipose tissue dysfunction. Cell Metab. 2013;18(4):470–7. doi: 10.1016/j.cmet.2013.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kiefer FW, Cohen P, Plutzky J. Fifty shades of brown: perivascular fat, thermogenesis, and atherosclerosis. Circulation. 2012;126(9):1012–5. doi: 10.1161/CIRCULATIONAHA.112.123521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cawthorn WP, Scheller EL, Macdougald OA. Adipose tissue stem cells meet preadipocyte commitment: going back to the future. J Lipid Res. 2012;53(2):227–46. doi: 10.1194/jlr.R021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Sethi JK. Activatin’ human adipose progenitors in obesity. Diabetes. 2010;59(10):2354–7. doi: 10.2337/db10-0923. [DOI] [PMC free article] [PubMed] [Google Scholar]