Abstract
Introduction
Odontogenic fibroma (OF), a rare odontogenic tumor of mesodermal origin, has been thought to originate from either dental follicle, periodontal ligament, or dental papilla [1]. Different studies reported high variability in the incidence rate as being between 3 and 23% of all odontogenic tumors [2,3]. OF manifests a dual character at the histopathological examination showing odontogenic epithelial structures mimicking those observed in biopsy of ameloblastoma and, in addition, peculiar fragments of cellular stroma. The clinical and radiological features of OF are similar to other odontogenic and/or non-odontogenic tumours and the differential diagnosis may first occur at fine-needle aspiration biopsy.
Presentation of case
In the case reported, a young patient showed a localized gingival enlargement involving radiologically the superior margin of the right angle of the mandible and associated with an un-erupted tooth. The morphological characteristics together with clinical and radiologic findings confirmed the tumor to be a central odontogenic fibroma (COF) with secondary gingival involvement.
Discussion and conclusion
Benign odontogenic tumors may be distinguished from other odontogenic/non-odontogenic neoplasias and from malignant tumours through a cytologic differential diagnosis as treatment differs accordingly.
Keywords: Odontogenic tumor, Differential diagnosis
Highlights
-
•
Attention to any gingival enlargement: it may be a clinical COF manifestation.
-
•
A wrong diagnosis may determine a serious delay in patient proper treatment.
-
•
It is highly recommended a periodic clinical and radiographic examination.
-
•
A careful cyto-histological examination of fibrous lesions of the jaw is necessary.
-
•
A proper differential diagnosis will allow the correct patient management.
1. Introduction
Odontogenic fibroma (OF) is a rare tumor of odontogenic origin, with variable percentages of incidence [2], [3], regarded by the World Health Organization (WHO) as a benign odontogenic neoplasm derived from mesenchymal odontogenic tissue [4], [5].
The lesion occurs most commonly in the mandible although several cases have been reported in the maxilla [6]. It has been reported in patients ranging in age from 11 to 80 years with a mean age of 34 years. Daley et al. reported in a literature review a slight female predominance [7]. The lesion grows slowly, in an asymptomatic manner. On radiographs OF can appear as a single well-defined radiolucency, or as a multilocular lesion frequently associated with unerupted or displaced teeth.
OF is characterized by varying amount of inactive-looking odontogenic epithelium embedded in a mature relatively dense collagenous fibrous stroma. Most pathologists believe that the absence of odontogenic epithelium does not preclude a diagnosis of odontogenic fibroma [1], [2], [8].
Wesley et al. had the merit to clarify the nature of the tumor, differentiating it from other pathological entities as odontogenic myxoma, hyperplastic dental follicle, and other fibro-osseous lesions [1].
Gardner has identified three histological separate variants of the OF: the hyperplastic dental follicle or fibrous hyperplasia, the epithelium-poor type (simple type) and the epithelium-rich type (complex or WHO-type) [8].
The hyperplastic dental follicle is composed of fibrous connective tissue with scattered focal nests of odontogenic epithelium, smaller than the epithelial islands found in ameloblastomas or ameloblastic fibromas and without stellate reticulum and signs of cellular polarization.
The simple type is minimally cellular with delicate collagen fibers, a considerable amount of fibromyxoid matrix and only scattered and small remnants of inactive-looking odontogenic epithelium. The WHO type is more complex and consists of cellular mature fibrous connective tissue in which sparse or often conspicuous strands of odontogenic epithelium are found, together with calcified tissue either in the form of dysplastic dentin, or cementum-like material.
Topographically, two variants can be distinguished: extraosseous or peripheral type (POF) and an intraosseous or central type (COF) [4], [5]. Table 1 summarizes the different classifications of OF [5], [8], [9], [10].
Table 1.
Different classifications of OF.
| Histological classifications | ||||
| Gardner, 1980 [8] | Hyperplastic dental follicle | Simple type fibrous neoplasm with collagenous fibrous connective tissue containing inactive-looking odontogenic epithelium | Complex or WHO-type lesion with dysplastic dentine or tissue like cementum, fibrous tissue with myxoid area and sparse or often conspicuous inactive looking odontogenic epithelium | |
| Lukinmaa PL et al., 1990 [9]; Langlais et al., 1995 [10] | Simple type | Complex or WHO type | Granular cell type | |
| WHO, 2005 [5] | Epithelium-poor type | Epithelium-rich type | ||
| Topographical classification | |
| Intra-osseous or central | Extra-osseous or peripheral |
Herein we presented the clinical, radiological and morphologic features of a particular case of central odontogenic fibroma (COF) with secondary gingival involvement, with the aim to reinforce the concept of a necessary multidisciplinary diagnostic approach for all gingival growths, also those ones seemingly as innocuous lesions.
2. Presentation of case
A 12-years-old boy presented with a slow-growing, asymptomatic gingival enlargement distal to the mandibular right first molar. His medical, surgical, family, and social histories were unremarkable. Oral examination showed a soft swelling at the lower right second molar region. The overlying mucosa was normal. There was no cervical lymph-node involvement.
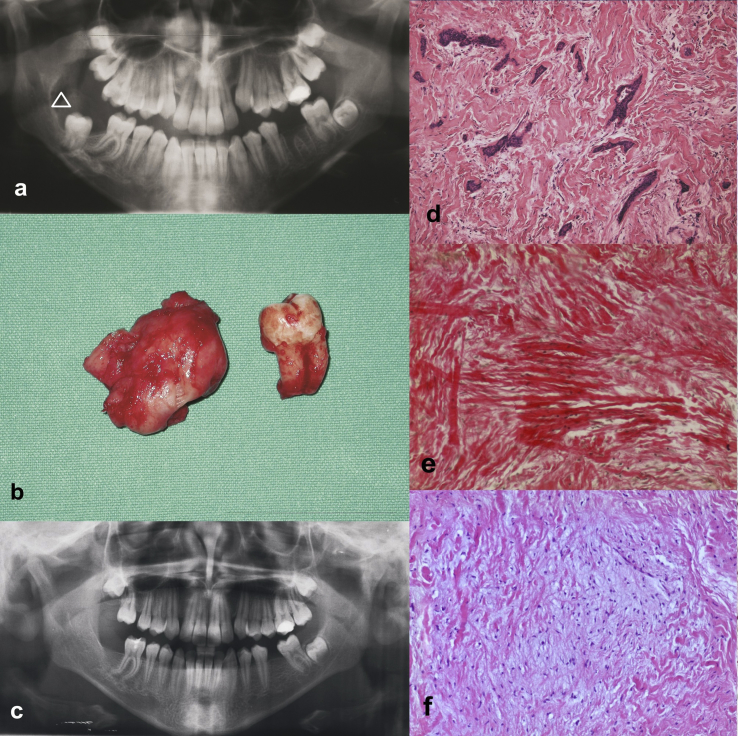
A panorex (Fig. 1A) showed a large uni-locular, radiolucent lesion localized at the superior margin of the right angle of the mandible, limited by a sclerotic border and in relation to an impacted second molar. A subsequent CT scan confirmed an heterogeneous mass eroding the superior margin of the right mandibular angle with a significant enlargement of the overlying mucosa. The patient was referred for a fine-needle aspiration (FNA) biopsy. This procedure was performed via several passes with a 22-gauge needle through transmucosal sampling of the mass. Both bloody and viscous tan material was obtained. Citological examination showed cohesive sheets of epithelial cells and stromal component consisting in of cellular fibroblasts with blurred borders. The diagnosis of possible benign odontogenic tumor was hypothesized, and, consequently, conservative enucleation of the lesion, extraction of the impacted molar and curettage of the surrounding tissues were performed under general anaesthesia. During the surgery, the lesion appears as an expansion of the cortical plate of the mandible, it was easily removed, not showing any adherence to bone, the inferior alveolar nerve was preserved and the patient retained normal sensation. In this case, no guided bone regeneration has been performed.
Fig. 1.
Radiographic and morphologic features of a case of central odontogenic fibroma with secondary gingival involvement. A. Panorex showing an uni-locular lesion localized at the superior margin of the right angle of the mandible and limited by sclerotic border (arrowhead). B. Resected lesion with impacted tooth. C. Panorex at 12 months after surgery showing bone healing with no evidence of recurrence. D. Tissue section showing tubules, nests and irregular branched strands of epithelial cells in an fibrous connective background with focal myxoid areas (haematoxylin-eosin stain, original magnification ×200). E. Van Gieson staining showing fibrous tissue (original magnification ×200). F. Haematoxylin-eosin stain showing areas of myxoid tissue (haematoxylin-eosin stain, original magnification ×200).
The surgical specimen was fixed in 10% neutral buffered formalin and sent for histological examination. Grossly, it measured 3 × 4 × 3 cm, and showed smooth whitish appearance with circumscribed outline (Fig. 1B).
Microscopically, tissue sections exhibited nests and irregular branched strands of inactive epithelial cells in a densely fibrous, moderately cellular, and only focally myxoid connective background (Fig. 1D, F). The epithelial tumor component was characterized by serpentine strands and islands of odontogenic epithelium, without palisading aspects and stellate reticulum formation, surrounded by fibrous stroma with fascicular configuration. Tumor stroma consisted of plump oval to spindle cells in prevalently mature collagen tissue highlighted by Van Gieson staining (Fig. 1E). Only focal trabeculae of calcified material or bone have been detected.
The microscopic characteristic together with clinical and radiologic findings confirmed the tumor to be central odontogenic fibroma (COF) with secondary gingival involvement during its expansion.
Healing and postoperative course were uneventful, the clinical examination showed a normal alignment of the teeth and the patient was discharged for further follow-up. One year after surgery, there were no signs of recurrence and both bone and soft tissue healing was satisfactory (Fig. 1C).
3. Discussion
Odontogenic fibroma is a rarely reported lesion of mesenchyme and odontogenic ecto-mesenchymal origin exhibiting a slow clinical growth. The World Health Organization (WHO) defined it as a benign odontogenic neoplasm of fibroblastic origin characterized by relative mature collagenous fibrous tissue with or without varying amounts of embedded inactive-looking odontogenic epithelium with potential to occur in either a central or an extraosseous location [5]. The extraosseous counterpart is designated as peripheral odontogenic fibroma (POF). The clinical-pathological differences and similarities between POF and COF are shown below.
3.1. Peripheral odontogenic fibroma (POF)
In the past, peripheral odontogenic fibroma (POF) has been improperly designed as odontogenic gingival epithelial hamartoma [11], peripheral fibroblastic dentinom [12], odontogenic epithelial hamartoma [13], peripheral ossifying fibroma.
In 1982 Gardner published a clarification in terminology and finally explained the histopathological and biological differences existing between POF and peripheral ossifying fibroma [14]. POF, as benign odontogenic neoplasm of the periodontal soft tissues, is more common than reported previously and even more frequent than its central counterpart with a 1.4:1 ratio, accounting for 51.1% of the total peripheral odontogenic tumors (POTs) [15].
Clinically POF presents as either a pedunculated or a sessile firm mass that is a similar in colour to the surrounding connective tissue and evolves as a slow-growing, non-ulcerated, asymptomatic exophytic elevated lesion attached to the gingival and covered by normal appearing mucosa, clinically indiscernible from other common fibrous gingival lesions [16]. Typically, POF is located on facial gingiva of mandible, as a solitary lesion arising mainly in the incisor-canine and premolar area. It does not involve the underlying bone and it seldom can cause displacement of teeth. Rarely, multifocal or diffuse lesions have also been reported also associated with ocular and skin lesions [17], [18]. Histologically, POF is a non-encapsulated tumor characterized by the admixture of connective tissue (acellular, loose, myxomatous or markedly cellular) and islands of odontogenic epithelium and associated dysplastic dentin, amorphous ovoid cementum like calcifications and trabeculae of osteoid [19]. Among histologic subtypes granular cell variant [20] and squamous variant [13] have been described.
The POF is treated by local excision and prognosis is excellent [21]. Finally, POF has a significant recurrence rate, varying from very low to as high as 38.9% [22]. For the possibility of recurrence [7], [23], radiographic, histopathologic and cytologic investigation is fundamental and follow-up is mandatory. Among the documented recurrences in literature, it is interesting to mention the biological behaviour of the case report described by Armas et al. [23]: a POF with three recurrence with the last one occurred after 11 years and in association with a COF.
3.2. Central odontogenic fibroma (COF)
COF shows histopathologic features similar to those ones above mentioned for POF, consisting of collagenous fibrous connective tissue containing varying amounts of odontogenic epithelium, sometime associated to a prominent giant cell granuloma-like histopathologic component [24]. This lesion appears as an asymptomatic expansion of the cortical plate of the mandible or maxilla with equal frequency. Generally the lesion causes the swelling of the jaw. The lesion may evolve from a dental germ (dental papilla or follicle) or from the periodontal membrane, and therefore is invariably related to the coronal or radicular portion of teeth. It should be pointed out that the most usual site of presentation in the mandible is the posterior area, while in the maxilla is the anterior region. It seems to arise in a wide age group with predilection for females.
COF radiologically presents as uni- or multilocular radiolucencies with well-defined borders. In some rare cases, it might present mixed radiolucent and radiopaque features and undefined borders. Root resorption and displacement have been reported in cases of more severe lesions. COF responds well to surgical enucleation or to vigorous curettage. Recurrence is relatively uncommon but there are still some examples of recurred COFs [25], [26], [27]. The tendency toward malignant change of the COF is not considered high. Long-term regular follow-up is nonetheless recommended [28]. According to Marx RE [29] and Chhabra V et al. [30], if recurrence is observed, the original pathological specimen as well as biopsy specimen should be reviewed for possible diagnostic mistake.
Current opinion regards the OF (both POF and COF) as a distinct entity with clinic-pathological patterns that separate it from inflammatory/reactive or traumatic lesions such as reactive fibrous hyperplastic tissue (which may also contain occasional inactive odontogenic cells) or hyperplastic dental follicle, and traumatic bone cyst, but also from numerous other lesions as fibroma, giant cell fibroma, peripheral ossifying fibroma, desmoplastic fibroma, ameloblastoma, ameloblastic fibroma, ameloblastic carcinoma, calcifying cystic odontogenic tumor (PCCOTs), calcifying epithelial odontogenic tumour, odontogenic keratocyst, central giant cell tumor, peripheral giant cell granuloma, pyogenic granuloma, odontogenic myxoma, and fibromyxoma.
Anyway, since the clinical, histological and radiologic features of OF are not often characteristic, a multidisciplinary clinical and diagnostic approach should be attempted.
Generally, although COF arises in a wide age range with a mean age of 34 years, with a slight predilection for females, in the case herein presented, the patient was a little twelve-year-old boy. From a clinical point of view the young patient showed a soft localized gingival enlargement, that could have been interpreted as a form of reactive lesion or also as a POT (peripheral odontogenic tumor). The radiographic images revealed the real central origin of the lesion, characterized by a bone localization at the superior margin of the right angle of the mandible and limited by a radiopaque sclerotic border. Intraoperative examination of the lesion revealed an incomplete fusion of the tumor mass with the overlying mucosa.
We retain that a fine needle aspiration (FNA), when possible, could be a valid diagnostic tool to perform a cytologic differential diagnosis from other non-neoplastic, benign and malignant lesions of the jaw. In our case the cytological examination guided the clinical case management, oriented the pathological diagnosis of tumor with an odontogenic origin, that was then confirmed by the histologic evaluation of the resected mass. Generally, a diagnosis of odontogenic tumor can be easily made by the presence of odontogenic epithelium; however in cases in which odontogenic epithelial structures are absent, this simple conclusion is not possible but it is necessary to consider a large host of other opportunities, in term of differential diagnosis.
4. Conclusion
It is important to note that a painless gingival enlargement or swelling may be a clinical manifestation also of a COF, that instead more frequently closely resembles an endodontic lesion. A wrong initial diagnosis may determine a serious and compromising delay, and in case the lesion is long standing, it may erode the surrounding bone. Consequently also the efficacy of proper treatment could be precluded. Therefore, this case report highlights the importance of recommending to the patient periodic diligent clinical and radiographic examinations, and underlines the merit of an adequate pathological expertise for a careful cyto-histological examination of fibrous lesions of the jaw and for a proper differential diagnosis among various types of odontogenic/non-odontogenic tumors.
Consent
Written informed consent was obtained from the patient' relatives for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Ethical approval
This study has been approved by Ethical Committee of the University of Foggia.
Sources of funding
We declare that there has been no significant financial support for this work that could have influenced its outcome.
Author contribution
RS conceived of the study; AS and GP have made substantial contribution to its design; LB and LLM have participated in the acquisition, analysis and clinic-pathological correlations of data; GP together with AS helped in the coordination of this work; AS has been involved in drafting the manuscript and in its sequences' alignment, too; in the end, LR and PB has revised it critically for important intellectual content, giving final approval of the version to be published.
Authors confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. They further confirm that the order of authors listed in the manuscript has been approved by all of us.
Conflict of interest statement
Authors wish to confirm that there are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
Authors confirm that the manuscript has been read and approved by all named authors and that there are no other persons who satisfied the criteria for authorship but are not listed. They further confirm that the order of authors listed in the manuscript has been approved by all of us.
Authors confirm that they have given due consideration to the protection of intellectual property associated with this work and that there are no impediments to publication, including the timing of publication, with respect to intellectual property. In so doing Authors confirm that they have followed the regulations of our institutions concerning intellectual property.
Research registry
Research Registry is: researchregistry219.
Guarantor
The Guarantors for this study are GP and RS.
Contributor Information
Angela Santoro, Email: angelasantoro1981@gmail.com.
Giuseppe Pannone, Email: giuseppepannone@virgilio.it.
Luca Ramaglia, Email: luca.ramaglia@unina.it.
Pantaleo Bufo, Email: pantaleo.bufo@unifg.it.
Lorenzo Lo Muzio, Email: llomuzio@tin.it.
Raffaele Saviano, Email: raffaele.saviano@tin.it.
References
- 1.Wesley R.K., Wysocki G.P., Mintz S.M. The central odontogenic-fibroma—clinical and morphological studies. Oral Surg. 1975;40:235. doi: 10.1016/0030-4220(75)90155-3. [DOI] [PubMed] [Google Scholar]
- 2.Bhaskar S.N. fifth ed. The CV Mosby Company; St. Louis: 1977. Synopsis of Oral Pathology; pp. 259–261. [Google Scholar]
- 3.LeDoussal V., Mahe E., Herbert H. Los fibromyxomes odontogenes. Arch. Anat. Cytol. Pathol. 1981;23:325–331. [PubMed] [Google Scholar]
- 4.Philipsen H.P., Reichart P.A. Classification of odontogenic tumours. A historical review. J. Oral Pathol. Med. 2006 Oct;35(9):525–529. doi: 10.1111/j.1600-0714.2006.00470.x. [DOI] [PubMed] [Google Scholar]
- 5.Philipsen H.P., Reichart P.A., Sciubba J.J., van der Waal I. Odontogenic fibroma. In: Barnes Leon, Eveson John W., Reichart Peter, Sidransky David., editors. World Health Organization Classification of Tumours. Pathology and Genetics of Head and Neck Tumours. IARC Press Lyon; 2005. Page 315. [Google Scholar]
- 6.Dahl E.C., Wolfson S.H., Haugen J.C. Central odontogenic fibroma: a review of literature and report of a case. J. Oral Surg. 1981;39:120. [PubMed] [Google Scholar]
- 7.Daley T.D., Wysocki G.P. Peripheral odontogenic fibroma. Oral Surg. Oral Med. Oral Pathol. 1994;78:329–336. doi: 10.1016/0030-4220(94)90064-7. [DOI] [PubMed] [Google Scholar]
- 8.Gardner D.G. The central odontogenic fibroma: an attempt at clarification. Oral Surg. 1980;50:425. doi: 10.1016/s0030-4220(80)80011-9. [DOI] [PubMed] [Google Scholar]
- 9.Lukinmaa P.L., Hietanee J., Anttinen J., Ahonen P. Continuous enlarged dental follicles with histologic features resembling the WHO type of odontogenic fibroma. Oral Surg. Oral Med. Oral Pathol. 1990;70:313–317. doi: 10.1016/0030-4220(90)90147-k. [DOI] [PubMed] [Google Scholar]
- 10.Langlais R.P., Langland O.E., Nortje C.J. Diagnostic Imaging of the Jaws. Williams & Wilkins; Baltimore: 1995. Multilocular radiolucencies; pp. 370–376. [Google Scholar]
- 11.Baden E., Moskow B.S., Moskow R. Odontogenic gingival epithelial hamartoma. J. Oral Surg. 1968;26:702–714. [PubMed] [Google Scholar]
- 12.Mckelvy B.D., Cherrick H.M. Peripheral ameloblastic fibrodentinoma. J. Oral Surg. 1976;34:826–829. [PubMed] [Google Scholar]
- 13.Sciubba J.J., Zola M.B. Odontogenic epithelial hamartoma. Oral Surg. Oral Med. Oral Pathol. 1978;45:261–265. doi: 10.1016/0030-4220(78)90093-2. [DOI] [PubMed] [Google Scholar]
- 14.Gardner D.G. The peripheral odontogenic fibroma: an attempt at clarification. Oral Surg. Oral Med. Pathol. 1982;54:40–48. doi: 10.1016/0030-4220(82)90415-7. [DOI] [PubMed] [Google Scholar]
- 15.Buchner A., Merrell P.W., Carpenter W.M. Relative frequency of central odontogenic tumours: a study of 1088 cases from northern California and comparison to studies from other parts of the world. J. Oral Maxillofac. Surg. 2006;64:1343–1352. doi: 10.1016/j.joms.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 16.Buchner A., Ficarra G., Hansen L.S. Peripheral odontogenic fibroma. Oral Surg. Oral Med. Oral Pathol. 1987 Oct;64(4):432–438. doi: 10.1016/0030-4220(87)90148-4. [DOI] [PubMed] [Google Scholar]
- 17.Neville B.W., Damm D.D., Allen C.M., Bouquot J.E. third ed. WB Saunders; Philadelphia: 2009. Oral and Maxillofacial Pathology. pp. 729–9. [Google Scholar]
- 18.Weber A., van Heerden W.F.P., Ligthelm A.J., Raubenheimer E.J. Diffuse peripheral odontogenic fibroma: report of 3 cases. J. Oral Pathol. Med. 1992;21:82–84. doi: 10.1111/j.1600-0714.1992.tb00984.x. [DOI] [PubMed] [Google Scholar]
- 19.Kenney J.N., Kaugars G.E., Abbey L.M. Comparison between the peripheral ossifying fibroma and peripheral odontogenic fibroma. J. Oral Maxillofac. Surg. 1989 Apr;47(4):378–382. doi: 10.1016/0278-2391(89)90339-x. [DOI] [PubMed] [Google Scholar]
- 20.Lownie J.F., Altini M., Shear M. Granular cell peripheral odontogenic fibroma. J. Oral Pathol. Med. 1976;5:295–304. doi: 10.1111/j.1600-0714.1976.tb01778.x. [DOI] [PubMed] [Google Scholar]
- 21.Baiju C.S., Sumidha Rohatgi. Peripheral odontogenic fibroma: a case report and review. J. Indian Soc. Periodontol. 2011 Jul-Sep;15(3):273–275. doi: 10.4103/0972-124X.85674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Michaelides P.L. Recurrent peripheral odontogenic fibroma of attached gingiva: a case report. J. Periodontol. 1992;63:645–647. doi: 10.1902/jop.1992.63.7.645. [DOI] [PubMed] [Google Scholar]
- 23.Armas J.M., Hunter K.D., Jenkins W. Odontogenic fibroma: an unusual presentation. J. Oral Maxillofac. Pathol. 2008;12:68–71. [Google Scholar]
- 24.Allen C.M., Hammond H.L., Stimson P.G. Central odontogenic fibroma, WHO type. A report of three cases with an unusual associated giant cell reaction. Oral Surg. Oral Med. Oral Pathol. 1992 Jan;73(1):62–66. doi: 10.1016/0030-4220(92)90156-k. [DOI] [PubMed] [Google Scholar]
- 25.Heimdal A., Isaacson G., Nilsson L. Recurrent odontogenic fibroma. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 1980;50:140–145. doi: 10.1016/0030-4220(80)90201-7. [DOI] [PubMed] [Google Scholar]
- 26.Svirsky J.A., Abbey L.M., Kaugars G.E. A clinical review of central odontogenic fibroma: with addition of 3 new cases. J. Oral Med. 1986;41:51–54. [PubMed] [Google Scholar]
- 27.Jones G.M., Eveson J.W., Shepherd J.P. Central odontogenic fibroma. A report of two controversial cases illustrating diagnostic dilemmas. Br. J. Oral Maxillofac. Surg. 1989;27:406–411. doi: 10.1016/0266-4356(89)90081-8. [DOI] [PubMed] [Google Scholar]
- 28.Chuang Han-Ping, Tsai Lo-Lin. Central odontogenic fibroma of mandible—a case report. Taiwan J. Oral Maxillofac. Surg. 2008 Sep;19:179–185. [Google Scholar]
- 29.Marx R.E. Quintessence Publishing Co, Inc.; 2007. Diane Stern, Oral and Maxillofacial Pathology: a Rationale for Diagnosis and Treatment; pp. 672–674. [Google Scholar]
- 30.Chhabra V., Chhabra A. Central odontogenic fibroma of the mandible. Contemp. Clin. Dent. 2012 Apr-Jun;3(2):230–233. doi: 10.4103/0976-237X.96845. [DOI] [PMC free article] [PubMed] [Google Scholar]