Summary
Two different subsets of naturally occurring regulatory T cells (nTregs), defined by their expression of the inducible co‐stimulatory (ICOS) molecule, are produced by the human thymus. To examine the differentiation of ICOS+ and ICOS−CD45RA+CD31+ recent thymic emigrant (RTE) Tregs during normal pregnancy and in the presence of pre‐eclampsia or haemolysis elevated liver enzymes low platelet (HELLP)‐syndrome, we used six‐colour flow cytometric analysis to determine the changes in the composition of the ICOS+ and ICOS− Treg pools with CD45RA+CD31+ RTE Tregs, CD45RA+CD31− mature naive (MN) Tregs, CD45RA−CD31+ and CD45RA−CD31− memory Tregs. With the beginning of pregnancy until term, we observed a strong differentiation of both ICOS+ and ICOS−CD45RA+CD31+ RTE, but not CD45RA+CD31− MN Tregs, into CD45RA−CD31− memory Tregs. At the end of pregnancy, the onset of spontaneous term labour was associated with a significant breakdown of ICOS+CD45RA−CD31− memory Tregs. However, in the presence of pre‐eclampsia, there was a significantly increased differentiation of ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs into CD45RA−CD31+ memory Tregs, wherein the lacking differentiation into CD45RA−CD31− memory Tregs was partially replaced by the increased differentiation of ICOS+ and ICOS−CD45RA+CD31− MN Tregs into CD45RA−CD31− memory Tregs. In patients with HELLP syndrome, this alternatively increased differentiation of CD45RA−CD31− MN Tregs seemed to be exaggerated, and presumably restored the suppressive activity of magnetically isolated ICOS+ and ICOS− Tregs, which were shown to be significantly less suppressive in pre‐eclampsia patients, but not in HELLP syndrome patients. Hence, our findings propose that the regular differentiation of both ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs ensures a healthy pregnancy course, while their disturbed differentiation is associated with the occurrence of pre‐eclampsia and HELLP syndrome.
Keywords: HELLP syndrome, ICOS+ and ICOS− Tregs, immune suppression, pre‐eclampsia, pregnancy
Introduction
In mammalian organisms, the maternal immune system must tolerate the semi‐allogeneic fetus during pregnancy. Numerous murine and human studies have shown that immunosuppressive regulatory T cells (Tregs) have an important role in protecting the fetus from immune‐mediated rejection 1, 2, 3. Interference with respect to their number, differentiation and suppressive function was shown to be responsible for certain pathological conditions, such as infertility 4, 5 and the occurrence of recurrent spontaneous abortions 6, 7, 8, 9. Meanwhile, aberrant Treg cell homeostasis was also linked to the pathophysiology of most human gestation‐associated diseases that occur in the third trimester and manifest as preterm labour 10, 11, 12, pre‐eclampsia 13, 14, 15, 16 and gestational diabetes 17.
Currently, two different Treg populations are described in the literature. The naturally occurring Tregs (nTregs) are induced in the thymus and were shown to suppress immune responses to self‐ and alloantigens 18. The peripherally induced Tregs (iTregs) are generated by conversion of conventional naive T cells under certain tolerogenic conditions and have the capacity to suppress immune responses towards foreign antigens 19, 20. As it is currently not possible to distinguish between nTregs and iTregs phenotypically, it is extremely difficult to recognize the contribution of each Treg population to tolerance induction during pregnancy and to understand their potential roles for the pathogenesis of the different gestation‐associated diseases. However, functional analysis of different Treg subsets, which differs with respect to their degree of differentiation, shows that naive CD45RA+ Tregs have lower suppressive activity than non‐activated human leucocyte antigen D‐related (HLA‐DR)−CD45RA− memory Tregs or activated HLA‐DR+CD45RA− memory Tregs 21. Astonishingly, this relation was found to be reversed in healthy pregnant women, whose naive Treg subset had very high suppressive activity, while the HLA‐DR+ memory Tregs were less suppressive 5. These findings suggest that the increase of the suppressive activity of the naive CD45RA+ Treg population may represent a key event in tolerance induction during pregnancy. This assumption is strengthened further by the fact that naive CD45RA+ Tregs were shown to be expanded in the periphery during the normal pregnancy course, but were found to be reduced significantly in the periphery of pregnant women suffering from most common pregnancy complications (preterm labour, pre‐eclampsia, gestational diabetes) 11, 12, 17. As naive CD45RA+ Tregs always represent nTregs, it seems that nTregs are crucial for a successful pregnancy course.
Recently, two different nTreg populations were detected in the human thymus, which differ concerning their expression of the inducible co‐stimulatory (ICOS) molecule. Their induction by dendritic cells, proliferation and survival were shown to be regulated differentially. It was confirmed that the murine as well as the human Treg pool consists of a predominant ICOS− Treg subset, which is much more sensitive to apoptosis, and a minor highly proliferative ICOS+ Treg subset which is resistant to activation‐induced cell death 22, 23.
Like conventional naive CD45RA+ T cells, both ICOS+ and ICOS− Tregs are released from the thymus as CD31+ recent thymic emigrants (RTE Tregs) and develop in a way comparable to conventional RTE T cells. It is known that RTE T cells undergo peripheral post‐thymic proliferation to form a long‐living CD31− mature naive (MN) T cell population, which has the capacity to maintain the naive T cell pool in elderly people. Thereby, both RTE and MN T cells can differentiate into memory T cells after stimulation with appropriate antigens 24. Recently we showed that the normal differentiation of RTE Tregs is changed with the onset of pregnancy. We found that RTE, but not MN Tregs, differentiated increasingly into memory Tregs and that these emerging memory Tregs were maintained during the whole pregnancy course, but disappeared with the onset of spontaneous term labour 25. In the presence of pre‐eclampsia, this differentiation seemed to be impaired, but replaced partly by the increased differentiation of MN Tregs into memory Tregs. Obviously, this phenomenon leads to diminished suppressive activity of the total Treg pool, which is reduced significantly in pre‐eclampsia patients 11. Currently, it is neither known whether the differentiation of ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs is regulated differentially during pregnancy, nor whether aberrant differentiation of ICOS+ or ICOS−CD45RA+CD31+ RTE Tregs is involved in the pathogenesis of pre‐eclampsia. Therefore, we examined the thymic output and the differentiation of ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs during the normal pregnancy course and in the presence of gestation‐associated diseases such as pre‐eclampsia and haemolysis elevated liver enzymes low platelet (HELLP) syndrome.
In this study, we show that normal pregnancy is characterized by the increased differentiation of both ICOS+ and ICOS−CD45RA+CD31+ RTE but not CD45RA+CD31− MN Tregs into CD45RA−CD31− memory Tregs. Spontaneous term labour was associated mainly with a sharp decline of ICOS+CD45RA−CD31− memory Tregs within the total CD4+CD127low+/ − forkhead box protein 3 (FoxP3)+ Treg pool. Compared to normal pregnancy, the differentiation of ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs into CD45RA−CD31− memory Tregs was impaired strongly in pre‐eclampsia patients. In contrast, the differentiation of ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs was relatively normal in patients with HELLP syndrome. However, there was a significantly increased generation of CD45RA−CD31− memory cells within the CD4+ICOS+CD127+FoxP3− responder T cell (Tresp) pool.
Materials and methods
Patient collectives and healthy volunteers
Peripheral blood samples were collected from 31 non‐pregnant fertile female volunteers (group 1), 128 pregnant women during the normal pregnancy course (groups 2–5), 41 women with spontaneous term labour (group 6), 45 women 1 day postpartum (group 7), 42 pregnant women affected by pre‐eclampsia (group 8) and 18 women affected with HELLP syndrome (group 9). The diagnosis of pre‐eclampsia was made in the case of blood pressure of more than 140/90 mm Hg occurring on two separate occasions, 6 h apart, along with significant proteinuria (>300 mg/l in a 24‐h collection or a dipstick reading of >1 on a voided random urine sample in the absence of urinary tract infection) in previously normotensive women. The diagnosis of HELLP syndrome was made on the basis of haemolysis, elevated liver enzyme levels (aspartate and alanine aminotransferase >30 U/l) and thrombocytopenia thrombocyte count <150 000/µl). The blood samples from healthy pregnancies (groups 2–5) were collected from women who had routine ultrasonography to exclude fetal malformations (groups 2–4), from women delivering by term elective caesarean section in the absence of labour (group 5), from women in the presence of spontaneous term labour before delivery (group 6) and from women who had delivered spontaneously 1 day before (group 7). The blood samples from women affected by pre‐eclampsia or HELLP syndrome (groups 8 and 9) were taken during their hospitalization. The clinical characteristics of all these women (groups 1–9) are summarized in Table 1. The study was approved by the Regional Ethics Committee. All women were fully informed of the aim of the study and informed consent was obtained from all participants.
Table 1.
Clinical characteristics
| Group | Number | Age, median (range) | Weeks’ gestation, median (range) | Diagnosis |
|---|---|---|---|---|
| 1 (non‐pregnant) | 31 | 25 (18–38) | – | Healthy |
| 2 (1st trimester) | 31 | 32 (32–39) | 13 (13–14) | Healthy |
| 3 (2nd trimester) | 31 | 31 (21–41) | 22 (20–23) | Healthy |
| 4 (3rd trimester) | 33 | 31 (19–42) | 31 (27–35) | Healthy |
| 5 (Term, caes. section) | 33 | 32 (20–39) | 38 (37–39) | Healthy |
| 6 (Term, spont. delivery) | 41 | 32 (19–38) | 40 (38–42) | Healthy |
| 7 (1 day postpartum) | 45 | 30 (19–41) | – | Healthy |
| 8 (Pre‐eclampsia) | 42 | 33 (21–45) | 35 (23–41) | Pre‐eclampsia |
| 9 (HELLP syndrome) | 18 | 32 (31–43) | 36 (30–40) | HELLP syndrome |
caes. = caesarean; spont. = spontaneous; HELLP = haemolysis elevated liver enzymes low platelet.
Changes in the composition of the total CD4+CD127low+/ −FoxP3+ Treg pool/CD4+CD127+FoxP3− Tresp pool with ICOS+ and ICOS− T cells were examined during the normal pregnancy course, in the presence of spontaneous term labour and in the presence of pre‐eclampsia or HELLP syndrome. To examine whether the differentiation of ICOS+ and ICOS− Tregs/Tresps was regulated differentially during normal pregnancy, or regulated aberrantly in the presence of pre‐eclampsia and HELLP syndrome, we calculated the percentages of CD45RA+CD31+ RTE cells, CD45RA+CD31− MN cells, CD45RA−CD31+ memory cells and CD45RA−CD31− memory cells, both within the total ICOS+ and ICOS− Treg/Tresp pools and within the total Treg pool. These measurements were performed by six‐colour flow cytometric analysis for all patient groups. Moreover, comparative analyses concerning the suppressive activity of separated ICOS+ and ICOS− Tregs were performed for non‐pregnant women (group 1), healthy pregnant women (groups 4 and 5) pre‐eclampsia patients (group 8) and women affected with HELLP syndrome (group 9).
Fluorescence activated cell sorter (FACS) staining
Venous blood samples (10 ml) were collected from all participants into ethylenediamine tetraacetic acid (EDTA)‐containing tubes. Whole peripheral blood mononuclear cells (PBMCs) were isolated by Lymphodex (Inno‐Train Diagnostik GMBH, Kronberg, Germany) gradient centrifugation and analysed by six‐colour flow cytometric analysis. Briefly, the PBMCs (8 × 106 cells) were surface‐stained with 10 µl peridinin chlorophyll (PerCP)‐conjugated anti‐CD4 (BD Bioscience, Heidelberg, Germany), 20 µl phycoerythrin (PE)‐conjugated anti‐ICOS (BD Bioscience), 5 µl PE cyanin (Cy)7‐conjugated anti‐CD127 (eBioscience, Frankfurt, Germany), 5 µl Alexa Fluor 647‐conjugated anti‐CD31 (BD Bioscience) and 5 µl allophycocyanin (APC)‐conjugated anti‐CD45RA (BD Bioscience) mouse monoclonal antibodies. Subsequently, intracellular staining was performed for the detection of FoxP3 using a fluorescein isothiocyanate (FITC)‐labelled anti‐human FoxP3 staining set (clone PCH101; eBioscience), according to the manufacturer's instructions. Negative control samples were incubated with isotype‐matched antibodies. Dead cells were excluded by forward‐ and side‐scatter characteristics. Cells were analysed by a FACS Canto cytometer (BD Bioscience). Statistical analysis was based on at least 100 000 gated CD4+ T cells.
Positive selection of CD4+CD127low+/−CD25+ Treg cells
Whole peripheral blood mononuclear cells (PBMCs) were isolated from peripheral blood (50 ml) drawn in EDTA tubes by Lymphodex (Inno‐Train Diagnostik GMBH) gradient centrifugation. CD4+CD127low+/ −CD25+ Treg cells were purified using the Regulatory T Cell Isolation Kit II (Miltenyi Biotec, Bergisch Gladbach, Germany), according to the manufacturer's instructions. First, CD4+CD127low+/ − T cells were isolated by magnetic depletion of non‐CD4+ and CD127high+ cells. In the second step, the CD4+CD127low+/ −CD25+ Tregs were isolated by positive selection over two consecutive columns. The CD4+CD127low+/ −CD25− T cells were obtained in the flow‐through fraction and used as Tresps. The CD4+CD127low+/ −CD25+ Tregs were subsequently retrieved from the columns.
Sorting and functional testing of Treg subsets
For the sorting of the isolated CD4+CD127low+/ −CD25+ Tregs into ICOS+ and ICOS− Tregs, cells were stained with 20 µl PE‐conjugated anti‐ICOS (BD Bioscience) and 20 µl FITC‐conjugated anti‐CD4 (BD Bioscience) mouse monoclonal antibodies. Total CD4+CD127low+/ −CD25+ Tregs were obtained from 11 non‐pregnant fertile women, 10 healthy pregnant women (groups 4 and 5), 11 pregnant women affected by pre‐eclampsia (group 8) and six pregnant women affected by HELLP syndrome (group 9). In all experiments, dead cells were excluded, while the remaining CD4+CD127low+/ −CD25+ Tregs were sorted using a FACS Vantage SE Sorter (BD Bioscience).
To analyse the suppressive activity of each isolated Treg population, 2 × 104 Tresps were co‐cultured with the purified Treg subsets at ratios of 1 : 2 to 1 : 256 in 96‐well U‐bottomed plates. Suppression assays were performed in a final volume of 100 μl/well of X‐VIVO15 medium (Lonza, Verviers, Belgium). For T cell stimulation, the medium was supplemented with 1 μg/ml anti‐CD3 and 2 μg/ml anti‐CD28 antibodies (eBioscience). As controls, CD4+CD127low+/ −CD25+ Tregs and Tresps alone were cultured both with and without stimulus. Cells were incubated at 37 °C in 5% CO2. After 4 days, 1 μCi [3H]‐thymidine (Hartmann Analytic, Braunschweig, Germany) was added to the cultures and cells were incubated for a further 16 h. Then, the cells were harvested and [3H] incorporation was measured by scintillation counting. According to the cell numbers achieved after cell sorting, the assays were performed as single or multiple determinations. In order to compare the suppressive activity of the different Treg subsets in non‐pregnant women, healthy pregnant women and pre‐eclampsia patients, the maximum suppressive activity (ratio of Tregs to Tresps 1/2) and the minimum ratio of Tregs to Tresps at which a suppression of at least 15% could be achieved were calculated 11.
Statistical analysis
Statistical comparison of the percentages of ICOS+ and ICOS− Tregs/Tresps within the total Treg/Tresp pool between the different patient groups was performed using the non‐parametric Wilcoxon–Mann–Whitney U‐test. This test was also used for statistical comparison of the percentages of CD45RA+CD31+ RTE cells, CD45RA+CD31− MN cells, CD45RA−CD31+ and CD45RA−CD31− memory cells within the ICOS+ and ICOS− Treg/Tresp pool. Comparison of the suppressive activity of the different Treg subsets between non‐pregnant women, healthy pregnant women and pre‐eclampsia or HELLP syndrome patients was also performed using the non‐parametric Wilcoxon–Mann–Whitney U‐test. P < 0·05 was considered significant. For all tests, the software package BiAS for Windows (version 10·06) was used.
Results
ICOS+ and ICOS− Tregs show similar differentiation during the normal pregnancy course
In this study we examined whether there were differences in the differentiation of ICOS+ and ICOS− Tregs/Tresps during the normal pregnancy course and whether the presence of pre‐eclampsia or HELLP syndrome was associated with deficient differentiation of either ICOS+ or ICOS− Tregs/Tresps compared to healthy pregnancies. Therefore, we first divided the total CD4+CD127low+/ −FoxP3+ Treg pool/CD4+CD127+FoxP3− Tresp pool into ICOS+ and ICOS− Tregs/Tresps and then determined the percentages of CD45RA+CD31+ RTE and CD45RA+CD31− MN Tregs/Tresps, as well as CD45RA−CD31+ and CD45RA−CD31− memory Tregs/Tresps within both the total ICOS+ and ICOS− Treg/Tresp pools. Figure 1a–h shows the gating strategy that was used in all experiments. These measurements were performed with venous blood samples of non‐pregnant fertile women (group 1), healthy non‐labouring pregnant women during the normal pregnancy course (groups 2–5), spontaneously term labouring women (group 6) and women having delivered spontaneously 1 day before (group 7). The data obtained from healthy non‐labouring third‐trimester women (groups 4 and 5) were compared with third‐trimester women affected by pre‐eclampsia (group 8) or HELLP syndrome (group 9). Table 1 summarizes the clinical characteristics of all participants in this study.
Figure 1.
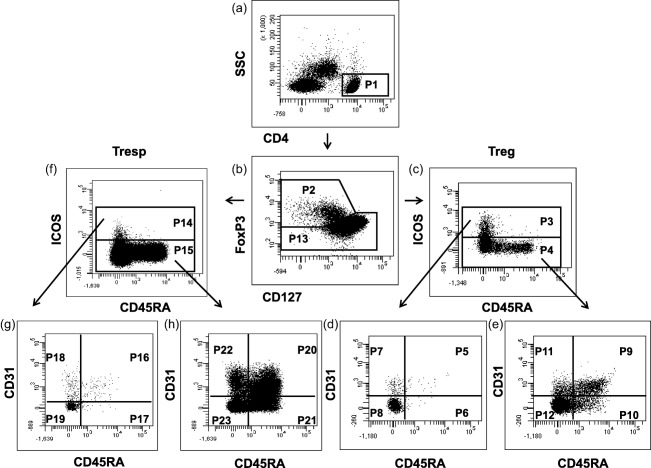
Gating strategy for six‐colour flow cytometric detection of CD45RA+CD31+ recent thymic emigrant (RTE), CD45RA+CD31−mature naive (MN) Tregs, CD45RA−CD31+ memory T cells and CD45RA−CD31− memory T cells within the inducible co‐stimulatory (ICOS)+ and ICOS− regulatory T cell (Treg) pool, as well as within the ICOS+ and ICOS− responder T cell (Tresp) pool. At first, CD4+ T cells (P1) were gated by fluorescence intensity of CD4 versus side‐scatter (SSC) (a). The CD4+CD127low+/− forkhead box protein 3 (FoxP3)+ Tregs (P2) and the CD4+CD127+FoxP3− Tresps (P13) were gated by fluorescence intensity of FoxP3 versus CD127 (b). The ICOS+ (P3) and ICOS− Tregs (P4) were gated by fluorescence intensity of CD45RA versus ICOS (c). The percentages of CD45RA+CD31+ RTE Tregs (P5, P9), CD45RA+CD31− MN Tregs (P6, P10), CD45RA− CD31+ memory Tregs (P7, P11) and CD45RA− CD31− memory Tregs (P8, P12) were estimated by analysing the total ICOS+ (P3) and ICOS− Treg pool (P4) for its expression of CD45RA and CD31 (d,e). Moreover, the ICOS+ (P14) and ICOS− Tresps (P15) were gated by fluorescence intensity of CD45RA versus ICOS (f). The percentages of CD45RA+CD31+ RTE Tresps (P16, P20), CD45RA+CD31− MN Tresps (P17, P21), CD45RA− CD31+ memory Tresps (P18, P22) and CD45RA− CD31− memory Tresps (P19, P23) were estimated by analysing the total ICOS+ (P14) and ICOS− Tresp pool (P15) for its expression of CD45RA and CD31 (g,h).
We found that the onset of normal pregnancy was associated with a significant decrease of CD45RA+CD31+ RTE (Fig. 2a,e), as well as CD45RA−CD31+ memory Tregs (Fig. 2c,g) within both the ICOS+ and the ICOS− Treg pools. The percentages of CD45RA+CD31− MN Tregs did not change, neither within the ICOS+ nor within the ICOS− Treg pool (Fig. 2b,f). However, there was a strong increase in the percentage of CD45RA−CD31− memory Tregs within both Treg pools (Fig. 2d,h). During the further pregnancy course (groups 2–5) this situation was almost maintained until term, when with the onset of normal spontaneous term labour (group 6) the original distribution of CD45RA+CD31+ RTE Tregs, CD45RA−CD31+ and CD45RA−CD31− memory Tregs was restored according with that of non‐pregnant women (group 1). Surprisingly, after spontaneous term delivery (group 7), there was a new, significant, decrease of CD45RA+CD31+ RTE and CD45RA−CD31+ memory Tregs (Fig. 2a,c) and a complementary increase of CD45RA−CD31− memory Tregs within the ICOS+ Treg pool (Fig. 2d). A similar decrease of CD45RA+CD31+ RTE and CD45RA−CD31+ memory Tregs was also observed within the ICOS− Treg pool (Fig. 2e,g), but instead of the CD45RA−CD31− memory Tregs, the CD45RA+CD31− MN Tregs increased complementary postpartum (Fig. 2f). Supporting information, Fig. S1 also shows the confidence intervals for the median for these results.
Figure 2.
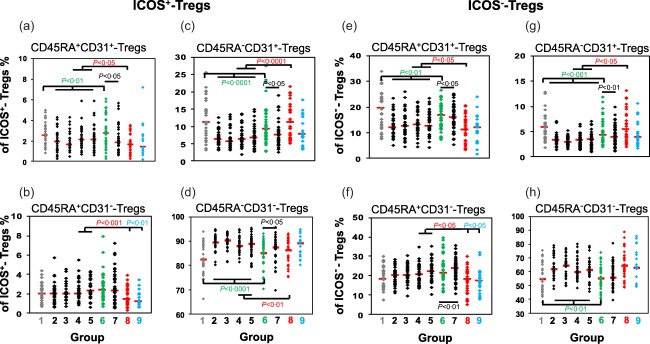
Changes in the differentiation of inducible co‐stimulatory (ICOS)+ and ICOS− regulatory T cells (Tregs) during the normal pregnancy course and in the presence of pre‐eclampsia and haemolysis elevated liver enzymes low platelet (HELLP) syndrome. The percentages of CD45RA+CD31+ recent thymic emigrant (RTE) Tregs (a,e), CD45RA+CD31−mature naive (MN) Tregs (b,f), CD45RA−CD31+ memory Tregs (c,g) and CD45RA−CD31− memory Tregs (d,h) within the ICOS+ and ICOS− Treg pool were estimated in healthy non‐pregnant fertile women (group 1,  ), healthy pregnant women during their pregnancy course (groups 2–5, ◆), spontaneously term labouring women (group 6,
), healthy pregnant women during their pregnancy course (groups 2–5, ◆), spontaneously term labouring women (group 6,  ), 1 day postpartum (group 7, ◆) and in women with pre‐eclampsia (group 8,
), 1 day postpartum (group 7, ◆) and in women with pre‐eclampsia (group 8,  ) or HELLP syndrome (group 9,
) or HELLP syndrome (group 9,  ). Significant differences concerning the percentages of the different Treg subsets were detected between women during the normal pregnancy course (groups 2–5) and non‐pregnant women (group 1) or women with spontaneous term labour (group 6). Significant differences were also observed between spontaneously term labouring women (group 6) and women 1 day postpartum (group 7). In addition, significant differences were detected between healthy third‐trimester women (groups 4 and 5) and women with pre‐eclampsia (group 8) or HELLP syndrome (group 9).
). Significant differences concerning the percentages of the different Treg subsets were detected between women during the normal pregnancy course (groups 2–5) and non‐pregnant women (group 1) or women with spontaneous term labour (group 6). Significant differences were also observed between spontaneously term labouring women (group 6) and women 1 day postpartum (group 7). In addition, significant differences were detected between healthy third‐trimester women (groups 4 and 5) and women with pre‐eclampsia (group 8) or HELLP syndrome (group 9).
In summary, these data show that the onset of pregnancy is associated with a decreased thymic distribution of both ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs and that the already distributed ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs differentiate increasingly into CD45RA−CD31− memory Tregs. This situation seems to be maintained during the entire healthy pregnancy course. With the onset of spontaneous term labour, the percentage of CD45RA−CD31− memory Tregs declined, while the percentage of CD45RA−CD31+ memory Tregs increased strongly. Furthermore, there was a significant redistribution of CD45RA+CD31+ RTE Tregs, both within the ICOS+ and ICOS− Treg pools.
The decrease of ICOS+CD45RA−CD31− but not ICOS−CD45RA−CD31− memory Tregs is crucial for the onset of spontaneous term labour
To examine whether the onset of normal spontaneous term labour was associated primarily with a loss of either ICOS+ or ICOS− Tregs, we estimated their percentages within the total CD4+CD127low+/ −FoxP3+ Treg pool during the whole pregnancy course. In addition, we examined whether there were considerable changes in the composition of the total CD4+CD127low+/ −FoxP3+ Treg pool with CD45RA+CD31+ RTE and CD45RA+CD31− MN Tregs, as well as CD45RA−CD31+ and CD45RA−CD31− memory Tregs, each of ICOS+ and ICOS− Tregs (Fig. 3). We found that total Tregs decreased continuously (Fig. 3a) and that the percentages of ICOS+ and ICOS− Tregs did not change markedly during the normal pregnancy course (Fig. 3b,c). However, the presence of spontaneous term labour was associated with a significant decrease of ICOS+ Tregs and a complementary increase of ICOS− Tregs (Fig. 3b,c). Thereby, the percentage of ICOS+CD45RA−CD31− memory Tregs decreased strongly (Fig. 3g), while the percentage of ICOS−CD45RA−CD31− memory Tregs did not change perceptibly (Fig. 3k). Instead, there was a significant increase in the percentages of ICOS−CD45RA+CD31+ RTE and ICOS−CD45RA−CD31+ memory Tregs (Fig. 3h,j). Supporting information, Fig. S2 also shows the confidence intervals for the median for these results. Therefore, it seems that the occurrence of normal spontaneous term labour is characterized mainly by a significant decrease of ICOS+CD45RA−CD31− memory Tregs and a significant redistribution of ICOS−CD45RA+CD31+ RTE Tregs within the total CD4+CD127low+/−FoxP3+ Treg pool.
Figure 3.
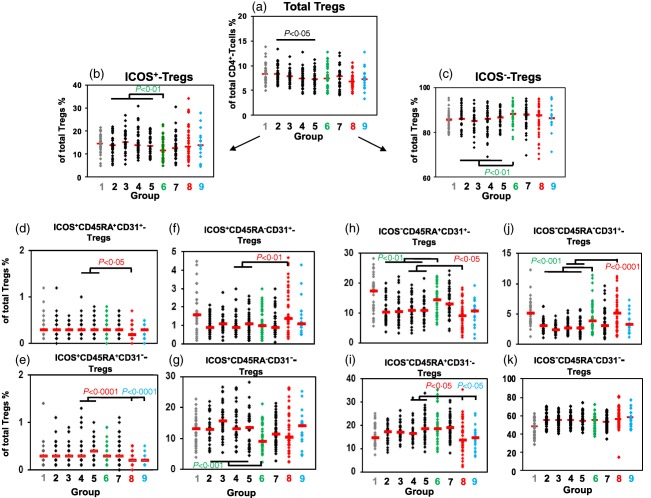
Detection of inducible co‐stimulatory (ICOS)+ and ICOS−CD45RA+CD31+ recent thymic emigrant (RTE) regulatory T cells (Tregs), ICOS+ and ICOS−CD45RA+CD31− mature naive (MN) Tregs, ICOS+ and ICOS−CD45RA−CD31+ memory Tregs and ICOS+ and ICOS−CD45RA−CD31− memory Tregs within the total CD4+CD127low+/−forkhead box protein 3 (FoxP3)+ Treg pool during the normal pregnancy course and in the presence of pre‐eclampsia and haemolysis elevated liver enzymes low platelet (HELLP) syndrome. The percentage of total CD4+CD127low+/−FoxP3+ Tregs within CD4+ T cells (a), the percentages of ICOS+ (b) and ICOS− Tregs (c), ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs (d,h), ICOS+ and ICOS−CD45RA+CD31− MN Tregs (e,i), ICOS+ and ICOS−CD45RA−CD31+ memory Tregs (f,j) and ICOS+ and ICOS−CD45RA−CD31− memory Tregs (g,k) within the total CD4+CD127low+/−FoxP3+ Treg pool were determined in healthy non‐pregnant fertile women (group 1,  ), healthy pregnant women during their pregnancy course (groups 2–5, ◆), spontaneously term labouring women (group 6,
), healthy pregnant women during their pregnancy course (groups 2–5, ◆), spontaneously term labouring women (group 6,  ), 1 day postpartum (group 7, ◆) and in women with pre‐eclampsia (group 8,
), 1 day postpartum (group 7, ◆) and in women with pre‐eclampsia (group 8,  ) or HELLP syndrome (group 9,
) or HELLP syndrome (group 9,  ). Significant differences concerning the percentages of the different Treg subsets were detected between women during the normal pregnancy course (groups 2–5) and non‐pregnant women (group 1) or women with spontaneous term labour (group 6). In addition, significant differences were detected between healthy third‐trimester women (groups 4 and 5) and women with pre‐eclampsia (group 8) or HELLP syndrome (group 9).
). Significant differences concerning the percentages of the different Treg subsets were detected between women during the normal pregnancy course (groups 2–5) and non‐pregnant women (group 1) or women with spontaneous term labour (group 6). In addition, significant differences were detected between healthy third‐trimester women (groups 4 and 5) and women with pre‐eclampsia (group 8) or HELLP syndrome (group 9).
Pre‐eclampsia patients show a significantly increased differentiation of both ICOS+ and ICOS−CD45RA+CD31+RTE Tregs into CD45RA−CD31+ instead of CD45RA−CD31− memory Tregs
To examine whether ICOS+ or ICOS− Tregs show deficient differentiation in the presence of pre‐eclampsia or HELLP syndrome, we determined the percentages of CD45RA+CD31+ RTE and CD45RA+CD31− MN Tregs, as well as CD45RA−CD31+ and CD45RA−CD31− memory Tregs, within the ICOS+ and the ICOS− Treg pool of both healthy third‐trimester women (groups 4 and 5) and patients affected with pre‐eclampsia (group 8) or HELLP syndrome (group 9). We found that the differentiation of ICOS+ and ICOS− Tregs was disturbed similarly in pre‐eclampsia patients (Fig. 2a–h). Compared to healthy pregnancies, the percentages of CD45RA+CD31+ RTE and CD45RA+CD31− MN Tregs (Fig. 2a,b,e,f) were reduced significantly, while the percentages of CD45RA−CD31+ memory Tregs (Fig. 2c,g) were increased significantly in both Treg pools. The only difference was found in the ICOS+ Treg pool, as the percentages of CD45RA−CD31− memory Tregs were decreased significantly in pre‐eclampsia patients (Fig. 2d), but unchanged in the ICOS− Treg pool (Fig. 2h). Surprisingly, compared to healthy pregnancies, HELLP syndrome patients showed only significantly decreased percentages of CD45RA+CD31− MN Tregs within the ICOS+ Treg pool as well as within the ICOS− Treg pool (Fig. 2b,f). The percentages of CD45RA+CD31+ RTE Tregs, CD45RA−CD31+ and CD45RA−CD31− memory Tregs again reached values in the range of healthy pregnancies in both Treg pools.
The total CD4+CD127low+/−FoxP3+ Treg pool was not decreased in the presence of pre‐eclampsia and HELLP syndrome (Fig. 3a). When detecting total ICOS+ and ICOS− Tregs within the total CD4+CD127low+/−FoxP3+ Treg pool, we found that the relationship between these two Treg subsets was not changed (Fig. 3b,c). Rather, we could see that the deviations in the differentiation of the ICOS+ and ICOS− Treg pool in pre‐eclampsia and HELLP syndrome patients were also observable when detecting ICOS+ and ICOS− Tregs as CD45RA+CD31+ RTE, CD45RA+CD31− MN, CD45RA−CD31+ memory and CD45RA−CD31− memory Tregs within the total CD4+CD127low+/−FoxP3+ Treg pool (Fig. 3d–k). The percentages of both ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs (Fig. 3d,h), as well as ICOS+ and ICOS−CD45RA+CD31− MN Tregs (Fig. 3e,i), were reduced significantly, while the percentages of ICOS+ and ICOS−CD45RA−CD31+ memory Tregs (Fig. 3f,j) were increased significantly in the presence of pre‐eclampsia. However, the percentages of ICOS+ and ICOS−CD45RA−CD31− memory Tregs remained unchanged within the total CD4+CD127low+/−FoxP3+ Treg pool. In the presence of HELLP syndrome, we only detected significantly reduced percentages of ICOS+ and ICOS−CD45RA+CD31− MN Tregs within the total CD4+CD127low+/−FoxP3+ Treg pool (Fig. 3e,i). All other Treg subsets again reached values in the range of healthy pregnancies. Supporting information, Figs S1 and S2 show the confidential intervals for these results.
These data suggest that the occurrence of pre‐eclampsia is associated with an increased differentiation of both ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs into CD45RA−CD31+ memory Tregs which presumably do not proliferate enough to increase CD45RA−CD31− memory Tregs. As the CD45RA−CD31− memory Tregs are not reduced in these patients, it seems that there is an alternatively increased differentiation of both ICOS+ and ICOS−CD45RA+CD31− MN Tregs into CD45RA−CD31− memory Tregsin the presence of pre‐eclampsia. With the occurrence of HELLP syndrome, this increased differentiation of ICOS+ and ICOS−CD45RA+CD31− MN Tregs into CD45RA−CD31− memory Tregs seems to be exaggerated, and presumably induces an increasingly enhanced differentiation of previously non‐activated ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs into CD45RA−CD31+ memory Tregs with stronger proliferative capacity. Thus, the percentages of CD45RA+CD31+ RTE Tregs, CD45RA−CD31+ and CD45RA−CD31− memory Tregs seem to adjust again to normal values detected in healthy pregnancies.
ICOS+ and ICOS− Tregs have similar suppressive activity that is reduced strongly in patients affected with pre‐eclampsia, but not HELLP syndrome
To examine whether the suppressive activity of ICOS+ and ICOS− Tregs decreases in the presence of pre‐eclampsia or HELLP syndrome, the total CD4+CD127low+/−CD25+ Treg pool obtained from 11 non‐pregnant fertile women (group 1), 10 healthy pregnant women (groups 4 and 5), 11 pregnant women affected by pre‐eclampsia (group 8) and six women affected by HELLP syndrome (group 9) was isolated by magnetic activated cell sorting (MACS) and sorted into ICOS+ and ICOS− Tregs (Fig. 4a). Subsequently, both ICOS+ (Fig. 4a, P1) and ICOS− Tregs (Fig. 4a, P2) obtained from each women were analysed separately for their suppressive capacity. Figure 4b–e shows the results of the maximum suppressive activity (ratio Tregs/Tresps = 1/2) and the ratio of the Tregs/Tresps up to which the Tregs could be diluted to achieve a minimum suppressive activity of at least 15%. Depending on the number of the separated ICOS+ and ICOS− Treg cells the suppression assays were performed as single or multiple determinations. Both parameters did not differ between ICOS+ and ICOS− Tregs, either in non‐pregnant or healthy pregnant women. There were also no differences concerning these parameters between non‐pregnant women and healthy pregnant women. However, ICOS+ and ICOS− Tregs showed a significantly decreased suppressive activity regarding both parameters in pre‐eclampsia patients compared to non‐pregnant and pregnant women. In contrast, both ICOS+ and ICOS− Tregs obtained from patients with HELLP syndrome showed a similar high suppressive activity as detected in healthy pregnant and non‐pregnant women (Fig. 4b–e).
Figure 4.
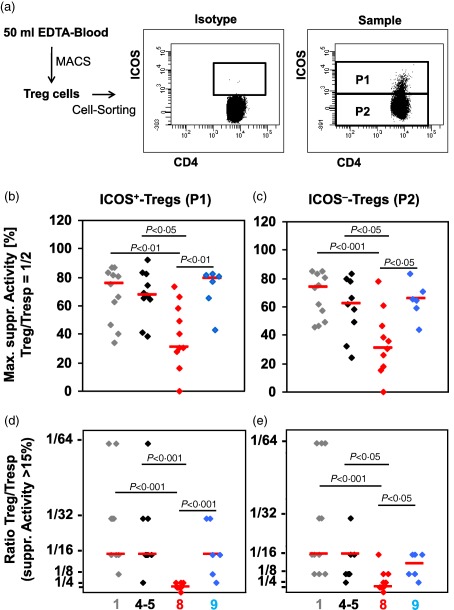
Cell sorting and suppressive activity of inducible co‐stimulatory (ICOS)+ and ICOS− regulatory T cells (Tregs). CD4+CD127low+/−CD25+ Tregs were isolated by magnetic activated cell sorting (MACS), stained with anti‐CD4 and anti‐ICOS‐specific monoclonal antibodies and sorted into ICOS+ (P1) and ICOS− Tregs (P2) (a). The suppressive activity of the different ICOS+ and ICOS− Treg subsets was estimated by suppression assays. Blood samples were obtained from non‐pregnant women ( ), healthy third‐trimester women (groups 4 and 5) (◆), pre‐eclampsia patients (
), healthy third‐trimester women (groups 4 and 5) (◆), pre‐eclampsia patients ( ) and women with haemolysis elevated liver enzymes low platelet (HELLP) syndrome (
) and women with haemolysis elevated liver enzymes low platelet (HELLP) syndrome ( ). The figure shows the individual and median values of the maximum suppressive activity (Treg/responder T cell (Tresp) = 1/2) (b,c) and of the ratio of Treg/Tresp up to which the purified Treg cells could be diluted to achieve a minimum suppressive activity of at least 15% (d,e). Compared to non‐pregnant and healthy third‐trimester women, the suppressive activity of both ICOS+ and ICOS− Tregs was decreased significantly in pre‐eclampsia patients, but not in HELLP syndrome patients.
). The figure shows the individual and median values of the maximum suppressive activity (Treg/responder T cell (Tresp) = 1/2) (b,c) and of the ratio of Treg/Tresp up to which the purified Treg cells could be diluted to achieve a minimum suppressive activity of at least 15% (d,e). Compared to non‐pregnant and healthy third‐trimester women, the suppressive activity of both ICOS+ and ICOS− Tregs was decreased significantly in pre‐eclampsia patients, but not in HELLP syndrome patients.
These findings suggest that the increased differentiation of ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs into CD45RA−CD31+ instead of CD45RA−CD31− memory Tregs has a negative influence on the suppressive activity of both the ICOS+ and ICOS− Treg pools. As the percentages of all Treg subsets, with the exception of the CD45RA+CD31− MN Tregs, were found to be in the normal range in patients with HELLP syndrome, it seems that the reinforced differentiation of ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs into sufficiently proliferating CD45RA−CD31+ memory Tregs causes the restoration of the suppressive activity of both Treg pools.
ICOS+ and ICOS− Tresp cells differentiate similar to ICOS+ and ICOS− Tregs during the normal pregnancy course
Regarding the differentiation of Tresps, we also could not detect any shift in the composition of the total CD4+CD127+FoxP3− Tresp pool with ICOS+ or ICOS− Tresps during the normal pregnancy course (Fig. 5a,b). We could even see a similar differentiation of the ICOS+ and ICOS− Tresps, whose CD45RA+CD31+ RTE and CD45RA−CD31+ memory T cells also decreased strongly with the beginning of pregnancy (Fig. 5c,g,e,i). Their percentages of CD45RA+CD31− MN T cells either did not change (Fig. 5d) or even rose (Fig. 5h), while their CD45RA−CD31− memory T cells increased significantly (Fig. 5f,j). Similar to Treg cell differentiation, this condition was maintained during the entire pregnancy course. However, with the onset of spontaneous term labour, the original distribution of CD45RA+CD31+ RTE, CD45RA−CD31+ and CD45RA−CD31− memory T cells was restored for ICOS− Tresps (Fig. 5g,i,j), but not for ICOS+ Tresps (Fig. 5c,e,f). These findings reveal that beside the increased generation of ICOS+ and ICOS−CD45RA−CD31− memory Tregs, there is also an increased generation of ICOS+ and ICOS−CD45RA−CD31− memory Tresps during the normal pregnancy course. With the beginning of spontaneous term labour, ICOS+ and ICOS−CD45RA−CD31− memory Tregs, as well as ICOS−CD45RA−CD31− memory Tresps, diminish while the ICOS+CD45RA−CD31− memory Tresps do not decrease. Therefore, it appears that the continued existence of ICOS+CD45RA−CD31− memory Tresps may account for the induction of normal spontaneous term labour.
Figure 5.
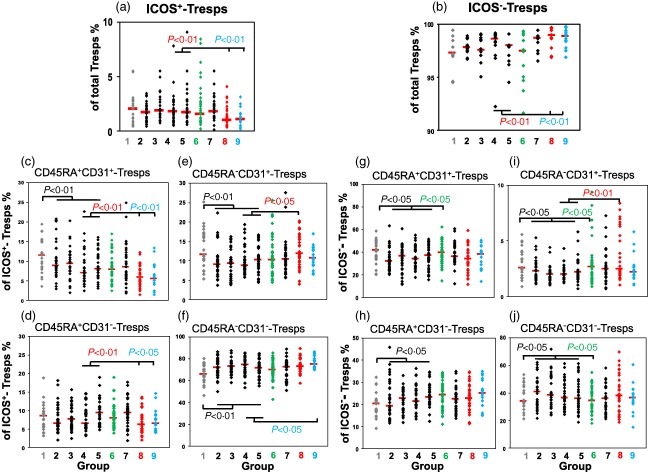
Changes in the differentiation of inducible co‐stimulatory (ICOS)+ and ICOS−responder T cells (Tresps) during the normal pregnancy course and in the presence of pre‐eclampsia and haemolysis elevated liver enzymes low platelet (HELLP) syndrome. The percentages of ICOS+ (a) and ICOS− Tresps (b) within the total Tresp pool, as well as CD45RA+CD31+RTE Tresps (c,g), CD45RA+CD31−MN Tresps (d,h), CD45RA−CD31+ memory Tresps (e,i) and CD45RA−CD31− memory Tresps (f,j) within the ICOS+ and ICOS− Tresp pool were estimated in healthy non‐pregnant fertile women (group 1,  ), healthy pregnant women during their pregnancy course (groups 2–5, ◆), spontaneously term labouring women (group 6,
), healthy pregnant women during their pregnancy course (groups 2–5, ◆), spontaneously term labouring women (group 6,  ), 1 day postpartum (group 7, ◆) and in women with pre‐eclampsia (group 8,
), 1 day postpartum (group 7, ◆) and in women with pre‐eclampsia (group 8,  ) or HELLP syndrome (group 9,
) or HELLP syndrome (group 9,  ). Significant differences concerning the percentages of the different Tresp subsets were detected between women during the normal pregnancy course (groups 2–5) and non‐pregnant women (group 1) or women with spontaneous term labour (group 6). In addition, significant differences were detected between healthy third‐trimester women (groups 4 and 5) and women with pre‐eclampsia (group 8) or HELLP syndrome (group 9).
). Significant differences concerning the percentages of the different Tresp subsets were detected between women during the normal pregnancy course (groups 2–5) and non‐pregnant women (group 1) or women with spontaneous term labour (group 6). In addition, significant differences were detected between healthy third‐trimester women (groups 4 and 5) and women with pre‐eclampsia (group 8) or HELLP syndrome (group 9).
Surprisingly, the presence of pre‐eclampsia and HELLP syndrome was associated with a clear shift in favour of ICOS− Tresps within the total CD4+CD127+FoxP3− Tresp pool (Fig. 5a,b). Thereby, it was striking that the changes concerning the composition of the total ICOS+ Tresp pool were the same as those found in the ICOS+ Treg pool. In the presence of pre‐eclampsia, the percentages of CD45RA+CD31+ RTE and CD45RA+CD31− MN T cells decreased, while the percentages of CD45RA−CD31+ memory T cells increased significantly (Fig. 5c–e). An increased percentage of CD45RA−CD31+ memory T cells was also found within the ICOS− Tresp pool (Fig. 5i). Therefore, it seems that particularly the ICOS+CD45RA+CD31+ RTE Tresps and presumably also the ICOS−CD45RA+CD31+ RTE Tresps show an increased differentiation into CD45RA−CD31+ instead of CD45RA−CD31− memory Tresps in the presence of pre‐eclampsia. In contrast, there was a significantly increased differentiation of ICOS+CD45RA+CD31+ RTE Tresps into ICOS+CD45RA−CD31− memory Tresps in the presence of HELLP syndrome (Fig. 5c,d,f). Supporting information, Fig. S3 also shows the confidential intervals for these results.
In summary, these findings suggest that an adequate differentiation of both Treg and Tresp cells may be necessary for a successful pregnancy course. Obviously, the continued or enhanced generation of especially ICOS+CD45RA−CD31− memory Tresps plays an important role in spontaneous term delivery or HELLP syndrome.
Discussion
Our previous reports demonstrated that thymus‐derived naturally occurring naive CD45RA+ Tregs are of potential importance for successful pregnancy course 5, 10, 11, 12, 17. Due to the fact that the human thymus contains two subsets of nTregs, defined by their expression of the co‐stimulatory molecule ICOS, we examined whether the thymic output of ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs and their subsequent differentiation in the periphery were regulated differentially during pregnancy and whether aberrant differentiation could be observed in the presence of pre‐eclampsia or HELLP syndrome. For that, we compared the changes in the composition of the ICOS+ and the ICOS− Treg pool with CD45RA+CD31+ RTE, CD45RA+CD31− MN, CD45RA−CD31+ memory and CD45RA−CD31− memory Tregs during normal and complicated pregnancy and examined whether the characteristic changes observed within the ICOS+ and the ICOS− Treg pool could be confirmed when detecting the different ICOS+ and ICOS− Treg subsets within the total CD4+CD127low+/−FoxP3+ Treg pool. In addition, we examined whether there were similar changes in the composition of the total CD4+CD127+FoxP3− Tresp pool with the corresponding Tresp subsets.
We found that both CD45RA+CD31+ RTE and CD45RA−CD31+ memory Tregs decreased strongly with the onset of pregnancy, both within the ICOS+ and ICOS− Treg pools, indicating that the thymic output of both ICOS+ and ICOS− RTE Tregswas greatly reduced. It is already known that the thymic activity is restricted severely during puberty in both men and women (thymic involution), due to the increased secretion of gonadal hormones 26. Therefore, it seems likely that the excessive production of pregnancy hormones may cause an additional reduction of the thymic activity, which seems to be limited temporally for the duration of pregnancy. In mice, such transient thymic involution during pregnancy has already been shown and it was demonstrated that progesterone and oestrogens had the capacity to decrease thymocyte proliferation at the DN stage without inducing apoptosis 26, 27.
Our findings show that the decrease of CD45RA+CD31+ RTE and CD45RA−CD31+ memory Tregs at the onset of pregnancy was accompanied by a concurrent increase of CD45RA−CD31− memory Tregs, while CD45RA+CD31− MN Tregs did not change, both within the ICOS+ and the ICOS− Treg pools. Therefore, we propose that both ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs, but not CD45RA+CD31− MN Tregs, differentiate increasingly into CD45RA−CD31− memory Tregs. Presumably, the restricted thymic release of RTE Tregs at the onset of pregnancy may cause their enhanced proliferation and force their direct differentiation into CD45RA−CD31− memory Tregs during the entire pregnancy course. At the end of pregnancy, these newly generated populations of both ICOS+ and ICOS−CD45RA−CD31− memory Tregs were found to break down with the onset of normal spontaneous term labour. This phenomenon may be explained by the fact that both naive ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs may not have the capacity to proliferate indefinitely. Therefore, both ICOS+ and ICOS−CD45RA−CD31− memory Tregs may decline at the end of pregnancy, when the ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs lose the capacity to proliferate any longer.
Although ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs differentiated similarly during the pregnancy course, we found a significant shift in the proportion of ICOS+ and ICOS− Tregs within the total CD4+CD127low+/−FoxP3+ Treg pool in favour of ICOS− Tregs with the onset of normal spontaneous term labour. This was due mainly to a strong decrease of ICOS+CD45RA−CD31− memory Tregs and a potential increase of ICOS−CD45RA+CD31+ RTE and ICOS−CD45RA−CD31+ memory Tregs. As the ICOS+ Tregs were shown to have a greater capacity for proliferation than ICOS− Tregs 22, 23, the loss of ICOS+CD45RA−CD31− memory Tregs may have a greater impact on the composition of the total CD4+CD127low+/−FoxP3+ Treg pool than the loss of ICOS−CD45RA−CD31− memory Tregs. Therefore, our findings may propose that spontaneous term delivery may be induced primarily by the breakdown of ICOS+CD45RA−CD31− memory Tregs, but not ICOS−CD45RA−CD31− memory Tregs. Clearly, there is a significant thymic redistribution of ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs with the onset of spontaneous term labour. Thereby, the redistribution of ICOS−CD45RA+CD31+ RTE Tregs may be much more noticeable than that of ICOS+CD45RA+CD31+ RTE Tregs, as these cells account for only a vanishingly small fraction within the total CD4+CD127low+/−FoxP3+ Treg pool.
Interestingly, after delivery, these newly released CD45RA+CD31+ RTE Tregs differentiated preferably into CD45RA+CD31− MN Tregs in the ICOS− Treg pool but into CD45RA−CD31− memory Tregs in the ICOS+ Treg pool. Such findings may propose that placental hormones such as oestrogens and progesterone may be necessary for the direct differentiation of ICOS−CD45RA+CD31+ RTE Tregs into ICOS−CD45RA−CD31− memory Tregs and that the postpartum loss of these hormones may, rather, favour the development of ICOS−CD45RA+CD31− MN Tregs instead of ICOS−CD45RA−CD31− memory Tregs. In contrast, it seems that there is a hormone‐independent differentiation of ICOS+CD45RA+CD31+ RTE Tregs into ICOS+CD45RA−CD31− memory Tregs. A hormone‐dependent differentiation was already reported for conventional T cells, as it was shown that there is a profound loss of naive T cells during puberty, accompanied by a strong expansion of T cells with memory phenotype 28, 29, 30, 31. In addition, it was shown in mice that ovarian hormone ablation leads to phenotypical alterations in the major peripheral blood lymphocyte (PBL) and splenic T cell subsets by diminishing the peripubertal changes in the frequency of RTE, MN and memory T cells 32.
In contrast to spontaneous term delivery, the presence of pre‐eclampsia or HELLP syndrome was not associated with a significant shift in the proportion of ICOS+ and ICOS− Tregs within the total CD4+CD127low+/−FoxP3+ Treg pool. However, there was an increased differentiation of both ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs into CD45RA−CD31+ memory Tregs instead of CD45RA−CD31− memory Tregs, indicating that both ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs may have deficiencies concerning their proliferative capacity. As these patients showed decreased percentages of both ICOS+ and ICOS−CD45RA+CD31− MN Tregs, the deficient differentiation of CD45RA+CD31+ RTE Tregs into CD45RA−CD31− memory Tregs may have been replaced partially by the increased differentiation of CD45RA+CD31− MN Tregs into CD45RA−CD31− memory Tregs. Nevertheless, pre‐eclampsia patients showed decreased suppressive activity, both of their ICOS+ and ICOS− Treg pools. In contrast, the suppressive activity of both Treg pools was in the normal range in patients with HELLP syndrome, a phenomenon which was confirmed when testing the suppressive activity of the total Treg pool of these patients 11. As these patients showed only decreased percentages of CD45RA+CD31− MN Tregs, both within the ICOS+ and the ICOS− Treg pool and within the total CD4+CD127low+/−FoxP3+ Treg pool, we propose that the alternatively increased differentiation of CD45RA+CD31− MN Tregs into CD45RA−CD31− memory Tregs in pre‐eclampsia patients may be exaggerated in patients with HELLP syndrome. The strong decrease of CD45RA+CD31− MN Tregs could induce the additional differentiation of so far non‐activated CD45RA+CD31+ RTE Tregs and could cause their further reinforced differentiation into CD45RA−CD31− memory Tregs with stronger proliferation capacity. Presumably, this reinforced differentiation of ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs into CD45RA−CD31− memory Tregs ensures that the suppressive activity of both Treg pools is restored in patients with HELLP syndrome. Nevertheless, such mechanisms may constitute an emergency response causing a large consumption of functional RTE Tregs.
In addition, our results reveal that the reduction of thymic activity during pregnancy also affects the differentiation of ICOS+ and ICOS−CD45RA+CD31+ RTE Tresp cells. It was striking that similar to Treg cells, both ICOS+ and ICOS−CD45RA+CD31+ RTE Tresps, but not ICOS+ and ICOS−CD45RA+CD31− MN Tresps, differentiated into ICOS+ and ICOS−CD45RA−CD31− memory Tresps during the normal pregnancy course. Currently, we are not able to explain how these mechanisms are realized. There are some indications in the literature that the CD31 molecule itself could play a role in the post‐thymic homeostatic proliferation of conventional Tresps, and presumably of Tregs as well 24. Similar to ICOS+ and ICOS−CD45RA−CD31− memory Tregs, the population of ICOS−CD45RA−CD31− memory Tresps, but not that of ICOS+CD45RA−CD31− memory Tresps, broke down with the onset of normal spontaneous term labour. Such findings suggest that ICOS+CD45RA+CD31+ RTE Tresps may have the ability to proliferate for longer than ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs or ICOS−CD45RA+CD31+ RTE Tresps. Presumably, at the end of pregnancy these conditions are responsible for the development of normal spontaneous term labour.
Regarding the presence of pre‐eclampsia, our results propose that the proliferation capacity of ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs as well as ICOS+ and ICOS−CD45RA+CD31+ RTE Tresps is impaired, so that both the suppressive effect of Tregs and the repellent effect of Tregs may be disturbed. However, in the presence of HELLP syndrome, there was a significant excessive production of ICOS+CD45RA−CD31+ memory Tresps, whose effector functions could, presumably, have caused the reinforced differentiation of ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs into CD45RA−CD31− memory Tregs. Meanwhile, it is known that the ICOS molecule is expressed on follicular helper T cells (Tfh). These cells are necessary for B cell antibody isotype‐switching, germinal centre (GC) formation and high‐affinity antibody production 33. Recently, the frequency of these cells was found to be increased significantly in patients with autoimmune diseases such as systemic lupus erythematosus (SLE) and ankylosing spondylitis 34, 35. In addition, ICOS signalling was shown to be required for the generation of both central and effector CD4+ memory T cell populations 36. Currently, it is not known whether ICOS+ and ICOS− Tresps also differ concerning their induction, proliferation and survival. Further studies may be necessary to clarify the special role of ICOS+CD45RA−CD31− memory Tregs/Tresps for tolerance induction during healthy and affected pregnancies.
Author contributions
M. W. and A. S. designed the study, M. W. and M. J. performed the study, J. S., M. S., K. M., S. M. and M. Z. contributed important methods and patients. M. W. and A. S. analysed the data and wrote the manuscript. All authors contributed to the final version of the manuscript and approved it.
Disclosure
None of the authors have any conflicts of interest related to this manuscript.
Supporting information
Additional Supporting information may be found in the online version of this article at the publisher's web site:
Fig. 1. Changes in the differentiation of inducible co‐stimulatory (ICOS)+ and ICOS− regulatory T cells (Tregs) during the normal pregnancy course and in the presence of pre‐eclampsia and haemolysis elevated liver enzymes low platelet (HELLP) syndrome. The percentages of CD45RA+CD31+ recent thymic emigrant (RTE) Tregs (a,e), CD45RA+CD31− mature naive (MN) Tregs (b,f), CD45RA−CD31+ memory Tregs (c,g) and CD45RA−CD31− memory Tregs (d,h) within the ICOS+ and ICOS− Treg pool were estimated in healthy non‐pregnant fertile women (group 1,  ), healthy pregnant women during their pregnancy course (groups 2–5, ◆), spontaneously term labouring women (group 6,
), healthy pregnant women during their pregnancy course (groups 2–5, ◆), spontaneously term labouring women (group 6,  ), 1 day postpartum (group 7, ◆) and in women with pre‐eclampsia (group 8,
), 1 day postpartum (group 7, ◆) and in women with pre‐eclampsia (group 8,  ) or HELLP syndrome (group 9,
) or HELLP syndrome (group 9,  ). The figures show the 95% confidence intervals and the median of all patient groups for each Treg subset.
). The figures show the 95% confidence intervals and the median of all patient groups for each Treg subset.
Fig. 2. Detection of inducible co‐stimulatory (ICOS)+ and ICOS−CD45RA+CD31+recent thymic emigrant (RTE) Tregs, ICOS+ and ICOS−CD45RA+CD31− mature naive (MN) Tregs, ICOS+ and ICOS−CD45RA−CD31+ memory Tregs and ICOS+ and ICOS−CD45RA−CD31− Tregs within the total CD4+CD127low+/−forkhead box protein 3 (FoxP3)+ Treg pool during the normal pregnancy course and in the presence of pre‐eclampsia and haemolysis elevated liver enzymes low platelet (HELLP) syndrome. The percentage of total CD4+CD127low+/−FoxP3+ Tregs within CD4+ T cells (a), the percentages of ICOS+ (b) and ICOS− Tregs (c), ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs (d,h), ICOS+ and ICOS−CD45RA+CD31− MN Tregs (e,i), ICOS+ and ICOS−CD45RA−CD31+ memory Tregs (f,j) and ICOS+ and ICOS−CD45RA−CD31− memory Tregs (g,k) within the total CD4+CD127low+/−FoxP3+ Treg pool were determined in healthy non‐pregnant fertile women (group 1,  ), healthy pregnant women during their pregnancy course (groups 2–5, ◆), spontaneously term labouring women (group 6,
), healthy pregnant women during their pregnancy course (groups 2–5, ◆), spontaneously term labouring women (group 6,  ), 1 day postpartum (group 7, ◆) and in women with pre‐eclampsia (group 8,
), 1 day postpartum (group 7, ◆) and in women with pre‐eclampsia (group 8,  ) or HELLP syndrome (group 9,
) or HELLP syndrome (group 9,  ). The figures show the 95% confidence intervals and the median of all patient groups for each Treg subset.
). The figures show the 95% confidence intervals and the median of all patient groups for each Treg subset.
Fig. 3. Changes in the differentiation of inducible co‐stimulatory (ICOS)+ and ICOS− responder T cells (Tresps) during the normal pregnancy course and in the presence of pre‐eclampsia and haemolysis elevated liver enzymes low platelet (HELLP) syndrome. The percentages of ICOS+ (a) and ICOS− Tresps (b) within the total Tresp pool, as well as CD45RA+CD31+ recent thymic emigrant (RTE) Tresps (c,g), CD45RA+CD31− mature naive (MN) Tresps (d,h), CD45RA−CD31+ memory Tresps (e,i) and CD45RA−CD31− memory Tresps (f,j) within the ICOS+ and ICOS− Tresp pool were estimated in healthy non‐pregnant fertile women (group 1,  ), healthy pregnant women during their pregnancy course (groups 2–5, ◆), spontaneously term labouring women (group 6,
), healthy pregnant women during their pregnancy course (groups 2–5, ◆), spontaneously term labouring women (group 6,  ), 1 day postpartum (group 7, ◆) and in women with pre‐eclampsia (group 8,
), 1 day postpartum (group 7, ◆) and in women with pre‐eclampsia (group 8,  ) or HELLP syndrome (group 9,
) or HELLP syndrome (group 9,  ). The figures show the 95% confidence intervals and the median of all patient groups for each Treg subset.
). The figures show the 95% confidence intervals and the median of all patient groups for each Treg subset.
Acknowledgements
This work was supported by the Dietmar Hopp Foundation, Sankt Leon‐Rot, Germany. The authors would like to thank the nursing staff of the Department of Obstetrics and Gynecology for arranging the collection of the blood samples. In addition, they would like to thank Dieter Stefan (Institute of Immunology, University of Heidelberg, Heidelberg, Germany) for professional help with FACS sorting of the Treg subsets, and Larissa Schoenhoff (Department of Medicine I (Nephrology), University of Heidelberg, Heidelberg, Germany) for excellent technical assistance.
References
- 1. Erlebacher A. Mechanisms of T cell tolerance towards the allogeneic fetus. Nat Rev Immunol 2013; 13:23–33. [DOI] [PubMed] [Google Scholar]
- 2. Williams N. Inducing tolerance to pregnancy. N Engl J Med 2012; 7:1159–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Jiang TT, Chaturvedi V, Ertelt JM et al Regulatory T cells: new keys for further unlocking the enigma of fetal tolerance and pregnancy complications. J Immunol 2014; 192:4949–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Zhou J, Wang Z, Zhao X, Wang J, Sun H, Hu Y. An increase of Treg cells in the peripheral blood is associated with a better in vitro fertilization treatment outcome. Am J Reprod Immunol 2012; 68:100–6. [DOI] [PubMed] [Google Scholar]
- 5. Schlossberger V, Schober L, Rehnitz J et al The success of assisted reproduction technologies in relation to composition of the total regulatory T cell (Treg) pool and different Treg subsets. Hum Reprod 2013; 28:3062–73. [DOI] [PubMed] [Google Scholar]
- 6. Yang H, Qiu L, Chen G, Ye Z, Lü C, Lin Q. Proportional change of CD4+CD25+ regulatory T cells in decidua and peripheral blood in unexplained recurrent spontaneous abortion patients. Fertil Steril 2008; 92:301–5. [DOI] [PubMed] [Google Scholar]
- 7. Winger EE, Reed JL. Low circulating CD4+ CD25+ Foxp3+ T regulatory cell levels predict miscarriage in newly pregnant women with a history of failure. Am J Reprod Immunol 2011; 66:320–8. [DOI] [PubMed] [Google Scholar]
- 8. Lee S, Kim JY, Lee M, Gilman‐Sachs A, Kwak‐Kim J. Th17 and regulatory T cells in women with recurrent pregnancy loss. Am J Reprod Immunol 2012; 67:311–8. [DOI] [PubMed] [Google Scholar]
- 9. Wang WJ, Liu FJ, Xin‐Liu Hao CF, Bao HC, Qu QL Liu XM. Adoptive transfer of pregnancy‐induced CD4+CD25+‐ regulatory T cells reverses the increase in abortion rate caused by interleukin 17 in the CBA/JxBALB/c mouse model. Hum Reprod 2014; 29:946–52. [DOI] [PubMed] [Google Scholar]
- 10. Kisielewicz A, Schaier M, Schmitt E et al A distinct subset of HLA‐DR+‐regulatory T cells is involved in the induction of preterm labor during pregnancy and in the induction of organ rejection after transplantation. Clin Immunol 2010; 137:209–20. [DOI] [PubMed] [Google Scholar]
- 11. Steinborn A, Schmitt E, Kisielewicz A et al Pregnancy‐associated diseases are characterized by the composition of the systemic regulatory T cell (Treg) pool with distinct subsets of Tregs. Clin Exp Immunol 2012; 167:84–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schober L, Radnai D, Schmitt E, Mahnke K, Sohn C, Steinborn A. Term and preterm labor: decreased suppressive activity and changes in composition of the regulatory T‐cell pool. Immunol Cell Biol 2012; 90:935–44. [DOI] [PubMed] [Google Scholar]
- 13. Santner‐Nanan B, Peek MJ, Khanam R et al Systemic increase in the ratio between Foxp3+ and IL‐17‐producing CD4+ T cells in healthy pregnancy but not in preeclampsia. J Immunol 2009; 183:7023–30. [DOI] [PubMed] [Google Scholar]
- 14. Laresgoiti‐Servitje E, Gomez‐Lopez N, Olson DM. An immunological insight into the origins of pre‐eclampsia. Hum Reprod Update 2010; 16:510–24. [DOI] [PubMed] [Google Scholar]
- 15. Darmochwal‐Kolarz J, Kludka‐Sternik M, Tabarkiewicz J et al The predominance of Th 17 lymphocytes and decreased number and function of Treg cells in preeclampsia. Reprod Immunol 2012; 93:75–81. [DOI] [PubMed] [Google Scholar]
- 16. Hsu P, Santner‐Nanan B, Dahlstrom JE et al Altered decidual DC‐SIGN+ antigen‐presenting cells and impaired regulatory T‐cell induction in preeclampsia. Am J Pathol 2012; 181:2149–60. [DOI] [PubMed] [Google Scholar]
- 17. Schober L, Radnai D, Spratte J et al The role of regulatory T cell (Treg) subsets in gestational diabetes mellitus. Clin Exp Immunol 2014; 177:76–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Aluvihare VR, Kallikourdis M, Betz AG. Regulatory T cells mediate maternal tolerance to the fetus. Nat Immunol 2004; 5:266–71. [DOI] [PubMed] [Google Scholar]
- 19. Samstein RM, Josefowicz SZ, Arvey A, Treuting PM, Rudensky AY. Extrathymic generation of regulatory T cells in placental mammals mitigates maternal–fetal conflict. Cell 2012; 150:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rowe JH, Ertelt JM, Xin L, Way SS. Pregnancy imprints regulatory memory that sustains anergy to fetal antigen. Nature 2012; 490:102–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Schaier M, Seissler N, Schmitt E et al DRhigh+CD45RA−‐Tregs potentially affect the suppressive activity of the total Treg pool in renal transplant patients. PLOS ONE 2012; 7:e34208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ito T, Hanabuchi S, Wang Y et al Two functional subsets of FOXP3+ regulatory T cells in human thymus and periphery. Immunity 2008; 28:870–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chen Y, Shen S, Gorentla BK, Gao J, Zhong XP. Murine regulatory T cells contain hyperproliferative and death‐prone subsets with differential ICOS expression. J Immunol 2012; 188:1698–707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kohler S, Thiel A. Life after the thymus: CD31+ and CD31 human naive CD4+ T‐cell subsets. Blood 2009; 113:769–74. [DOI] [PubMed] [Google Scholar]
- 25. Wagner MI, Mai C, Schmitt E et al The role of recent thymic emigrant‐regulatory T cell (RTE‐Treg) differentiation during pregnancy. Immunol Cell Biol 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 26. Dooley J, Liston A. Molecular control over thymic involution: from cytokines and microRNA to aging and adipose tissue. Eur J Immunol 2012; 42:1073–79. [DOI] [PubMed] [Google Scholar]
- 27. Zoller AL, Schnell FJ, Kersh GJ. Murine pregnancy leads to reduced proliferation of maternal thymocytes and decreased thymic emigration. Immunology 2007; 121:207–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Clambey ET, Kappler JW, Marrack P. CD8 T cell clonal expansions and aging: a heterogeneous phenomenon with a common outcome. Exp Gerontol 2007; 42:407–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Czesnikiewicz‐Guzik M, Lee WW, Cui D et al T cell subset‐specific susceptibility to aging. Clin Immunol 2008; 127:107–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mason D, Powrie F. Memory CD4+ T cells in man form two distinct subpopulations, defined by their expression of isoforms of the leucocyte common antigen, CD45. Immunology 1990; 70:427–33. [PMC free article] [PubMed] [Google Scholar]
- 31. Ericsson PO, Linden O, Dohlsten M, Sjogren HO, Hedlund G. Functions of rat CD4+ T cell subsets defined by CD45RB: CD45RB‐ cells have a much stronger response to recall antigens, whereas polyclonally activated cells of both subsets are equally efficient producers of IFN in the presence of exogenous IL‐2. Cell Immunol 1991; 132:391–99. [DOI] [PubMed] [Google Scholar]
- 32. Perisic M, Stojic‐Vukanic Z, Pilipovic I et al Role of ovarian hormones in T‐cell homeostasis: from the thymus to the periphery. Immunobiology 2013; 218:353–67. [DOI] [PubMed] [Google Scholar]
- 33. Akiba H, Takeda K, Kojima Y et al The role of ICOS in the CXCR5+ follicular B helper T cell maintenance in vivo . J Immunol 2005; 175:2340–48. [DOI] [PubMed] [Google Scholar]
- 34. Choi JY, Ho JH, Pasoto SG et al Circulating follicular helper‐like T cells in systemic lupus erythematosus: association with disease activity. Arthritis Rheumatol 2015; 67:988–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Wu S, Yang T, Pan F et al Increased frequency of circulating follicular helper T cells in patients with ankylosing spondylitis. Mod Rheumatol 2015; 25:110–5. [DOI] [PubMed] [Google Scholar]
- 36. Marriott CL, Carlesso G, Herbst R, Withers DR. ICOS is required for the generation of both central and effector CD4+ memory T‐cell populations following acute bacterial infection. Eur J Immunol 2015; 45:1706–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Additional Supporting information may be found in the online version of this article at the publisher's web site:
Fig. 1. Changes in the differentiation of inducible co‐stimulatory (ICOS)+ and ICOS− regulatory T cells (Tregs) during the normal pregnancy course and in the presence of pre‐eclampsia and haemolysis elevated liver enzymes low platelet (HELLP) syndrome. The percentages of CD45RA+CD31+ recent thymic emigrant (RTE) Tregs (a,e), CD45RA+CD31− mature naive (MN) Tregs (b,f), CD45RA−CD31+ memory Tregs (c,g) and CD45RA−CD31− memory Tregs (d,h) within the ICOS+ and ICOS− Treg pool were estimated in healthy non‐pregnant fertile women (group 1,  ), healthy pregnant women during their pregnancy course (groups 2–5, ◆), spontaneously term labouring women (group 6,
), healthy pregnant women during their pregnancy course (groups 2–5, ◆), spontaneously term labouring women (group 6,  ), 1 day postpartum (group 7, ◆) and in women with pre‐eclampsia (group 8,
), 1 day postpartum (group 7, ◆) and in women with pre‐eclampsia (group 8,  ) or HELLP syndrome (group 9,
) or HELLP syndrome (group 9,  ). The figures show the 95% confidence intervals and the median of all patient groups for each Treg subset.
). The figures show the 95% confidence intervals and the median of all patient groups for each Treg subset.
Fig. 2. Detection of inducible co‐stimulatory (ICOS)+ and ICOS−CD45RA+CD31+recent thymic emigrant (RTE) Tregs, ICOS+ and ICOS−CD45RA+CD31− mature naive (MN) Tregs, ICOS+ and ICOS−CD45RA−CD31+ memory Tregs and ICOS+ and ICOS−CD45RA−CD31− Tregs within the total CD4+CD127low+/−forkhead box protein 3 (FoxP3)+ Treg pool during the normal pregnancy course and in the presence of pre‐eclampsia and haemolysis elevated liver enzymes low platelet (HELLP) syndrome. The percentage of total CD4+CD127low+/−FoxP3+ Tregs within CD4+ T cells (a), the percentages of ICOS+ (b) and ICOS− Tregs (c), ICOS+ and ICOS−CD45RA+CD31+ RTE Tregs (d,h), ICOS+ and ICOS−CD45RA+CD31− MN Tregs (e,i), ICOS+ and ICOS−CD45RA−CD31+ memory Tregs (f,j) and ICOS+ and ICOS−CD45RA−CD31− memory Tregs (g,k) within the total CD4+CD127low+/−FoxP3+ Treg pool were determined in healthy non‐pregnant fertile women (group 1,  ), healthy pregnant women during their pregnancy course (groups 2–5, ◆), spontaneously term labouring women (group 6,
), healthy pregnant women during their pregnancy course (groups 2–5, ◆), spontaneously term labouring women (group 6,  ), 1 day postpartum (group 7, ◆) and in women with pre‐eclampsia (group 8,
), 1 day postpartum (group 7, ◆) and in women with pre‐eclampsia (group 8,  ) or HELLP syndrome (group 9,
) or HELLP syndrome (group 9,  ). The figures show the 95% confidence intervals and the median of all patient groups for each Treg subset.
). The figures show the 95% confidence intervals and the median of all patient groups for each Treg subset.
Fig. 3. Changes in the differentiation of inducible co‐stimulatory (ICOS)+ and ICOS− responder T cells (Tresps) during the normal pregnancy course and in the presence of pre‐eclampsia and haemolysis elevated liver enzymes low platelet (HELLP) syndrome. The percentages of ICOS+ (a) and ICOS− Tresps (b) within the total Tresp pool, as well as CD45RA+CD31+ recent thymic emigrant (RTE) Tresps (c,g), CD45RA+CD31− mature naive (MN) Tresps (d,h), CD45RA−CD31+ memory Tresps (e,i) and CD45RA−CD31− memory Tresps (f,j) within the ICOS+ and ICOS− Tresp pool were estimated in healthy non‐pregnant fertile women (group 1,  ), healthy pregnant women during their pregnancy course (groups 2–5, ◆), spontaneously term labouring women (group 6,
), healthy pregnant women during their pregnancy course (groups 2–5, ◆), spontaneously term labouring women (group 6,  ), 1 day postpartum (group 7, ◆) and in women with pre‐eclampsia (group 8,
), 1 day postpartum (group 7, ◆) and in women with pre‐eclampsia (group 8,  ) or HELLP syndrome (group 9,
) or HELLP syndrome (group 9,  ). The figures show the 95% confidence intervals and the median of all patient groups for each Treg subset.
). The figures show the 95% confidence intervals and the median of all patient groups for each Treg subset.


