Summary
Recent studies have reported that calcitonin gene‐related peptide (CGRP) contributes to joint pain. However, regulation of the CGRP/CGRP receptor signalling in osteoarthritis (OA) is not fully understood. To investigate the regulation of CGRP/CGRP receptor signalling by macrophages in the synovial tissue (ST) of OA joints, we characterized the gene expression profiles of CGRP and CGRP receptors in the ST of OA mice (STR/Ort). In addition, we examined whether macrophage depletion by the systemic injection of clodronate‐laden liposomes affected the expression of CGRP and CGRP receptors in ST. CD11c+ macrophages in the ST of STR/Ort and C57BL/6J mice were analysed by flow cytometry. Real‐time polymerase chain reaction (PCR) was used to evaluate the expression of interleukin (IL)‐1β, CGRP, calcitonin receptor‐like receptor (CLR) and receptor activity‐modifying protein 1 (RAMP1) in F4/80+ and F4/80− cells. The effects of IL‐1β on the expression of CGRP and CLR by cultured synovial cells were also examined. The percentage of CD11c+ macrophages in the ST of STR/Ort was higher than that in C57/BL6J mice. Notably, the F4/80+ cell fraction expressed IL‐1β highly, whereas the F4/80− cell fraction expressed CGRP, CLR, and RAMP1 highly. In addition, expression of the IL‐1β and CLR genes was increased in ST, but was decreased upon macrophage depletion, and the IL‐1β treatment of cultured synovial cells up‐regulated CLR. Taken together, the present findings suggest that synovial macrophages are the major producers of IL‐1β and regulators of CLR in OA mice. Therefore, macrophages and IL‐1β may be suitable therapeutic targets for treating OA pain.
Keywords: calcitonin receptor, interleukin 1β, osteoarthritis
Introduction
Osteoarthritis (OA), which is the most common form of arthritis, is characterized by cartilage breakdown, synovial fibrosis and osteophyte formation. Chronic pain is the main clinical symptom of OA and is treated predominantly using analgesic drugs, such as non‐steroidal anti‐inflammatory drugs and weak opioids. However, because these drugs provide only variable symptomatic pain relief, and because few effective disease‐modifying drugs are available for OA, there is a demand for more effective analgesics. To this end, a better understanding is required of the mechanisms that drive OA pain.
Calcitonin gene‐related peptide (CGRP) appears to be involved in pain transmission and neurogenic inflammation. CGRP binds to functional CGRP receptors, which consist of calcitonin receptor‐like receptor (CLR) and receptor activity‐modifying protein 1 (RAMP1) 1, 2. Several clinical trials have shown that CGRP receptor antagonists are effective in treating acute migraine 3, 4. In addition, inhibition of CGRP receptors abolishes joint nociceptor sensitization in mouse models of OA pain 5, 6. Although CGRP/CGRP receptor signalling plays a critical role in the modulation of OA pain, the factors regulating CGRP/CGRP receptor signalling in OA remain unclear.
We have reported previously that inflammatory macrophages are increased in the synovial tissue (ST) of osteoarthritic mice 7. Recent studies have also shown that macrophages produce a number of inflammatory cytokines, particularly interleukin (IL)‐1β, IL‐6 and tumour necrosis factor (TNF)‐α, which contribute to pain and the progression of OA 8, 9, 10. In addition, Hill et al. 11 used magnetic resonance imaging (MRI) imaging to demonstrate that synovitis correlates with OA pain. Based on these findings, we hypothesized that synovial macrophages regulate CGRP/CGRP receptor signalling in ST and contribute to OA pain.
Here, we characterized the expression profiles of CGRP and CGRP receptors in the ST of OA mice (STR/Ort). In addition, we investigated whether macrophage depletion by systemic injection of clodronate‐laden liposomes affects the levels of CGRP and CGRP receptors in ST.
Materials and methods
Animals
Male STR/Ort mice aged 9 months and age‐ and sex‐matched C57BL/6J control mice (Charles River Laboratories, Inc., Yokohama, Japan) were used in this study. Specific‐pathogen free colonies of STR/Ort and C57BL/6J mice were maintained at Nippon Charles River Laboratories (Kanagawa, Japan). The mice were housed in a semi‐barrier system with a controlled environment (temperature: 23 ± 2°C; humidity: 55% ± 10%; lighting: 12‐h light/dark cycle) throughout the study. All the experimental protocols were approved by the Kitasato University School of Medicine Animal Care Committee.
Flow cytometric analysis of leucocytes from ST of STR/ort mice
C57BL/6J and STR/Ort mice were killed by deep anaesthesia with a mixture of medetomidine, midazolam and butorphanol tartrate by intramuscular injection, and skin was then removed with a scalpel for the harvesting of ST. The harvested ST was digested with 1 mg/ml type I collagenase for 2 h at 37°C. The released cells were stained with antibodies against F4/80, CD11b and CD11c, and 7‐amino actinomycin D (7‐AAD) staining was used to identify dead cells.
Real‐time polymerase chain reaction (PCR)
Total RNA was extracted from harvested ST using TRIzol (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions, and was used as template for first‐strand cDNA synthesis using SuperScript III RT (Invitrogen). The PCR reaction mixtures consisted of 2 μl cDNA, specific primer set (0·2 μM final concentration) and 12·5 μl SYBR Premix Ex Taq (Takara, Kyoto, Japan) in a final volume of 25 μl. The primers were designed using Primer Blast software and were synthesized by Hokkaido System Science Co., Ltd (Sapporo, Japan). Melt curve analysis was performed to confirm the specificity of the amplified products. The sequences of the PCR primer pairs are listed in Table 1. Quantitative PCR was performed using a real‐time PCR detection system (CFX‐96; Bio‐Rad, Hercules, CA, USA). The PCR cycle parameters consisted of an initial denaturation at 95°C for 1 min, followed by 40 cycles of 95°C for 5 s, and 60°C for 30 s. mRNA expression was normalized to the levels of glyceraldehyde 3‐phosphate dehydrogenase (GAPDH) mRNA.
Table 1.
Sequences of the primers used in this study.
| Gene | Direction | Primer sequence (5′–3′) | Product size (bp) |
|---|---|---|---|
| IL‐1β | F | GCAACTGTTCCTGAACTCAACT | 89 |
| R | ATCTTTTGGGGTCCGTCAACT | ||
| CGRP | F | CCCTTTCCTGGTTGTCAGCA | 88 |
| R | TGGGCTGCTTTCCAAGATTGA | ||
| CLR | F | GTAGTTAGTGCTCCTCGGGC | 113 |
| R | CTCTCCTCGCCTTCGTTGTT | ||
| RAMP1 | F | GGACCCTGACTATGGGACTC | 92 |
| R | AGTCACACCATAGCGTCTTCC | ||
| GAPDH | F | AACTTTGGCATTGTGGAAGG | 223 |
| R | ACACATTGGGGGTAGGAACA |
IL = interleukin; CGRP = calcitonin gene‐related peptide, RAMP1 = receptor activity‐modifying protein 1; CLR = calcitonin receptor‐like receptor; GAPDH = glyceraldehyde 3‐phosphate dehydrogenase.
Isolation of F4/80‐positive cells from ST
Ten ST samples were harvested from the bilateral knees of five 9‐month‐old STR/Ort mice. Mononuclear cells were isolated from ST by digestion with type I collagenase for 2 h at 37°C. ST‐derived mononuclear cells were suspended in 500 μl phosphate‐buffered saline (PBS) containing biotinylated anti‐F4/80 antibody and were then incubated for 30 min at 4°C. The cells were washed once with PBS, mixed with streptavidin‐labelled magnetic particles (BD IMag Streptavidin Particles Plus‐DM; BD Biosciences, Tokyo, Japan), and the tube containing the cells was then placed on an IMag separation system (BD Biosciences). After a further incubation on ice for 30 min, warmed (37°C) α‐minimum essential culture medium (MEM) was added to the cell suspension to collect unbound (F4/80‐negative) cells. The tube was removed from the magnetic support and an additional 3 ml α‐MEM was added to collect F4/80‐positive cells. The collected F4/80‐positive and ‐negative cells were centrifuged at 300 g for 10 min and the resulting supernatants were removed. The obtained cell pellets were cultured in α‐MEM in six‐well plates. IL‐1β, CGRP, CLR and RAMP1 expression in the collected cells was analysed by reverse transcription–polymerase chain reaction (RT–PCR). The experiment was performed three times.
Macrophage depletion by injection of clodronate‐laden liposomes
To investigate the effects of macrophages on IL‐1β, CGRP, CLR and RAMP1 expression in ST, clodronate liposomes were administered systemically to STR/Ort mice by intraperitoneal injection. After 24 h, splenocytes were stained with antibodies against F4/80 and CD11b to examine macrophage depletion. ST were harvested as described above and the expression of IL‐1β, CGRP, CLR and RAMP1 was analysed by real‐time PCR.
Effect of IL‐1β on ST‐derived cells
Cells in the ST of STR/Ort mice were harvested as described above. ST‐derived mononuclear cells were suspended in 500 μl phosphate‐buffered saline (PBS) containing biotinylated anti‐F4/80 antibody and then incubated for 30 min at 4°C. The cells were washed once with PBS, resuspended in 200 μl streptavidin‐labelled magnetic particles, and then added to tubes held in an IMag separation system (BD Biosciences). Warmed (37°C) α‐MEM culture medium was added to the tube to collect unbound (F480‐negative) cells, which were then cultured in α‐MEM in six‐well plates. After 1 week at 37°C in a 5% CO2 incubator, synovial fibroblasts were incubated with 0 (control group) or 50 ng/ml mouse recombinant IL‐1β (IL‐1β group) (Biolegend, San Diego, CA, USA) for 24 h. Cells were then harvested for RNA isolation, as described above, and CGRP, CLR and RAMP1 expression was analysed by RT–PCR. The experiment was performed three times.
Statistical analysis
All statistical analyses were performed using spss software version 11·0 (SPSS, Inc., Chicago, IL, USA). The Mann–Whitney U‐test was used to examine differences between C57BL/6J and STR/Ort mice and PBS‐injected STR/Ort and clodronate‐liposome injected STR/Ort mice. A P‐value of < 0·05 was considered statistically significant.
Results
Flow cytometric analysis of macrophage populations in ST
Proinflammatory macrophages in synovium express CD11c 7. Here, the proportion of CD11c+ macrophages in ST of STR/Ort mice was investigated by flow cytometry. The number of F4/80+CD11b+ (Fig. 1a,b,e) and CD11c+ F4/80+CD11b+ macrophages (Fig. 1c,d,f) in the ST of STR/Ort mice was significantly higher compared to that found in the ST of C57BL/6J mice.
Figure 1.
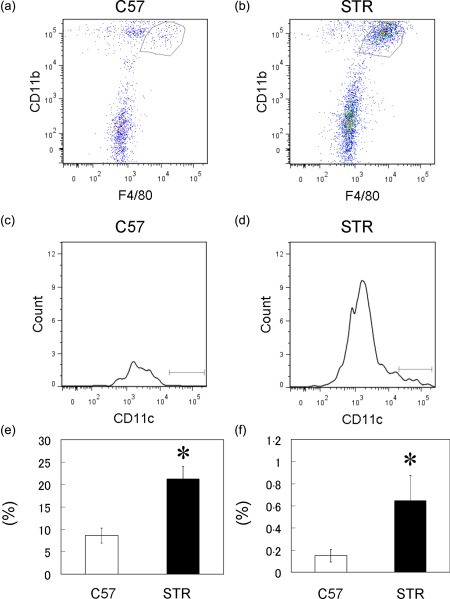
Flow cytometric analysis of CD11c+F4/80+CD11b macrophage cells in the synovial tissue (ST) of C57BL/6J (C57) and STR/Ort (STR) mice. (a,b) Dot‐plot analysis of F4/80+CD11b+ cells in ST of C57 (a) and STR mice (b); x‐axis, F4/80; y‐axis, CD11b. (c,d) Histogram analysis of CD11c+ cells in the gated regions in the dot‐plots of ST cells isolated from C57 (c) and STR mice (d). Percentages of F4/80‐ and CD11b‐positive cells (e) and CD11c+ cells in F480‐ and CD11b‐positive gated regions in ST of C57 and STR mice (f) (n = 5). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Expression of IL‐1β, CGRP, CLR and RAMP1 in ST
Real‐time PCR analysis of the genes encoding IL‐1β, CGRP, CLR and RAMP1 showed that the expression levels of IL‐1β and CLR were elevated significantly in the ST of STR/Ort mice compared to those in control C57BL/6J mice (Fig. 2a,c). In contrast, no differences in the expression of the genes encoding CGRP and RAMP1 were detected between C57BL/6J and STR/Ort mice (Fig. 2b,d).
Figure 2.
Real‐time polymerase chain reaction (PCR) analysis for the expression of interleukin (IL)‐1β, calcitonin gene‐related peptide (CGRP), receptor activity‐modifying protein 1 (RAMP1) and calcitonin receptor‐like receptor (CLR) genes in synovial tissues (a–d) of C57BL/6J (C57) and STR/Ort (STR) mice. *Statistically significant difference between C57 and STR mice. All data are presented as the mean ± standard error (n = 10).
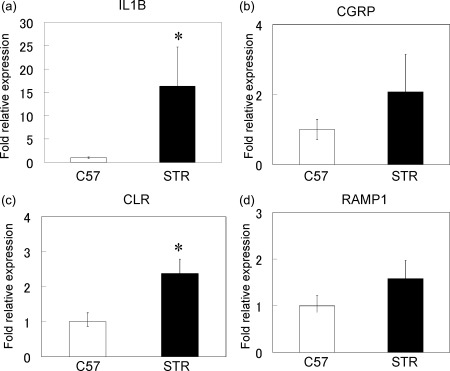
Activated macrophages reportedly produce higher levels of IL‐1β than tissue‐resident macrophages 12. Here, to evaluate whether macrophages in ST also produce IL‐1β, the expression of the gene encoding IL‐1β in F4/80‐positive cells isolated from the ST of STR/Ort mice was examined by real‐time PCR (Fig. 3). IL‐1β gene expression in F4/80‐positive cell fractions was threefold higher than that in F4/80‐negative cells (Fig. 3a). In contrast, the expression of the genes encoding CGRP, CLR and RAMP1 in the F4/80‐positive cell fraction was lower than that in the F4/80‐negative cell fraction (Fig. 3b–d).
Figure 3.
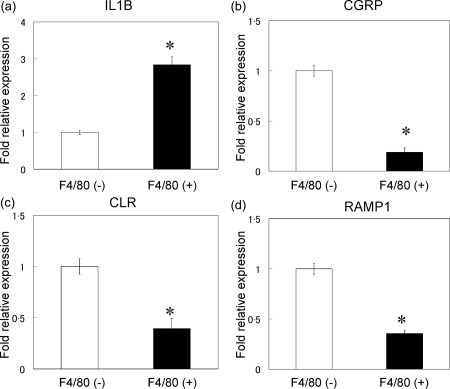
Real‐time polymerase chain reaction (PCR) analysis for the expression of interleukin (IL)‐1β, calcitonin gene‐related peptide (CGRP), calcitonin receptor‐like receptor (CLR) and receptor activity‐modifying protein 1 (RAMP1) genes in F4/80‐negative and ‐positive cell fractions of synovial tissues of STR/Ort mice. All data are presented as the mean ± standard error of three experiments (n = 3).
Effect of clodronate liposome injection on the expression of IL‐1β, CGRP, CLR and RAMP1
The induction of macrophage depletion by the injection of liposomal clodronate decreased the number of F4/80+CD11b+ cells significantly in the spleens of liposomal clodronate‐injected STR/Ort mice compared to PBS‐injected STR/Ort mice (Fig. 4a–c). Macrophage depletion also led to a significant decrease of IL‐1β and CLR gene expression in the ST of STR/Ort mice (Fig. 5a,c). In contrast, macrophage depletion had no significant effects on the expression of CGRP or RAMP1 in ST (Fig. 5b,d).
Figure 4.
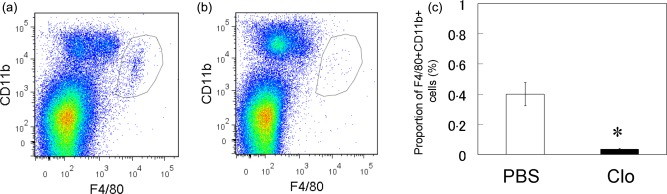
Flow cytometric analysis of F4/80+CD11b macrophage cells in the spleen of phosphate‐buffered saline (PBS)‐injected (PBS) and clodronate‐injected STR/Ort mice (Clo). (a,b) Dot‐plot analysis of F4/80+CD11b+ cells in the spleens of PBS (a) and Clo (b) mice; x‐axis, F4/80; y‐axis, CD11b. (c) Percentage of F4/80‐ and CD11b‐positive cells in the spleens of PBS and Clo mice (n = 10). [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 5.
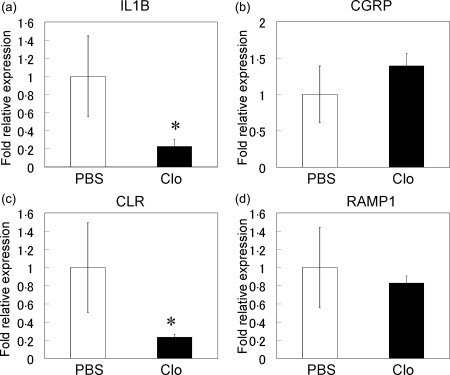
Real‐time polymerase chain reaction (PCR) analysis for interleukin (IL)‐1β, calcitonin gene‐related peptide (CGRP), calcitonin receptor‐like receptor (CLR) and receptor activity‐modifying protein 1 (RAMP1) gene expression in the synovial tissue (a–d) of phosphate‐buffered saline (PBS)‐injected (PBS) and clodronate‐injected STR/Ort (Clo) mice. *Statistically significant difference between PBS and Clo mice. All data are presented as the mean ± standard error (n = 10).
Effect of IL‐1β on expression of CGRP, CLR and RAMP1 in synovial cells
Real‐time PCR analysis revealed that the gene expression of CLR increased significantly in synovial cells in the presence of exogenously added IL‐1β compared to untreated control cells (Fig. 6). In contrast, the expression of the genes encoding CGRP and RAMP1 were not affected in IL‐1β‐treated synovial cells (Fig. 6).
Figure 6.
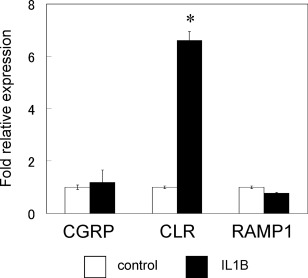
Effect of interleukin (IL)‐1β on calcitonin gene‐related peptide (CGRP), calcitonin receptor‐like receptor (CLR) and receptor activity‐modifying protein 1 (RAMP1) gene expression in cultured synovial cells. All real‐time polymerase chain reaction (PCR) data are presented as the mean ± standard error of four experiments (n = 3). F4/80(−) cells stimulated with 50 ng/ml IL‐1β were compared with untreated (0 ng/ml IL‐1β) F4/80(−) cells as a control.
Discussion
In the present study investigating the mechanisms underlying OA pain, CD11c+ populations of macrophages were found to be elevated in the ST of STR/Ort mice. Higher expression of the genes encoding IL‐1β and CLR was also observed in the ST of STR/Ort mice compared to C57BL/6J mice. However, macrophage depletion by the treatment of mice with clodronate‐laden liposomes decreased IL‐1β and CLR gene expression in ST. In addition, the treatment of cultured synovial cells with IL‐1β stimulated CLR expression. Taken together, these findings suggest that IL‐1β‐producing macrophages regulate CLR expression in synovial cells and may contribute to the pain in OA patients.
The cytokines produced by synovial macrophages, particularly IL‐1β and TNF‐α, contribute to inflammation and OA progression 13, 14, 15. A recent study reported that macrophages display one of two distinct phenotypes: classically activated M1 macrophages and alternatively activated M2 macrophages 16. CD11c is a marker of M1 macrophages, which produce high levels of IL‐1β 12. In the present study, the number of CD11c+ macrophages was increased in the synovium of STR/Ort mice. Consistent with this finding, higher expression of IL‐1β was observed in synovial macrophages which, when depleted by the treatment of mice with liposomal clodronate, led to decreased IL‐1β gene expression in ST. Taken together, these findings indicate that IL‐1β is produced mainly by macrophages in synovium.
Numerous studies have implicated the involvement of CGRP in the peripheral mechanisms of OA pain 6, 17, 18, 19, 20, 21, 22. For example, suppression of the expression of CGRP in the synovium of an adjuvant‐induced arthritis rat model has been shown to attenuate the pain 17. In addition, in a monosodium iodoacetate‐induced OA model, the gene expression of calcitonin receptor increased and the administration of CGRP receptor antagonist resulted in reduced pain 6. Here, we found that CLR gene expression was increased in the synovium of STR/Ort mice, particularly in the F4/80‐negative fraction of synovial cells. However, macrophage depletion led to decreased CLR and IL‐1β gene expression in ST of STR/Ort mice. In addition, the treatment of ST cells with IL‐1β increased CLR gene expression. These results suggest that macrophage‐produced IL‐1β regulates CLR expression in ST and may induce CGRP/CGRP receptor signalling, thereby contributing to the development of pain in OA.
Two limitations of the present study warrant mention. First, the mechanism proposed in this study was based on cross‐sectional analysis, and therefore no data on the effects of the observed changes on the progression of OA are available. Secondly, we revealed that IL‐1β and CLR gene expression was elevated in the ST of osteoarthritic mice; however, it remains to be determined if the elevation of IL‐1β and CLR levels contributes to OA pain.
In conclusion, synovial macrophages are the main IL‐1β producer and regulator of CLR in OA mice. Thus, macrophages and IL‐1β may regulate CGRP/CGRP receptor signalling in OA joints and be suitable therapeutic targets for treating OA pain.
Disclosure
The authors declare that there is no disclosure regarding the publication of this paper.
Author contributions
All authors read the manuscript and had input into revising it for intellectual content and style. For experimental design: K. U. and M. T.; for acquisition of data: S. T. K. U., M. M., G. I., A. J., H. F., A. M., M. S. and K. I.; for analysis and interpretation of data: S. T., K. U., A. M., M. S. and K. I. For drafting the manuscript: S. T., K.U. and M. T.
Acknowledgements
This investigation was supported in part by JSPS KAKENHI Grant no. 15K20016, the Uehara Memorial Foundation, a Kitasato University Research Grant for Young Researchers, and research grants from the Parents’ Association of Kitasato University School of Medicine.
References
- 1. Aiyar J, Rizzi JP, Gutman GA, Chandy KG. The signature sequence of voltage‐gated potassium channels projects into the external vestibule. J Biol Chem 1996; 271:31013–6. [DOI] [PubMed] [Google Scholar]
- 2. McLatchie LM, Fraser NJ, Main MJ et al RAMPs regulate the transport and ligand specificity of the calcitonin‐receptor‐like receptor. Nature 1998; 393:333–9. [DOI] [PubMed] [Google Scholar]
- 3. Doods H, Arndt K, Rudolf K, Just S. CGRP antagonists: unravelling the role of CGRP in migraine. Trends Pharmacol Sci 2007; 28:580–7. [DOI] [PubMed] [Google Scholar]
- 4. Ho TW, Connor KM, Zhang Y et al Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology 2014; 83:958–66. [DOI] [PubMed] [Google Scholar]
- 5. Benschop RJ, Collins EC, Darling RJ et al Development of a novel antibody to calcitonin gene‐related peptide for the treatment of osteoarthritis‐related pain. Osteoarthritis Cartilage 2014; 22:578–85. [DOI] [PubMed] [Google Scholar]
- 6. Bullock CM, Wookey P, Bennett A, Mobasheri A, Dickerson I, Kelly S. Peripheral calcitonin gene‐related peptide receptor activation and mechanical sensitization of the joint in rat models of osteoarthritis pain. Arthritis Rheumatol 2014; 66:2188–200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uchida K, Satoh M, Inoue G et al CD11c(+) macrophages and levels of TNF‐alpha and MMP‐3 are increased in synovial and adipose tissues of osteoarthritic mice with hyperlipidaemia. Clin Exp Immunol 2015; 180:551–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ohtori S, Orita S, Yamauchi K et al Efficacy of direct injection of etanercept into knee joints for pain in moderate and severe knee osteoarthritis. Yonsei Med J 2015; 56:1379–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schaible HG, von Banchet GS, Boettger MK et al The role of proinflammatory cytokines in the generation and maintenance of joint pain. Ann NY Acad Sci 2010; 1193:60–9. [DOI] [PubMed] [Google Scholar]
- 10. Shimura Y, Kurosawa H, Sugawara Y et al The factors associated with pain severity in patients with knee osteoarthritis vary according to the radiographic disease severity: a cross‐sectional study. Osteoarthritis Cartilage 2013; 21:1179–84. [DOI] [PubMed] [Google Scholar]
- 11. Hill CL, Hunter DJ, Niu J et al Synovitis detected on magnetic resonance imaging and its relation to pain and cartilage loss in knee osteoarthritis. Ann Rheum Dis 2007; 66:1599–603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim HY, Lee HJ, Chang YJ et al Interleukin‐17‐producing innate lymphoid cells and the NLRP3 inflammasome facilitate obesity‐associated airway hyperreactivity. Nat Med 2014; 20:54–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lu S, Xiao X, Cheng M. Matrine inhibits IL‐1beta‐induced expression of matrix metalloproteinases by suppressing the activation of MAPK and NF‐kappaB in human chondrocytes in vitro . Int J Clin Exp Pathol 2015; 8:4764–72. [PMC free article] [PubMed] [Google Scholar]
- 14. Kapoor M, Martel‐Pelletier J, Lajeunesse D, Pelletier JP, Fahmi H. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol 2011; 7:33–42. [DOI] [PubMed] [Google Scholar]
- 15. Bondeson J, Wainwright SD, Lauder S, Amos N, Hughes CE. The role of synovial macrophages and macrophage‐produced cytokines in driving aggrecanases, matrix metalloproteinases, and other destructive and inflammatory responses in osteoarthritis. Arthritis Res Ther 2006; 8:R187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lumeng CN, Bodzin JL, Saltiel AR. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J Clin Invest 2007; 117:175–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ahmed AS, Li J, Ahmed M et al Attenuation of pain and inflammation in adjuvant‐induced arthritis by the proteasome inhibitor MG132. Arthritis Rheum 2010; 62:2160–9. [DOI] [PubMed] [Google Scholar]
- 18. Fernihough J, Gentry C, Bevan S, Winter J. Regulation of calcitonin gene‐related peptide and TRPV1 in a rat model of osteoarthritis. Neurosci Lett 2005; 388:75–80. [DOI] [PubMed] [Google Scholar]
- 19. Hirsch S, Corradini L, Just S, Arndt K, Doods H. The CGRP receptor antagonist BIBN4096BS peripherally alleviates inflammatory pain in rats. Pain 2013; 154:700–7. [DOI] [PubMed] [Google Scholar]
- 20. Kalff KM, El MM, van EJ et al Pre‐treatment with capsaicin in a rat osteoarthritis model reduces the symptoms of pain and bone damage induced by monosodium iodoacetate. Eur J Pharmacol 2010; 641:108–13. [DOI] [PubMed] [Google Scholar]
- 21. Saito T, Koshino T. Distribution of neuropeptides in synovium of the knee with osteoarthritis. Clin Orthop Relat Res 2000; 172–82. [DOI] [PubMed] [Google Scholar]
- 22. Saxler G, Loer F, Skumavc M, Pfortner J, Hanesch U. Localization of SP‐ and CGRP‐immunopositive nerve fibers in the hip joint of patients with painful osteoarthritis and of patients with painless failed total hip arthroplasties. Eur J Pain 2007; 11:67–74. [DOI] [PubMed] [Google Scholar]


