Summary
B cells undergo a number of different developmental stages, from initial formation of their B cell receptor (BCR) genes to differentiation into antibody‐secreting plasma cells. Because the BCR is vital in these differentiation steps, autoreactive and exogenous antigen binding to the BCR exert critical selection pressures to shape the B cell repertoire. Older people are more prone to infectious disease, less able to respond well to vaccination and more likely to have autoreactive antibodies. Here we review evidence of changes in B cell repertoires in older people, which may be a reflection of age‐related changes in B cell selection processes.
Keywords: ageing, antibodies, B cell, repertoire, selection
Introduction
As with many biological systems, the B cell repertoire exists in a state of equilibrium. It is beneficial to have the widest diversity of potential antigen specificities as possible, in order to have the best chance of recognizing any potential challenge to the organism. At the same time, any B cells with receptors that recognize self have to be eliminated or controlled to avoid potential autoreactive pathology. Evidence from our laboratory, and others, has been accumulating to suggest that the aged immune system is less efficient as a result of dysregulation of this balance, rather than a simple failure to respond.
‘B cell repertoire’ is a phrase that can be used at both the cellular and molecular levels. At the cellular level, there are a number of different phases of B cell development, from immature B cells to memory cells and antibody‐secreting plasma cells. Given the finite size of the B cell ‘space’, and the findings that the total number of B cells changes little throughout most of life, changes in the different types of B cells represented in the population will have important consequences. At the molecular level, the specificity of an individual B cell is defined by the variable regions of immunoglobulin genes. The functionality of antibodies produced by these cells can be changed by class‐switching of the constant regions of immunoglobulin (Ig) genes. Thus, a study of Ig genes can determine the breadth of the repertoire in terms of B cell receptor/antibody specificity. As the Ig genes determine the specificity of a B cell, selective forces imposed by events dependent upon antigen specificity will be reflected in the Ig gene repertoire of a population.
Generation of B cell repertoire diversity
Initial B cell Ig gene diversity is created during early B cell development in the bone marrow. The Ig gene locus comprises a number of different types of genes that make up the variable region of the antibody. A process of random gene assortment to recombine IGHV,IGHD,IGHJ gene segments for heavy chain (and IGKV,IGKJ or IGLV,IGLJ segments for kappa or lambda light chains) can facilitate the creation of thousands of different variable regions from just a few hundred different gene segments 1. The subsequent random assortment of heavy and light chains increases the diversity further, to more than 4 million different possible combinations (Fig. 1). These numbers are hypothetical, as factors such as proximity of gene segments to each other or recombination signal sequence preference can influence gene choice, and this skews the recombination slightly 2. In addition, and a much greater influence, is the increase in diversity because the joining of the different segments is imprecise and includes random extra nucleotide addition by the action of an enzyme called terminal deoxynucleotidyl transferase (TdT) 3. Therefore, there will probably be many more than 4 million combinations. However, not all combinations of V(D)J genes will survive early development and enter the mature B cell repertoire. Only the heavy chain rearrangements that are functional and bind effectively with surrogate light chain and kappa or lambda light chain will be able to send back survival signals to ensure that the cell progresses further in its development pathway 4. This is the first selection step in altering the shape of the repertoire (Fig. 2). The second influence over the shaping of the B cell repertoire is the process of central tolerance. One of the inevitable consequences of generating such a huge diversity of different B cell specificities is that some heavy/light chain pairs will produce a B cell receptor (BCR) that will recognize self‐antigens. These will be removed from the repertoire at the immature stage by a poorly understood process of negative selection 5. Some cells avoid negative selection by editing their receptors, replacing the light chain with a different light chain in an effort to change the receptor specificity to something more acceptable 6. Transitional cells leaving the bone marrow may be subjected to a further round of negative selection, which involves competition for the B cell survival factor [B cell activating factor (BAFF)] 7. The surviving mature B cells form the naive B cell compartment, which is a highly diverse pool available to react against challenge. Upon challenge, the naive cells that recognize the antigen will expand, so the repertoire is again affected by positive selection. Further genetic diversity of the activated B cell repertoire can be introduced by the processes of somatic hypermutation and class‐switching in the germinal centre of secondary lymphoid follicles 8. Both these processes are initiated by the deamination of cytosine to uracil in DNA by the enzyme activation‐induced cytidine deaminase (AID) 9. B cells carrying Ig genes where hypermutation has resulted in a significant advantage in antigen binding will out‐compete other B cells for survival signals in the germinal centre, a selection process reminiscent of Darwinian evolution 8. The B cells can undergo class‐switching to change the class of antibody from IgM/IgD to IgG/IgA/IgE, which keeps the variable region binding qualities but changes the potential function of the BCR/antibody.
Figure 1.
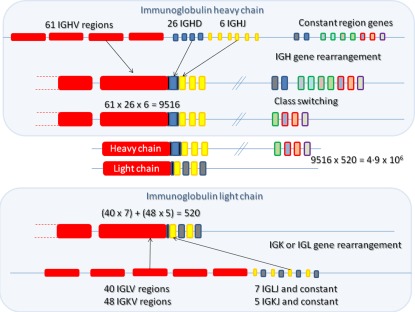
Generation of antibody diversity. A number of different genes exist in three loci in the genome, for heavy chain [immunoglobulin (IG)H], kappa light chain (IGK) and for lambda light chain (IGL). Gene rearrangement occurs between the variable (IGHV), diversity (IGHD) and joining (IGHJ) regions of heavy chain, such that one of each type of gene is brought together with the help of recombination activating genes (RAG1 and RAG2). Similarly, the light chain genes are rearranged, either kappa or lambda, but without any diversity regions. Random recombination of heavy and light chain genes results in 9516 or 520 different combination possibilities, respectively. Random assortment of heavy and light chain gene rearrangements further increases the number of possibilities, in a multiplicative manner, to 4·9 × 106. Inaccurate joining of the V(D)J regions further increases the possibility for diversity. Antibody CDR3 regions are at the junction of the different genes, and therefore the CDR3 regions have the highest diversity.
Figure 2.
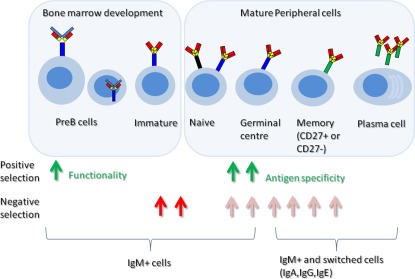
Selection events in B cell development. B cells undergo a number of developmental stages in the bone marrow and in the periphery. At various stages there will be positive selection to enrich for B cells with attributes useful for binding exogenous antigens (green arrows) and negative selection to ensure that autoreactive B cells do not survive (red arrows).
Cellular repertoire of B cells
Mature B cells in the periphery are not of a uniform type. As well as there being a distinction between naive and memory, and between B cells of different classes of BCR/antibody, there are further distinctions of B cells by their phenotype. Classical memory cells produced in the T‐dependent germinal centre response are recognized by the presence of memory marker CD27 on their surface in conjunction with the loss of IgD in favour of IgG/IgA/IgE 10. The presence of CD27 on the surface of an IgD‐positive B cell distinguishes a population of so‐called ‘IgM memory’ cells 11. While this population contains some cells that are precursors to the classical switched memory cells (IgD–, CD27+) 12, a larger proportion of this compartment is thought to be activated by a different pathway, possibly in a T‐independent antibody response to antigens such as bacterial polysaccharides 13, 14, 15. Toll‐like receptor (TLR) signalling pathways, in particular TLR‐10, are thought to be important for the development and/or survival of ‘IgM memory’ cells 16. Regulatory B cells are also present in the ‘IgM memory’ population 17. Another population separated by IgD/CD27 is the population that is negative for both markers and which seems to comprise memory cells 18. However, there are also reports that this population contains tissue‐trafficking B cells that can produce granzyme B 19 and some of the IgA+ CD27– cells may be T‐independent responders, carrying polyreactive BCRs which bind readily to bacterial antigens 20, so the population is probably of mixed function.
Age‐related changes
The older immune system is less able to respond effectively to infectious challenge. Pneumococcal disease is perhaps the most well‐known instance of immune failure. Very young and elderly people have less resistance to Streptococcus pneumoniae, older people being three times more susceptible to pneumonia than young adults and having a greater morbidity and risk of mortality 21. The 23 valent pneumococcal polysaccharide vaccine is also less efficient at protecting very young and elderly people, although in the case of young people this has been circumvented by conjugating the polysaccharide to a protein antigen 22. There are many more infections, with perhaps a lower profile, that increase in incidence and morbidity with age 23; for example, urinary tract infections are up to ×20 more common in older people 24. Recent data on the mortality rates from the recent Ebola virus disease epidemic in West Africa also shows a greater mortality in the very young and the older population compared to young adults 25.
One of the most common ways of measuring the extent of an immune response is by measuring antibody efficiency after immunization. Whether this is performed by enzyme‐linked immunosorbent assay (ELISA) or by functional studies such as opsonophagocytic or haemagglutination inhibition assays, it is clear that the efficacy of the human antibody response is decreased with age 26, 27. The total number of B cells remains constant throughout most of life 28. Only in extreme old age does a decline occur 29, long after the loss of immune system efficacy is first seen. Levels of serum and salivary antibody are quite variable between individuals, but there has been no consistently reported increase/decline in antibody quantity with age 30. Hence, it is generally accepted that it is the quality of the B cell/antibody repertoire that changes.
Cellular repertoire
There are some age‐related changes in the peripheral blood B cell composition. There is a general decrease of memory and plasma cells as defined by CD27 and CD38 surface markers 31. The IgM memory compartment has been reported to decrease with age 32, 33, and may well be responsible for the decreased reactivity to polysaccharide antigens and increased susceptibility of older people to bacterial infections such as pneumococcal disease 21, 33, 34, particularly in light of the fact that removal of IgM from serum shows a decrease in opsonophagocytic activity 35 and ELISA analysis of polysaccharide‐specific IgM shows an age‐related decrease after pneumococcal vaccination 36. Furthermore, we have also shown that there is a great deal of variability in IgM memory cells with respect to the quantity of IgM on the surface of the cell. In particular, we could identify two populations that differed in a number of respects – one that was IgM medium, IgD high CD27+ and the other being IgM high, IgD medium CD27+. The latter population was increased in age at the expense of the IgD high population 32, so the repertoire of B cells available to respond to T‐independent challenge may well be compromised in old age.
The other major change in the peripheral blood B cell population is an increase in IgD– memory cells lacking the activation marker CD27 37. These cells are also increased in chronic viral infection, and in autoimmune conditions such as systemic lupus erythematosus (SLE) 38, 39, 40. Studies looking at levels of somatic hypermutation in conjunction with replication history in normal and CD40 ligand‐deficient patients suggest a T‐independent origin for these cells, at least for the CD27–IgA+ subset 20. In our laboratory we also see lower levels of somatic hypermutation in the CD27– cells in the population as a whole, but we find a very similar B cell repertoire between the CD27– and CD27+ populations and evidence of members of the same clonal expansion of B cells differing by CD27 expression 18. This would indicate that lack of CD27 on the cell surface is not always a feature of lineage. Taken together, this information, coupled with the fact that older people have more autoreactive antibody in their serum, makes it tempting to speculate that an increase in CD27‐ memory cells may be partially a result of down‐regulated activation markers as part of a peripheral tolerance mechanism, i.e. an insufficient numbers of appropriate T cells in a T‐dependent reaction may result in some members of a B cell clone missing out on the CD40 interactions required to maintain activation status. There is as yet no evidence to support this, and further characterization of the function of CD27– memory B cells will be required to interpret correctly the observed age‐related changes.
The B cell environment is paramount to their survival and function. Information on tissue‐resident B cells is scarce. Although there is evidence in mice that germinal centres decrease in size and number with age 41, and there is a report of human tonsillar germinal centre changes 42, previous studies on human splenic, lymph node and gut‐associated follicles did not show any significant age‐related changes in the size of the different B cell compartments 43, 44. The bone marrow environment is crucial for early B cell development, and undergoes substantial age‐related changes, with decreased cellularity and increased fatty tissue 45. Whether this causes decreased naive B cell output, as in mice, has not been shown formally but is assumed in many hypotheses of ageing lymphocyte studies. A decrease in the numbers of antigen‐specific plasma cells in the bone marrow has been reported recently and will probably affect the quality of serum antibody 31.
Immunoglobulin gene repertoire
Because the quality of the immune response with age is called into question, studies of the Ig gene repertoire have been undertaken to look for indications that the repertoire might change with age 46. None of our studies have indicated that the mechanism of somatic hypermutation of memory cells is altered in any way. Within single germinal centres the rate of hypermutation is unaltered, and the AID‐targeting motifs do not vary 43, 47. Our first indication that a change in selection of the B cell repertoire might occur was in a study of individual germinal centres. B cells were microdissected out of germinal centres and their Ig sequences were assembled into lineage trees to determine how the high‐affinity B cells were selected. We found that that the B cell selection processes in the gut appeared to weaken with age, although there was no equivalent change in the splenic germinal centres 47. Later studies of repertoire diversity, using spectratype analysis of the Ig CDRH3 region, indicated that an increase in expanded clones of B cells occurs with age and correlates with ill health and poor survival in the very elderly 48.
The advent of high‐throughput sequencing (HTS) has enabled a more detailed and comprehensive study of changes in the Ig gene repertoire. An HTS study of identical twin pairs indicates that Ig gene segment usage has a strong genetic determinant and is quite resilient to environmental influences, so any observed changes in Ig gene segment use with age or disease may be confounded by interindividual variation. The CDRH3 region, however, is much more reflective of environmental effects 36, 49. Further confounders of HTS results are the variations in Ig gene repertoire that can be seen between different subsets of B cells 13. Because, as mentioned above, the composition of B cell subtypes in the blood may change with age, repertoire changes seen in whole blood may partially reflect the change in cellular repertoire. Nevertheless, a great deal of useful information is to be gained from whole peripheral blood analyses, especially from studies looking at immune challenge. HTS of the repertoire before and after vaccine challenge has highlighted the diversity of an immune response at all ages, with many different types of genes expanding upon challenge 50, 51. It has shown that older people have switched memory cells with a higher load of mutation prior to vaccination, and confirms in detail that some people have pre‐expanded population of B cells in the repertoire 51. Where relative proportions of IgM versus IgG antibodies change after challenge to reflect creation of more IgG memory, this is not always as apparent in older people 51. Detailed analyses of the different subclasses of antibody are possible, and have indicated that some age‐related changes are class‐specific. In particular, the IgM and IgA polysaccharide response in elderly people is diminished, coincident with a reduced expansion of IgM and IgA genes in the repertoire 36, 50, highlighting the fact that different types of antibody can be selected by different antigens and this selection may fail with age. Chronic viral infections such as cytomegalovirus (CMV) and Epstein–Barr virus (EBV) can have a selective effect on the B cell repertoire, affecting the presence of clonal expansions (EBV) or the levels of mutation of antibodies (CMV), so it is useful to know the donor status with respect to these viruses in future studies on ageing 52.
We can identify differences in gene usage during B cell repertoire development in young subjects. As well as our studies showing differences between different mature B cell subsets 13, we can see changes in gene use between immature and naive B cells (data not shown). Notable is a decrease in the use of IGHV3 family between immature and naive, and between naive and class‐switched memory, although IGHV3 family use increases in IgM memory cells 13. In view of the fact that immunoglobulin repertoire changes with the type of B cell, we have conducted a study of repertoire in people of different ages after sorting cells with respect to IgD and CD27 expression, and according to subclass usage. Some differences can be seen in older people, such as an increased use of IgA2 over IgA1, and of IgG2 over IgG1 and IgG3 in CD27+ memory cells. Moreover, a repertoire distinction that existed between IgG2 and IgG1/3 in younger subjects was no longer seen in older individuals, the low IGHV1 family/high IGHV3 family characteristic of IgG2 in the young being the predominant pattern for all IgG in older samples 53. This, and other changes in CDRH3 characteristics, indicates that the activation factors involved in repertoire selection in the young have changed with time, such that older people may have a repertoire more influenced by IgG2‐type activation. As IgG2 is the subclass involved in T‐independent responses, such as to polysaccharide antigen, one hypothesis would be that the older repertoire is selected in a more T‐independent fashion.
Aside from measuring expansions of genes or the usage of gene segments, a study of the CDRH3 region of antibodies is critical. The CDRH3 region has the largest influence over the antigen‐binding site and is therefore the most likely to be affected by positive or negative selection events 49. Both the spectratype and the high‐throughput sequencing methods have shown that the repertoire of antibodies upon challenge has a smaller CDRH3 size 36, 50, 52, implying that smaller CDRH3 have an advantage in the response. This appears to be independent of specific antigen, as it is a general feature of memory cells compared with naive cells 36, 52, 53, 54. Peptide analysis also shows an increased hydrophilic characteristic after challenge 50. While these differences occur at all ages, the challenge‐related decrease in size is less marked in the samples from older individuals, implying that the selection of repertoire for smaller CDRH3 size is diminished with age 36. Hydrophobicity of CDRH3 after challenge does not appear to change with age in a broad overview of sequences, although when cells are separated by CD27 and IgG subclass before sequencing there are some significant age‐related differences in the physical characteristics of the CDRH3, particularly CD27– IgG2 cells 52, 53.
At first sight, it might seem that a decrease in CDRH3 size is an advantage for positive selection during challenge by exogenous antigen. However, an alternative explanation is that a larger, hydrophobic, CDRH3 may be a disadvantage for survival in the face of negative selection mechanisms that ensure tolerance towards self‐antigens. This latter hypothesis was prompted by the observations, using both Sanger sequencing and HTS methods, that naive B cells have shorter, less hydrophobic, CDRH3 regions than immature B cells in the bone marrow (data not shown 55). HTS analysis of DNA sequences that can be divided into productive versus non‐productive sequences has also shown that there is a general selection for shorter hydrophilic sequences in the functional repertoire 56, so there is a selection against longer hydrophobic CDRH3 regions coincident with the negative selection processes of central tolerance. The older samples show a greater CDRH3 size in the naive repertoire, indicating that these putative negative selection processes are also less effective with age 52, 53. Coupled with the fact that older people have more autoreactive antibodies, we therefore propose the hypothesis that at all stages of B cell development, including during a response to exogenous challenge, there are negative selection tolerance processes that are less efficient with age, and where the size and physicochemical characteristics of immunoglobulin CDRH3 are major determinants of the tolerance process. These changes in selection may be partially responsible for the altered repertoire in different B cell subsets of the aged repertoire.
In summary, there are both positive and negative selection processes in the immune system which depend largely upon antigen specificity, and are therefore reflected in the repertoire of Ig genes in the B cell population. Knowledge of the mechanisms of selection processes and how they affect the repertoire, together with repertoire comparisons between people of different ages, should help to elucidate the age‐related changes in B cell function that contribute to immune senescence.
Disclosure
There are no relevant disclosures.
Acknowledgements
The author would like to acknowledge the following support for our ageing research: The Dunhill Medical Trust, Research into Ageing, The Rosetrees Trust, The Human Frontiers Science Program, MRC and BBSRC.
References
- 1. Alt FW, Blackwell TK, Yancopoulos GD. Development of the primary antibody repertoire. Science 1987; 238:1079–87. [DOI] [PubMed] [Google Scholar]
- 2. Degner‐Leisso SC, Feeney AJ. Epigenetic and 3‐dimensional regulation of V(D)J rearrangement of immunoglobulin genes. Semin Immunol 2010; 22:346–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. VanDyk L, Meek K. Assembly of IgH CDR3: mechanism, regulation, and influence on antibody diversity. Int Rev Immunol 1992; 8:123–33. [DOI] [PubMed] [Google Scholar]
- 4. Mårtensson IL, Keenan RA, Licence S. The pre‐B‐cell receptor. Curr Opin Immunol 2007; 19:137–42. [DOI] [PubMed] [Google Scholar]
- 5. Rowland SL, Tuttle K, Torres RM, Pelanda R. Antigen and cytokine receptor signals guide the development of the naïve mature B cell repertoire. Immunol Res 2013; 55:231–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Hertz M, Nemazee D. Receptor editing and commitment in B lymphocytes. Curr Opin Immunol 1998; 10:208–13. [DOI] [PubMed] [Google Scholar]
- 7. Stadanlick JE, Cancro MP. BAFF and the plasticity of peripheral B cell tolerance. Curr Opin Immunol 2008; 20:158–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. McHeyzer‐Williams LJ, McHeyzer‐Williams MG. Antigen‐specific memory B cell development. Annu Rev Immunol 2005; 23:487–513. [DOI] [PubMed] [Google Scholar]
- 9. Lee GS, Brandt VL, Roth DB. B cell development leads off with a base hit: dU:dG mismatches in class switching and hypermutation. Mol Cell 2004; 16:505–8. [DOI] [PubMed] [Google Scholar]
- 10. Sanz I, Wei C, Lee FE, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol 2008; 20:67–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Tangye SG, Good KL. Human IgM+CD27+ B cells: memory B cells or ‘memory’ B cells? J Immunol 2007; 179:13–9. [DOI] [PubMed] [Google Scholar]
- 12. Seifert M, Küppers R. Molecular footprints of a germinal center derivation of human IgM+(IgD+)CD27+ B cells and the dynamics of memory B cell generation. J Exp Med 2009; 206:2659–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Wu YC, Kipling D, Leong HS, Martin V, Ademokun AA, Dunn‐Walters DK. High‐throughput immunoglobulin repertoire analysis distinguishes between human IgM memory and switched memory B‐cell populations. Blood 2010; 116:1070–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Scheeren FA, Nagasawa M, Weijer K et al T cell‐independent development and induction of somatic hypermutation in human IgM+ IgD+ CD27+ B cells. J Exp Med 2008; 205:2033–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Weill JC, Weller S, Reynaud CA. Human marginal zone B cells. Annu Rev Immunol 2009; 27:267–85. [DOI] [PubMed] [Google Scholar]
- 16. Weller S, Bonnet M, Delagreverie H et al IgM+IgD+CD27+ B cells are markedly reduced in IRAK‐4‐, MyD88‐, and TIRAP‐ but not UNC‐93B‐deficient patients. Blood 2012; 120:4992–5001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Khoder A, Sarvaria A, Alsuliman A et al Regulatory B cells are enriched within the IgM memory and transitional subsets in healthy donors but are deficient in chronic GVHD. Blood 2014; 124:2034–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wu YC, Kipling D, Dunn‐Walters DK. The relationship between CD27 negative and positive B cell populations in human peripheral blood. Front Immunol 2011; 2:81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Bulati M, Buffa S, Martorana A et al Trafficking phenotype and production of granzyme B by double negative B cells (IgG(+)IgD(−)CD27(−)) in the elderly. Exp Gerontol 2014; 54:123–9. [DOI] [PubMed] [Google Scholar]
- 20. Berkowska MA, Schickel JN, Grosserichter‐Wagener C et al Circulating human CD27‐IgA+ memory B cells recognize bacteria with polyreactive Igs. J Immunol 2015; 195:1417–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marrie TJ. Community‐acquired pneumonia in the elderly. Clin Infect Dis 2000; 31:1066–78. [DOI] [PubMed] [Google Scholar]
- 22. Steens A, Vestrheim DF, Aaberge IS et al A review of the evidence to inform pneumococcal vaccine recommendations for risk groups aged 2 years and older. Epidemiol Infect 2014; 142:2471–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Gavazzi G, Krause KH. Ageing and infection. Lancet Infect Dis 2002; 2:659–66. [DOI] [PubMed] [Google Scholar]
- 24. Nicolle LE. Urinary tract infection in geriatric and institutionalized patients. Curr Opin Urol 2002; 12:51–5. [DOI] [PubMed] [Google Scholar]
- 25. WHO Ebola Response Team ; Agua‐Agum J, Ariyarajah A, Blake IM et al Ebola virus disease among children in West Africa. N Engl J Med 2015; 372:1274–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Siegrist CA, Aspinall R. B‐cell responses to vaccination at the extremes of age. Nat Rev Immunol 2009; 9:185–94. [DOI] [PubMed] [Google Scholar]
- 27. Del Giudice G, Weinberger B, Grubeck‐Loebenstein B. Vaccines for the elderly. Gerontology 2015; 61:203–10. [DOI] [PubMed] [Google Scholar]
- 28. Tavares SM, Junior Wde L, Lopes E Silva MR. Normal lymphocyte immunophenotype in an elderly population. Rev Bras Hematol Hemoter 2014; 36:180–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ferguson FG, Wikby A, Maxson P, Olsson J, Johansson B. Immune parameters in a longitudinal study of a very old population of Swedish people: a comparison of survivors and nonsurvivors. J Gerontol Biol Sci 1995; 50:378–82. [DOI] [PubMed] [Google Scholar]
- 30. Dunn‐Walters DK, Ademokun AA. B cell repertoire and ageing. Curr Opin Immunol 2010; 22:514–20. [DOI] [PubMed] [Google Scholar]
- 31. Pritz T, Lair J, Ban M et al Plasma cell numbers decrease in bone marrow of old patients. Eur J Immunol 2015; 45:738–46. [DOI] [PubMed] [Google Scholar]
- 32. Martin V, Wu YC, Kipling D, Dunn‐Walters DK. Age‐related aspects of human IgM+ B cell heterogeneity. Ann NY Acad Sci 2015. doi:10.1111/nyas.12823 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shi Y, Yamazaki T, Okubo Y, Uehara Y, Sugane K, Agematsu K. Regulation of aged humoral immune defense against pneumococcal bacteria by IgM memory B cell. J Immunol 2005; 175:3262–7. [DOI] [PubMed] [Google Scholar]
- 34. Moberley SA, Holden J, Tatham DP, Andrews RM. Vaccines for preventing pneumococcal infection in adults. Cochrane Database Syst Rev 2008; 1:CD000422. [DOI] [PubMed] [Google Scholar]
- 35. Park S, Nahm MH. Older adults have a low capacity to opsonize pneumococci due to low IgM antibody response to pneumococcal vaccinations. Infect Immun 2011; 79:314–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Ademokun A, Wu YC, Martin V et al Vaccination‐induced changes in human B‐cell repertoire and pneumococcal IgM and IgA antibody at different ages. Aging Cell 2011; 10:922–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Colonna‐Romano G, Bulati M, Aquino A et al A double‐negative (IgD‐CD27‐) B cell population is increased in the peripheral blood of elderly people. Mech Ageing Dev 2009; 130:681–90. [DOI] [PubMed] [Google Scholar]
- 38. Wei C, Anolik J, Cappione A et al A new population of cells lacking expression of CD27 represents a notable component of the B cell memory compartment in systemic lupus erythematosus. J Immunol 2007; 178:6624–33. [DOI] [PubMed] [Google Scholar]
- 39. Moir S, Ho J, Malaspina A et al Evidence for HIV‐associated B cell exhaustion in a dysfunctional memory B cell compartment in HIV‐infected viremic individuals. J Exp Med 2008; 205:1797–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Rojas OL, Narvaez CF, Greenberg HB, Angel J, Franco MA. Characterization of rotavirus specific B cells and their relation with serological memory. Virology 2008; 380:234–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zheng B, Han S, Takahashi Y, Kelsoe G. Immunosenescence and germinal center reaction. Immunol Rev 1997; 160:63–77. [DOI] [PubMed] [Google Scholar]
- 42. Kolar GR, Mehta D, Wilson PC, Capra JD. Diversity of the Ig repertoire is maintained with age in spite of reduced germinal centre cells in human tonsil lymphoid tissue. Scand J Immunol 2006; 64:314–24. [DOI] [PubMed] [Google Scholar]
- 43. Lazuardi L, Jenewein B, Wolf AM, Pfister G, Tzankov A, Grubeck‐Loebenstein B. Age‐related loss of naive T cells and dysregulation of T‐cell/B‐cell interactions in human lymph nodes. Immunology 2005; 114:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Banerjee M, Sanderson JD, Spencer J, Dunn‐Walters DK. Immunohistochemical analysis of ageing human B and T cell populations reveals an age‐related decline of CD8 T cells in spleen but not gut‐associated lymphoid tissue (GALT). Mech Ageing Dev 2000; 115:85–99. [DOI] [PubMed] [Google Scholar]
- 45. Pritz T, Weinberger B, Grubeck‐Loebenstein B. The aging bone marrow and its impact on immune responses in old age. Immunol Lett 2014; 162:310–5. [DOI] [PubMed] [Google Scholar]
- 46. Boyd SD, Liu Y, Wang C, Martin V, Dunn‐Walters DK. Human lymphocyte repertoires in ageing. Curr Opin Immunol 2013; 25:511–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Banerjee M, Mehr R, Belelovsky A, Spencer J, Dunn‐Walters DK. Age‐ and tissue‐specific differences in human germinal center B cell selection revealed by analysis of IgVH gene hypermutation and lineage trees. Eur J Immunol 2002; 32:1947–57. [DOI] [PubMed] [Google Scholar]
- 48. Gibson KL, Wu YC, Barnett Y et al B‐cell diversity decreases in old age and is correlated with poor health status. Aging Cell 2009; 8:18–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Glanville J, Kuo TC, von Büdingen HC et al Naive antibody gene‐segment frequencies are heritable and unaltered by chronic lymphocyte ablation. Proc Natl Acad Sci USA 2011; 108:20066–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Wu YC, Kipling D, Dunn‐Walters DK. Age‐related changes in human peripheral blood IGH repertoire following vaccination. Front Immunol 2012; 3:193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Jiang N, He J, Weinstein JA et al Lineage structure of the human antibody repertoire in response to influenza vaccination. Sci Transl Med 2013; 5:171ra19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Wang C, Liu Y, Xu LT et al Effects of aging, cytomegalovirus infection, and EBV infection on human B cell repertoires. J Immunol 2014; 192:603–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Martin V, Bryan Wu YC, Kipling D, Dunn‐Walters D. Ageing of the B cell repertoire. Phil Trans R Soc B 2015; 370:20140237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Rosner K, Winter DB, Tarone RE, Skovgaard GL, Bohr VA, Gearhart PJ. Third complementarity‐determining region of mutated VH immunoglobulin genes contains shorter V, D, J, P, and N components than non‐mutated genes. Immunology 2001; 103:179–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science 2003; 301:1374–7. [DOI] [PubMed] [Google Scholar]
- 56. Larimore K, McCormick MW, Robins HS, Greenberg PD. Shaping of human germline IgH repertoires revealed by deep sequencing. J Immunol 2012; 189:3221–30. [DOI] [PubMed] [Google Scholar]


