Significance
Diastolic dysfunction is characteristic of patients with cardiomyopathy. Evidence indicates that diseased hearts show basal sarcomeric activation capable of impairing diastolic performance. By activating human cardiomyopathy muscle in ADP-containing solutions without Ca2+, we showed that actin–myosin blockade is disrupted. This may be caused by the presence of mutations and/or the reduced phosphorylation of myofilament proteins. Our mechanistic study supports the novel idea that protein kinase A-target phosphorylation and myosin-binding protein C regulate the OFF–ON transition of the thin filaments. ADP increased myofilament force and stiffness in the presence of Ca2+ in cardiomyopathy samples, suggesting this condition limits muscle relaxation through increased actin–myosin interactions. We conclude that ADP-stimulated contraction can be used to reveal conformational changes in the three-state model of thin-filament activation.
Keywords: hypertrophy, idiopathic dilated, ADP, three-state model, cardiomyopathy
Abstract
Diastolic dysfunction is general to all idiopathic dilated (IDCM) and hypertrophic cardiomyopathy (HCM) patients. Relaxation deficits may result from increased actin–myosin formation during diastole due to altered tropomyosin position, which blocks myosin binding to actin in the absence of Ca2+. We investigated whether ADP-stimulated force development (without Ca2+) can be used to reveal changes in actin–myosin blockade in human cardiomyopathy cardiomyocytes. Cardiac samples from HCM patients, harboring thick-filament (MYH7mut, MYBPC3mut) and thin-filament (TNNT2mut, TNNI3mut) mutations, and IDCM were compared with sarcomere mutation-negative HCM (HCMsmn) and nonfailing donors. Myofilament ADP sensitivity was higher in IDCM and HCM compared with donors, whereas it was lower for MYBPC3. Increased ADP sensitivity in IDCM, HCMsmn, and MYH7mut was caused by low phosphorylation of myofilament proteins, as it was normalized to donors by protein kinase A (PKA) treatment. Troponin exchange experiments in a TNNT2mut sample corrected the abnormal actin–myosin blockade. In MYBPC3trunc samples, ADP sensitivity highly correlated with cardiac myosin-binding protein-C (cMyBP-C) protein level. Incubation of cardiomyocytes with cMyBP-C antibody against the actin-binding N-terminal region reduced ADP sensitivity, indicative of cMyBP-C’s role in actin–myosin regulation. In the presence of Ca2+, ADP increased myofilament force development and sarcomere stiffness. Enhanced sarcomere stiffness in sarcomere mutation-positive HCM samples was irrespective of the phosphorylation background. In conclusion, ADP-stimulated contraction can be used as a tool to study how protein phosphorylation and mutant proteins alter accessibility of myosin binding on actin. In the presence of Ca2+, pathologic [ADP] and low PKA-phosphorylation, high actin–myosin formation could contribute to the impaired myocardial relaxation observed in cardiomyopathies.
Heart failure (HF) is a syndrome clinically defined as the inability of the heart to sufficiently supply blood to organs and tissues (1). Systolic dysfunction is present in approximately one-half of the HF population, whereas diastolic dysfunction is a common feature in almost all HF patients (2). Moreover, in hypertrophic cardiomyopathy (HCM), which is caused by mutations in genes encoding thin- and thick-filament proteins, impaired diastolic function is frequently observed (3). Impaired relaxation of the heart may be caused by high myofilament Ca2+ sensitivity. This increased sensitivity for Ca2+ would result in residual myofilament activation at diastolic [Ca2+], which may delay the onset of ventricular relaxation and limit proper filling of the heart. High myofilament Ca2+ sensitivity has been observed in both acquired and genetic forms of cardiomyopathy (3, 4). In human idiopathic dilated cardiomyopathy (IDCM), high myofilament Ca2+ sensitivity has been associated with reduced β-adrenergic receptor-mediated phosphorylation by protein kinase A (PKA) (4). Reduced PKA phosphorylation of cardiac troponin I (cTnI) and cardiac myosin-binding protein C (cMyBP-C) increases myofilament Ca2+ sensitivity (5–8). Likewise, high myofilament Ca2+ sensitivity is a common characteristic of HCM and may be caused by the mutant protein or by reduced PKA-mediated protein phosphorylation secondary to HCM disease progression (3, 9).
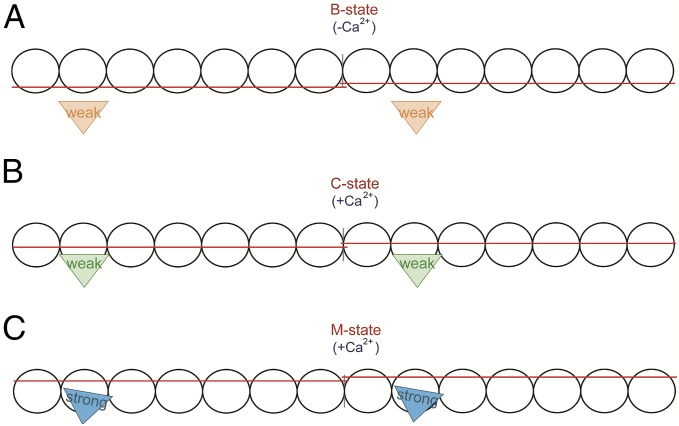
Contractile performance of the heart muscle may thus be perturbed by mutation-induced and phosphorylation-mediated protein changes that affect thin-filament transitions. Ca2+-induced cardiac muscle contraction is tightly modulated by the troponin–tropomyosin complex that regulates the interactions between the actin thin filament and myosin thick filament (i.e., cross-bridge formation). Accordingly, the myofilaments oscillate between three transitions termed the blocked (B-state), closed (C-state), and open (M-state) states of thin-filament regulation that represent the distinct position of tropomyosin on actin (10–12) (Fig. 1). In the absence of Ca2+ (B state), tropomyosin sterically blocks the myosin-binding sites on actin (Fig. 1A). Upon electrical activation of cardiomyocytes, the rise of cytosolic [Ca2+] alters the conformation of the troponin–tropomyosin complex, which moves tropomyosin on actin and exposes myosin-binding sites (C state). Weakly bound cross-bridges (myosin-ADP-Pi) populate the C state (10, 12) (Fig. 1B). Transition to the M state involves release of inorganic phosphate (Pi) from the cross-bridge and strong-binding cross-bridge formation (myosin-ADP) that induces additional movement of tropomyosin, resulting in myofilament contraction and sliding (Fig. 1C).
Fig. 1.
Three-state model of thin-filament activation. Seven actin monomers (circles), spanned by one tropomyosin dimer (red strand), together with the troponin complex (not depicted) comprise one functional unit (A7TmTn). Two functional units are depicted, and individual myosins are shown as triangles (weak, weak-binding cross-bridges; strong, strong-binding cross-bridges). (A) B state (blocked); when ATP is present and cytoplasmic [Ca2+] is low and is not bound to cardiac troponin C (cTnC), tropomyosin is sterically blocking the myosin-binding sites on actin. (B) C state (Ca2+-induced); upon rise in cytoplasmic [Ca2+], Ca2+ binds to cTnC, inducing conformational changes of the troponin complex, resulting in a ∼25° movement of tropomyosin on the thin filament, thereby exposing myosin-binding sites on actin. In the C state, the myofilament is not yet activated as non–tension-generating cross-bridges bind weakly to actin. (C) M state (myosin induced); the strong binding of tension-generating cross-bridges induces a ∼10° movement of tropomyosin on actin, resulting in myofilament activation and contraction.
The three-state model of cross-bridge interaction implies that the main task of Ca2+ is to uncover myosin-binding sites on actin and that formation of myosin-ADP represents the main regulator of force development and contraction. Notably, solution (10) and cryo-electron microscopy (13) studies have shown that in the absence of Ca2+ the myofilaments are not entirely blocked, as ∼5% of the thin filaments have tropomyosin localized in the C-state position. This observation suggests that conditions that promote myosin-ADP formation can trigger myofilament contraction in Ca2+-free conditions and thereby impair relaxation. Indeed, in membrane-permeabilized rabbit skeletal muscle fibers (14), bovine myocardium (15, 16) and human cardiac muscle (17) millimolar levels of ADP stimulate force development in the absence of Ca2+.
Because ADP-stimulated contraction is due to myosin-ADP binding to the nonblocked sites of the thin filament in the absence of Ca2+, it provides an experimental tool to assess changes in tropomyosin’s position in acquired and genetic cardiomyopathies in which altered protein phosphorylation and mutant proteins may alter myofilament activation. In addition, it could represent a pathomechanism underlying the diastolic dysfunction seen in both disease states. Solution studies with mutant troponin proteins, which are known to cause HCM, showed a reduction in the B state at low-Ca2+ conditions compared with wild-type troponin proteins (18, 19). Mutation-induced irregularities in troponin–tropomyosin interactions disrupt the B state and shift the thin filament to the C state, increasing the available myosin-binding sites on actin.
In addition to Ca2+-induced changes of the thin filament, tropomyosin location may also be altered by the thick-filament protein cMyBP-C. Recent evidence supports that the N-terminal extension of cMyBP-C binds the low-Ca2+–state (B-state) position of tropomyosin on actin and interferes with tropomyosin–actin interactions, dislocating tropomyosin into the C-state position (i.e., the presence of cMyBP-C sensitizes the thin filament to Ca2+) (20, 21). Because it was previously shown that in Ca2+-free conditions (B state) ∼5% of the thin filaments (lacking cMyBP-C) have tropomyosin localized in the C-state position (10), more myofilaments may be in the C state in the presence of cMyBP-C. We (22) and others (23) have shown that cMyBP-C mutations, which are a major cause of HCM, have a reduced level of healthy cMyBP-C protein compared with nonfailing hearts (i.e., haploinsufficiency), which may alter tropomyosin position on the thin filament.
To verify whether ADP-stimulated contraction provides an experimental tool to assess mutation-induced and phosphorylation-mediated changes in thin-filament transitions, which precede Ca2+ activation of myofilaments, we tested the following hypotheses: (i) that IDCM and HCM samples with thin-filament mutations are more sensitive to ADP, as a result of a higher accessibility of myosin-binding sites on actin, whereas (ii) cMyBP-C haploinsufficient HCM myocardium has a reduced ADP sensitivity (i.e., less cMyBP-C causes reduced displacement of tropomyosin from the B state) compared with cells from nonfailing hearts. To answer our hypotheses, we activated membrane-permeabilized human cardiomyocytes in ADP containing Ca2+-free solutions. Cells were isolated from HCM patients with mutations in genes encoding thick-filament (MYH7, MYBPC3) and thin-filament (TNNT2, TNNI3) proteins and patients with IDCM and compared with cells from sarcomere mutation-negative HCM (HCMsmn) and nonfailing donors. Finally, we investigated whether the ADP level as observed in diseased hearts, in the presence of Ca2+, increases myofilament force development in cardiomyocytes from human cardiomyopathy hearts.
We conclude that, in HCM with thin-filament mutations, tropomyosin’s ability to block myosin-binding sites on actin is reduced. This effect is exacerbated in HCM samples by the low PKA phosphorylation of myofilament proteins, which is also observed in human IDCM. In contrast, cMyBP-C HCM-causing mutations reduce accessibility of myosin for actin. The findings in this study provide evidence that ADP-mediated activation can be used as an experimental tool to reveal mutation- and phosphorylation-mediated changes in tropomyosin location on the thin filament.
Results
ADP Sensitivity in IDCM and HCM.
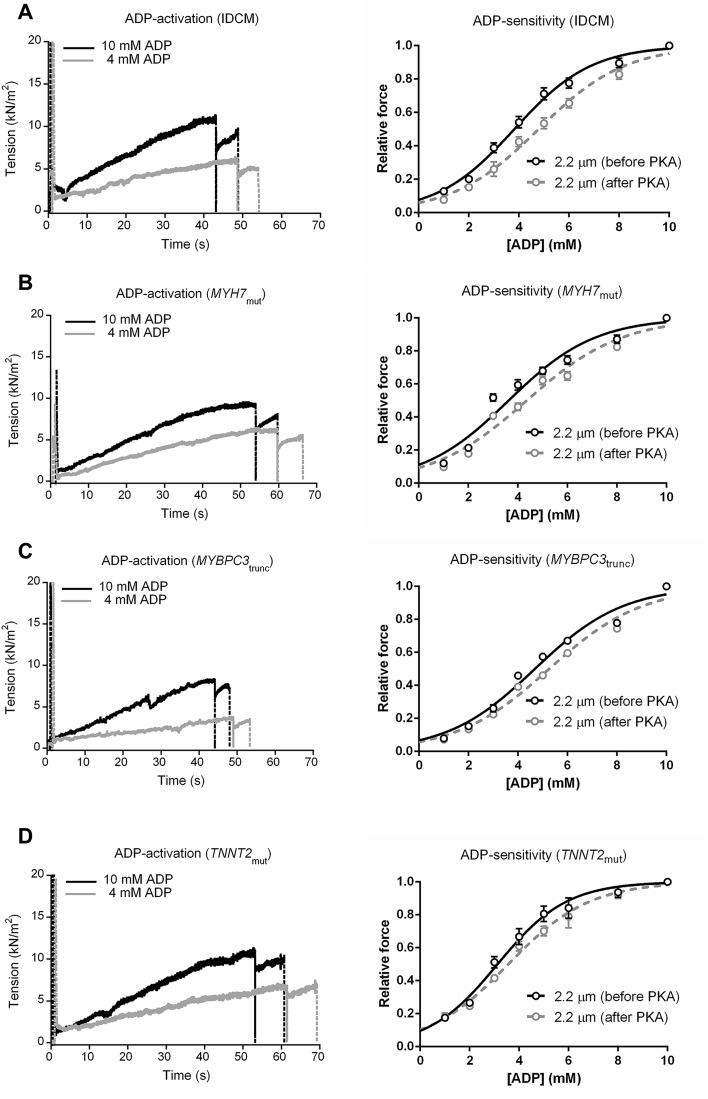
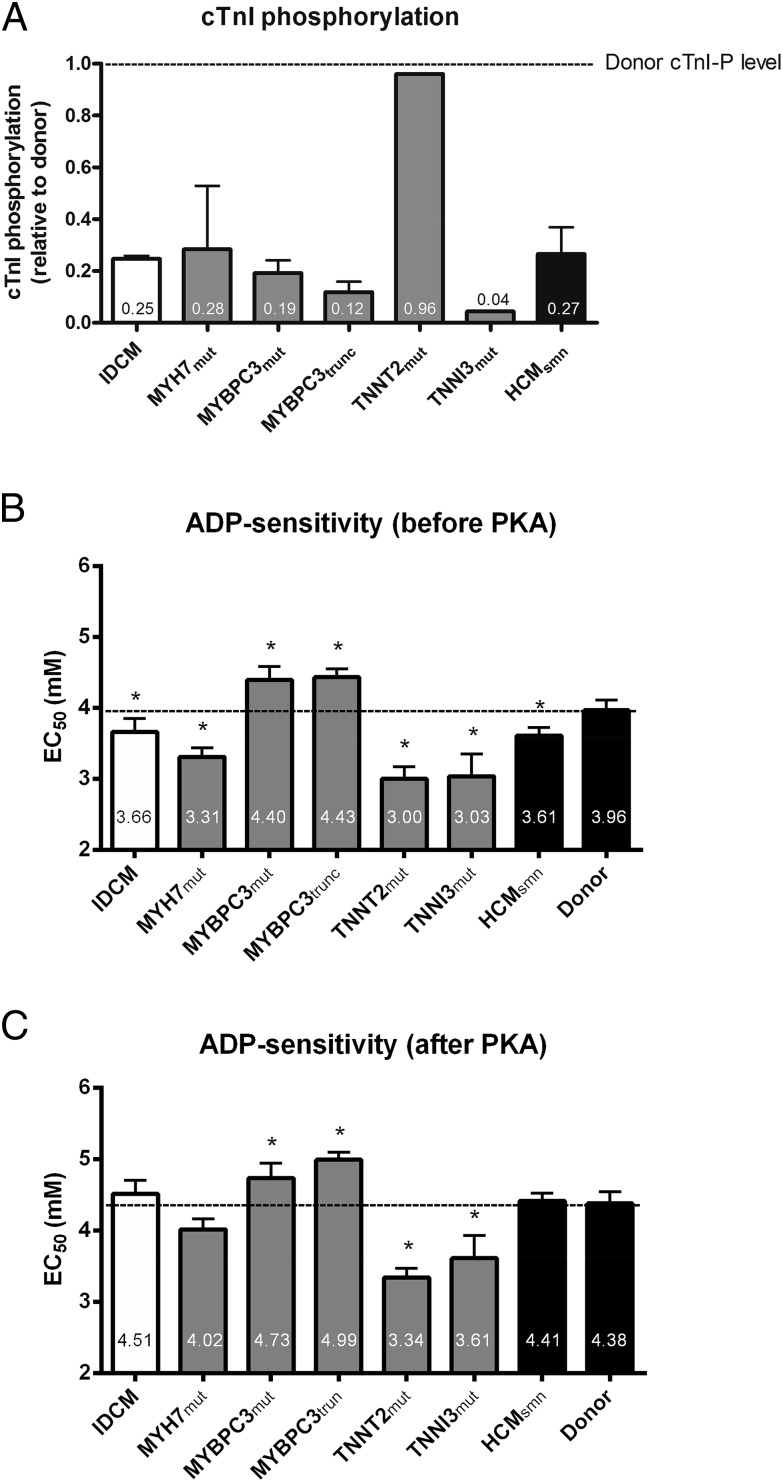
To evaluate the accessibility of myosin-binding sites on actin, force production of single membrane-permeabilized cardiomyocytes was measured at varying ADP concentrations, in the absence of Ca2+. Tissue samples from human IDCM and HCM hearts were selected and compared with those from nonfailing donors (Table S1). Typical tension tracings of single cardiomyocytes from IDCM, MYH7mut, MYBPC3trunc, and TNNT2mut samples at 2.2-μm sarcomere length during ADP-maximal (10 mM) and -submaximal (4 mM) activation are shown in Fig. S1. We have previously shown that all IDCM and HCM samples, except for the HCM TNNT2mut sample, have lower phosphorylation of PKA target proteins in the myofilament compared with donor myocardium (4, 9). Fig. 2A shows that the phosphorylation level of the PKA target protein cTnI is lower in the IDCM and HCM samples relative to nonfailing donor, which was set to 1. One exception is the homozygous TNNT2mut sample, which showed similar cTnI phosphorylation as in donor myocardium. To determine the effect of low myofilament phosphorylation, force–ADP relations were constructed for all samples before and after PKA treatment (Fig. S1, Right). As shown in Fig. 2B, ADP sensitivity (denoted as EC50) was higher in IDCM and several HCM patient groups, including MYH7mut, TNNT2mut, TNNI3mut, and HCMsmn, whereas it was lower in MYBPC3mut (missense mutations) and MYBPC3trunc (truncating mutations) compared with donors (Table S2). PKA normalized ADP sensitivity to donor levels in IDCM, MYH7mut, and HCMsmn, whereas TNNT2mut and TNNI3mut remained more sensitive to ADP (Fig. 2C and Table S2). Interestingly, after PKA, MYBPC3mut and MYBPC3trunc were still less sensitive to ADP than donor cells (Fig. 2C and Table S2).
Table S1.
HCM samples information
| Samples | Mutation | Type | |
| MYBPC3trunc | Cardiac myosin-binding protein C (cMyBP-C) | Truncating mutations | |
| 1 | p.Y340X | Termination codon | |
| 2 | p.R493X | Termination codon | |
| 3 | c.927-2A > G | Splice site | |
| 4 | c.927-2A > G | Splice site | |
| 5 | c.1458-1G > C | Splice site | |
| 6 | c.2373duplicationG | Insertion | |
| 7 | c.2373duplicationG | Insertion | |
| 8 | c.2373duplicationG | Insertion | |
| 9 | c.2864.2865delCT | Deletion | |
| 10 | c.3407.3409del | Deletion | |
| MYBPC3mut | Cardiac myosin-binding protein C (cMyBP-C) | Missense mutations | |
| 11 | p.E258K | Missense | |
| 12 | p.G531R | Missense | |
| 13 | p.R597Q | Missense | |
| MYH7mut | Myosin-heavy chain (MyHC) | ||
| 14 | p.R403Q | Missense | |
| 15 | p.R403Q | Missense | |
| 16 | p.V606M | Missense | |
| TNNT2mut | Cardiac troponin T (cTnT) | ||
| 17 | p.K280N | Missense | |
| TNNI3mut | Cardiac troponin I (cTnI) | ||
| 18 | p.R145W | Missense |
c, codon region; mut, missense mutation; p, protein amino residue; trunc, truncating mutation; X, stop codon.
Fig. S1.
Myofilament ADP activation and ADP sensitivity in idiopathic dilated cardiomyopathy (IDCM) and hypertrophic cardiomyopathy (HCM) membrane-permeabilized cardiomyocytes. Left panels show typical tension recordings of a single membrane-permeabilized cardiomyocytes at 2.2-μm sarcomere length during ADP maximal (10 mM) and submaximal activation (4 mM) at 15 °C and 1 mM ATP for IDCM (A), MYH7mut (B), MYBPC3trunc (C), and TNNT2mut (D). Right panels show normalized force–ADP relationship for IDCM (A), MYH7mut (B), MYBPC3trunc (C), and TNNT2mut (D) cardiomyocytes before and after protein kinase A (PKA) treatment. MYBPC3trunc, myosin-binding protein-C (truncating) mutations; MYH7mut, myosin heavy-chain mutations; TNNT2mut, cardiac troponin T mutation.
Fig. 2.
(A) Idiopathic dilated cardiomyopathy (IDCM) and hypertrophic cardiomyopathy (HCM) samples showed reduced phosphorylation of the protein kinase A (PKA) target protein cardiac troponin I (cTnI) relative to nonfailing donor, which was set to 1. Only the homozygous TNNT2mut sample showed relatively high cTnI phosphorylation similar as observed in donor tissue. Myofilament ADP sensitivity in IDCM and HCM cardiomyopathy membrane-permeabilized cardiomyocytes. ADP sensitivity (EC50) before (B) and after (C) PKA treatment in IDCM, HCM, and donor samples. Data were compared using multilevel analysis. Detailed statistics, sample numbers, and number of cardiomyocytes used are shown in Table S2. HCMsmn, sarcomere mutation-negative HCM samples; MYBPC3mut, myosin-binding protein-C (missense) mutations; MYBPC3trunc, myosin-binding protein-C (truncating) mutations; MYH7mut, myosin heavy-chain mutations; TNNI3mut, cardiac troponin I mutation; TNNT2mut, cardiac troponin T mutation.
Table S2.
ADP sensitivity (EC50) of nontreated and PKA-treated cardiomyocytes
| Samples | Vs. donor | P value | 95% CI, mmol/L | ns |
| Nontreated | ||||
| IDCM | *P = 0.041 | −0.83 to −0.018 | n = 4, n = 14 | |
| MYH7mut | *P = 0.003 | −1.08 to −0.23 | n = 3, n = 11 | |
| MYBPC3mut (missense) | *P = 0.010 | 0.14–0.98 | n = 3, n = 12 | |
| MYBPC3trunc (truncating) | *P = 0.004 | 0.16–0.85 | n = 10, n = 28 | |
| TNNT2mut | *P = 0.002 | −1.57 to −0.36 | n = 1, n = 4 | |
| TNNI3mut | *P = 0.003 | −1.54 to −0.32 | n = 1, n = 4 | |
| HCMsmn | *P = 0.038 | −0.77 to 0.02 | n = 5, n = 18 | |
| PKA-treated | ||||
| IDCM | P = 0.895 | −0.44 to 0.39 | n = 4, n = 14 | |
| MYH7mut | P = 0.106 | −0.80 to −0.08 | n = 3, n = 11 | |
| MYBPC3mut (missense) | *P = 0.032 | −0.04 to 0.92 | n = 3, n = 12 | |
| MYBPC3trunc (truncating) | *P = 0.001 | 0.26–0.96 | n = 10, n = 28 | |
| TNNT2mut | *P = 0.001 | −1.67 to −0.41 | n = 1, n = 4 | |
| TNNI3mut | *P = 0.017 | −1.40 to −0.14 | n = 1, n = 4 | |
| HCMsmn | P = 0.870 | −0.35 to 0.42 | n = 5, n = 18 |
Multilevel analysis. *P < 0.05 was considered significant. N, number of samples; n, number of cardiomyocytes. Sixteen cardiomyocytes from four nonfailing hearts (donor) served as controls. HCMsmn, sarcomere mutation negative; IDCM, idiopathic dilated cardiomyopathy; MYBPC3, myosin-binding protein-C mutations; MYH7mut, myosin heavy-chain mutations; PKA, protein kinase A; TNNI3mut, cardiac troponin I mutation; TNNT2mut, cardiac troponin T mutation.
These results indicate that PKA target phosphorylation of the myofilaments reduces accessibility of myosin-binding sites on actin in the absence of Ca2+. At baseline (before PKA administration), the myofilaments from cardiomyopathy samples are more sensitive to ADP activation, except for the low sensitivity to ADP of MYBPC3mut and MYBPC3trunc. PKA phosphorylation normalized sensitivity to ADP in IDCM, MYH7mut, and HCMsmn, suggesting that any putative disruption of tropomyosin’s B-state position (and the greater accessibility of myosin-binding sites on actin) in these samples may be due to the low baseline phosphorylation of PKA target proteins.
cMyBP-C Regulates ADP Sensitivity.
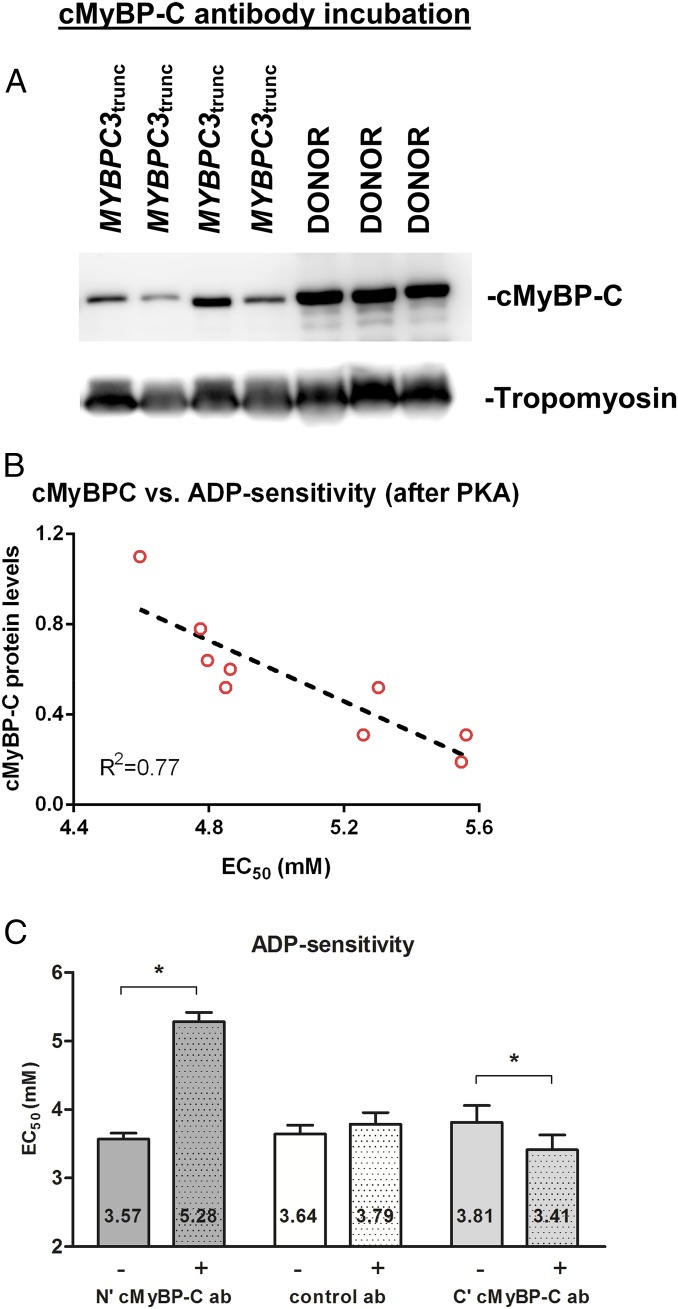
We (22) and others (23) have shown that cMyBP-C truncating mutations lack the presence of the smaller truncated protein, but have a lower total level of healthy full-length cMyBP-C (i.e., haploinsufficiency). To assess whether the expression level of cMyBP-C in MYBPC3trunc correlates with ADP sensitivity [as cMyBP-C modulates thin-filament transitions (20, 21)], cMyBP-C expression was determined. Total cMyBP-C protein level (relative to tropomyosin) was 45 ± 9% reduced in MYBPC3trunc compared with controls (Fig. 3A). To correlate cMyBP-C level to thin-filament activation, the level of cMyBP-C per sample was plotted against its ADP sensitivity value obtained after PKA treatment (to normalize for differences in phosphorylation). Indeed, we observed a strong correlation between cMyBP-C protein level and ADP sensitivity (Fig. 3B), indicating that a reduction in cMyBP-C diminishes the number of available myosin-binding sites on actin (i.e., favors the B-state formation).
Fig. 3.
Cardiac myosin-binding protein C (cMyBP-C) regulates ADP sensitivity. (A) cMyBP-C protein levels were assessed in MYBPC3trunc (n = 9) and donor (n = 3) samples. Western blots were stained with specific antibodies for total cMyBP-C and tropomyosin (loading control). No traces of truncated cMyBP-C proteins were found in MYBPC3trunc samples. (B) Expression level of cMyBP-C was significantly lower in MYBPC3trunc than in donors. cMyBP-C protein levels from MYBPC3trunc samples were plotted against their corresponding sample average for ADP sensitivity [after protein kinase A (PKA) treatment]. (C) Single membrane-permeabilized cardiomyocytes isolated from IDCM heart samples were incubated with a specific cMyBP-C N′-terminal (mouse monoclonal) antibody, cMyBP-C C′-terminal (rabbit polyclonal), or a control (mouse monoclonal) antibody against nucleophosmin (antibodies used at 1:100 dilutions). Myofilament ADP sensitivity (EC50) before (−) and after (+) treatment with cMyBP-C antibody (N′ or C′ cMyBP-C ab) or nucleophosmin antibody (control ab). Myofilament ADP sensitivity was significantly reduced after N′ cMyBP-C ab treatment, but not after C′ cMyBP-C ab or control ab. Data were compared using paired-samples t test for each specific group of antibodies. Eight to 11 cardiomyocytes from three to four failing heart samples were used per group.
To confirm and provide the proof-of-concept that cMyBP-C indeed regulates thin-filament transitions via its N-terminal region, IDCM samples were incubated for 90 min with an antibody that binds the C0 domain of cMyBP-C (the domain involved in thin-filament binding). We expected that antibody binding to cMyBP-C decreased cMyBP-C affinity for the thin filament. ADP sensitivity was reduced after incubation with specific N-terminal cMyBP-C antibody, suggesting that antibody competition for cMyBP-C resulted in less cMyBP-C affinity for the thin filament and, hence, a reduced accessibility of myosin-binding sites (Fig. 3C). Maximal ADP–Fact significantly reduced upon cMyBP-C N-terminal antibody incubation, whereas it did not affect passive tension (ADP–Fpass) (Table 1). To control for protocol artifacts (e.g., unspecific antibody binding), another set of cardiomyocytes was incubated for 90 min with an antibody against the C10 domain of cMyBP-C (domain involved in cMyBP-C anchoring to the thick filament) or a control antibody (against nucleophosmin, a nucleolar protein). Increased responsiveness to ADP was found for the C10 antibody associated with a small drop in ADP–Fpass, whereas no alterations in ADP sensitivity, maximal force, and ADP–Fpass were found using nucleolar protein antibody (Fig. 3C and Table 1). These data support that the effects seen for the C0 antibody were specific to the N-terminal region of cMyBP-C.
Table 1.
Response of ADP-stimulated contraction with cMyBPC and control antibodies
| Sample | Before | After | ns |
| N′ cMyBPC ab (2.2 μm) | N′ cMyBPC ab (2.2 μm) | ||
| IDCM | n = 4, n = 11 | ||
| ADP–Fact, kN/m2 | 8.4 ± 1.0 | 6.6 ± 0.9* | |
| ADP–Fpass, kN/m2 | 0.36 ± 0.1 | 0.33 ± 0.1 | |
| Nucleolar ab (2.2 μm) | Nucleolar ab (2.2 μm) | n = 3, n = 8 | |
| IDCM | |||
| ADP–Fact, kN/m2 | 9.3 ± 1.1 | 9.0 ± 1.0 | |
| ADP–Fpass, kN/m2 | 0.57 ± 0.1 | 0.50 ± 0.1 | |
| C′ cMyBPC ab (2.2 μm) | C′ cMyBPC ab (2.2 μm) | n = 3, n = 11 | |
| IDCM | |||
| ADP–Fact, kN/m2 | 11.7 ± 1.9 | 10.8 ± 2.0 | |
| ADP–Fpass, kN/m2 | 1.5 ± 0.3 | 1.2 ± 0.2* |
Paired-samples t test. P < 0.05 was considered significant; *vs. before antibodies. C′ cMyBPC or nucleolar antibody served as controls for unspecific binding. ab, antibody; ADP–Fact, ADP total tension (10 mM ADP); ADP–Fpass, ADP passive tension; cMyBPC, cardiac myosin-binding protein C; IDCM, idiopathic dilated cardiomyopathy; N, number of samples; n, number of cardiomyocytes; 2.2-μm sarcomere length.
Finally, haploinsufficiency was also reported in HCM with MYBPC3 missense mutations (23), including the mutation investigated in our study (E258K). Indeed, samples with MYBPC3mut missense mutations in our study showed a 22 ± 9% reduction in cMyBP-C protein level compared with donors.
Correction of ADP Sensitivity in HCM with Mutant cTnT by Human Recombinant Wild-Type Troponin.
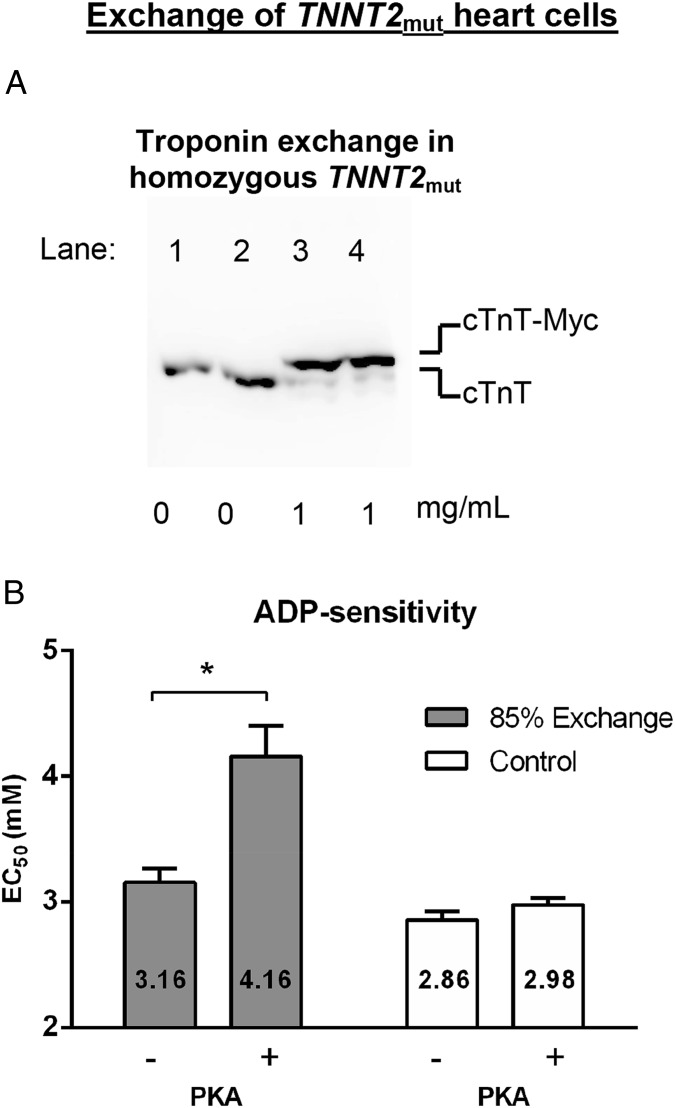
To evaluate whether mutant thin-filament protein directly causes destabilization of the B state and changes sensitivity to ADP, troponin exchange experiments were performed in cardiomyocytes from the TNNT2mut sample. Because the homozygous TNNT2mut results in 100% mutant cTnT protein, it provides the unique opportunity to control the amount of wild-type human troponin complex incorporated. Exchange was performed with 1 mg/mL wild-type human troponin complex and resulted in 85 ± 2% troponin exchange based on Western blot analyses of endogenous and myc-tag–labeled wild-type cTnT (Fig. 4A). We have previously shown that this protocol does not affect other sarcomeric (phospho)proteins (8, 9). In exchanged cells without PKA treatment, replacement of mutant troponin with recombinant unphosphorylated wild-type troponin did not restore the ADP sensitivity (Fig. 4B, 3.16 ± 0.16 mM ADP) to donor levels (Fig. 2B, 3.96 ± 0.15 mM ADP). Notably, PKA treatment in exchanged cells containing 85% wild-type troponin complex restored ADP sensitivity (Fig. 4B, 4.16 ± 0.24 mM ADP) to donor levels that were PKA treated (Fig. 2C, 4.38 ± 0.16 mM ADP). Control TNNT2mut cardiomyocytes, in the presence of exchange buffer without wild-type troponin complex, showed similar ADP sensitivity (Fig. 4B, 2.86 ± 0.07 mM ADP before PKA and 2.98 ± 0.05 mM ADP after PKA) as TNNT2mut cardiomyocytes without the exchange procedure (Fig. 2 B and C, 3.00 ± 0.17 mM ADP before PKA and 3.34 ± 0.13 mM ADP after PKA). Together, these results support that this specific cTnT mutant protein disrupts tropomyosin’s B-state position on actin and, hence, is highly susceptible for ADP-activated myosin binding.
Fig. 4.
Cardiac troponin T (cTnT) regulates ADP sensitivity. Endogenous mutant cTnT in TNNT2mut cardiomyocytes was exchanged by exogenous recombinant human wild-type troponin complex. TNNT2mut cells were incubated with 1 mg/mL wild-type human troponin complex or without troponin complex (control). (A) Quantification of troponin exchange in cardiomyocytes from a TNNT2mut heart. Immunoblots stained with a specific antibody against cTnT that recognizes both endogenous mutant cTnT (lower band) and recombinant myc-tag wild-type cTnT (cTnT-myc; upper band). TNNT2mut without recombinant troponin complex (lanes 1 and 2; control cells) and with 1 mg/mL recombinant wild-type troponin complex (lanes 3 and 4). Exchange resulted in 85 ± 2% troponin exchange based on Western blot analyses of endogenous and myc-tag–labeled wild-type cTnT. (B) Myofilament ADP sensitivity (EC50) before (−) and after (+) protein kinase A (PKA) treatment. ADP sensitivity was significantly reduced after PKA treatment in troponin-exchanged cardiomyocytes. No change in myofilament ADP sensitivity was observed in control cardiomyocytes from the TNNT2mut heart. Data were compared using two-way ANOVA followed by Bonferroni post hoc test. Four cardiomyocytes from one TNNT2mut heart were used per group.
Physiological ADP Levels Increase Ca2+ Sensitivity in IDCM and HCM.
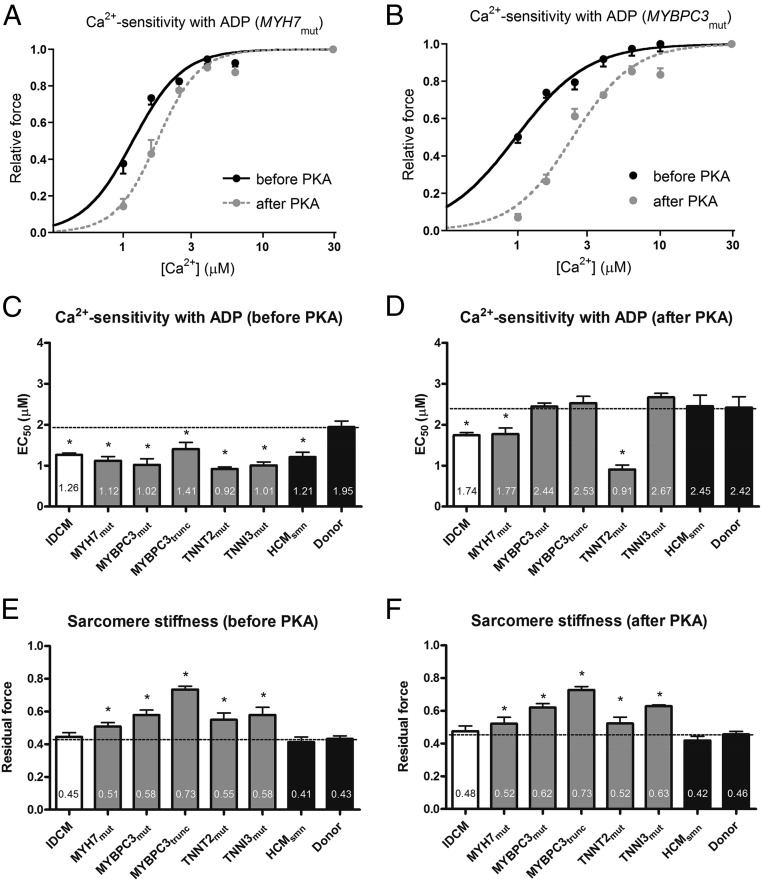
We recently provided evidence that elevation of myocardial ADP levels, even in the micromolar range, in the presence of diastolic Ca2+, contributes to diastolic dysfunction by increasing residual actin–myosin interactions and elevating myofilament Ca2+ sensitivity (17). This leads to high cardiomyocyte stiffness and abnormal relaxation, associated with limited ventricular compliance (17). In HCM animal models, an increased [ADP] was observed from ∼60 μM in controls to ∼100 μM in diseased hearts (24–26). We therefore investigated whether an ADP level as reported in diseased hearts (100 μM) in concert with Ca2+ increases force generation in human membrane-permeabilized cardiomyocytes from IDCM and HCM hearts compared with nonfailing donor myocardium. Fig. 5 A and B show normalized force–Ca2+ relations in the presence of 100 μM ADP, before and after PKA treatment, for MYH7mut and MYBPC3mut samples. In the presence of 100 μM ADP, myofilament Ca2+ sensitivity was significantly higher in IDCM and HCM samples compared with donors (Fig. 5C). Because we have previously demonstrated that the high myofilament Ca2+ sensitivity in human cardiomyopathy samples correlates well with the low phosphorylation of PKA myofilament targets (4, 9), we repeated the measurements after treatment with exogenous PKA. PKA treatment normalized the high Ca2+ sensitivity in HCM with MYBPC3 and TNNI3mut mutations and HCMsmn, whereas Ca2+ sensitivity remained higher in IDCM, MYH7mut, and TNNT2mut samples (Fig. 5D). In addition, we observed augmented sarcomere stiffness for all sarcomere mutation-positive HCM samples evidenced by high residual force compared with nonfailing donors (Fig. 5E). Residual force values in IDCM and HCMsmn were similar to those observed in nonfailing donor cells before and after PKA treatment (Fig. 5 E and F). The increase in sarcomere stiffness suggests an increased strain for each individual cross-bridge and/or an enhanced number of actin–myosin interactions for HCM samples with sarcomeric mutant proteins.
Fig. 5.
Myofilament Ca2+ sensitivity and sarcomere stiffness, in the presence of 100 µM ADP, in idiopathic dilated cardiomyopathy (IDCM) and hypertrophic cardiomyopathy (HCM) membrane-permeabilized cardiomyocytes. Normalized force–Ca2+ relationships before and after protein kinase A (PKA) in the presence of ADP for MYH7mut (A) and MYBPC3mut (B) heart samples. Myofilament Ca2+ sensitivity in the presence of ADP (EC50) before (C) and after (D) PKA treatment in IDCM, HCM, and donor samples. Residual force, a measure of sarcomere stiffness, at high Ca2+ with 100 µM ADP (EC50) before (E) and after (F) PKA treatment in IDCM, HCM, and donor samples. Data were compared using multilevel analysis. N, number of samples; n, number of cardiomyocytes. HCMsmn, sarcomere mutation-negative HCM samples (n = 3, n = 8); IDCM (n = 4, n = 18); MYBPC3mut, myosin-binding protein-C (missense) mutations (n = 3, n = 10); MYBPC3trunc, myosin-binding protein-C (truncating) mutations (n = 4, n = 12); MYH7mut, myosin heavy-chain mutations (n = 3, n = 10); TNNI3mut, cardiac troponin I mutation (n = 1, n = 4); TNNT2mut, cardiac troponin T mutation (n = 1, n = 4). Fourteen cardiomyocytes from five nonfailing hearts (donor) served as controls.
Discussion
In this study, we show that ADP-stimulated contraction can be used as experimental tool to assess changes in thin-filament transitions induced by sarcomeric mutations and phosphorylation-mediated protein alterations. These mutation- and phosphorylation-related changes in ADP sensitivity are explained by changes in tropomyosin’s position and the number of accessible myosin-binding sites on actin. In accordance with our first hypothesis, we show that HCM cardiomyocytes containing troponin mutations have high ADP sensitivity, irrespective of the phosphorylation background. High ADP sensitivity was corrected to control values upon incorporation of healthy troponin in HCM cardiomyocytes harboring a homozygous TNNT2mut mutation. These data indicate that the troponin mutations investigated in the present study disrupt the steric blockade imposed by tropomyosin, which favors accessibility of myosin-binding sites on actin. The high responsiveness to ADP observed in IDCM, MYH7mut, and HCMsmn is, in this context, solely explained by reduced PKA phosphorylation of myofilament proteins. This is consistent with the idea that PKA phosphorylation of the myofilaments stabilizes the B-state thin-filament formation, which reduces myosin-binding sites on actin. Moreover, in agreement with our second hypothesis, HCM samples with MYBPC3 mutations were shown to have less sensitivity to ADP compared with cells from nonfailing hearts, in support for the enhanced stabilization of the steric blockade of tropomyosin.
Finally, a pathophysiologic level of ADP elevated myofilament Ca2+ sensitivity in all disease samples. Furthermore, under these conditions, high myofilament stiffness was observed in samples from HCM patients with sarcomeric mutations. The high myofilament Ca2+ sensitivity in the presence of ADP was corrected to control values by PKA in all HCM samples, except for the samples with MYH7 and TNNT2 mutations, whereas myofilament stiffness was unaffected by PKA in all mutation-positive HCM samples. These data illustrate the complex interaction between changes in protein phosphorylation, Ca2+, and ADP.
Overall, our data support the notion that increased actin–myosin interactions during the diastolic phase may occur in environments where the myofilaments are highly sensitized by the complex interactions of low phosphorylation of PKA targets, elevated in vivo levels of ADP and of diastolic Ca2+, which may limit muscle relaxation.
Modulation of ADP Sensitivity by PKA-Mediated Phosphorylation.
We showed that PKA-mediated phosphorylation of the myofilaments reduced the responsiveness to ADP, supporting the stabilization of the B-state formation (Fig. 2C). In the intact heart, regulation of [Ca2+]i transients is accomplished via sympathetic stimulation of β-adrenergic receptors, which results in positive inotropic (contraction), lusitropic (relaxation), and chronotropic (frequency) effects (27). At the myofilament level, PKA main targets are cTnI, cMyBP-C, and titin. Multiple roles have been assigned to increased PKA phosphorylation of myofilament proteins, such as reduced myofilament Ca2+ sensitivity, increased length-dependent activation (cellular basis of the Frank–Starling relation), and enhanced cross-bridge cycling kinetics via phosphorylation of cTnI and cMyBP-C (5–8).
The reduced ADP sensitivity following PKA incubation implies strengthening of the B-state localization of tropomyosin. There are likely mechanisms, which may act synergistically, that could explain this effect.
First, the reduction in ADP sensitivity may be explained by cTnI phosphorylation. Two sites on cTnI are known to be target of PKA-induced phosphorylation in human, serines 23 and 24 (28, 29). Phosphorylation of these sites is associated with reduced Ca2+ affinity of troponin C (cTnC) (30) due to structural changes of cTnC that diminish its Ca2+ affinity (31–33). Although cTnC changes are likely sufficient to account for the reduction of myofilament Ca2+ sensitivity upon PKA activation, they do not necessarily apply to the present conditions where Ca2+ is absent. It has been shown that the C-terminal half of cTnI docks the troponin–tropomyosin complex onto the outer domain of actin at low cytoplasmic [Ca2+] (34), thereby stabilizing the formation of the B state. The reduced ADP sensitivity after PKA treatment likely reflects potentiation of the inhibitory function of cTnI-tropomyosin on actin as PKA decreases the affinity of cTnI for cTnC. The PKA-mediated conformational changes would increase tropomyosin-mediated blockade of myosin-binding sites on actin.
Second, PKA-mediated phosphorylation of cMyBP-C may underlie the observed reduction in ADP sensitivity. PKA phosphorylation of cMyBP-C occurs at the M domain where it interacts with actin (35). It was shown that M-domain phosphorylation reduces the N-terminal affinity of cMyBP-C for actin (36), consistent with less activation of the thin filament (37). One can speculate that cMyBP-C phosphorylation reduces competition between cMyBP-C and tropomyosin on actin, and subsequently decreases the number of myosin-binding sites on actin.
Modulation of ADP Sensitivity by the N′ and C′ Terminus of cMyBP-C.
In the heart, up to nine transverse stripes (half-sarcomere) of cMyBP-C are observed in the C zone, where it is recently been proposed to modulate myofilament activation (38). The C terminus of cMyBP-C is important for proper localization and anchoring onto the thick filament and the rod region of myosin (39, 40). The N-terminal region has been shown to directly interact with actin in in vitro studies (41, 42) and in situ 3D reconstructions, where it was shown to regulate thin-filament transitions via direct competition for the low-Ca2+–state (B-state) position with tropomyosin (20, 21). Here, we provide further evidence for N-terminal cMyBP-C’s interaction with tropomyosin by showing that cMyBP-C regulates thin-filament transitions via its N-terminal region (Fig. 3C). Preincubation of IDCM myofilaments with a N-terminal cMyBP-C antibody is expected to decrease cMyBP-C’s affinity to bind and compete with tropomyosin and shift it toward the C state. Indeed, N-terminal cMyBP-C antibody preincubation decreased ADP sensitivity in membrane-permeabilized cardiomyocytes, consistent with less myosin-ADP binding to actin. In addition to its effect on the thin filament, there is general agreement that cMyBP-C acts as brake on myosin cross-bridge cycling (43–45). This effect is mediated by the interaction of cMyBP-C’s N′ terminus (C1–C2) with subfragment 2 (S2) of myosin (46), whereby cMyBP-C is able to slow cross-bridge cycling kinetics. Interestingly, incubation of membrane-permeabilized cardiomyocytes with specific antibody binding to the C′ terminus of cMyBP-C increased the responsiveness to ADP (Fig. 3C). This might be due to allosteric protein changes that translate their effects from the C-terminal domain of cMyBP-C to its N-terminal region, which release part of the brake on myosin.
Haploinsufficiency of cMyBP-C Reduces ADP Sensitivity of Myofilaments.
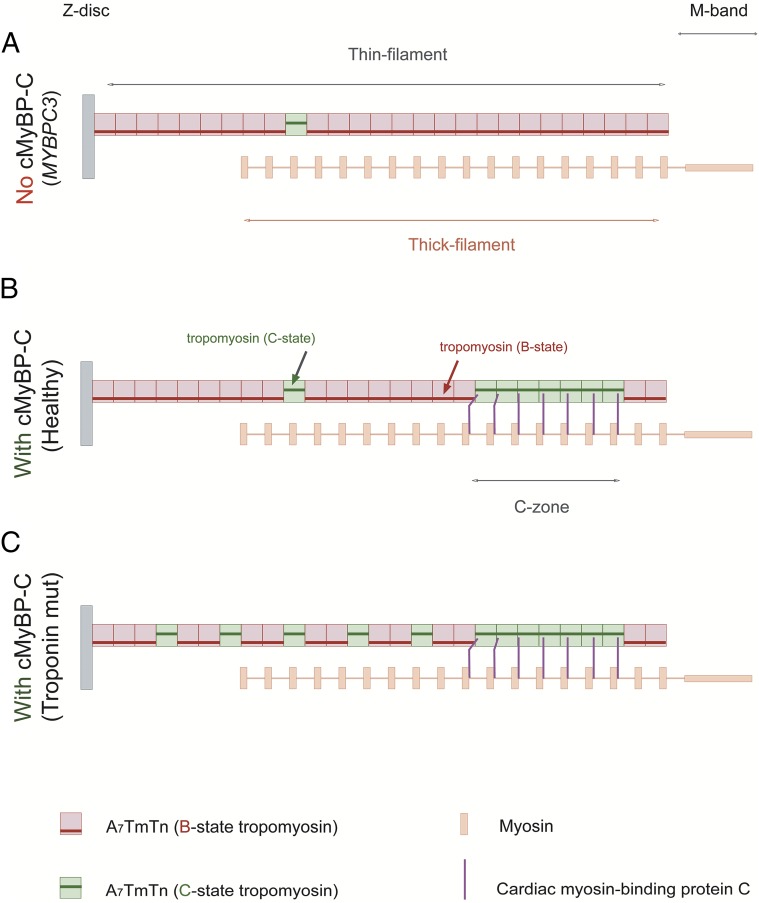
Our data indicate that loss of cMyBP-C full-length protein (i.e., haploinsufficiency) in HCM with truncating cMyBP-C mutations weakens cMyBP-C–tropomyosin interactions and results in a reduced accessibility of myosin-binding sites on actin, evident from a reduced ADP sensitivity (Fig. 2 B and C). This is consistent with the recent observation that the N-terminal region of cMyBP-C binds to the low-Ca2+–state position of tropomyosin (B state), which is sufficient to interfere and compete with tropomyosin–actin interactions, moving tropomyosin into the C-state position (20, 21). A schematic picture is presented in Fig. 6A to illustrate that the loss of cMyBP-C full-length protein weakens the overall cMyBP-C–tropomyosin interaction resulting in reduced accessibility of myosin-binding sites on the actin filament in the absence of Ca2+. In contrast, the presence of cMyBP-C competes with tropomyosin for the B-state position on actin and increases the accessibility of myosin-binding sites on the actin filament (Fig. 6B). Reduced ADP sensitivity was also observed in cMyBP-C HCM samples with missense mutations, where loss of full-length protein may similarly decrease the availability of myosin-binding sites on actin. However, in these samples, cMyBP-C poison peptide effects on muscle function cannot be excluded. The mutant protein could directly interfere with cMyBP-C–tropomyosin interactions. One can speculate that in particular the E258K mutation [localized on the actin-binding site (47)] has the potential to reduce its actin affinity and thereby alter cMyBP-C–tropomyosin competition.
Fig. 6.
Schematic diagram of a half-sarcomere illustrating thin-filament transitions, in the absence of Ca2+. The average length of the thin filament (half-sarcomere) is 1.05 μm (60, 61), which consists of a 38.5-nm periodicity of the actin–tropomyosin–troponin (A7TmTn, functional unit) complex (62, 63), i.e., each half-thin filament consists of 27 A7TmTn units (red and green rectangular boxes depict functional units). The length of the thick filament is ∼1.63 μm. After subtracting the thick-filament bare zone (M-band region that is deprived of myosin heads; 0.16 μm), the thick-filament length per half-sarcomere is ∼0.74 μm (64). Because the distance between myosin helical repeats is 42.9 nm (on the same axis layer) (65), ∼18 myosins occupy each half-thick filament. (A) Solution studies indicate that ∼95% of the myofilaments in the free Ca2+ state are blocked (B state) in the absence of cardiac myosin-binding protein C (cMyBP-C) (10). This indicates that ∼26 A7TmTn units are blocked (depicted in red) and only a single unit of A7TmTn is in the C-state position (depicted in green) per half-thin filament. The single “C”-state functional unit is randomly drawn in the scheme. This situation parallels cMyBP-C truncating mutations, where reduced total cMyBP-C protein levels have been found (22, 23). Please note that for clarity no cMyBP-C is shown (i.e., mimicking full lack of cMyBP-C). (B) In the presence of cMyBP-C, more myosin-binding sites are available on actin, because the N-terminal extension of cMyBP-C dislocates tropomyosin into the C-state position (20, 21) (depicted as seven green functional units). Assuming that cardiac muscle contains a minimum of seven cMyBP-C at regular intervals of 43 nm starting from “stripe” 5–11 in the C zone (215 nm from the M band) (66), cMyBP-C would coincide with the second to third myosin counting from the M band in the scheme. This situation mimics healthy donor samples. (C) Mutations in troponin subunits disrupt troponin–tropomyosin interactions, which causes the thin filament to be less blocked. As shown in the picture, more functional units are in the C state.
Increased Diastolic Actin–Myosin Interactions in cMyBP-C Haploinsufficient Tissue.
Although ADP responsiveness of myofilaments from HCM with MYBPC3 mutations is reduced (Fig. 2B), myofilament sensitivity to Ca2+ is increased in the presence of pathophysiological levels of ADP (Fig. 5C). In the absence of Ca2+ and with low levels of cMyBP-C, an increased pool of myosins is less restrained by cMyBP-C at the thick-filament C-zone area. However, the myosin-binding sites on actin are less accessible due to the higher steric blockade as a result of reduced cMyBP-C–tropomyosin interactions (Fig. 6A). Hence, fewer actin–myosin interactions are formed. In the presence of Ca2+, Ca2+ binding to troponin causes the movement of tropomyosin on actin and leads to the uncovering of myosin-binding sites. The reduced restraining effect on myosin caused by the low levels of cMyBP-C in combination with increased accessibility of myosin-binding sites on actin cause an increased myofilament Ca2+ sensitization. Based on our findings, this suggests that even low levels of Ca2+, as those that exist in vivo during the diastolic phase, are sufficient to increase actin–myosin interactions in HCM heart with MYBPC3 mutations and may be capable of limiting relaxation during diastole. This is indeed observed in cMyBP-C KO mice with a HCM phenotype, where cross-bridge cycling is accelerated in membrane-permeabilized cardiomyocytes (43, 44), consistent with impaired relaxation of intact cardiomyocytes (48). Pohlmann et al. (48) observed that cardiomyocytes with total cMyBP-C ablation contracted at very low Ca2+ levels and had lower diastolic sarcomere length, which was associated with high actin–myosin interactions (i.e., diastolic length was partially normalized to controls following addition of the cross-bridge inhibitor BDM). This is also consistent with the augmented sarcomere stiffness observed in MYBPC3 mutations (Fig. 5E) that could not be rescued after PKA treatment (Fig. 5F). Although counterintuitive with the findings in the absence of Ca2+ (Fig. 2), where cross-bridge formation is reduced, it likely reflects the complex interaction of the factors discussed above, including thin-filament accessibility and Ca2+ responsiveness. Overall, the results support the concept that cMyBP-C restrains actin–myosin interactions at low Ca2+ to allow complete relaxation and filling during diastole.
Troponin Mutants Increase ADP Sensitivity of Myofilaments.
Here, we provide evidence that mutations in troponin subunits may increase the availability of myosin-binding sites on actin, illustrated by higher myofilament ADP sensitivity (Fig. 2B). A schematic picture is presented in Fig. 6C to illustrate that more myosin-binding sites are accessible due to the greater disruption of the B state caused by troponin mutations. Notably, troponin exchange experiments provide the direct proof that the endogenous mutant cTnT, used in this study, impairs tropomyosin’s B-state position (Fig. 4B). This is in good agreement with solution studies with reconstituted HCM mutant troponin–tropomyosin proteins [in cTnI (19) and cTnT (18)] that support for a disrupted B state. The C-terminal half of cTnI (residues 137–210) docks the troponin–tropomyosin complex onto the outer domain of actin at low [Ca2+] and thereby maintains formation of the B state (34). It is likely that the present cTnI mutation (R145W) disrupts cTnI–tropomyosin interactions, which increases the availability of myosin-binding sites on actin. Likewise, cTnT via its interaction with tropomyosin stabilizes the thin filament in the B state (49, 50), supporting that at least the current cTnT mutation may confer a greater availability of myosin-binding sites on actin. Altogether, these findings indicate that mutations in troponin proteins may disrupt the blockade of tropomyosin on actin, which precedes the rise of cytosolic Ca2+, and are able to augment actin–myosin interactions during the diastolic phase in HCM patients.
Clinical Implications.
Our study shows that the high basal sarcomeric activation previously observed in animals and humans with HCM (9, 51–53) with thin-filament mutations may be partly due to the diminished steric blockade of myosin-binding sites on actin by tropomyosin that precedes the rise in cytosolic Ca2+ (Fig. 2B). Our data illustrate that this effect is exacerbated by the diminished PKA protein phosphorylation in HCM and IDCM hearts (Fig. 2C). The high myofilament Ca2+ sensitivity in patient samples with low PKA phosphorylation in the presence of pathologic ADP levels (Fig. 5) may be sufficient to slow ventricular relaxation. Additionally, sarcomere mutation-positive HCM samples show augmented sarcomere stiffness, which is not caused by low PKA phosphorylation (Fig. 5 E and F). The increased myofilament Ca2+ sensitivity and high sarcomere stiffness in the presence of ADP is in line with our previous study, where we showed that the complex interactions of Ca2+ and ADP increased myofilament Ca2+ sensitivity and stiffness in membrane-permeabilized rat cardiomyocytes, limited restoration of diastolic length in intact rat cardiomyocytes, and diminished ventricular compliance in whole rat hearts (17). Altogether, these myofilament changes have the potential to delay the onset of ventricular relaxation and limit proper filling, which are seen in patients (54, 55) and animal models (51, 52) of HCM, and human IDCM (4).
Conclusion
We conclude that a rather straightforward activation assay with ADP, in a membrane-permeabilized muscle preparation, can be used to reveal conformational changes in the three-state model of thin-filament activation induced by mutations and posttranslational modifications such as phosphorylation. The ADP-stimulated contraction assay indicates that increased force at diastolic Ca2+ levels, as observed in cardiomyopathies, involves increased cross-bridge formation, and is not restricted to changes in Ca2+-binding affinity of cTnC. In addition, we show that pathologic ADP levels increase myofilament Ca2+ sensitivity and stiffness, which may together contribute to the diastolic dysfunction seen in acquired and genetics forms of cardiomyopathy in humans.
Methods
Myocardial Human Samples.
Human left ventricular (LV) tissue was obtained during open heart surgery from end-stage failing (n = 4; IDCM) and HCM patients. HCM heart samples harboring thick- and thin-filament gene mutations were obtained during myectomy surgery to relieve LV outflow obstruction. Our study included patients carrying heterozygous mutations in MYBPC3 (n = 13), MYH7 (n = 3; MYH7mut), and TNNI3 (n = 1; TNNI3mut), and five HCM patients in whom no mutation was found after screening of eight genes (sarcomere mutation-negative HCM; HCMsmn). The MYBPC3 group consisted of patients with truncating (n = 10; MYBPC3trunc) and missense (n = 3; MYBPC3mut) mutations. Data for these two MYBPC3 groups are presented separately. Tissue was also obtained from one end-stage failing HCM patient carrying a homozygous TNNT2 mutation (TNNT2mut). Specific gene mutation information of HCM patients is listed in Table S1. Heart tissue from four nonfailing donors served as controls. IDCM and nonfailing donor samples were collected in cardioplegic solution. Tissue was immediately frozen and stored in liquid nitrogen. Samples were obtained after written informed consent, and the study protocol was approved by the local ethical committee of the Institute for Cardiovascular Research at the Vrije Universiteit Medisch Centrum. The investigation conforms with the principles outlined in the Declaration of Helsinki (1997).
Experimental Solutions for Isometric Force Measurements in Membrane-Permeabilized Cardiomyocytes.
The composition of all solutions was calculated based on a computer program similar to that described previously (56). The pH of all solutions was adjusted to 7.1 at 15 °C by KOH, and ionic strength was adjusted to 180 mM with KCl. The relaxing solution contained 2 mM free Mg2+, 1 mM MgATP, 20 mM EGTA, 10 mM N,N-bis(2-hydroxyethyl)-2-aminoethanesulfonic acid (BES), and 14.5 mM phosphocreatine (PCr). ADP-activating solutions contained 2 mM free Mg2+, 1 mM MgATP, 20 mM EGTA, 10 mM BES, and MgADP in increasing concentrations from 1 to 10 mM. Nominal free Ca2+ was present. Ca2+- activating solutions in the presence of 100 µM ADP consisted of 2 mM free Mg2+, 1 mM MgATP, 20 mM EGTA, 10 mM BES, and free Ca2+ varied from 1 to 32 μM. PCr was omitted from the activating solutions to preserve the MgADP/MgATP ratio. All force measurements were performed at 15 °C. We maintained [ATP] constant at 1 mM to avoid rigor cross-bridge formation [reported to occur when [ATP] falls below 0.1 mM (57)].
Isometric Force Measurements.
Small human cardiac tissue samples were thawed in relaxing solution and cardiomyocytes were mechanically isolated by tissue disruption as described previously (58). Cardiomyocytes were membrane permeabilized by incubation for 5 min in relaxing solution containing 0.5% (vol/vol) Triton X-100 and glued between a force transducer and a piezoelectric motor. The sarcomere length of the preparation was measured in relaxing solution and adjusted to 2.2 μm. Average sarcomere lengths were determined by means of a spatial Fourier transformation as described previously (58). The force measurements started with a maximal activation at ADP concentration of 10 mM (ADP-Ftotal). After a series of seven activations at submaximal ADP concentrations, another measurement was carried out at maximal ADP activation of 10 mM. Total ADP–force was measured from the zero force value obtained from slacking the cardiomyocyte to 70% of its original length within 1 ms, after the plateau of force was reached. Force was corrected for myocyte deterioration by linear interpolation between maximal force values. The intermediate results, obtained at higher ADP values, were normalized to the interpolated maximal force values. The measurements were continued until a full ADP–force curve was obtained. Cells were excluded from the study when the final force measurement at maximal ADP was less than 80% compared with the first maximal control measurement. To obtain passive force (Fpass), the cardiomyocyte was slackened to 70% of its original length for 10 s in relaxing solution by means of the piezoelectric motor from the initial sarcomere length of 2.2 μm. The decrease in force during this procedure equals the passive force at 2.2-μm sarcomere length. Active tension (ADP–Fact) was calculated as Fact = Ftotal − Fpass. Absolute tensions were normalized to cardiomyocyte cross-sectional area and expressed as developed tension (in kilonewtons per square meter). Similarly, Ca2+–force measurements in the presence of 100 µM ADP were determined as described previously (17). Residual force measurements, an indication of sarcomere stiffness, were measured after a slack–restretch procedure at high Ca2+ (32 μM) with 100 µM ADP as follows: after steady-state force was reached, cardiomyocytes were shortened to 70% of their original length within 1 ms and then restretched to the original length after 30 ms. Residual force was calculated from the initial force recovery reached after the length change and normalized to each total steady-state force reached before length change. Measurements are normalized to each corresponding total force as described previously (17). Finally, force measurements were performed following exogenous PKA treatment of cells for 40 min at 20 °C in relaxing solution containing the catalytic subunit of PKA (100 U per incubation; Sigma). To control for protocol artifacts (e.g., incubation time, buffer solution), cardiomyocytes harvested from failing hearts were incubated with PKA buffer solution but without PKA. No statistical changes were observed between the absence (3.59 ± 0.17 mM ADP) and presence of PKA buffer (3.65 ± 0.20 mM ADP).
Data Analysis Isometric Force Measurements.
Ca2+- and/or ADP–force relations were determined by a nonlinear fit procedure using a modified Hill equation using KaleidaGraph, version 3.6 (Synergy Software) as follows:
where P is steady-state force. P0 denotes the steady isometric force at either high [ADP] and saturating [Ca2+] with ADP, nH describes the steepness of the relationship, and K represents the [Ca2+] and/or [ADP] at which force is half-maximal (0.5 × P0). Myofilament Ca2+ and/or ADP sensitivity is denoted as EC50.
Exchange of Recombinant Human Wild-Type Troponin Complex in Single Cardiomyocytes.
Troponin exchange protocol.
Troponin exchange was performed as described previously (8, 9). In brief, single cardiomyocytes from the TNNT2mut heart were mechanically isolated by tissue disruption in ice-cold rigor solution (132 mM NaCl, 5 mM KCl, 1 mM MgCl2, 10 mM Tris, 5 mM EGTA, 1 mM Na-azide, pH 7.1). Cardiomyocytes were membrane permeabilized by incubation for 5 min in rigor solution containing 0.5% (vol/vol) Triton X-100. After permeabilization, cardiomyocytes were washed twice with rigor solution followed by washing in exchange solution (10 mM imidazole, 200 mM KCl, 5 mM MgCl2, 2.5 mM EGTA, 1 mM DTT, pH 6.9). Next, single cardiomyocytes were incubated overnight at 4 °C in exchange solution containing the appropriate concentration of recombinant human wild-type troponin complex (1.0 mg/mL) with the addition of 4 mM CaCl2, 4 mM DTT, 5 μL/mL protease inhibitor mixture (PIC; Sigma; P8340) and 10 μL/mL phosphatase inhibitor mixture 2 and 3 (PhIC; Sigma; P2850, P5726) (pH 6.9). The next day, cells were washed twice in rigor solution followed by washing in relaxing solution.
Determination of troponin exchange percentage.
One-half of the cardiomyocyte suspension was used for isometric force measurements, whereas the other half was used to analyze troponin exchange percentage. This half was treated with 2D-clean-up kit (GE Healthcare), homogenized in sample buffer [15% glycerol, 62.5 mM Tris (pH 6.8), 1% (wt/vol) SDS, and 2% (wt/vol) DTT], and protein concentration was measured with RCDC Protein Assay kit II (Bio-Rad).
To determine the degree of exchange of endogenous mutant troponin by recombinant wild-type cardiac troponin, Western blotting was performed. Recombinant wild-type cTnT contains a myc-tag, which allowed differentiation between endogenous and recombinant cardiac troponin complex. Proteins were separated on a 1D SDS/PAGE and blotted onto a nitrocellulose membrane. A specific monoclonal antibody was used against cTnT (clone JLT-12; Sigma) to detect endogenous and recombinant cTnT by chemiluminescence (ECL; Amersham Biosciences) as described previously.
Myofilament Protein Phosphorylation and Levels.
Phosphorylation of cTnI.
ProQ-Diamond–stained 1D gels were used to assess phosphorylation of the myofilament protein cTnI as previously described (59).
Western blot analysis of cMyBP-C total protein.
Total protein of cMyBP-C was assessed using specific antibodies in Western blots (E-7; sc-137180; Santa Cruz Biotechnology).
Incubation of Failing Cardiomyocytes with Specific cMyBP-C Antibodies.
Single cardiomyocytes from four IDCM samples were incubated for 90 min with a 1:100 dilution of either specific N′ cMyBP-C antibody (mouse monoclonal E-7; sc-137180; Santa Cruz Biotechnology), C′ cMyBP-C antibody (generously provided by Sakthivel Sadayappan, Loyola University Chicago, Maywood, IL), or an antibody against nucleolar protein nucleophosmin (mouse monoclonal; sc-32256; Santa Cruz Biotechnology). The C′ cMyBP-C antibody or nucleolar phosphoprotein antibody (control antibody) served as the incubation negative controls.
Statistical Analysis.
Data analysis and statistics were performed using Prism, version 6.0 (Graphpad Software) and SPSS, version 15.0 (IBM). Data are presented as mean ± SEM of all single cardiomyocytes per patient group [eight groups, i.e., one IDCM group, the five HCM sarcomere mutation-positive groups (HCMmut), HCMsmn, and nonfailing donor]. To take into account the repeated sample assessments within patient/donor groups, multilevel analysis was performed as shown previously (9). Comparison between all groups was performed for ADP sensitivity and Ca2+–ADP sensitivity at 2.2-μm sarcomere length before and after PKA treatment.
All data were tested for normality using the Shapiro–Wilk test. Normality was assumed when P > 0.05 and the variances were equal. Paired-samples t test was conducted when one variable was tested between two groups of data. When more than one variable was tested in more than two groups, a two-way ANOVA was conducted followed by a Bonferroni post hoc test if significant differences were found. Each test is clearly specified in the tables or figure legends. The level of significance was set at P < 0.05.
Acknowledgments
We thank Prof. David M. Warshaw (Department of Molecular Physiology and Biophysics, University of Vermont) for the valuable insightful comments and discussion, and Prof. Sakthivel Sadayappan (Department of Cell and Molecular Physiology, Loyola University Chicago) for generously providing antibody material. We acknowledge support from the Netherlands Organization for Scientific Research (NWO; VIDI Grant 91711344) and the Netherlands Cardiovascular Research Initiative, an initiative with support of the Dutch Heart Foundation, CVON-ARENA.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1513843112/-/DCSupplemental.
References
- 1.Dickstein K, et al. ESC Committee for Practice Guidelines (CPG) ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2008: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2008 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association of the ESC (HFA) and endorsed by the European Society of Intensive Care Medicine (ESICM) Eur Heart J. 2008;29(19):2388–2442, and correction (2010) 31(5):624. doi: 10.1093/eurheartj/ehn309. [DOI] [PubMed] [Google Scholar]
- 2.Borlaug BA, Paulus WJ. Heart failure with preserved ejection fraction: Pathophysiology, diagnosis, and treatment. Eur Heart J. 2011;32(6):670–679. doi: 10.1093/eurheartj/ehq426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tardiff JC, et al. Targets for therapy in sarcomeric cardiomyopathies. Cardiovasc Res. 2015;105(4):457–470. doi: 10.1093/cvr/cvv023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hamdani N, et al. Sarcomeric dysfunction in heart failure. Cardiovasc Res. 2008;77(4):649–658. doi: 10.1093/cvr/cvm079. [DOI] [PubMed] [Google Scholar]
- 5.Cazorla O, et al. Length and protein kinase A modulations of myocytes in cardiac myosin binding protein C-deficient mice. Cardiovasc Res. 2006;69(2):370–380. doi: 10.1016/j.cardiores.2005.11.009. [DOI] [PubMed] [Google Scholar]
- 6.Konhilas JP, et al. Troponin I in the murine myocardium: Influence on length-dependent activation and interfilament spacing. J Physiol. 2003;547(Pt 3):951–961. doi: 10.1113/jphysiol.2002.038117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stelzer JE, Patel JR, Moss RL. Protein kinase A-mediated acceleration of the stretch activation response in murine skinned myocardium is eliminated by ablation of cMyBP-C. Circ Res. 2006;99(8):884–890. doi: 10.1161/01.RES.0000245191.34690.66. [DOI] [PubMed] [Google Scholar]
- 8.Wijnker PJM, et al. Length-dependent activation is modulated by cardiac troponin I bisphosphorylation at Ser23 and Ser24 but not by Thr143 phosphorylation. Am J Physiol Heart Circ Physiol. 2014;306(8):H1171–H1181. doi: 10.1152/ajpheart.00580.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sequeira V, et al. Perturbed length-dependent activation in human hypertrophic cardiomyopathy with missense sarcomeric gene mutations. Circ Res. 2013;112(11):1491–1505. doi: 10.1161/CIRCRESAHA.111.300436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McKillop DF, Geeves MA. Regulation of the interaction between actin and myosin subfragment 1: Evidence for three states of the thin filament. Biophys J. 1993;65(2):693–701. doi: 10.1016/S0006-3495(93)81110-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lehman W, et al. Tropomyosin and actin isoforms modulate the localization of tropomyosin strands on actin filaments. J Mol Biol. 2000;302(3):593–606. doi: 10.1006/jmbi.2000.4080. [DOI] [PubMed] [Google Scholar]
- 12.Geeves MA, Lehrer SS. Dynamics of the muscle thin filament regulatory switch: The size of the cooperative unit. Biophys J. 1994;67(1):273–282. doi: 10.1016/S0006-3495(94)80478-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pirani A, et al. Single particle analysis of relaxed and activated muscle thin filaments. J Mol Biol. 2005;346(3):761–772. doi: 10.1016/j.jmb.2004.12.013. [DOI] [PubMed] [Google Scholar]
- 14.Shimizu H, Fujita T, Ishiwata S. Regulation of tension development by MgADP and Pi without Ca2+. Role in spontaneous tension oscillation of skeletal muscle. Biophys J. 1992;61(5):1087–1098. doi: 10.1016/S0006-3495(92)81918-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fukuda N, Fujita H, Fujita T, Ishiwata S. Spontaneous tension oscillation in skinned bovine cardiac muscle. Pflugers Arch. 1996;433(1-2):1–8. doi: 10.1007/s004240050241. [DOI] [PubMed] [Google Scholar]
- 16.Fukuda N, Fujita H, Fujita T, Ishiwata S. Regulatory roles of MgADP and calcium in tension development of skinned cardiac muscle. J Muscle Res Cell Motil. 1998;19(8):909–921. doi: 10.1023/a:1005437517287. [DOI] [PubMed] [Google Scholar]
- 17.Sequeira V, et al. Synergistic role of ADP and Ca2+ in diastolic myocardial stiffness. J Physiol. 2015;593(17):3899–3916. doi: 10.1113/JP270354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burhop J, Rosol M, Craig R, Tobacman LS, Lehman W. Effects of a cardiomyopathy-causing troponin t mutation on thin filament function and structure. J Biol Chem. 2001;276(23):20788–20794. doi: 10.1074/jbc.M101110200. [DOI] [PubMed] [Google Scholar]
- 19.Kobayashi T, Solaro RJ. Increased Ca2+ affinity of cardiac thin filaments reconstituted with cardiomyopathy-related mutant cardiac troponin I. J Biol Chem. 2006;281(19):13471–13477. doi: 10.1074/jbc.M509561200. [DOI] [PubMed] [Google Scholar]
- 20.Mun JY, et al. Electron microscopy and 3D reconstruction of F-actin decorated with cardiac myosin-binding protein C (cMyBP-C) J Mol Biol. 2011;410(2):214–225. doi: 10.1016/j.jmb.2011.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mun JY, et al. Myosin-binding protein C displaces tropomyosin to activate cardiac thin filaments and governs their speed by an independent mechanism. Proc Natl Acad Sci USA. 2014;111(6):2170–2175. doi: 10.1073/pnas.1316001111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Dijk SJ, et al. Cardiac myosin-binding protein C mutations and hypertrophic cardiomyopathy: Haploinsufficiency, deranged phosphorylation, and cardiomyocyte dysfunction. Circulation. 2009;119(11):1473–1483. doi: 10.1161/CIRCULATIONAHA.108.838672. [DOI] [PubMed] [Google Scholar]
- 23.Marston S, et al. Evidence from human myectomy samples that MYBPC3 mutations cause hypertrophic cardiomyopathy through haploinsufficiency. Circ Res. 2009;105(3):219–222. doi: 10.1161/CIRCRESAHA.109.202440. [DOI] [PubMed] [Google Scholar]
- 24.Javadpour MM, Tardiff JC, Pinz I, Ingwall JS. Decreased energetics in murine hearts bearing the R92Q mutation in cardiac troponin T. J Clin Invest. 2003;112(5):768–775. doi: 10.1172/JCI15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Spindler M, et al. Diastolic dysfunction and altered energetics in the alphaMHC403/+ mouse model of familial hypertrophic cardiomyopathy. J Clin Invest. 1998;101(8):1775–1783. doi: 10.1172/JCI1940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.He H, Javadpour MM, Latif F, Tardiff JC, Ingwall JS. R-92L and R-92W mutations in cardiac troponin T lead to distinct energetic phenotypes in intact mouse hearts. Biophys J. 2007;93(5):1834–1844. doi: 10.1529/biophysj.107.107557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bers DM. Cardiac excitation-contraction coupling. Nature. 2002;415(6868):198–205. doi: 10.1038/415198a. [DOI] [PubMed] [Google Scholar]
- 28.Swiderek K, Jaquet K, Meyer HE, Heilmeyer LMG. Cardiac troponin I, isolated from bovine heart, contains two adjacent phosphoserines. A first example of phosphoserine determination by derivatization to S-ethylcysteine. Eur J Biochem. 1988;176(2):335–342. doi: 10.1111/j.1432-1033.1988.tb14286.x. [DOI] [PubMed] [Google Scholar]
- 29.Swiderek K, et al. Sites phosphorylated in bovine cardiac troponin T and I. Characterization by 31P-NMR spectroscopy and phosphorylation by protein kinases. Eur J Biochem. 1990;190(3):575–582. doi: 10.1111/j.1432-1033.1990.tb15612.x. [DOI] [PubMed] [Google Scholar]
- 30.Robertson SP, et al. The effect of troponin I phosphorylation on the Ca2+-binding properties of the Ca2+-regulatory site of bovine cardiac troponin. J Biol Chem. 1982;257(1):260–263. [PubMed] [Google Scholar]
- 31.Dong W-J, et al. Phosphorylation-induced distance change in a cardiac muscle troponin I mutant. Biochemistry. 1997;36(22):6754–6761. doi: 10.1021/bi9622276. [DOI] [PubMed] [Google Scholar]
- 32.Finley N, et al. NMR analysis of cardiac troponin C-troponin I complexes: Effects of phosphorylation. FEBS Lett. 1999;453(1-2):107–112. doi: 10.1016/s0014-5793(99)00693-6. [DOI] [PubMed] [Google Scholar]
- 33.Ward DG, et al. NMR and mutagenesis studies on the phosphorylation region of human cardiac troponin I. Biochemistry. 2004;43(19):5772–5781. doi: 10.1021/bi036310m. [DOI] [PubMed] [Google Scholar]
- 34.Murakami K, et al. Structural basis for Ca2+-regulated muscle relaxation at interaction sites of troponin with actin and tropomyosin. J Mol Biol. 2005;352(1):178–201. doi: 10.1016/j.jmb.2005.06.067. [DOI] [PubMed] [Google Scholar]
- 35.Gautel M, Zuffardi O, Freiburg A, Labeit S. Phosphorylation switches specific for the cardiac isoform of myosin binding protein-C: A modulator of cardiac contraction? EMBO J. 1995;14(9):1952–1960. doi: 10.1002/j.1460-2075.1995.tb07187.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shaffer JF, Kensler RW, Harris SP. The myosin-binding protein C motif binds to F-actin in a phosphorylation-sensitive manner. J Biol Chem. 2009;284(18):12318–12327. doi: 10.1074/jbc.M808850200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Previs MJ, et al. Dephosphorylated cardiac myosin-binding protein C (cMyBP-C) activates native cardiac thin filaments within the C-zone of native cardiac thick filaments. Biophys J. 2013;104:186a. [Google Scholar]
- 38.Previs MJ, et al. Myosin-binding protein C corrects an intrinsic inhomogeneity in cardiac excitation-contraction coupling. Sci Adv. 2015;1(1):e1400205. doi: 10.1126/sciadv.1400205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Starr R, Offer G. The interaction of C-protein with heavy meromyosin and subfragment-2. Biochem J. 1978;171(3):813–816. doi: 10.1042/bj1710813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gilbert R, Kelly MG, Mikawa T, Fischman DA. The carboxyl terminus of myosin binding protein C (MyBP-C, C-protein) specifies incorporation into the A-band of striated muscle. J Cell Sci. 1996;109(Pt 1):101–111. doi: 10.1242/jcs.109.1.101. [DOI] [PubMed] [Google Scholar]
- 41.Moos C, Mason CM, Besterman JM, Feng INM, Dubin JH. The binding of skeletal muscle C-protein to F-actin, and its relation to the interaction of actin with myosin subfragment-1. J Mol Biol. 1978;124(4):571–586. doi: 10.1016/0022-2836(78)90172-9. [DOI] [PubMed] [Google Scholar]
- 42.Yamamoto K, Moos C. The C-proteins of rabbit red, white, and cardiac muscles. J Biol Chem. 1983;258(13):8395–8401. [PubMed] [Google Scholar]
- 43.Stelzer JE, Dunning SB, Moss RL. Ablation of cardiac myosin-binding protein-C accelerates stretch activation in murine skinned myocardium. Circ Res. 2006;98(9):1212–1218. doi: 10.1161/01.RES.0000219863.94390.ce. [DOI] [PubMed] [Google Scholar]
- 44.Stelzer JE, Fitzsimons DP, Moss RL. Ablation of myosin-binding protein-C accelerates force development in mouse myocardium. Biophys J. 2006;90(11):4119–4127. doi: 10.1529/biophysj.105.078147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Previs MJ, Beck Previs S, Gulick J, Robbins J, Warshaw DM. Molecular mechanics of cardiac myosin-binding protein C in native thick filaments. Science. 2012;337(6099):1215–1218. doi: 10.1126/science.1223602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gruen M, Gautel M. Mutations in β-myosin S2 that cause familial hypertrophic cardiomyopathy (FHC) abolish the interaction with the regulatory domain of myosin-binding protein-C. J Mol Biol. 1999;286(3):933–949. doi: 10.1006/jmbi.1998.2522. [DOI] [PubMed] [Google Scholar]
- 47.Sequeira V, Witjas-Paalberends ER, Kuster DWD, van der Velden J. Cardiac myosin-binding protein C: Hypertrophic cardiomyopathy mutations and structure-function relationships. Pflugers Arch. 2014;466(2):201–206. doi: 10.1007/s00424-013-1400-3. [DOI] [PubMed] [Google Scholar]
- 48.Pohlmann L, et al. Cardiac myosin-binding protein C is required for complete relaxation in intact myocytes. Circ Res. 2007;101(9):928–938. doi: 10.1161/CIRCRESAHA.107.158774. [DOI] [PubMed] [Google Scholar]
- 49.Tobacman LS, et al. The troponin tail domain promotes a conformational state of the thin filament that suppresses myosin activity. J Biol Chem. 2002;277(31):27636–27642. doi: 10.1074/jbc.M201768200. [DOI] [PubMed] [Google Scholar]
- 50.Gollapudi SK, Mamidi R, Mallampalli SL, Chandra M. The N-terminal extension of cardiac troponin T stabilizes the blocked state of cardiac thin filament. Biophys J. 2012;103(5):940–948. doi: 10.1016/j.bpj.2012.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schober T, et al. Myofilament Ca sensitization increases cytosolic Ca binding affinity, alters intracellular Ca homeostasis, and causes pause-dependent Ca-triggered arrhythmia. Circ Res. 2012;111(2):170–179. doi: 10.1161/CIRCRESAHA.112.270041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tardiff JC, et al. Cardiac troponin T mutations result in allele-specific phenotypes in a mouse model for hypertrophic cardiomyopathy. J Clin Invest. 1999;104(4):469–481. doi: 10.1172/JCI6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Knollmann BC, et al. Inotropic stimulation induces cardiac dysfunction in transgenic mice expressing a troponin T (I79N) mutation linked to familial hypertrophic cardiomyopathy. J Biol Chem. 2001;276(13):10039–10048. doi: 10.1074/jbc.M006745200. [DOI] [PubMed] [Google Scholar]
- 54.Germans T, et al. How do hypertrophic cardiomyopathy mutations affect myocardial function in carriers with normal wall thickness? Assessment with cardiovascular magnetic resonance. J Cardiovasc Magn Reson. 2010;12(1):13. doi: 10.1186/1532-429X-12-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nagueh SF, et al. Tissue Doppler imaging consistently detects myocardial abnormalities in patients with hypertrophic cardiomyopathy and provides a novel means for an early diagnosis before and independently of hypertrophy. Circulation. 2001;104(2):128–130. doi: 10.1161/01.cir.104.2.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Papp Z, Szabó A, Barends JP, Stienen GJM. The mechanism of the force enhancement by MgADP under simulated ischaemic conditions in rat cardiac myocytes. J Physiol. 2002;543(Pt 1):177–189. doi: 10.1113/jphysiol.2002.022145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cooke R, Bialek W. Contraction of glycerinated muscle fibers as a function of the ATP concentration. Biophys J. 1979;28(2):241–258. doi: 10.1016/S0006-3495(79)85174-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.van der Velden J, et al. Force production in mechanically isolated cardiac myocytes from human ventricular muscle tissue. Cardiovasc Res. 1998;38(2):414–423. doi: 10.1016/s0008-6363(98)00019-4. [DOI] [PubMed] [Google Scholar]
- 59.Zaremba R, et al. Quantitative analysis of myofilament protein phosphorylation in small cardiac biopsies. Proteomics Clin Appl. 2007;1(10):1285–1290. doi: 10.1002/prca.200600891. [DOI] [PubMed] [Google Scholar]
- 60.Page SG, Huxley HE. Filament lengths in striated muscle. J Cell Biol. 1963;19(2):369–390. doi: 10.1083/jcb.19.2.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Robinson TF, Winegrad S. The measurement and dynamic implications of thin filament lengths in heart muscle. J Physiol. 1979;286:607–619. doi: 10.1113/jphysiol.1979.sp012640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Tobacman LS. Thin filament-mediated regulation of cardiac contraction. Annu Rev Physiol. 1996;58:447–481. doi: 10.1146/annurev.ph.58.030196.002311. [DOI] [PubMed] [Google Scholar]
- 63.White SP, Cohen C, Phillips GN., Jr Structure of co-crystals of tropomyosin and troponin. Nature. 1987;325(6107):826–828. doi: 10.1038/325826a0. [DOI] [PubMed] [Google Scholar]
- 64.Higuchi H, Yanagida T, Goldman YE. Compliance of thin filaments in skinned fibers of rabbit skeletal muscle. Biophys J. 1995;69(3):1000–1010. doi: 10.1016/S0006-3495(95)79975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Huxley HE. The structural basis of muscular contraction. Proc R Soc Lond B Biol Sci. 1971;178(1051):131–149. doi: 10.1098/rspb.1971.0057. [DOI] [PubMed] [Google Scholar]
- 66.Luther PK, et al. Direct visualization of myosin-binding protein C bridging myosin and actin filaments in intact muscle. Proc Natl Acad Sci USA. 2011;108(28):11423–11428. doi: 10.1073/pnas.1103216108. [DOI] [PMC free article] [PubMed] [Google Scholar]