Significance
Using functional MRI, we examined reading and speech perception in four highly contrasting languages: Spanish, English, Hebrew, and Chinese. With three complementary analytic approaches, we demonstrate that in spite of striking dissimilarities among writing systems, successful literacy acquisition results in a convergence of the speech and orthographic processing systems onto a common network of neural structures. These findings have the major theoretical implication that the reading network has evolved to be universally constrained by the organization of the brain network underlying speech.
Keywords: cross-language invariance, word recognition, functional MRI
Abstract
We propose and test a theoretical perspective in which a universal hallmark of successful literacy acquisition is the convergence of the speech and orthographic processing systems onto a common network of neural structures, regardless of how spoken words are represented orthographically in a writing system. During functional MRI, skilled adult readers of four distinct and highly contrasting languages, Spanish, English, Hebrew, and Chinese, performed an identical semantic categorization task to spoken and written words. Results from three complementary analytic approaches demonstrate limited language variation, with speech–print convergence emerging as a common brain signature of reading proficiency across the wide spectrum of selected languages, whether their writing system is alphabetic or logographic, whether it is opaque or transparent, and regardless of the phonological and morphological structure it represents.
Although all orthographies have evolved to convey meaning through written forms, the world’s writing systems differ in a wide array of characteristics and dimensions. Decades of behavioral research have shown that such extensive cross-linguistic differences result in substantial variability in the behavioral phenotype of reading [e.g., the trajectory for reading proficiency (1), grain size of computed phonological representation (2), sensitivity to letter order (3), and extent and type of lexically driven phonology (4)]. Recent theoretical advances, however, argue that there are higher-order cognitive operations that are invariant across writing systems—reading universals (5, 6).
This ongoing controversy is reflected in heated debates regarding the universality of the neural bases of reading. For example, whereas some neuroimaging studies focused on print processing argue that the same core network (7–9) underlies reading in different languages and orthographies with subtle cross-linguistic differences in the relative weighting of the network’s components (10–12), other studies contest language invariance, demonstrating that certain cortical constituents of the reading network are language-specific (13, 14). Here we address this controversy by tracking and comparing brain signatures of native proficient written and spoken word processing in four distinct and highly contrasting languages: Spanish, English, Hebrew, and Chinese. We show for the first time, to our knowledge, that the convergence of the print- and speech-processing networks emerges as an invariant and universal signature of literacy proficiency despite extensive differences among these writing systems.
The theoretical assumption guiding this investigation is that reading is best understood as fundamentally a linguistic act rather than the mere recognition of orthographic forms (5, 15). Therefore, in any language, regardless of its writing system, reading would not only recruit the neural circuits best suited for processing its orthographic symbols (which could show some front-end variation due to visuospatial differences) but would fundamentally depend on access to existing neurocircuits implicated in processing meaningful spoken words (16). By this view, a universal hallmark of successful literacy acquisition would be the emergence of a reading network that is strongly constrained by the brain network underlying the processing of spoken words (a network itself likely to be largely universal across languages), regardless of how these words are represented orthographically (17, 18).
We examined the extent of convergence of neural networks involved in spoken and written word recognition in 84 right-handed, healthy, and skilled adult readers in Spanish, English, Hebrew, and Chinese (n = 21 per language; see Table S1 for details on group matching). These languages were selected because they provide contrasts of transparent vs. opaque orthographies with alphabetic vs. logographic writing systems, which map into monomorphemic and monosyllabic words vs. morphologically complex and multisyllabic words, having concatenated linear morphology vs. nonconcatenated nonlinear morphology, with visually simple vs. complex print, which map into tonal vs. nontonal spoken forms. Because a primary goal of reading is to extract abstract meaning from the encoded symbols, we selected an ecologically valid task in which responses to printed or spoken forms required semantic judgments. During functional MRI (fMRI), participants performed an identical semantic categorization task in the four sites (judging whether spoken or written words referred to living things).
Table S1.
Descriptive statistics for the 21 participants in each language group, including mean proportion correct and reaction times for the fMRI task
| Language | Age (y) | Sex (male, female) | IQ (WASI T) | Accuracy (auditory) | Accuracy (visual) | RT (auditory) | RT (visual) |
| English | 23.38 | 11, 10 | 58.37 | 0.93 | 0.941 | 1,288 | 1,081 |
| Hebrew | 24.86 | 9, 12 | 55.67 | 0.87 | 0.953 | 1,420 | 907 |
| Spanish | 22.67 | 10, 11 | 56.52 | 0.92 | 0.925 | 1,216 | 1,003 |
| Chinese | 23.48 | 9, 12 | 56.29 | 0.92 | 0.954 | 1,186 | 850 |
RT, reaction time; WASI, Wechsler Abbreviated Scale of Intelligence. T scores have a mean of 50 and a standard deviation of 10.
Results
fMRI Task Performance.
Separate 2 (modality: print, speech) × 4 (language: Spanish, English, Hebrew, Chinese) mixed-factors ANOVAs were conducted on accuracy and reaction times to correct responses. Mean accuracy and response latencies for each language are shown in Table S1. Accuracy was uniformly high with no language differences; however, there was a significant main effect of language on reaction time [F(1,80) = 4.491, P = 0.006], which post hoc Tukey tests revealed was due to faster reaction times for Chinese than for English and Hebrew. A significant modality by language interaction was observed on reaction times [F(1,80) = 10.224, P < 0.0001] and on accuracy [F(1,80) = 4.528, P = 0.006]. Inspection of means shows that this is due to larger accuracy and latency advantage for print than speech in Hebrew relative to the other languages. Neuroimaging analyses excluded error responses from analysis, and separate models were run including reaction times as a covariate, which verified that the neuroimaging results were not qualified by accuracy or response latencies.
Analytic Approach.
We used three complementary analytic approaches to assess the degree of convergence between activation for print and speech, both in each language and across languages. First, we used conjunction logic to create intersect maps of regions within each language that were significantly active across subjects for both print and speech (as well as for print only and speech only). We then created intersect-of-intersect maps to identify speech–print convergence regions (as well as print-only and speech-only regions) that were significantly active across all languages. Our second approach used ANOVA to assess whether there were differences in the mean activation of regions engaged for print and speech across languages as well as whether there were any language differences within print and speech that might be of general interest to the field. Third, we used a voxel-wise correlation analyses to assess whether the magnitude of print activation and speech activation was correlated across subjects for each language. This provided unique information regarding how print-related activation covaried with speech-related activation across participants, allowing us to gauge the extent of individual differences in the four languages.
Intersect Analysis.
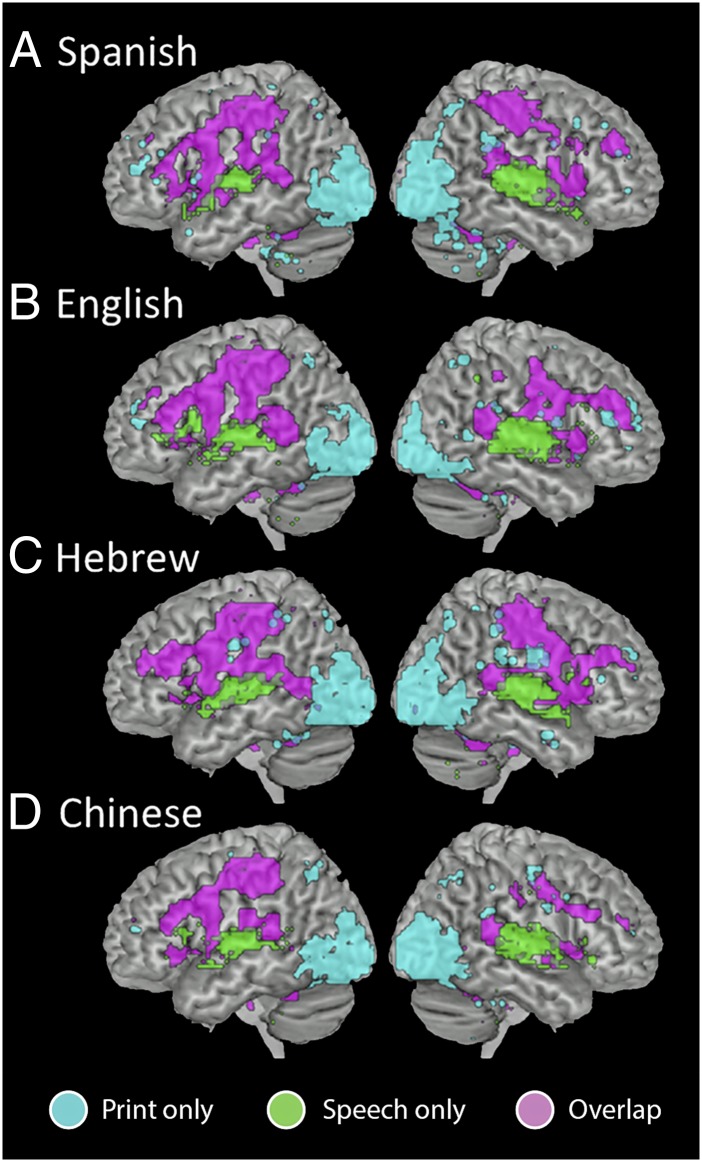
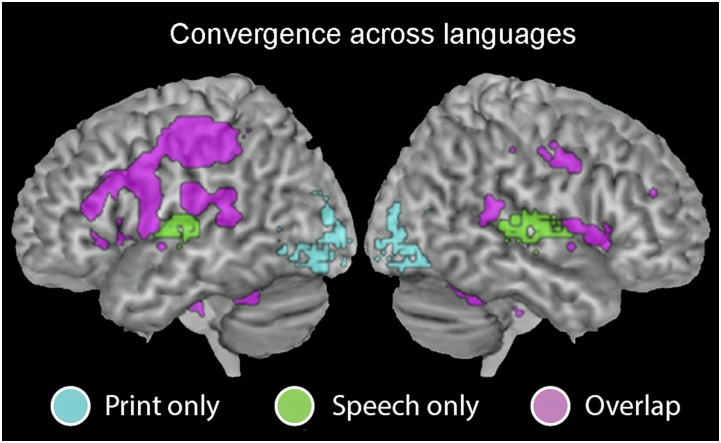
As shown in Figs. 1 and 2, the results of the intersect analysis revealed that printed and spoken word recognition engaged similar networks both within and across languages. In each language, bilateral striate and extrastriate regions were significantly active only for print, and anterior aspects of the superior temporal gyrus (STG) were active only for speech. Most striking, however, is the extensive convergence of printed and spoken language processing in many areas, including both cortical [bilateral inferior frontal gyrus (IFG), bilateral middle temporal gyrus (MTG) to STG, left inferior parietal lobule (IPL)] and subcortical (bilateral insula, putamen, thalamus) regions associated with both phonological and semantic processing.
Fig. 1.
Intersect maps showing brain regions that are active for print only (cyan), speech only (green), or both print and speech (violet) (threshold for each modality q < 0.001, FDR-corrected) for each language. (A) Spanish. (B) English. (C) Hebrew. (D) Chinese.
Fig. 2.
Intersect maps showing brain regions that are active for print only (cyan), speech only (green), or both print and speech (violet) across all four languages (threshold for each modality q < 0.001, FDR-corrected; conjoint probability of activation for both speech and print across all languages is q < 0.001^8).
ANOVA.
For the interaction of language by modality, there were no activated voxels at a significance level of q < 0.05, corrected for false discovery rate. That is, despite the strong activations revealed for print and speech in each language, regional activation did not differ by modality across languages. This again suggests a common network engaged for print and speech for all four writing systems. To examine language differences within print and within speech, we conducted separate 1 × 4 ANOVAs. In both ANOVAs, we observed small differences in regions outside the canonical reading and speech networks—bilateral postcentral gyrus and cingulate gyrus. Whereas our primary analyses mitigate scanner-related differences by comparing within-subject measures (print and speech) across languages, these direct language comparisons are potentially more susceptible to cross-scanner differences and hence should be interpreted with caution.
Correlation.
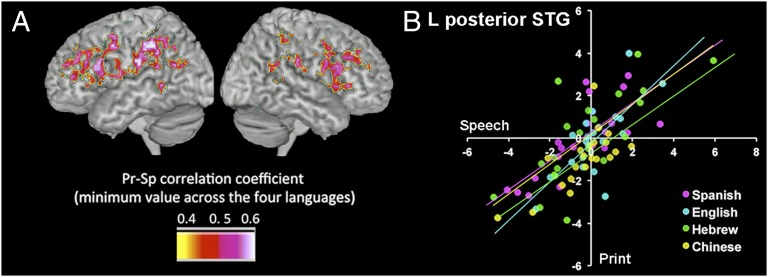
We computed the Pearson correlation coefficient across subjects, between each subject’s regression parameter estimate for print processing and speech processing. Results of this analysis revealed frontal and dorsal regions, including bilateral IFG, right supramarginal gyrus (SMG), left middle frontal gyrus, and left insula, in which activations for print and speech were strongly and similarly correlated in each of the languages (r > 0.45, P < 0.04 for each language independently; Fig. 3).
Fig. 3.
Print and speech convergence common across all languages. (A) Areas in which print (Pr) and speech (Sp) activation were correlated in the four languages. (Values indicate the minimum r value across all languages.) (B) Scatter plot of the correlation between print and speech activation in a representative area for each language (posterior superior temporal gyrus; MNI coordinates X −47, Y −62, Z 21).
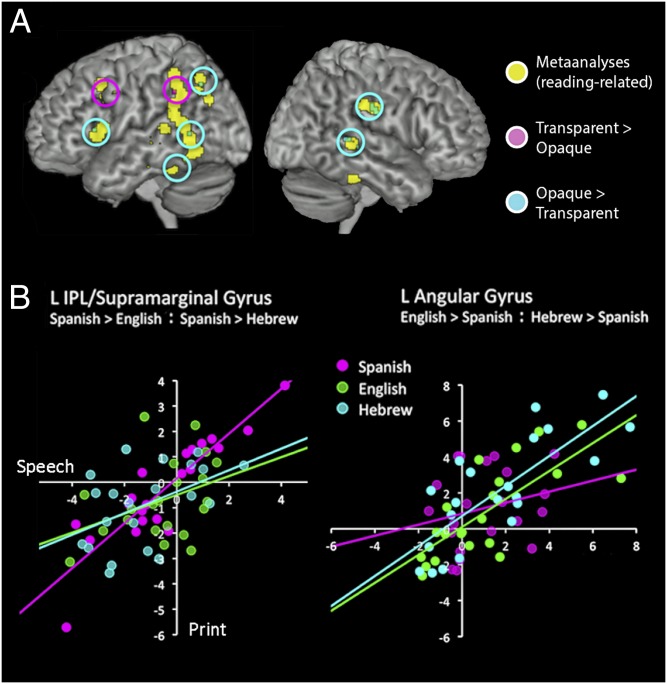
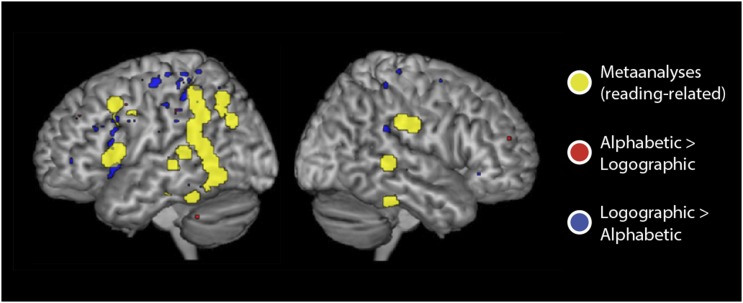
Next, we examined how orthographic depth [ambiguity in the coding of phonology by spelling (4)] modulated speech–print overlap within the three alphabetic languages. Although this issue has been discussed extensively in behavioral research (19–21), studies of functional neuroanatomy are fewer, typically limited to two-language comparisons. To limit statistical correction and maximize our ability to detect differences among languages, this analysis was guided by meta-analyses of reading studies (22–24). We restricted analysis to voxels within the core reading circuit by combining 10-mm-diameter spheres centered on all reported peaks of reading-related activation in these meta-analyses. We found that the transparent orthography (Spanish) showed significantly greater positive correlation between print and speech in the left hemisphere dorsal pathways in left SMG, IFG/precentral gyri compared independently with each of the opaque (English, Hebrew) orthographies. In contrast, speech–print correlations were higher for the opaque orthographies compared with the transparent orthography in left angular gyrus, left posterior MTG, left fusiform/inferior temporal gyri (ITG), and left IFG (pars triangularis) (Fig. 4 and Table S2). Right hemisphere SMG and posterior STG also showed stronger correlations for the comparison of opaque versus transparent orthographies.
Fig. 4.
Print and speech convergence as a factor of orthographic depth. (A) Areas in yellow show the reading circuit identified by published meta-analyses. Speech–print correlations were higher for transparent (Spanish) than opaque orthographies (English and Hebrew compared independently, P < 0.05 for each comparison) in areas shown in pink. Areas shown in light blue show higher correlations for the opaque orthographies compared with the transparent orthography, P < 0.05. (B) Scatter plots of the correlation between print and speech activation in representative areas showing greater convergence for more transparent languages in left inferior parietal lobule, supramarginal gyrus (Left), and left angular gyrus for opaque languages (Right). Fisher’s R-to-Z transform was performed to calculate statistical significance.
Table S2.
Brain regions in which speech–print correlations for alphabetic languages differed as a function of orthographic depth
| Brain region | MNI coordinates | Volume, mm3 | ||
| X | Y | Z | ||
| Spanish > English : Spanish > Hebrew | ||||
| L IFG Op | −32 | 11 | 33 | 27 |
| L MFG | −26 | 38 | −4 | 27 |
| L SMG | −53 | −44 | 39 | 27 |
| English > Spanish : Hebrew > Spanish | ||||
| L IFG Tri | −50 | 20 | 9 | 27 |
| L IFG, PreCG | −38 | −2 | 36 | 27 |
| L IPL, SPL | −35 | −65 | 45 | 27 |
| L MTG | −47 | −56 | 9 | 54 |
| L MTG, ITG | −50 | −47 | −13 | 27 |
| L precuneus, MOG | −35 | −68 | 36 | 54 |
| R insula | 41 | −20 | −1 | 27 |
| R PostCG, PreCG, SMG | 53 | −20 | 33 | 243 |
| R MTG, STG | 56 | −38 | 6 | 189 |
| R MTG | 53 | −32 | −1 | 27 |
Analyses were conducted only on voxels within a composite of reading regions identified from meta-analyses of reading skill. IFG, inferior frontal gyrus; IPL, inferior parietal lobule; ITG, inferior temporal gyrus; L, left; MFG, middle frontal gyrus; MOG, middle occipital gyrus; MTG, middle temporal gyrus; Op, opercularis; PostCG, postcentral gyrus; PreCG, precentral gyrus; R, right; SMG, supramarginal gyrus; SPL, superior parietal lobule; STG, superior temporal gyrus; Tri, triangularis.
We also examined specifically whether there were significant differences in speech–print correlation between alphabetic and logographic languages. For this comparison, analyses were conducted on a whole-brain voxel-wise basis rather than with the reading circuit identified from published meta-analyses of reading that include a disproportionate number of studies of alphabetic languages. Results of this analysis revealed small clusters in which speech–print correlation was greater for Chinese than for each of the alphabetic languages; however, the clusters were neither in the canonical alphabetic reading regions nor in the regions claimed to be selectively activated in Chinese (e.g., right fusiform, left middle frontal, and superior parietal gyri; Fig. 5). The reverse effect was observed in a small number of voxels in areas such as left SMG, bilateral cerebellum, and bilateral middle frontal gyri.
Fig. 5.
Areas in which speech–print convergence differed for alphabetic orthographies (Spanish, English, Hebrew) compared independently with the logographic orthography (Chinese), each comparison P < 0.05, corrected. Areas shown in blue showed greater convergence for the logographic than the alphabetic orthographies; areas in red showed the opposite pattern. Areas in yellow provide a reference to the reading circuit identified by published meta-analyses.
Converging Measures of Speech–Print Convergence.
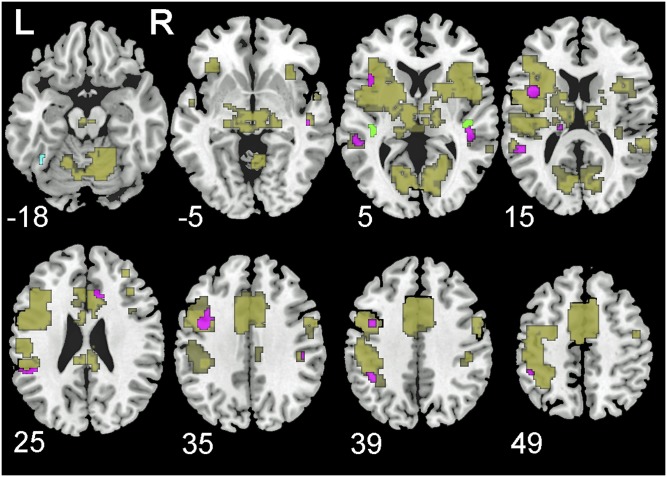
Thus far, we have presented three analyses suggesting that speech–print convergence emerges as a common brain signature across the wide spectrum of the selected languages. An ANOVA failed to reveal any region where the effect of modality on activation was modulated by language. More importantly, both the intersection and correlation analyses provided positive evidence that a number of regions were engaged in both print and speech processing in each of the four languages. However, the intersection and correlation approaches to isolating speech–print overlap are quite different in many respects, and therefore it is important to identify the subset of regions where speech–print overlap is common to both. Thus, in Fig. 6 (also see Table 1), we display those regions that were identified as speech–print convergence regions by both the intersection and the correlation analyses. Because some of these regions likely support general task functions such as motor response or attentional control, we further identify the subset of regions within the core reading network (as defined above by three meta-analyses) that were classified as speech–print overlap regions by both analyses. As the figure illustrates, the intersection and correlation analyses converge in identifying key regions within the reading network that are engaged by both speech and print in all four languages. (Several small regions within the reading network are also displayed that were active across languages for print or speech only in the intersect analysis and for which print and speech were not significantly correlated.) These overlap regions include primarily left hemisphere clusters in anterior (e.g., IFG/insula), posterior dorsal (e.g., STG, SMG, IPL), and posterior ventral (e.g., MTG) sites. The extent of speech–print correspondence found using both methods provides strong support for the conclusion that speech–print overlap is a universal marker of reading.
Fig. 6.
Overlap in print, speech, and speech–print convergence regions in relation to reading-related regions. Brain regions in olive represent speech–print convergence regions, namely areas common across speech-related activation (speech), print-related activation (print), and correlated activation between speech and print (correlation). Brain regions in violet represent reading-related regions with speech–print convergence, namely areas common across print, speech, and correlation, and also the known reading circuit defined by prior meta-analyses of fMRI studies (reading). The brain region in cyan represents reading-related regions with print specificity, namely an area common across print and reading but not speech or correlation. Brain regions in green represent reading-related regions with speech specificity, namely areas common across speech and reading but not print or correlation. L, left; R, right.
Table 1.
Brain activation patterns common across all four languages
| Brain region | MNI coordinates | Volume, mm3 | ||
| X | Y | Z | ||
| Print–speech convergence regions* | ||||
| L IFG Tri, PreCG, MFG, insula, IPL, IFG Op, thalamus, putamen | 6 | −20 | −8 | 86,238 |
| Bilateral SFG, MFG, SMA, anterior cingulate | 6 | 26 | 18 | 34,803 |
| Bilateral cerebellum | 22 | −70 | −40 | 21,627 |
| Bilateral calcarine, cuneus, lingual gyrus | −12 | −80 | 0 | 12,375 |
| L STG, SMG, PostCG, MTG, insula | −48 | −42 | 6 | 9,423 |
| R PreCG, PostCG, MFG, IFG Op | 58 | 0 | 34 | 1,719 |
| Posterior cingulate | 6 | −40 | 22 | 1,593 |
| R STG, MTG, insula | 64 | −46 | 6 | 1,548 |
| R IPL, PostCG, SMG | 46 | −28 | 34 | 1,053 |
| R STG, MTG | 52 | −18 | −8 | 900 |
| Posterior cingulate | −2 | −34 | 24 | 675 |
| R insula, STG | 36 | −34 | 16 | 567 |
| R MFG | 36 | 42 | 22 | 243 |
| R MFG, IFG Tri | 40 | 26 | 24 | 243 |
| L cerebellum | −8 | −80 | −34 | 207 |
| R STG | 54 | 0 | −6 | 153 |
| R STG | 48 | −46 | 10 | 99 |
| L STG | −52 | −6 | −6 | 90 |
| Reading-related regions with print–speech convergence† | ||||
| L IFG Op, PreCG | 38 | 0 | 30 | 1,008 |
| L insula, L IFG Op | 38 | −4 | 8 | 720 |
| R STG | −48 | 22 | −6 | 441 |
| L STG | 52 | 46 | 12 | 423 |
| L MTG, STG | 50 | 40 | 6 | 306 |
| L IFG Tri, IFG Orb, insula | 42 | −14 | 0 | 270 |
| Anterior cingulate | −10 | −26 | 20 | 261 |
| L IPL | 38 | 48 | 40 | 162 |
| L thalamus | 16 | 20 | 8 | 90 |
| Reading-related regions with print specificity‡ | ||||
| L fusiform | −40 | −54 | −20 | 90 |
| Reading-related regions with speech specificity§ | ||||
| R STG, insula | 42 | −22 | 0 | 288 |
| L STG | −40 | −32 | 4 | 252 |
IFG, inferior frontal gyrus; IPL, inferior parietal lobule; L, left; MFG, middle frontal gyrus; MTG, middle temporal gyrus; Op, opercularis; Orb, orbitalis; PostCG, postcentral gyrus; PreCG, precentral gyrus; R, right; SFG, superior frontal gyrus; SMA, supplementary motor area; SMG, supramarginal gyrus; STG, superior temporal gyrus; Tri, triangularis.
Areas common across speech-related activation (speech), print-related activation (print), and correlated activation between speech and print (correlation).
Areas common across speech, print, and correlation within the reading circuit (reading).
Areas common across print and reading but not speech or correlation.
Areas common across speech and reading but not print or correlation.
Discussion
In this study, we show for the first time, to our knowledge, that reading in four contrasting languages, Spanish, English, Hebrew, and Chinese, results in a strikingly similar neural organization despite dramatic differences in their writing systems. Considering results from three types of data-analytic approaches, our findings demonstrate that speech–print convergence emerges as a common brain signature across the wide spectrum of selected languages, whether their writing system is alphabetic or logographic, whether it is transparent or opaque, and regardless of the phonological and morphological structure it represents. Our findings thus provide support for speech–print convergence as a universal principle of brain organization resulting from (i) the biological constraints imposed by perisylvian specialization for speech and natural language processing, and (ii) the cognitive imperative to leverage these biologically specialized systems for supporting print comprehension in any language (5).
Our aim in the present study was to examine speech–print convergence in an ecologically valid reading task that not only taps the front-end processing of orthography but mimics the end purpose of reading: access to meaning. By focusing on tasks that force lexical semantic processing and by specifically targeting speech–print convergence to identify core regions, we provide clear evidence of speech–print integration in all four languages throughout the perisylvian region, including Broca’s area in the IFG and Wernicke’s area in the STG, which has been suggested to play a diminished role in the reading of Chinese (14). This comparable pattern of convergence is particularly important, because Chinese and its logographic monomorphemic writing system is often taken as a critical test case for nonuniversality in reading (25, 26). The convergence of speech and reading appears, then, to be a universal principle.
Our findings indicate that the general topology for speech–print convergence is invariant across all four languages; however, correlational analyses revealed subtle differences in the strength of this coupling in several regions of interest. Specifically, speech–print convergence was higher for (transparent) Spanish than (opaque) English and Hebrew in two left hemisphere regions, the SMG and supplementary motor area. By contrast, speech–print convergence was higher for English and Hebrew relative to Spanish in left angular gyrus and in several ventral left hemisphere regions including the fusiform gyrus, MTG, and ITG, along with right STG and MTG. It is noteworthy that the sites with stronger speech–print coupling in orthographically transparent Spanish (SMG and supplementary motor area) are generally associated with phonological processing (27), whereas the sites with stronger speech–print coupling in orthographically deep English and Hebrew (including angular gyrus, MTG, and ITG) are more closely associated with semantic processing (28). However, note that all four languages show convergence in all of these regions, with observed differences representing relatively minor variation in the degree of convergence, demonstrating how the reading network is deeply constrained by the organization of the brain network underlying speech.
Several points of discussion regarding the implications of our results should be raised at this point. First, although it is generally assumed that shared activation equals shared functionality at some level, the fact that the same brain regions are engaged in reading and understanding spoken words in different languages does not necessarily imply that identical computations are used across these languages. Indeed, language-specific computations have been shown to be shaped by the idiosyncratic properties of different writing systems (e.g., the grain size and reliability of the orthographic–phonological correspondences embodied by a given writing system, etc.) (2). Second, previous studies have demonstrated both task-dependent and task-invariant activation in reading tasks (29, 30). Compared with perceptual judgment tasks, or lexical tasks such as lexical decision and naming, the animacy judgment task we used in the present study is arguably more similar across languages in terms of computations, and matched our aim to probe the full lexical processing system engaged in reading. It is possible, however, that had we used other tasks, some language-specific differences might have emerged, although the core convergence should be task-invariant. Finally, the language groups in our study were defined by the native languages of the participants. It is likely that some of these participants (particularly those in the Hebrew, Spanish, and Chinese samples) are bilingual or multilingual. Although we argue that the convergence of speech and reading on a common neural network reflects universal neurocomputational principles, the degree to which this convergence is modulated by the acquisition of a second or third language is a matter for future research.
In conclusion, our findings reveal that, regardless of how spoken forms and their meanings are represented in a given writing system, proficient reading entails the convergence of speech and orthographic processing systems onto a common network of neural structures. In general, all orthographies have evolved to provide readers with maximal cues about spoken words and their meaning in any given language. This communality invariantly leads the neural bases of reading to be organized around speech–print convergence rather than simply to neural activation evoked by print alone.
Materials and Methods
Participants.
Twenty-one right-handed native speakers of each language (total of 84 participants) participated in the experiment in exchange for payment. All participants reported normal or corrected-to-normal vision, normal hearing, and no history of neurological or language impairment. Across each language group, participants were matched on mean age, gender, and IQ (Table S1). All procedures were approved by local institutional review boards in Spain (Basque Center on Cognition, Brain and Language), the United States (Yale University School of Medicine), Israel (The Hebrew University of Jerusalem), and Taiwan (National Yang-Ming University). Informed consent was obtained in compliance with human subjects protection and Helsinki Declaration guidelines.
Stimuli and Task.
The stimuli for each language consisted of concrete/imageable nouns, which were presented visually or auditorily. Within each modality, half of the words referred to nonliving objects (e.g., WINDOW) and half referred to living objects (WOMAN). Word frequency was equated across the four languages.
Participants made semantic judgments (living/nonliving) in eight functional runs. In each run, only print stimuli or auditory stimuli were presented, and all runs began with 20 s of silent fixation to improve estimation of the resting baseline in functional analyses. Each run of written words comprised six blocks of eight trials each, separated by six 20-s rest blocks. Each run of spoken words comprised six blocks of 20 auditory trials, separated by six 20-s rest blocks. In each block, an equal number of living and nonliving items was presented. There were 288 print stimuli in total presented over eight runs, including 192 stimuli (four additional conditions) that are not the focus of this report. For the analyses reported below, we therefore included data from only the two of the six print blocks per run (96 printed word trials in total) that were directly comparable to the auditory condition. The auditory condition included 80 auditorily presented items over two functional runs.
fMRI Acquisition Parameters.
fMRI data at each site were acquired on Siemens 3T scanners using the same single-shot gradient echo echo-planar imaging sequence [flip angle (FA), 80°; echo time (TE), 30 ms; repetition time (TR), 2,000 ms; field of view (FOV), 22 × 22 cm; slice thickness, 4 mm, no gap; matrix size, 64 × 64; number of excitations (NEX), 1]. Trials with each activation block were presented at jittered trial durations (4–7 s) to permit event-related analyses, and also included occasional longer durations (i.e., null trials) to increase baseline estimation; visual targets remained on the screen for 2 s. Scan length for the auditory runs was 304 s, and scan length for the visual runs was 360 s.
fMRI Data Processing and Analysis.
fMRI data were processed using Analysis of Functional NeuroImages (AFNI) (31). Data were first aligned in time to correct for offsets of slice time acquisition, and next data were aligned to the high-resolution anatomical scan, corrected for motion, and transformed to Montreal Neurological Institute (MNI) space using a single interpolation warp, which concatenated a rigid-body transform for motion, a 12-parameter affine transform for coregistration, and a combination of affine and nonlinear warp for normalization to MNI space. Following recommendations from the Functional Bioinformatics Research Network (FBIRN), data from each site were blurred to a uniform final full width at half maximum (FWHM) value of 8 mm to control for multiple scanner comparisons (32) and scaled to percent signal change. Final voxel size for analysis was 3 mm3. Single-subject data were submitted to an event-related general linear model (GLM) analysis modeling the print and speech conditions using restricted maximum likelihood (REML) estimation and autoregressive moving average (ARMA) covariance structure. The model included nuisance regressors to control for motion parameters (three rotation, three translation). Additionally, 0.89% of TRs were considered outliers and censored from the model due to containing more than 10% of voxels with a point-to-point movement greater than 3 mm or 3°. To ensure that neuroimaging results were not qualified by accuracy or response latencies, error responses were excluded from analysis and separate models were run including reaction times as a covariate.
For group analyses, single-subject regression parameter estimates for print and speech conditions were entered into a 4 (language: Chinese, English, Hebrew, Spanish) × 2 (modality: print, speech) repeated-measures ANOVA performed using 3dMVM (33). To further correct scanner differences, we included signal-to-fluctuation-noise ratio (SFNR) as a voxel-wise covariate in the model (34). Alpha level for the ANOVA was set at q < 0.05, corrected for false discovery rate [FDR (35)].
Intersect maps were constructed using the output of these group analyses. Based on conjunction null logic (36), we conducted analyses in which we identified voxels that were significantly active across subjects at P < 0.001, FDR-corrected, for print only or speech only, or were active for both print and speech for (i) each language and (ii) across all languages.
For the voxel-wise correlation analysis, we computed the Pearson correlation coefficient across subjects, between each subject’s regression parameter estimate for print processing and speech processing. Alpha level for correlational analyses was set at r > 0.45 (P = 0.04) for each language separately.
Acknowledgments
This work was supported by National Institutes of Health Grants P01 HD001994, R01 HD078351, and R01 HD067364; Israeli Science Foundation Grant 214/17; Grant 102-2410-H-010-004-MY2 from the Ministry of Science and Technology, Taiwan; Grants PSI2012-32093 and RYC-2014-15440 from the Spanish Ministry of Economy and Competitiveness (MINECO; to P.M.P.-A.); PSI2012-32123 from the MINECO (to J.A.D.); Grant PSI2012-31448 from the MINECO (to M.C.); and the European Research Council (ERC-2011-ADG-295362; to M.C.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The functional data reported in this paper have been deposited on the Haskins servers at haskins.yale.edu/datasharing. Descriptions of the data structure, preprocessing, and group analyses steps are included.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509321112/-/DCSupplemental.
References
- 1.Seymour PHK, Aro M, Erskine JM. Foundation literacy acquisition in European orthographies. Br J Psychol. 2003;94(Pt 2):143–174. doi: 10.1348/000712603321661859. [DOI] [PubMed] [Google Scholar]
- 2.Ziegler JC, Goswami U. Reading acquisition, developmental dyslexia, and skilled reading across languages: A psycholinguistic grain size theory. Psychol Bull. 2005;131(1):3–29. doi: 10.1037/0033-2909.131.1.3. [DOI] [PubMed] [Google Scholar]
- 3.Velan H, Frost R. Cambridge University versus Hebrew University: The impact of letter transposition on reading English and Hebrew. Psychon Bull Rev. 2007;14(5):913–918. doi: 10.3758/bf03194121. [DOI] [PubMed] [Google Scholar]
- 4.Frost R, Katz L, Bentin S. Strategies for visual word recognition and orthographical depth: A multilingual comparison. J Exp Psychol Hum Percept Perform. 1987;13(1):104–115. doi: 10.1037//0096-1523.13.1.104. [DOI] [PubMed] [Google Scholar]
- 5.Frost R. Towards a universal model of reading. Behav Brain Sci. 2012;35(5):263–279. doi: 10.1017/S0140525X11001841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Perfetti CA, Liu Y, Tan LH. The lexical constituency model: Some implications of research on Chinese for general theories of reading. Psychol Rev. 2005;112(1):43–59. doi: 10.1037/0033-295X.112.1.43. [DOI] [PubMed] [Google Scholar]
- 7.Price CJ. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. Neuroimage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pugh KR, et al. Neurobiological studies of reading and reading disability. J Commun Disord. 2001;34(6):479–492. doi: 10.1016/s0021-9924(01)00060-0. [DOI] [PubMed] [Google Scholar]
- 9.Wandell BA, Rauschecker AM, Yeatman JD. Learning to see words. Annu Rev Psychol. 2012;63:31–53. doi: 10.1146/annurev-psych-120710-100434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakamura K, et al. Universal brain systems for recognizing word shapes and handwriting gestures during reading. Proc Natl Acad Sci USA. 2012;109(50):20762–20767. doi: 10.1073/pnas.1217749109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paulesu E, et al. A cultural effect on brain function. Nat Neurosci. 2000;3(1):91–96. doi: 10.1038/71163. [DOI] [PubMed] [Google Scholar]
- 12.Zhao J, et al. Neural division of labor in reading is constrained by culture: A training study of reading Chinese characters. Cortex. 2014;53:90–106. doi: 10.1016/j.cortex.2014.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hu W, et al. Developmental dyslexia in Chinese and English populations: Dissociating the effect of dyslexia from language differences. Brain. 2010;133(Pt 6):1694–1706. doi: 10.1093/brain/awq106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tan LH, et al. The neural system underlying Chinese logograph reading. Neuroimage. 2001;13(5):836–846. doi: 10.1006/nimg.2001.0749. [DOI] [PubMed] [Google Scholar]
- 15.Perfetti CA. The universal grammar of reading. Sci Stud Read. 2003;7(1):3–24. [Google Scholar]
- 16.Dehaene S, et al. How learning to read changes the cortical networks for vision and language. Science. 2010;330(6009):1359–1364. doi: 10.1126/science.1194140. [DOI] [PubMed] [Google Scholar]
- 17.Frost SJ, et al. Phonological awareness predicts activation patterns for print and speech. Ann Dyslexia. 2009;59(1):78–97. doi: 10.1007/s11881-009-0024-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shankweiler D, et al. Reading differences and brain: Cortical integration of speech and print in sentence processing varies with reader skill. Dev Neuropsychol. 2008;33(6):745–775. doi: 10.1080/87565640802418688. [DOI] [PubMed] [Google Scholar]
- 19.Caravolas M, Lervåg A, Defior S, Seidlová Málková G, Hulme C. Different patterns, but equivalent predictors, of growth in reading in consistent and inconsistent orthographies. Psychol Sci. 2013;24(8):1398–1407. doi: 10.1177/0956797612473122. [DOI] [PubMed] [Google Scholar]
- 20.Caravolas M, et al. Common patterns of prediction of literacy development in different alphabetic orthographies. Psychol Sci. 2012;23(6):678–686. doi: 10.1177/0956797611434536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ziegler JC, et al. Orthographic depth and its impact on universal predictors of reading: A cross-language investigation. Psychol Sci. 2010;21(4):551–559. doi: 10.1177/0956797610363406. [DOI] [PubMed] [Google Scholar]
- 22.Linkersdörfer J, Lonnemann J, Lindberg S, Hasselhorn M, Fiebach CJ. Grey matter alterations co-localize with functional abnormalities in developmental dyslexia: An ALE meta-analysis. PLoS One. 2012;7(8):e43122. doi: 10.1371/journal.pone.0043122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maisog JM, Einbinder ER, Flowers DL, Turkeltaub PE, Eden GF. A meta-analysis of functional neuroimaging studies of dyslexia. Ann N Y Acad Sci. 2008;1145:237–259. doi: 10.1196/annals.1416.024. [DOI] [PubMed] [Google Scholar]
- 24.Richlan F, Kronbichler M, Wimmer H. Functional abnormalities in the dyslexic brain: A quantitative meta-analysis of neuroimaging studies. Hum Brain Mapp. 2009;30(10):3299–3308. doi: 10.1002/hbm.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Siok WT, Perfetti CA, Jin Z, Tan LH. Biological abnormality of impaired reading is constrained by culture. Nature. 2004;431(7004):71–76. doi: 10.1038/nature02865. [DOI] [PubMed] [Google Scholar]
- 26.Siok WT, Niu Z, Jin Z, Perfetti CA, Tan LH. A structural-functional basis for dyslexia in the cortex of Chinese readers. Proc Natl Acad Sci USA. 2008;105(14):5561–5566. doi: 10.1073/pnas.0801750105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stoeckel C, Gough PM, Watkins KE, Devlin JT. Supramarginal gyrus involvement in visual word recognition. Cortex. 2009;45(9):1091–1096. doi: 10.1016/j.cortex.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Boukrina O, Graves WW. Neural networks underlying contributions from semantics in reading aloud. Front Hum Neurosci. 2013;7:518. doi: 10.3389/fnhum.2013.00518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carreiras M, Mechelli A, Estévez A, Price CJ. Brain activation for lexical decision and reading aloud: Two sides of the same coin? J Cogn Neurosci. 2007;19(3):433–444. doi: 10.1162/jocn.2007.19.3.433. [DOI] [PubMed] [Google Scholar]
- 30.Wang X, et al. Language differences in the brain network for reading in naturalistic story reading and lexical decision. PLoS One. 2015;10(5):e0124388. doi: 10.1371/journal.pone.0124388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cox RW. AFNI: Software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29(3):162–173. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 32.Friedman L, Glover GHG, Krenz D, Magnotta V. FIRST BIRN Reducing inter-scanner variability of activation in a multicenter fMRI study: Role of smoothness equalization. Neuroimage. 2006;32(4):1656–1668. doi: 10.1016/j.neuroimage.2006.03.062. [DOI] [PubMed] [Google Scholar]
- 33.Chen G, Adleman NE, Saad ZS, Leibenluft E, Cox RW. Applications of multivariate modeling to neuroimaging group analysis: A comprehensive alternative to univariate general linear model. Neuroimage. 2014;99:571–588. doi: 10.1016/j.neuroimage.2014.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Friedman L, Glover GH. FBIRN Consortium Reducing interscanner variability of activation in a multicenter fMRI study: Controlling for signal-to-fluctuation-noise-ratio (SFNR) differences. Neuroimage. 2006;33(2):471–481. doi: 10.1016/j.neuroimage.2006.07.012. [DOI] [PubMed] [Google Scholar]
- 35.Genovese CR, Lazar NA, Nichols T. Thresholding of statistical maps in functional neuroimaging using the false discovery rate. Neuroimage. 2002;15(4):870–878. doi: 10.1006/nimg.2001.1037. [DOI] [PubMed] [Google Scholar]
- 36.Friston KJ, Penny WD, Glaser DE. Conjunction revisited. Neuroimage. 2005;25(3):661–667. doi: 10.1016/j.neuroimage.2005.01.013. [DOI] [PubMed] [Google Scholar]