Significance
Experience-dependent plasticity is the capacity of the brain to undergo changes following environmental input and use, and is a primary means through which the adult brain enables new behavior. In the current study, we provide evidence that enhancing signaling at the glutamate N-methyl-d-aspartate receptor (NMDAR) can enhance the mechanism underlying many forms of experience-dependent plasticity (i.e., long-term potentiation of synaptic currents) and also enhance experience-dependent learning in healthy adult humans. This suggests exciting possibilities for manipulating plasticity in adults and has implications for treating neurological and neuropsychiatric disorders in which experience-dependent plasticity is impaired.
Keywords: d-cycloserine, NMDA receptor, neuroplasticity, long-term potentiation, learning
Abstract
Experience-dependent plasticity is a fundamental property of the brain. It is critical for everyday function, is impaired in a range of neurological and psychiatric disorders, and frequently depends on long-term potentiation (LTP). Preclinical studies suggest that augmenting N-methyl-d-aspartate receptor (NMDAR) signaling may promote experience-dependent plasticity; however, a lack of noninvasive methods has limited our ability to test this idea in humans until recently. We examined the effects of enhancing NMDAR signaling using d-cycloserine (DCS) on a recently developed LTP EEG paradigm that uses high-frequency visual stimulation (HFvS) to induce neural potentiation in visual cortex neurons, as well as on three cognitive tasks: a weather prediction task (WPT), an information integration task (IIT), and a n-back task. The WPT and IIT are learning tasks that require practice with feedback to reach optimal performance. The n-back assesses working memory. Healthy adults were randomized to receive DCS (100 mg; n = 32) or placebo (n = 33); groups were similar in IQ and demographic characteristics. Participants who received DCS showed enhanced potentiation of neural responses following repetitive HFvS, as well as enhanced performance on the WPT and IIT. Groups did not differ on the n-back. Augmenting NMDAR signaling using DCS therefore enhanced activity-dependent plasticity in human adults, as demonstrated by lasting enhancement of neural potentiation following repetitive HFvS and accelerated acquisition of two learning tasks. Results highlight the utility of considering cellular mechanisms underlying distinct cognitive functions when investigating potential cognitive enhancers.
Experience-dependent neuroplasticity is the capacity of the brain to change in response to environmental input, learning, and use. It is a fundamental property of the brain and is critical for everyday functioning. It allows us to learn and remember patterns, predict and obtain reward, and refine and accelerate response selection for adaptive behavior (1). During development, experience-dependent plasticity interacts with genetic programming to organize neurons into the structurally and functionally connected circuits that characterize a mature brain. Although this basic circuitry is established by early adulthood, experience-dependent plasticity continues to shape connectivity within these circuits such that important inputs and action outputs are represented by larger and more coordinated populations of neurons. Given that these changes are the primary means through which the adult brain enables new behavior and that such plasticity is impaired in a range of neurological and psychiatric disorders (2), identifying manipulations that can harness experience-dependent plasticity offers exciting possibilities. Here, we tested whether augmenting N-methyl-d-aspartate receptor (NMDAR) activity could enhance experience-dependent plasticity in the adult human brain.
The classical mechanism underlying experience-dependent plasticity is long-term potentiation (LTP) or depression (LTD) of synaptic strength. The brain encodes external and internal events through spatiotemporal patterns of activity generated by populations of neurons. Lasting changes in synaptic strength via LTP and LTD shapes these patterns of activity and are thought to be the primary cellular mechanism for representing new information in the brain (1, 3). In animals, LTP is identified electrophysiologically as an enduring increase in postsynaptic cellular currents using single-cell or local field recordings and is observed following high-frequency electrical stimulation or new learning. In mature animals, LTP has been observed at subcortical and sensory cortex synapses, including in the amygdala, hippocampus, and striatum, as well as in visual, auditory, and somatosensory cortex (1–6). Although a lack of noninvasive methods has traditionally limited our ability to investigate LTP in humans, recent research indicates that protocols using high-frequency, repetitive presentation of visual or auditory stimuli provide a naturalistic method for inducing LTP in humans and animals. Studies in rodents demonstrated that changes in neural responses following repetitive sensory stimulation show the cardinal features of synaptic LTP, including persistence (>1 h), input specificity, and NMDAR dependency (7, 8). Furthermore, these LTP-like changes can be measured noninvasively as changes in sensory evoked potentials, which are stimulus-synchronized electroencephalograph (EEG) signals that result from postsynaptic potentials in populations of sensory neurons. High-frequency sensory stimulation has thus been shown to induce lasting potentiation of visual and auditory evoked potentials in human adults (9, 10) and has been used to demonstrate that LTP-like processes are impaired in patients with depression (11), bipolar disorder (12), and schizophrenia (13, 14). Sensory LTP protocols therefore provide a valuable window into the cellular mechanism thought to underlie many forms of experience-dependent plasticity.
One potential method for promoting experience-dependent plasticity is to augment NMDAR signaling. The NMDAR is a primary glutamate receptor and is critical for triggering LTP at many synapses in the brain. This role stems from the receptor’s unique biophysical properties, including that (i) NMDARs are blocked by a magnesium ion at rest such that they are dually voltage and ligand gated and therefore detect coincident presynaptic and postsynaptic activity; (ii) NMDARS are calcium permeable and therefore initiate signaling cascades when activated, leading to structural synaptic changes such as α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic (AMPA) receptor up-regulation and enlarged dendritic spines on which synapses are localized; and (iii) NMDARs have slow excitatory postsynaptic potential (EPSP) decay kinetics that facilitate temporal summation of EPSPs and sustained neural excitation (15, 16). Studies of transgenic and knockout mice showed that blocking NMDARs impairs LTP as well as learning and memory performance. Conversely, enhancing NMDAR activity enhanced LTP and the acquisition and retention of information (17). Given the role of NMDARs in triggering the cellular machinery that supports experience-dependent plasticity, augmenting NMDAR signaling may offer a powerful means to promote LTP and learning in humans.
In the current study, we used the NMDAR agonist d-cycloserine (DCS) to examine how augmenting NMDAR signaling affects LTP-like processes and learning in the adult human brain. NMDARs are tetramers composed of two NR1 and two NR2 subunits. Activation requires binding of glutamate to the NR2 subunit and concurrent binding of glycine or d-serine to the NR1 subunit (18). Although direct enhancement of NMDAR signaling via the glutamate site can produce excitotoxicity, indirect stimulation via the glycine site offers a safer method for facilitating activity. DCS is a partial agonist at the glycine site that readily crosses the blood–brain barrier, is approved by the Food and Drug Administration for daily use as an antituberculosis drug, and has few side effects at low doses. Thus, DCS offers a safe means to augment NMDAR signaling at low doses.
Using a double-blind design, we randomized healthy adults to receive DCS or placebo. We examined the effects of augmenting NMDAR signaling on two indices of experience-dependent plasticity: (i) LTP and (ii) incremental learning. Participants completed the visual LTP task using high-frequency visual stimulation (HFvS) to induce potentiation of visual cortex neurons, followed by a weather prediction task (WPT) (19), an information integration task (IIT) (20), and an n-back task. The WPT and IIT are incremental learning tasks in which stimulus–feedback associations are thought to be encoded by LTP at corticostriatal synapses (21, 22). The n-back is a spatial working memory task. Working memory relies on reverberating activity in cortical microcircuits over short delays to maintain information in the absence of stimuli and, thus, does not rely on LTP (23). To facilitate dissociation of the effects of DCS on experience-dependent plasticity versus working memory, the n-back task was designed to be identical to the IIT in stimuli and trial structure. Thus, the only difference participants experienced between the tasks was whether they were asked to learn about the stimuli (i.e., for the IIT) or recall whether stimuli were in the same location on the screen as recently shown stimuli (i.e., for the n-back). To assess potential delayed effects of DCS, participants returned to the laboratory the following day to repeat cognitive testing. No drug or placebo was administered on the second day. Although the idea of using NMDAR agonists to enhance cognition is not new, past studies examining diverse cognitive domains have yielded mixed results (24–36). Difficulty reconciling divergent effects has limited our ability to harness NMDAR agonists as cognitive enhancers. To our knowledge, this is the first human study to systematically test the hypothesis that increasing NMDAR signaling enhances experience-dependent plasticity, and the first study to combine behavioral measures with assessment of a mechanism thought to underlie experience-dependent plasticity. We hypothesized that participants who received DCS would show (i) enhanced neural potentiation following HFvS on the LTP task; (ii) enhanced performance on the WPT and IIT; and (iii) similar performance on the n-back task, compared with Placebo participants.
Results
Participants.
Sixty-five healthy adults enrolled in the study and received DCS (n = 32) or placebo (n = 33). Randomization yielded groups that were well-matched in age [t(63) = 0.16, P = 0.87], gender [χ2 = 0.01, P = 0.91], and IQ [t(63) = −0.08, P = 0.94] (Table 1).
Table 1.
Demographic characteristics of Placebo and DCS participants
| Group | n | Age (SD) | Sex | WASI IQ (SD) |
| Placebo | 33 | 20.55 (2.41) | 19 F/14 M | 120.42 (9.33) |
| DCS | 32 | 20.59 (2.69) | 18 F/14 M | 120.78 (8.23) |
F, female; M, male; WASI, Weschler Abbreviated Scale of Intelligence.
Visual Evoked Potential Responses.
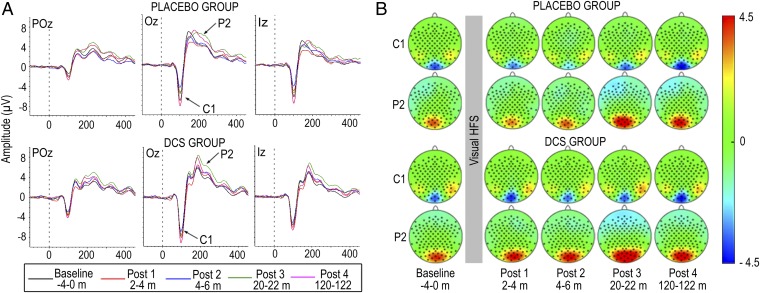
To examine the effects of enhancing NMDAR signaling on LTP-like processes in the human brain, we compared changes in visual evoked potentials (VEPs) to a black-and-white checkerboard stimulus following HFvS in participants who received DCS versus those who received placebo. VEPs were assessed for 4 min immediately before HFvS to establish baseline neural responses, and during four post-HFvS blocks that occurred at 2–4, 4–6, 20–22, and 120–122 min following HFvS (SI Methods). The VEP complex was prominent at midline parietal-occipital channels and included a negative component, C1, that peaked at Oz at 100.79 ms (SD = 7.20) in Placebo participants and 102.06 ms (SD = 9.07) in DCS participants, and a positive component, P2, that peaked at Oz at 195.40 ms (SD = 29.03) in Placebo participants and 197.39 ms (SD = 24.10) in DCS participants (Fig. 1 A and B). C1 and P2 latencies did not differ between groups. For description of the time course of C1 and P2 plasticity following HFvS, see SI Results.
Fig. 1.
(A) Grand average VEPs elicited by the standard checkerboard stimulus in example midline parietal-occipital channels for Placebo (Top) and DCS (Bottom) participants across VEP assessment blocks. (B) Scalp topography of C1 and P2 for Placebo (Top) and DCS (Bottom) participants across VEP assessment blocks. C1 analyses were based on average peak amplitudes from four channels surrounding Oz and Iz (i.e., Oz, Iz, O1, OI1h); P2 analyses were based on average peak amplitudes from six channels surrounding POz, Oz, and Iz (i.e., POz, Oz, Iz, POO1, O1, OI1h).
DCS Enhanced Potentiation of VEP Components.
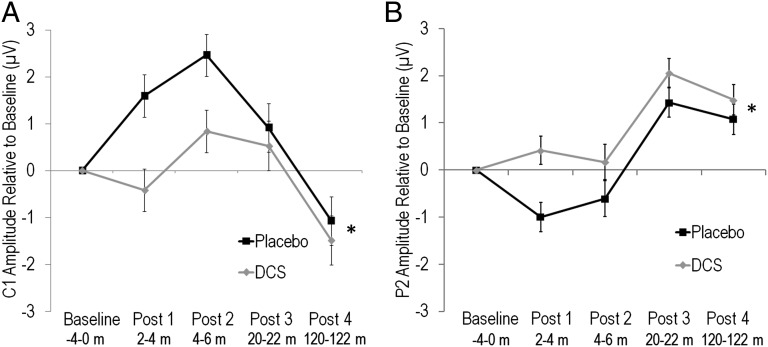
There were no differences in baseline amplitude of C1 [t(63) = 0.39, P = 0.70] or P2 [t(63) = 0.24, P = 0.81] between DCS and Placebo participants, indicating that DCS did not affect general neural excitability (SI Results). HFvS modulated C1 and P2 in both groups (SI Results), however, DCS significantly enhanced potentiation of both C1 and P2 following HFvS. Repeated-measures ANOVA on C1 amplitude change from baseline across the four post-HFvS blocks revealed that DCS participants showed greater potentiation overall compared with Placebo [F(1,63) = 4.92, P = 0.03], due to less depression of C1 during early post-HFvS blocks and greater potentiation of C1 during the last post-HFvS block (Fig. 2A). The Group by Block interaction was not significant.
Fig. 2.
(A) Mean ± SE. C1 amplitude change from baseline for Placebo and DCS participants. *DCS participants showed enhanced potentiation of C1 across post-HFvS blocks compared with Placebo (P = 0.03). (B) Mean ± SE. P2 amplitude change from baseline for Placebo and DCS participants. *DCS participants showed enhanced potentiation of P2 across post-HFvS blocks compared with Placebo (P = 0.02).
Similarly for P2, repeated-measures ANOVA revealed a significant effect of Group overall, due to the DCS group showing greater potentiation of P2 across all post-HFvS blocks compared with Placebo [F(1,63) = 6.08, P = 0.02] (Fig. 2B). The Group by Block interaction was not significant. Parallel analyses using C1–P2 peak-to-peak amplitude also showed enhanced potentiation across post-HFvS blocks in the DCS group (SI Results). These results indicate that enhancing NMDAR signaling augmented potentiation of neural responses for 2 h following HFvS compared with Placebo, consistent with our first hypothesis.
DCS Enhanced Experience-Dependent Learning.
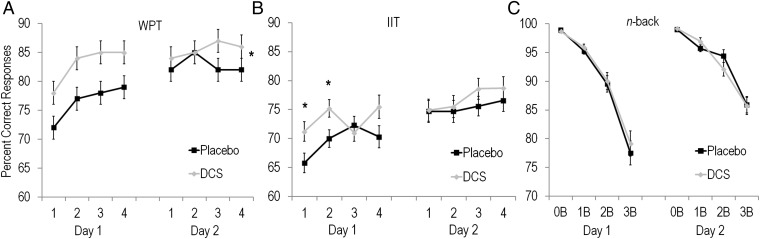
The WPT is a probabilistic classification learning task in which participants viewed combinations of cues that probabilistically predicted sun or rain. Following each response, participants were shown feedback regarding the actual outcome for each trial (SI Methods). Successful learning of associations between cues and probabilistic outcomes is thought to depend on LTP at corticostriatal synapses (21, 22). Both groups showed successful learning on the WPT, as indicated by improved performance over trial blocks [F(3,180) = 10.35, P < 0.001] and days [F(1,180) = 20.05, P < 0.001]. However, the DCS group showed enhanced performance overall [F(1,60) = 5.60, P = 0.02]. Improved performance in the DCS group was evident within the first block of trials, indicating that DCS participants learned more rapidly and maintained gains over Placebo, despite no drug being given on the second day (Fig. 3A). No interactions of Group with Block or Day were significant.
Fig. 3.
(A) Mean ± SE percent correct responses per 80-trial blocks of the weather prediction task (WPT) for Placebo (n = 31) and DCS participants (n = 31). *DCS participants performed significantly better than Placebo participants overall (P = 0.02). (B) Mean ± SE percent correct responses per 80-trial blocks of the information integration task (IIT) for Placebo (n = 29) and DCS participants (n = 30). *DCS participants performed significantly better than Placebo participants during blocks 1 (P = 0.03,) and 2 (P = 0.02). (C) Mean ± SE percent correct responses per 80-trial blocks for the 0-back (0B), 1-back (1B), 2-back (2B), and 3-back (3B) conditions for Placebo (n = 29) and DCS participants (n = 32). There were no group differences between Placebo and DCS participants on the n-back.
The IIT is a classification learning task in which participants viewed sine-wave grating stimuli that varied in bar width and orientation. Participants were instructed to integrate the two dimensions and use auditory feedback to learn whether stimuli belonged to category A or B (SI Methods). Modeling of participant responses confirmed that the majority of participants (95%) used the optimal information integration decision strategy to learn the IIT (SI Results). Similar to the WPT, although both groups showed learning across trial blocks [F(3,171) = 7.48, P < 0.001] and days [F(1,171) = 27.03, P < 0.001], participants who received DCS showed enhanced learning compared with participants who received placebo. Enhanced performance in the DCS group was particularly evident during early learning, as indicated by a significant Group by Block by Day interaction [F(3,171) = 4.76, P = 0.003], due to the DCS group showing significantly enhanced performance during the first (P = 0.03) and second trial blocks on day 1 (P = 0.02). The DCS group also showed a trend toward enhanced performance during the fourth trial block on day 1 (P = 0.06) (Fig. 3B). Although correct responses remained higher for DCS than Placebo participants on the second day of testing when no drug was administered, this effect was not significant. Thus, consistent with our second hypothesis, DCS significantly enhanced acquisition of two incremental learning tasks.
In contrast to the effects of DCS on the IIT and WPT, DCS did not affect performance on the n-back. The n-back was a spatial working memory task with four memory loads (0- to 3-back). Both groups performed better at lower working memory loads [F(3,177) = 146.26, P < 0.001] and showed practice effects over testing days [F(1,177) = 35.33, P < 0.001]. However, consistent with our third hypothesis, DCS and Placebo participants did not differ in working memory performance at any load. Thus, the main effect of Group and interactions of Group with Day and memory load were not significant (values of P > 0.05) (Fig. 3C). This lack of effect of DCS on the n-back task was evident despite identical stimuli, trial structure, and auditory feedback to the IIT (SI Methods). Furthermore, there were no group differences in reaction times on any task (SI Results).
SI Methods
Inclusion Criteria.
Participants were eligible for the study if they were between 18 and 30 y of age; were comfortable reading in English; had no history of seizures, neurologic disease, or allergies to antibiotics; had normal vision and hearing; were not prescribed psychotropic medication; were not pregnant; had not used recreational drugs in the past month; and had an IQ > 70, as assessed by the vocabulary and matrix reasoning tests of the Weschler Abbreviated Scale of Intelligence (WASI) (52). Participants completed a brief medical screen and were asked to abstain from alcohol use for 24 h before testing.
For data analyses, participants were excluded from analysis of a given cognitive test if they missed more than 5% of trials on a testing day. This resulted in exclusion of two Placebo and one DCS participants for the IIT; two Placebo and one DCS participants for the WPT; and four Placebo participants for the n-back. One Placebo participant who performed poorly on the IIT (51.6% correct on day 1) reported that he had not understood the instructions; one Placebo participant performed below chance on the IIT and was considered an outlier (28.4% correct overall); and one DCS participant was erroneously administered a rule-based version of the IIT; data for these participants on the IIT were excluded. No participants were excluded from LTP data analyses.
DCS Properties and Administration Schedule.
The administration schedule of DCS was chosen based on pharmaceutical information that peak plasma levels are reached in 3–4 h (King’s Pharmaceuticals), and is consistent with other studies showing beneficial effects of DCS when administered 3–4 h before learning or exposure therapy (e.g., refs. 26 and 28). However, it is possible that an alternate administration schedule would have yielded stronger effects of DCS. For example, a recent metaanalysis of DCS and fear extinction indicated that beneficial effects of DCS were most reliable when DCS was administered 1–2 h before exposure sessions (30). Nevertheless, given that the half-life of DCS is 8–12 h (King’s Pharmaceuticals), it is likely that maximal or near-maximal effects of DCS were present during testing in the current study.
It is also important to note that the effects of DCS can differ at low versus high doses. This dose–response pattern may reflect the differential efficacy of DCS at NMDARs with different subunit compositions. Specifically, DCS has been shown to increase the channel open time and open probability of NMDARs containing the NR2C subunit with ∼200% efficacy compared with glycine. However, at NMDARs containing NR2A or 2B subunits, DCS has ∼50% efficacy compared glycine (36). Thus, although DCS is a potent agonist at NMDARs with NR2C subunits regardless of dose, at NMDARs with NR2B and NR2A subunits, DCS may act as an agonist at low doses (e.g., 50–250 mg) by stimulating unoccupied glycine sites, but as an antagonist at high doses (e.g., 1,000 mg) (53) by displacing endogenous glycine from the glycine site. Given this activity profile, the current study used a low dose of 100 mg of DCS that has been shown to be an agonist in other studies (e.g., refs. 25 and 37).
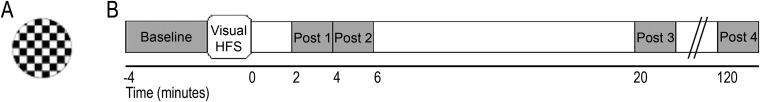
LTP Task.
VEP assessment blocks during the LTP task consisted of a pseudorandom oddball sequence of 90% standard and 10% target stimuli presented for 33 ms (jittered 1- to 1.33-s stimulus onset asynchrony). The standard stimulus was a circle filled with a black-and-white checkerboard pattern, presented at 0.83 Hz. To maintain attention, participants were asked to press a button whenever they saw a target square containing a blue-and-white checkerboard pattern. Unrelated auditory and resting tasks were performed between the HFvS and post-HFvS blocks 1 through 3. The cognitive tasks were performed between post-HFvS blocks 3 and 4. The standard stimulus and time course of the LTP task are shown in Fig. S1.
Fig. S1.
(A) Standard circle black-and-white checkerboard stimulus presented at 0.83 Hz during visual evoked potential (VEP) assessment blocks and at ∼8.87 Hz during high-frequency visual stimulation (HFvS). (B) Time course of the long-term potentiation (LTP) paradigm. Adapted from ref. 13.
Continuous EEG was amplified and recorded using a Biosemi Active-Two system (BioSemi, Amsterdam) employing 128 electrodes in an elastic cap and arranged according to the extended 10–20 EEG system. Horizontal eye movements were measured via two electrodes located 1 cm lateral to the lateral canthi of the right and left eyes, and vertical eye movements and blinks were measured by two electrodes located 1 cm above and below the orbit of the right eye. Due to recording error, data from the 64 right hemisphere channels were not retained for the first 33 participants; however, posterior midline electrodes were available for all participants.
EEG data were processed using Brain Vision Analyzer (Brain Products) and custom MATLAB (Mathworks) scripts. Continuous data were rereferenced to nose, bandpass filtered at 0.5–50 Hz, and 600-ms (−100 to 500 ms) epochs time-locked to the standard stimulus onsets were extracted for each VEP block. Epochs were baseline corrected using the prestimulus period and subjected to ocular correction using the Gratton et al. (54) algorithm. Epochs with voltage exceeding ±100 µV between 0 and 250 ms after stimulus onset at parietal or occipital sites were excluded. VEP blocks following artifact rejection contained a minimum of 57 segments (Placebo, M = 89.15, SD = 2.37; DCS, M = 89.43, SD = 1.70).
Epochs were averaged generating VEPs for the two baseline and four post-HFvS blocks. A custom MATLAB script identified the negative peak with the greatest amplitude between 80 and 120 ms for C1 and the positive peak with the greatest amplitude between 150 and 250 ms for P2 for each participant, averaged across VEP blocks. C1 and P2 latencies and peak amplitudes for each VEP block were extracted.
Cognitive Tasks.
WPT.
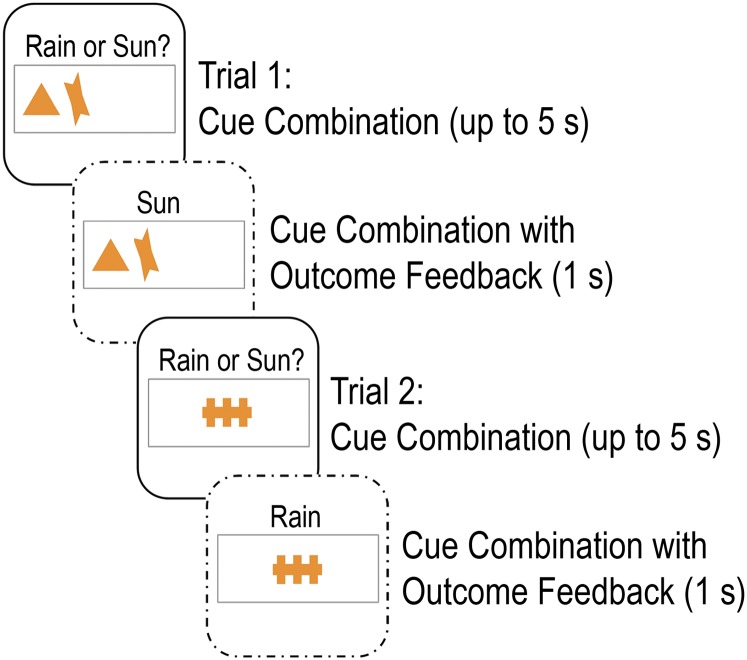
On each trial of the WPT, between one and three out of four possible cues appeared, yielding 14 different combinations. Cues were presented for a maximum of 5 s, and participants were instructed to respond using buttons for “rain” or “sun.” Following response, feedback showing the cue combination and actual outcome for the trial was presented for 1 s (Fig. S2). The probabilities for different cue combinations predicting rain or sun ranged from 0.875 to 0.125. Thus, if a given cue combination was 0.875 associated with sun, the cue combination was associated with sun for 87.5% of trials and with rain for 12.5% of trials. The probability structure of the WPT is summarized in Table S1. Responses were counted as correct if the most likely outcome was selected.
Fig. S2.
Two example weather prediction task (WPT) trials.
Table S1.
Probability structure of WPT
| Combination | Cue 1 | Cue 2 | Cue 3 | Cue 4 | P (Combination) | P (Sun|| Combination) |
| 1 | 1 | 0 | 0 | 0 | 0.14 | 0.857 |
| 2 | 0 | 0 | 1 | 0 | 0.06 | 0.333 |
| 3 | 1 | 0 | 1 | 0 | 0.08 | 0.75 |
| 4 | 0 | 1 | 0 | 0 | 0.06 | 0.667 |
| 5 | 1 | 1 | 0 | 0 | 0.08 | 0.75 |
| 6 | 0 | 1 | 1 | 0 | 0.04 | 0.5 |
| 7 | 1 | 1 | 1 | 0 | 0.04 | 0.5 |
| 8 | 0 | 0 | 0 | 1 | 0.16 | 0.125 |
| 9 | 1 | 0 | 0 | 1 | 0.04 | 0.5 |
| 10 | 0 | 0 | 1 | 1 | 0.14 | 0.143 |
| 11 | 1 | 0 | 1 | 1 | 0.02 | 1 |
| 12 | 0 | 1 | 0 | 1 | 0.04 | 0.5 |
| 13 | 1 | 1 | 0 | 1 | 0.04 | 0.5 |
| 14 | 0 | 1 | 1 | 1 | 0.06 | 0.333 |
| P (Sun|| Overall) | 0.727 | 0.556 | 0.409 | 0.280 |
For each cue combination, each card could be present (1) or absent (0). The bottom row lists the overall probability of Sun for the four cues across all cue combinations.
IIT.
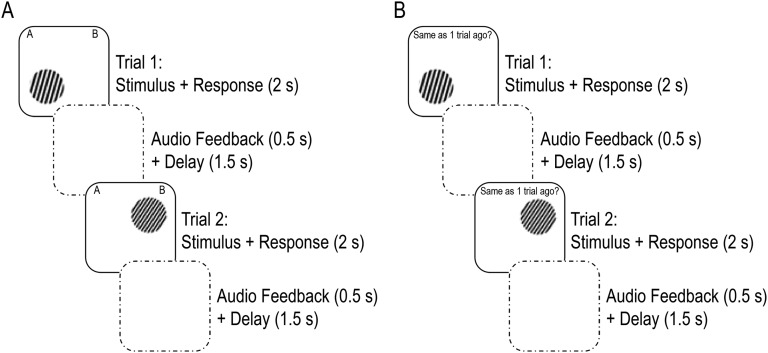
On each trial of the IIT, a single stimulus was presented in one of four quadrants of the screen for 2 s. IIT stimuli consisted of circular sine-wave gratings that varied across trials on bar width and bar orientation. Stimuli belonged to category A or B and were defined by a set of points (x,y) randomly sampled from a 100 × 100 stimulus space and converted to a disk stimulus by defining frequency as f = 3(x/100) − 1, and orientation as o = (3π/8)(y/100) + (π/11). Category A stimuli were generated from a multivariate normal distribution with the following parameters (55): μA = (43,57); ƩΑ = {155 145; 145 155}. The same sampling method was used to generate category B stimuli: μB = (57, 43); ƩA = ƩB. Participants were instructed to integrate information about the two dimensions and decide whether each stimulus belonged to category A or B by pressing the corresponding keys. Following response, participants were provided auditory feedback for 500 ms using a pure tone at 262 Hz for a correct response and a sawtooth (harsher) tone centered at 440 Hz for an incorrect response. Auditory feedback was followed by a 1,500-ms delay before the next trial (Fig. S3A). If participants did not respond during stimulus presentation, “please respond faster” was displayed for 1,500 ms. Participants were told that they would learn the categories by attending to the auditory feedback, that perfect accuracy was possible, and that stimulus location on the screen did not matter. Participants completed four practice trials before testing to ensure that they understood the task.
Fig. S3.
(A) Two example information integration task (IIT) trials and (B) two example trials for the 1-back condition of the n-back task. The IIT and n-back task were identical in stimuli, trial structure, and feedback such that the only difference participants experienced was whether they were asked to learn about the stimuli (i.e., for the IIT) or recall whether stimuli were in the same location on the screen as recently shown stimuli (i.e., for the n-back).
n-back.
In the n-back task, participants viewed a sequence of 80 stimuli at each of four working memory loads (0- to 3-back), and on each trial, were asked to respond “yes” or “no,” whether the stimulus was in the same quadrant on the screen as the stimulus they saw n trials earlier. For the 0-back control condition, participants were instructed to press “yes” when the stimulus appeared on the left side of the screen, and “no” when the stimulus appeared on the right side of the screen. Load conditions were separated by a 10-s rest during which instructions were displayed to inform participants of the upcoming condition. Participants were provided auditory feedback for 500 ms following each response, parallel to the IIT (Fig. S3B). Participants completed 13 practice trials per block in ascending memory load difficulty before testing to ensure that they understood the task.
IIT Model-Based Analyses.
The WPT and IIT are most commonly thought of as striatal-dependent implicit learning tasks. However, debate remains regarding the extent to which individuals use explicit strategies to learn these tasks, and accordingly, the extent to which executive prefrontal and medial temporal lobe structures are also involved. Given that the optimal multidimensional rules that govern the WPT and IIT are more difficult to learn and verbalize than one-dimensional rules, it is thought that multidimensional rules are more likely learned implicitly, whereas one-dimensional rules are more likely learned explicitly (44). Interestingly, on the WPT, the rate of positive feedback that participants receive if they use a multicue strategy is not much higher than the rate of positive feedback received if they ignore all cues other than the single most predictive cue. For example, in the current study, a participant who integrated information from all four cues would receive positive feedback at a rate of 74%, whereas a participant who ignored all cues except cue 1 would receive positive feedback at a rate of 72.7% (Table S1). Thus, it has been suggested that participants may be likely to use a single-cue strategy on the WPT rather than the more difficult multicue strategy, given the small difference in positive feedback between these strategies (44). In contrast, on the IIT, the rate at which participants receive positive feedback if they use a one-dimensional strategy is much lower than the rate of positive feedback if they learn the optimal information integration strategy (e.g., 71% versus 100% in the current study). One method for assessing whether participants use implicit or explicit strategies is therefore to model participant responses to discern whether responses are dominated by multidimensional or one-dimensional rules. Such modeling is difficult to complete on the WPT given the complexity of task design, probabilistic nature of feedback, and the fact that a variety of decision-making strategies yield similar performance levels. However, decision-bound modeling has been reliably used to provide insight into the strategies being used by participants to solve the IIT (20, 56). Thus, we used decision-bound modeling to test whether participant responses on the IIT were dominated by one-dimensional versus multidimensional rules.
Briefly, decision-bound models assume that participants partition the perceptual space into response regions, determine which region the percept is in for each trial, and respond accordingly. In the current study, four models were fit to the each participant’s data across testing days and for each testing day separately: (i) a unidimensional X model; (ii) a unidimensional Y model; (iii) a guessing model; and (iv) a general linear classifier model (GLC), the optimal multidimensional model. The unidimensional X and unidimensional Y models assume that participants set a criterion on one perceptual dimension (i.e., spatial frequency or line orientation, respectively) and make category A versus B decisions based on explicit evaluations of the stimulus on that dimension. The guessing model assumes that participants guess on each trial. The GLC is a multidimensional model that assumes that participants divide the stimulus space using a linear decision bound that integrates the frequency and orientation dimensions (i.e., the line x = y). The GLC was the optimal IIT strategy in the current study. For detailed information on decision-bound modeling, please see ref. 56.
The model parameters were estimated using maximum likelihood and the goodness-of-fit statistic was the Bayesian information criterion (BIC), which is defined as follows:
where N is the sample size, r is the number of free parameters, and L is the likelihood of the model given the data. The best model was identified as the model with the smallest BIC score.
SI Results
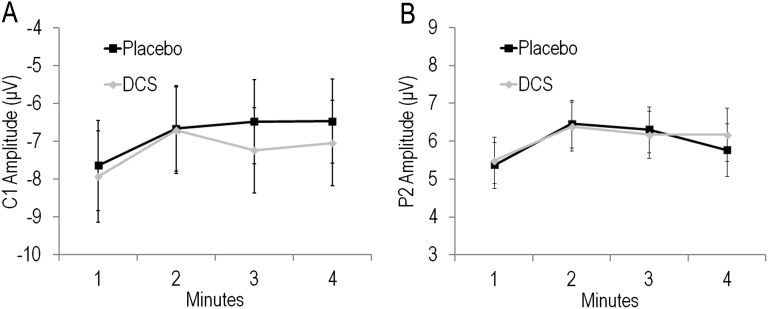
No Significant Effect of DCS on Baseline Excitability.
To further assess potential effects of DCS on baseline excitability, we extracted average C1 and P2 amplitude for each of the 4 min of baseline. Repeated-measures ANOVA for C1 indicated no significant effect of Group [F(1,63) = 0.07, P = 0.79] or Group by Block interaction [F(3,189) = 0.50, P = 0.65] (Fig. S4A). Similarly, for P2, there was no significant effect of Group [F(1,63) = 0.01, P = 0.92] nor Group by Block interaction [F(3,189) = 0.49, P = 0.66] (Fig. S4B). Thus, DCS did not affect basal synaptic transmission even though it is likely that peak plasma levels of DCS were present during the baseline assessment.
Fig. S4.
Mean ± SE (A) C1 and (B) P2 amplitude for Placebo and DCS participants across 4 min of baseline assessment (i.e., pre-HFvS).
Time Course of C1, P2, and C1–P2 Peak-to-Peak Plasticity.
HFvS modulated the C1 and P2 components in both groups. Repeated-measures ANOVA to characterize the time course of HFvS effects on C1 revealed a significant effect of Block [F(4,252) = 16.96, P < 0.001] and a significant Block by Group interaction [F(4,252) = 2.79, P = 0.04]; the main effect of Group was not significant. Follow-up tests within groups showed a significant Block effect for both groups (values of P < 0.001). Tests of simple contrast to baseline indicated that Placebo participants showed depression of C1 during the early post-HFvS blocks from 2 to 4 and 4 to 6 min (values of P < 0.01) and tended toward potentiation from 120 to 122 min post-HFvS (P = 0.07). In contrast, DCS participants showed significant potentiation of C1 at 120–122 min (P = 0.003) and only showed depression at 4–6 min post-HFvS (P = 0.03).
For P2, there was also a significant effect of Block [F(4,252) = 26.83, P < 0.001]; the main effect of Group and Group by Block interaction were not significant. Follow-up contrasts comparing post-HFvS blocks to baseline indicated that Placebo and DCS participants showed significant potentiation of P2 at 20–22 min and 120–122 min post-HFvS (values of P < 0.001).
Exploratory analyses also assessed modulation of C1–P2 peak-to-peak amplitude following HFvS. C1–P2 peak-to-peak amplitude was calculated by subtracting the amplitude of C1 from P2 for baseline and each post-HFvS block. Channel Oz was used, given that both components showed maximal amplitude at Oz. Repeated-measures ANOVA showed a significant effect of Block [F(4,252) = 19.53, P < 0.001] and a significant Block by Group interaction [F(4,252) = 3.18, P = 0.03]. Follow-up tests within each group showed a significant Block effect for both groups (values of P < 0.001). Simple contrasts to baseline indicated that Placebo participants showed depression of C1–P2 during the early post-HFvS blocks from 2 to 4 and 4 to 6 min (values of P = 0.001) and significant potentiation at 120–122 min post-HFvS (P = 0.04). In contrast, DCS participants showed significant potentiation of C1–P2 at 20–22 (P = 0.02) and 120–122 min (P < 0.001), and only showed depression at 2–4 min post-HFvS (P = 0.04).
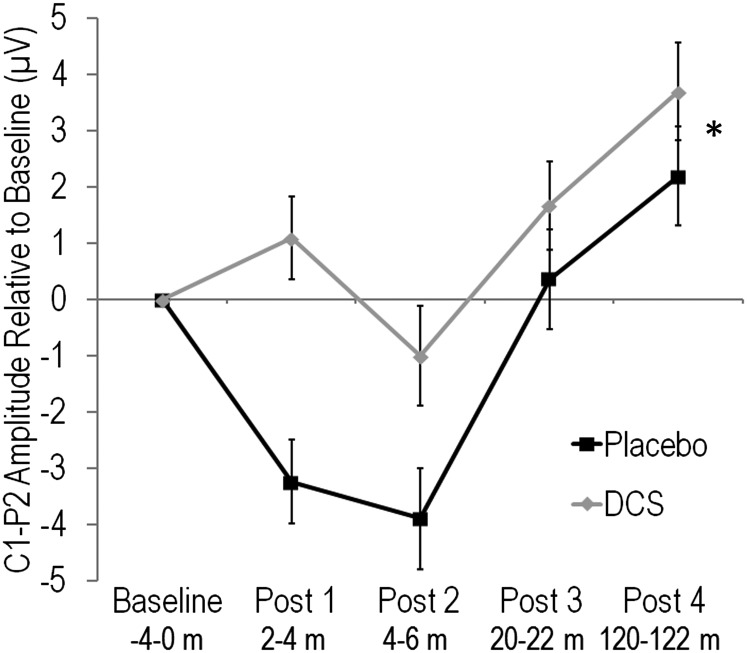
DCS Enhanced Potentiation of C1–P2 Peak-to-Peak Amplitude.
There were no group differences in baseline C1–P2 peak-to-peak amplitude (i.e., before exposure to HFvS) [t(63) = 0.49, P = 0.63]. However, repeated-measures ANOVA for C1–P2 amplitude change from baseline across the four post-HFvS blocks revealed that DCS participants showed greater potentiation overall compared with Placebo [F(1,63) = 8.75, P = 0.004] (Fig. S5). The Group by Block interaction was not significant (P > 0.05). Thus, potentiation of C1–P2 peak-to-peak amplitude was enhanced in the DCS group for 2 h following HFvS, similar to results for the C1 and P2 components separately.
Fig. S5.
Mean ± SE. C1–P2 peak-to-peak amplitude change relative to baseline for Placebo and DCS participants. *DCS participants showed enhanced potentiation of C1–P2 peak-to-peak amplitude across post-HFvS blocks (P = 0.004).
IIT Model-Based Results.
When IIT responses were modeled across testing days, the GLC model provided the best fit for ∼95% of participants, which corresponded to 26 of 29 Placebo participants and 30 of 30 DCS participants. The remaining three Placebo participants were best fit by a unidimensional X model. When responses were modeled for each testing day separately, the best-fitting model was more variable. On day 1, 26 Placebo and 27 DCS participants were best fit by the GLC model, 3 Placebo and 2 DCS participants were best fit by a unidimensional X model, and 1 DCS participant was best fit by a unidimensional Y model. On day 2, 24 Placebo and 27 DCS participants were best fit by the GLC model, 2 Placebo and 1 DCS participants were best fit by a unidimensional X model, 1 Placebo and 2 DCS participants were best fit by a unidimensional Y model, and 1 Placebo participant was best fit by a guessing model. Thus, overall, participant responses were dominated by the optimal information integration strategy. However, there was greater variability in the best-fitting model when participant responses were modeled across individual testing sessions, suggesting some switching between decision-making strategies during task acquisition. Analysis of the Group effect on day 1 of the IIT confined to participants who were best fit by the GLC model yielded results that were similar to the analysis with all included participants. Thus, among participants who relied primarily on the optimal information integration decision strategy on day 1, DCS participants performed significantly better than Placebo participants during block 2 [F(1,51) = 5.88, P = 0.02] and tended toward better performance compared with Placebo participants during blocks 1 [F(1,51) = 3.33, P = 0.07] and 4 [F(1,51) = 3.95, P = 0.05].
Reaction Time on Cognitive Tasks.
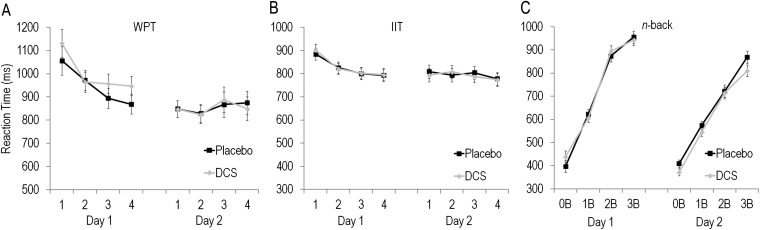
ANOVA for reaction time for each task revealed no significant main effect of Group or interactions involving Group for the IIT or WPT (values of P > 0.47). On the n-back, there was a significant interaction of Day by Group [F(1,177) = 4.26, P = 0.04]; however, follow-up tests for each testing day showed no significant effects of Group for day 1 or 2 (values of P > 0.18). Thus, reaction times for each task were similar between groups (Fig. S6).
Fig. S6.
Mean ± SE reaction time per 80-trial blocks across testing days for Placebo and DCS participants for the (A) weather prediction task (WPT), (B) information integration task (IIT), and (C) n-back task.
Relationships Between Plasticity Measures.
Exploratory correlational analyses were conducted to assess potential relationships between the EEG and cognitive measures of neuroplasticity. To minimize the number of comparisons, summary plasticity scores were used for each task. Thus, mean amplitude change across post-HFvS blocks relative to baseline was computed and used for each LTP component (i.e., C1, P2, and C1–P2 peak-to-peak amplitude), and mean percent correct responses across testing days was used for the IIT and WPT. Although plasticity measures within the LTP task were significantly related (values of P < 0.01), there was only one statistically significant correlation between measures of plasticity across tasks. Increased C1 amplitude relative to baseline positively predicted performance on the WPT [r(62) = 0.25, P = 0.049], suggesting that depression of C1 predicted better WPT performance. However, this relationship did not survive correction for multiple comparisons. No other relationships between the learning tasks or between the LTP and learning tasks were significant (Table S2). Given that the EEG measure of plasticity assessed LTP-like processes in visual cortex neurons and that acquisition of the WPT and IIT are thought to depend on plasticity at corticostriatal synapses, it may not be surprising that a clear relationship between the EEG and cognitive measures of plasticity was not detected. However, it is surprising that performance on the two learning tasks were not related.
Table S2.
Pearson correlation coefficients between plasticity on the LTP and learning tasks
| Measure | 1 | 2 | 3 | 4 |
| 1. Mean C1 plasticity | — | |||
| 2. Mean P2 plasticity | −0.35** | — | ||
| 3. Mean C1–P2 plasticity | −0.87*** | 0.72*** | — | |
| 4. WPT percent correct responses | 0.25* | 0.14 | −0.12 | — |
| 5. IIT percent correct responses | −0.08 | 0.20 | 0.17 | 0.17 |
*P < 0.05, **P < 0.01, ***P < 0.001.
Discussion
Augmenting NMDAR signaling using the partial agonist DCS enhanced experience-dependent plasticity as shown by persisting enhancement of neural potentiation following repetitive HFvS on the LTP task and enhanced acquisition of two incremental learning tasks. Thus, DCS augmented potentiation of the C1 and P2 components following HFvS, without affecting baseline neural excitability. DCS also improved acquisition of the WPT and IIT without affecting performance on the n-back working memory task. Together, these results suggest that DCS enhanced both a mechanism (i.e., LTP) and behavioral correlates of experience-dependent plasticity (i.e., incremental learning), and provide compelling evidence that enhancing NMDAR signaling can boost experience-dependent plasticity in the adult human brain.
LTP of synaptic currents is the most well-studied form of activity-dependent plasticity. It persists into adulthood, is frequently NMDAR dependent, and has been observed at subcortical and sensory cortex synapses (1–6). Although classical LTP studies used high-frequency electrical stimulation to induce LTP, HFvS also induces lasting potentiation of neural responses (8–14), and potentiated neural responses following HFvS show cardinal features of synaptic LTP (7, 8). In the current study, participants who received DCS showed greater potentiation of the VEP following HFvS compared with participants who received placebo. This is consistent with prior findings that DCS augmented increases in motor cortex excitability following anodal transcranial direct-current stimulation in humans (37) and augmented LTP in rat hippocampus following high-frequency electrical stimulation (38, 39). Our finding is also consistent with preclinical studies demonstrating that potentiation of the VEP following HFvS is NMDAR dependent (8).
It is interesting to note that both Placebo and DCS participants showed depression of C1 in the early minutes following HFvS, before showing potentiation at 2 h post-HFvS. C1 is the first major visual event-related potential component and is generated by neurons in primary visual cortex (40). Although one study using a similar LTP paradigm in humans found that C1 was potentiated up to 22 min following HFvS (13), additional studies found no change or a trend toward reduced C1 amplitude up to 28 min following HFvS (11, 12). Given that VEPs measured by EEG capture the electrical discharge of populations of neurons, this variable direction of C1 plasticity may reflect interactive effects of homosynaptic LTP and heterosynaptic LTD across visual cortex synapses, where homosynaptic synapses are those synapses that are directly activated by electrical stimulation and heterosynaptic synapses are nearby synapses that are not directly activated by stimulation. Traditional investigations of LTP have focused on homosynaptic plasticity. However, a growing body of research indicates that the same procedures that induce plasticity in one direction in a given synaptic pathway frequently induce inverse plasticity at adjacent synapses (41–43). Thus, stimulation that results in LTP at tetanized, homosynaptic synapses has been shown to produce LTD at nontetanized, heterosynaptic synapses, such that minimal change in total synaptic weight occurs over a population of neurons (42). Such heterosynaptic plasticity is thought to represent a homeostatic mechanism to provide stability at the neural system level (for review, see ref. 41). Importantly, whereas homosynaptic plasticity is associative and usually NMDAR dependent, heterosynaptic plasticity is nonassociative and usually depends on non-NMDAR mechanisms such as retrograde signaling by nitric oxide and endocannabinoids. Few studies have investigated the time course of heterosynaptic plasticity in neocortical regions of mature animals; however, at least one study found that heterosynaptic depression induced in somatosensory neurons following high-frequency stimulation was more transient (<10 min) than the induced homosynaptic LTP (6). Although speculative, we suggest that the variable direction of C1 plasticity during early post-HFvS blocks in the current study may reflect the net effect of homosynaptic potentiation and heterosynaptic depression at the neuron population level. Nevertheless, our finding that DCS participants showed enhanced potentiation of C1 and P2 across post-HFvS assessments compared with Placebo is consistent with evidence that homosynaptic LTP is usually NMDAR dependent, and suggests that enhancing NMDAR signaling resulted in enhanced homosynaptic LTP in visual cortex neurons.
Enhancing NMDAR signaling also enhanced performance on the WPT and the IIT. The WPT and IIT both depend on incremental, feedback-based learning that is generally mediated by corticostriatal circuits, although prefrontal and medial temporal lobe structures may also be involved (21, 22, 44, 45). In particular, rapid acquisition of stimulus–outcome contingencies that coincides with early gains in learning is thought to be mediated by NMDAR-dependent LTP at dorsomedial striatal (DMS) synapses (21, 22, 46). As stimulus–outcome contingencies are consolidated and motor responses are increasingly automatized during late learning, performance asymptotes. In contrast to early learning, later expression of automatized responses is thought to be supported by LTD at dorsolateral striatal synapses following metabotropic glutamate and D2 dopamine receptor activation (22, 46). In the current study, enhanced performance in the DCS group was evident within the first block of 80 trials for both the IIT and the WPT. This suggests that augmenting NMDAR signaling facilitated the encoding of stimulus–outcome contingencies, possibly by accelerating or augmenting LTP at DMS synapses. Our findings of enhanced acquisition of the WPT and IIT are consistent with other findings of enhanced incremental learning following DCS administration, including on category learning (28), motor learning (25), and mental rotation learning tasks (47).
In contrast to the WPT and IIT, DCS participants did not differ from Placebo participants on the n-back, despite identical stimuli and trial structure between the IIT and n-back. This dissociation of cognitive effects is consistent with an emerging neurobiological framework that suggests that transient plasticity that underlies working memory is modulated in a fundamentally different way than lasting plasticity changes that support learning and memory consolidation (23, 48). Thus, whereas experience-dependent learning is thought to be mediated by lasting structural changes at dendritic spines following NMDAR-induced signaling cascades, short-term representation of stimuli for spatial working memory is thought to be mediated by transient, persistent firing of dorsolateral prefrontal cortex (dlPFC) microcircuits over brief delays. These working memory microcircuits involve glutamatergic pyramidal neurons in layer III dlPFC with similar spatial tuning that excite each other via AMPA and NMDA receptors, and GABAergic interneurons that inhibit neurons with dissimilar spatial tuning. Sufficient NMDAR activity is necessary to generate reverberating activity; however, it is lateral inhibition from GABAergic interneurons and dynamic modulation from acetylcholine and dopamine that is thought to enhance neuron firing for preferred directions, reduce firing for nonpreferred directions, and sculpt network activity to define the specifics of mental representation (23). This framework suggests that, given the minimum NMDAR activation necessary to produce transient, persistent firing, further NMDAR activation should have relatively limited effect on working memory performance. Our finding of similar working memory performance between DCS and Placebo participants is consistent with this theory and is in line with prior studies that failed to find effects of DCS on working memory in healthy volunteers or patient groups (27, 34–36). This dissociation of effects of DCS on experience-dependent learning versus working memory highlights the importance of considering the cellular mechanisms underlying distinct cognitive functions and demonstrates how investigating targeted hypotheses that capitalize on basic cognitive neuroscience can help reconcile discrepant effects of potential cognitive-enhancing drugs.
Some limitations to the current study should be noted. First, the current investigation was limited to healthy young adults. Studies in healthy participants provide critical information about potential mechanisms through which procognitive drugs exert their effects, as well as early feedback on how drugs affect specific cognitive domains and ideas for how effects may best be investigated in patient groups (49). Nevertheless, given that a key motivation for investigating effects of enhancing NMDAR signaling is to identify manipulations that can ameliorate plasticity deficits in patient populations, parallel studies in older adults and patient groups are an obligatory next step. Additionally, investigation of prolearning effects of DCS in the current study was limited to classification learning tasks that depend primarily on corticostriatal circuits. NMDAR-dependent LTP is thought to underlie distinct forms of learning in distributed circuits. For example, fear learning is thought to be encoded by LTP at lateral amygdala synapses (4), whereas motor sequence learning is thought to be encoded by LTP in motor cortex synapses (2). Other studies have shown that DCS can enhance learning mediated by other circuits, including fear (24) and motor learning (25), and indeed, some of the most reliable effects of DCS have been found when administered before cognitive behavioral therapy (CBT) exposure sessions to enhance fear extinction (ref. 29; but see also refs. 30 and 31). Future studies that systematically assess the effects of DCS on learning across multiple neural circuits will strengthen our understanding of the distributed effects of augmenting NMDAR signaling on experience-dependent plasticity. Finally, the current study did not assess how varying the parameters of DCS administration affects the efficacy of DCS. For example, some evidence suggests that the NMDAR complex may show desensitization when DCS is administered repeatedly (50). Although beyond the scope of the current study, future studies that characterize the optimal time window for DCS administration and examine whether effects of DCS persist with chronic dosing will be critical before practical applications of DCS can be realized.
Taken together, the current results provide compelling evidence that enhancing NMDAR signaling can augment experience-dependent plasticity in the adult human brain. Effects of DCS were seen across tasks probing both the mechanism thought to underlie many forms of experience-dependent plasticity (i.e., LTP) and behavioral correlates of experience-dependent plasticity (i.e., acquisition of incremental learning). These findings suggest exciting possibilities for using NMDAR agonists to help ameliorate plasticity deficits in neurodegenerative and psychiatric disorders. Our results complement a growing literature that suggests that DCS can enhance new learning during CBT interventions (29, 32) and cognitive training programs (33, 34). The dissociation of effects of DCS on incremental learning versus working memory highlights the importance of capitalizing on progress in basic cognitive neuroscience to develop more specific hypotheses for targets of cognitive-enhancing drugs.
Methods
Participants and Procedures.
Healthy volunteers between 18 and 30 y completed the study (SI Methods). Study procedures were approved by the University of California, Los Angeles, Human Participants Institution Review Board, and written informed consent was obtained from all participants.
Testing consisted of a 2-d, randomized, double-blind 100-mg DCS versus placebo design. On the first day, DCS or placebo was administered as encapsulated pills. EEG testing began 3 h later, followed by cognitive testing (SI Methods). To explore potential delayed effects of DCS on memory consolidation (51), participants returned to the laboratory the next day to repeat the cognitive tasks. No drug or placebo was administered on the second day.
LTP Task.
The LTP task was adapted from Cavuş et al. (13) and assessed VEPs in 2-min blocks before and after exposure to HFvS. VEPs were assessed to a standard circle stimulus filled with a black-and-white checkerboard pattern, presented at 0.83 Hz. The HFvS block consisted of repeated presentation of the standard circle at ∼8.87 Hz for 2 min. For additional details on the task and on EEG recording and processing, see SI Methods.
Cognitive Tasks.
The WPT was a probabilistic classification task adapted from Wagshal et al. (19). The WPT consisted of 320 trials during which participants predicted the weather based on cue combinations that probabilistically related to “sun” and “rain.” Cues were presented for a maximum of 5 s. Following response, feedback showing the cue combination and actual outcome for the trial was presented for 1 s. The IIT was a category learning task adapted from Waldschmidt and Ashby (20). The IIT consisted of four blocks of 80 trials separated by 10-s rest periods, for 320 total trials. Stimuli belonged to category A or B, and consisted of circular sine-wave gratings that varied in bar width and bar orientation. On each trial, a single stimulus was presented in one of four quadrants of the screen for 2 s. Participants were instructed to integrate information about the two dimensions and choose whether stimuli belonged to category A or B; following response, participants were provided auditory feedback for 500 ms indicating whether they made a correct or incorrect response. The n-back was a spatial working memory task with four memory loads and was designed to be identical in stimuli and trial structure to the IIT. Thus, participants viewed a sequence of 80 stimuli at each memory load, and on each trial, were asked to respond “yes” or “no,” whether the stimulus was in the same quadrant on the screen as the stimulus they saw n trials earlier. See SI Methods for additional details on the cognitive tasks.
Statistical Analyses.
Statistical analyses were conducted with SPSS, version 22.0 (SPSS). Independent-samples t tests assessed group differences in age and IQ, and a χ2 test assessed differences in gender.
LTP analyses.
C1 and P2 components were identified at midline occipital channels and were analyzed using the channels available for all participants that showed the largest amplitude for each component. Thus, C1 analyses used Oz, Iz, O1, OI1h, and P2 analyses used POz, Oz, Iz, POO1, O1, OI1h. As initial analyses showed no interactions of channel with Group or Block, average amplitude across the four C1 channels and six P2 channels were used for all analyses. Paired-samples t tests showed no differences between the two baseline VEP blocks for either group for C1 or P2; thus, amplitudes for the two baseline blocks were averaged to yield one baseline. To first characterize the time course of HFvS effects, component amplitudes were investigated using Group by Block repeated-measures ANOVA, followed by tests of simple contrast to baseline (SI Results). Next, to obtain a measure of potentiation induced by HFvS, baseline VEP amplitude was subtracted from each post-HFvS amplitude. Group differences in potentiation were assessed using Group by Block repeated-measures ANOVA.
Cognitive analyses.
Percent correct responses per 80-trial blocks were calculated for each cognitive test. For the WPT, trials for cue combinations that equally predicted sun and rain (i.e., probability of 0.5) were excluded. Group differences in accuracy and reaction time were assessed using Group by Block by Day repeated-measures ANOVA. An α value of 0.05 was used to determine significance for all analyses.
Acknowledgments
We thank Dr. Gregory Ashby and Dr. Vivian Valentin for consultation on implementation and modeling of the IIT and Dr. Barbara Knowlton for consultation on implementation of the WPT. We also thank Elissa Ye, Cheryl Li, Heather Hansen, Devin Deer, and Chantelle Kinzel for assistance with data collection, data processing, and/or programming. This work was supported by the Della Martin Foundation and a National Science Foundation Graduate Research Fellowship (to J.K.F.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. K.F. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1509262112/-/DCSupplemental.
References
- 1.Feldman DE. Synaptic mechanisms for plasticity in neocortex. Annu Rev Neurosci. 2009;32:33–55. doi: 10.1146/annurev.neuro.051508.135516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ganguly K, Poo MM. Activity-dependent neural plasticity from bench to bedside. Neuron. 2013;80(3):729–741. doi: 10.1016/j.neuron.2013.10.028. [DOI] [PubMed] [Google Scholar]
- 3.Citri A, Malenka RC. Synaptic plasticity: Multiple forms, functions, and mechanisms. Neuropsychopharmacology. 2008;33(1):18–41. doi: 10.1038/sj.npp.1301559. [DOI] [PubMed] [Google Scholar]
- 4.Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147(3):509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yin HH, et al. Dynamic reorganization of striatal circuits during the acquisition and consolidation of a skill. Nat Neurosci. 2009;12(3):333–341. doi: 10.1038/nn.2261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castro-Alamancos MA, Donoghue JP, Connors BW. Different forms of synaptic plasticity in somatosensory and motor areas of the neocortex. J Neurosci. 1995;15(7 Pt 2):5324–5333. doi: 10.1523/JNEUROSCI.15-07-05324.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cooke SF, Bear MF. Visual experience induces long-term potentiation in the primary visual cortex. J Neurosci. 2010;30(48):16304–16313. doi: 10.1523/JNEUROSCI.4333-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Clapp WC, Eckert MJ, Teyler TJ, Abraham WC. Rapid visual stimulation induces N-methyl-d-aspartate receptor-dependent sensory long-term potentiation in the rat cortex. Neuroreport. 2006;17(5):511–515. doi: 10.1097/01.wnr.0000209004.63352.10. [DOI] [PubMed] [Google Scholar]
- 9.Kirk IJ, et al. Long-term potentiation (LTP) of human sensory-evoked potentials. Wiley Interdiscip Rev Cogn Sci. 2010;1(5):766–773. doi: 10.1002/wcs.62. [DOI] [PubMed] [Google Scholar]
- 10.Teyler TJ, et al. Long-term potentiation of human visual evoked responses. Eur J Neurosci. 2005;21(7):2045–2050. doi: 10.1111/j.1460-9568.2005.04007.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Normann C, Schmitz D, Fürmaier A, Döing C, Bach M. Long-term plasticity of visually evoked potentials in humans is altered in major depression. Biol Psychiatry. 2007;62(5):373–380. doi: 10.1016/j.biopsych.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 12.Elvsåshagen T, et al. Evidence for impaired neocortical synaptic plasticity in bipolar II disorder. Biol Psychiatry. 2012;71(1):68–74. doi: 10.1016/j.biopsych.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 13.Cavuş I, et al. Impaired visual cortical plasticity in schizophrenia. Biol Psychiatry. 2012;71(6):512–520. doi: 10.1016/j.biopsych.2012.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mears RP, Spencer KM. Electrophysiological assessment of auditory stimulus-specific plasticity in schizophrenia. Biol Psychiatry. 2012;71(6):503–511. doi: 10.1016/j.biopsych.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hunt DL, Castillo PE. Synaptic plasticity of NMDA receptors: Mechanisms and functional implications. Curr Opin Neurobiol. 2012;22(3):496–508. doi: 10.1016/j.conb.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lüscher C, Malenka RC. NMDA receptor-dependent long-term potentiation and long-term depression (LTP/LTD) Cold Spring Harb Perspect Biol. 2012;4(6):a005710. doi: 10.1101/cshperspect.a005710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lee YS, Silva AJ. The molecular and cellular biology of enhanced cognition. Nat Rev Neurosci. 2009;10(2):126–140. doi: 10.1038/nrn2572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Javitt DC. Glutamate as a therapeutic target in psychiatric disorders. Mol Psychiatry. 2004;9(11):984–997. doi: 10.1038/sj.mp.4001551. [DOI] [PubMed] [Google Scholar]
- 19.Wagshal D, et al. Evidence for corticostriatal dysfunction during cognitive skill learning in adolescent siblings of patients with childhood-onset schizophrenia. Schizophr Bull. 2014;40(5):1030–1039. doi: 10.1093/schbul/sbt147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Waldschmidt JG, Ashby FG. Cortical and striatal contributions to automaticity in information-integration categorization. Neuroimage. 2011;56(3):1791–1802. doi: 10.1016/j.neuroimage.2011.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yin HH, Knowlton BJ. The role of the basal ganglia in habit formation. Nat Rev Neurosci. 2006;7(6):464–476. doi: 10.1038/nrn1919. [DOI] [PubMed] [Google Scholar]
- 22.Kreitzer AC, Malenka RC. Striatal plasticity and basal ganglia circuit function. Neuron. 2008;60(4):543–554. doi: 10.1016/j.neuron.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: Flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76(1):223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kalisch R, et al. The NMDA agonist d-cycloserine facilitates fear memory consolidation in humans. Cereb Cortex. 2009;19(1):187–196. doi: 10.1093/cercor/bhn076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kuriyama K, Honma M, Koyama S, Kim Y. d-Cycloserine facilitates procedural learning but not declarative learning in healthy humans: A randomized controlled trial of the effect of d-cycloserine and valproic acid on overnight properties in the performance of non-emotional memory tasks. Neurobiol Learn Mem. 2011;95(4):505–509. doi: 10.1016/j.nlm.2011.02.017. [DOI] [PubMed] [Google Scholar]
- 26.Scholl J, et al. A role beyond learning for NMDA receptors in reward-based decision-making—a pharmacological study using d-cycloserine. Neuropsychopharmacology. 2014;39(12):2900–2909. doi: 10.1038/npp.2014.144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Otto MW, et al. Effects of d-cycloserine administration on weekly nonemotional memory tasks in healthy participants. Psychother Psychosom. 2009;78(1):49–54. doi: 10.1159/000172620. [DOI] [PubMed] [Google Scholar]
- 28.Onur OA, et al. The N-methyl-d-aspartate receptor co-agonist d-cycloserine facilitates declarative learning and hippocampal activity in humans. Biol Psychiatry. 2010;67(12):1205–1211. doi: 10.1016/j.biopsych.2010.01.022. [DOI] [PubMed] [Google Scholar]
- 29.Norberg MM, Krystal JH, Tolin DF. A meta-analysis of d-cycloserine and the facilitation of fear extinction and exposure therapy. Biol Psychiatry. 2008;63(12):1118–1126. doi: 10.1016/j.biopsych.2008.01.012. [DOI] [PubMed] [Google Scholar]
- 30.Hofmann SG, Sawyer AT, Asnaani A. d-Cycloserine as an augmentation strategy for cognitive behavioral therapy for anxiety disorders: An update. Curr Pharm Des. 2012;18(35):5659–5662. doi: 10.2174/138161212803530916. [DOI] [PubMed] [Google Scholar]
- 31.Ori R, et al. Augmentation of cognitive and behavioural therapies (CBT) with d-cycloserine for anxiety and related disorders. Cochrane Database Syst Rev. 2015;5:CD007803. doi: 10.1002/14651858.CD007803.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gottlieb JD, et al. d-Cycloserine facilitation of cognitive behavioral therapy for delusions in schizophrenia. Schizophr Res. 2011;131(1-3):69–74. doi: 10.1016/j.schres.2011.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Behar E, McHugh RK, Peckham A, Otto MW. d-Cycloserine for the augmentation of an attentional training intervention for trait anxiety. J Anxiety Disord. 2010;24(4):440–445. doi: 10.1016/j.janxdis.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 34.Cain CK, et al. d-Cycloserine augmentation of cognitive remediation in schizophrenia. Schizophr Res. 2014;153(1-3):177–183. doi: 10.1016/j.schres.2014.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Duncan EJ, et al. Effects of d-cycloserine on negative symptoms in schizophrenia. Schizophr Res. 2004;71(2-3):239–248. doi: 10.1016/j.schres.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 36.Goff DC. d-Cycloserine: An evolving role in learning and neuroplasticity in schizophrenia. Schizophr Bull. 2012;38(5):936–941. doi: 10.1093/schbul/sbs012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nitsche MA, et al. Consolidation of human motor cortical neuroplasticity by d-cycloserine. Neuropsychopharmacology. 2004;29(8):1573–1578. doi: 10.1038/sj.npp.1300517. [DOI] [PubMed] [Google Scholar]
- 38.Rouaud E, Billard JM. d-Cycloserine facilitates synaptic plasticity but impairs glutamatergic neurotransmission in rat hippocampal slices. Br J Pharmacol. 2003;140(6):1051–1056. doi: 10.1038/sj.bjp.0705541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Billard JM, Rouaud E. Deficit of NMDA receptor activation in CA1 hippocampal area of aged rats is rescued by d-cycloserine. Eur J Neurosci. 2007;25(8):2260–2268. doi: 10.1111/j.1460-9568.2007.05488.x. [DOI] [PubMed] [Google Scholar]
- 40.Luck SJ. An Introduction to the Event-Related Potential Technique. MIT Press; Cambridge, MA: 2014. [Google Scholar]
- 41.Chistiakova M, Bannon NM, Bazhenov M, Volgushev M. Heterosynaptic plasticity: Multiple mechanisms and multiple roles. Neuroscientist. 2014;20(5):483–498. doi: 10.1177/1073858414529829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Royer S, Paré D. Conservation of total synaptic weight through balanced synaptic depression and potentiation. Nature. 2003;422(6931):518–522. doi: 10.1038/nature01530. [DOI] [PubMed] [Google Scholar]
- 43.Huang Y, Yasuda H, Sarihi A, Tsumoto T. Roles of endocannabinoids in heterosynaptic long-term depression of excitatory synaptic transmission in visual cortex of young mice. J Neurosci. 2008;28(28):7074–7083. doi: 10.1523/JNEUROSCI.0899-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ashby FG, Spiering BJ. The neurobiology of category learning. Behav Cogn Neurosci Rev. 2004;3(2):101–113. doi: 10.1177/1534582304270782. [DOI] [PubMed] [Google Scholar]
- 45.Shohamy D, Myers CE, Kalanithi J, Gluck MA. Basal ganglia and dopamine contributions to probabilistic category learning. Neurosci Biobehav Rev. 2008;32(2):219–236. doi: 10.1016/j.neubiorev.2007.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lovinger DM. Neurotransmitter roles in synaptic modulation, plasticity and learning in the dorsal striatum. Neuropharmacology. 2010;58(7):951–961. doi: 10.1016/j.neuropharm.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bailey JE, Papadopoulos A, Lingford-Hughes A, Nutt DJ. d-Cycloserine and performance under different states of anxiety in healthy volunteers. Psychopharmacology (Berl) 2007;193(4):579–585. doi: 10.1007/s00213-007-0817-9. [DOI] [PubMed] [Google Scholar]
- 48.Wang M, et al. NMDA receptors subserve persistent neuronal firing during working memory in dorsolateral prefrontal cortex. Neuron. 2013;77(4):736–749. doi: 10.1016/j.neuron.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Millan MJ, et al. Cognitive dysfunction in psychiatric disorders: Characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov. 2012;11(2):141–168. doi: 10.1038/nrd3628. [DOI] [PubMed] [Google Scholar]
- 50.Boje KM, Wong G, Skolnick P. Desensitization of the NMDA receptor complex by glycinergic ligands in cerebellar granule cell cultures. Brain Res. 1993;603(2):207–214. doi: 10.1016/0006-8993(93)91239-o. [DOI] [PubMed] [Google Scholar]
- 51.Shimizu E, Tang Y-P, Rampon C, Tsien JZ. NMDA receptor-dependent synaptic reinforcement as a crucial process for memory consolidation. Science. 2000;290(5494):1170–1174. doi: 10.1126/science.290.5494.1170. [DOI] [PubMed] [Google Scholar]
- 52.Wechsler D. Wechsler Abbreviated Scale of Intelligence (WASI) Harcourt Assessment; San Antonio, TX: 1999. [Google Scholar]
- 53.Krystal JH, et al. Characterization of the interactive effects of glycine and d-cycloserine in men: Further evidence for enhanced NMDA receptor function associated with human alcohol dependence. Neuropsychopharmacology. 2011;36(3):701–710. doi: 10.1038/npp.2010.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Gratton G, Coles MG, Donchin E. A new method for off-line removal of ocular artifact. Electroencephalogr Clin Neurophysiol. 1983;55(4):468–484. doi: 10.1016/0013-4694(83)90135-9. [DOI] [PubMed] [Google Scholar]
- 55.Ashby FG, Gott RE. Decision rules in the perception and categorization of multidimensional stimuli. J Exp Psychol Learn Mem Cogn. 1988;14(1):33–53. doi: 10.1037//0278-7393.14.1.33. [DOI] [PubMed] [Google Scholar]
- 56.Maddox WT, Ashby FG. Comparing decision bound and exemplar models of categorization. Percept Psychophys. 1993;53(1):49–70. doi: 10.3758/bf03211715. [DOI] [PubMed] [Google Scholar]