Significance
Within a dominance hierarchy, low social status strongly reduces individual well-being. In socially living species, rank in a hierarchy is determined through competitive encounters. Despite the numerous health consequences, the ability of personality traits to predispose individuals to a particular social rank remains largely unclear. Our work identifies trait anxiety as a predisposing factor to a subordinate rank. We demonstrate that mitochondrial function in the nucleus accumbens, a brain region relevant for motivation and depression, is a critical mediating factor in the subordinate status displayed by high-anxious rats. These findings highlight a role for cerebral energy metabolism in social behavior and point to mitochondrial function in the nucleus accumbens as a potential marker and avenue of treatment for mood disorders.
Keywords: anxiety, mitochondria, nucleus accumbens, social dominance, social competition
Abstract
Dominance hierarchies are integral aspects of social groups, yet whether personality traits may predispose individuals to a particular rank remains unclear. Here we show that trait anxiety directly influences social dominance in male outbred rats and identify an important mediating role for mitochondrial function in the nucleus accumbens. High-anxious animals that are prone to become subordinate during a social encounter with a low-anxious rat exhibit reduced mitochondrial complex I and II proteins and respiratory capacity as well as decreased ATP and increased ROS production in the nucleus accumbens. A causal link for these findings is indicated by pharmacological approaches. In a dyadic contest between anxiety-matched animals, microinfusion of specific mitochondrial complex I or II inhibitors into the nucleus accumbens reduced social rank, mimicking the low probability to become dominant observed in high-anxious animals. Conversely, intraaccumbal infusion of nicotinamide, an amide form of vitamin B3 known to enhance brain energy metabolism, prevented the development of a subordinate status in high-anxious individuals. We conclude that mitochondrial function in the nucleus accumbens is crucial for social hierarchy establishment and is critically involved in the low social competitiveness associated with high anxiety. Our findings highlight a key role for brain energy metabolism in social behavior and point to mitochondrial function in the nucleus accumbens as a potential marker and avenue of treatment for anxiety-related social disorders.
In most socially living species, the social rank of an individual is established during competitive encounters with conspecifics. The outcome of these encounters determines the allocation of territory, resources and access to reproduction (1), and greatly influences physiology and health (2). Winning a social competition is rewarding, enhances rank in social hierarchies, and increases the probability of winning future contests (3, 4). In contrast, losing a social encounter typically undermines one’s social rank. In humans, a low social status predicts morbidity and survival (5) and has also been linked to the development of psychopathologies (6). Despite the important consequences of social rank on health, little is known regarding the mechanisms underlying the establishment of social hierarchies.
One of the main reasons for the paucity of neurobiological mechanisms determining social hierarchies may be that social competition involves interacting subjects that both need to be taken into account. The competitors may exhibit different features such as size, age, gender, as well as previous social experience, all known to influence social competitiveness. However, when subjects are matched for these characteristics, the impact of innate personality traits on social competitiveness may be investigated. One such personality trait, anxiety, may have important consequences for the outcome of social competitions. In humans, high-anxious individuals often display a subordinate status and report feelings of being overlooked and rejected (7), and their competitive self-confidence becomes undermined under stress (8). Thus, interindividual differences in anxiety could predetermine the outcome of a competitive encounter and, as such, trait anxiety may have important consequences for social status. However, the neural mechanisms whereby anxiety might affect social hierarchy formation are largely unknown.
We addressed this question by examining social competitiveness between male rats characterized for trait anxiety. Recent work has highlighted the potential for individual differences in mitochondrial function and, more broadly, energy metabolism to influence vulnerability to develop psychopathological disorders, such as anxiety and depression (9–14). Here, we demonstrate that trait anxiety is a determining factor of social rank that is mediated by brain region-specific mitochondrial function. We show that manipulation of mitochondrial function in the nucleus accumbens (NAc) is sufficient to influence social rank, highlighting a key role for brain mitochondrial function in social behavior.
Results
High Trait Anxiety Is a Predisposing Factor to Social Subordination.
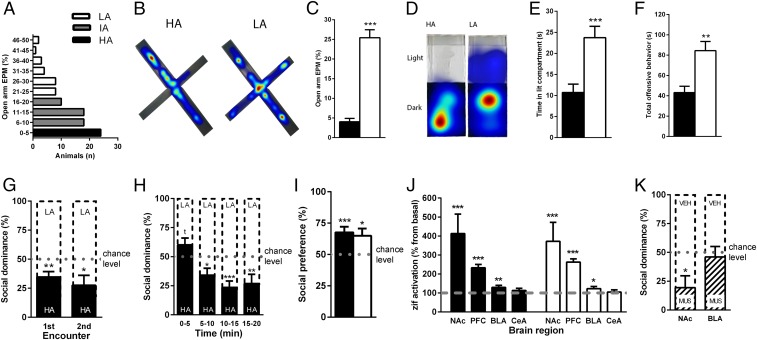
To establish the relationship between anxiety and the outcome of a social competition, male outbred rats were first classified as high-, intermediate-, or low-anxious based on natural variation for anxiety-like behavior on the elevated plus maze (Fig. 1 A–C and SI Appendix, Fig. S1A). Their anxiety profiles were confirmed in another validated test for anxiety, the light–dark box (Fig. 1 D and E and SI Appendix, Fig. S1 B–D). Then, high-anxious and low-anxious rats were matched for body weight, age, and social experience, and allowed to compete for a new territory. High-anxious rats exhibited reduced offensive behavior during the social encounter (Fig. 1F and SI Appendix, Fig. S1 E–G), which is reflected by a social dominance level below chance (50%, Fig. 1G). Importantly, the subordinate character of high-anxious rats was not apparent from the onset of the interaction, but developed throughout the social encounter, indicating no lack of motivation to compete in these animals (Fig. 1H). The low competitive success of high-anxious rats was also evident during a subsequent encounter that took place 1 wk later (Fig. 1G), emphasizing the pervasive impact of anxiety on social rank. To examine whether this anxiety-related difference in social competitiveness was related to “social anxiety,” we performed a follow-up study of social preference and found that both high- and low-anxious rats similarly exhibited a preference to explore a juvenile rat over an inanimate object (Fig. 1I). Thus, the differences in social competitiveness are not related to overall differences in social motivation or sociability. Because self-confidence is affected by stress (8), we investigated whether differences in the stress response might explain the low competitive success of high-anxious rats. We examined corticosterone levels in high- and low-anxious rats under basal conditions and following social competition. Although social competition did significantly increase corticosterone compared with baseline levels, there were no significant differences between anxiety groups at either time point (SI Appendix, Fig. S1H).
Fig. 1.
High anxiety predisposes for social submission. (A) Classification for anxiety-profiles was based on the elevated plus maze (EPM; HA, high-anxious; IA, intermediate-anxious; LA, low-anxious). Time spent in the open arm of the EPM (B and C) and in the lit compartment of the light–dark test (D and E) was lower for HA animals, n = 24 per group. When competing against an LA rat, HA rats display reduced offensive behavior (F) and show low social dominance (G) emerging throughout time (H), n = 24 pairs. (I) Both HA and LA rats display similar levels of social preference. (J) Levels of zif268 were increased following social competition in the nucleus accumbens (NAc), prefrontal cortex (PFC), and basolateral (BLA), but not central (CeA), amygdala in both groups, n = 10–20 per group. (K) Local inactivation with muscimol in the NAc, but not BLA, reduced social dominance, n = 11–13 pairs. Data are mean ± SEM (*P < 0.05, **P < 0.01, ***P < 0.001, Student’s t test or one-sample t test against chance, 50%, level).
The Nucleus Accumbens Is Critically Involved in the Establishment of a Social Hierarchy.
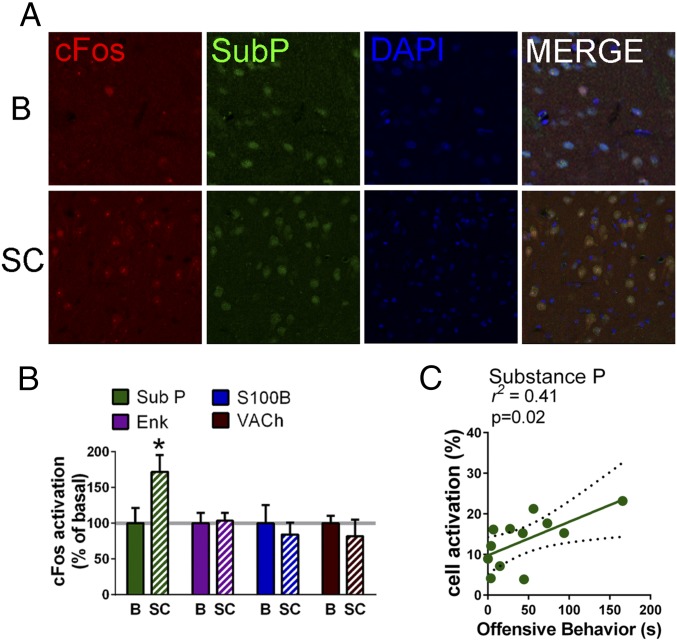
Several brain regions [e.g., the prefrontal cortex (15), NAc (16), and the amygdala (17, 18)] have been involved in the establishment of social hierarchies in rodents, with the ventral striatum (including the NAc) being consistently highlighted in human imaging studies of social status (19, 20) and competition (21). We confirmed the activation of the nucleus accumbens (along with that of the prefrontal cortex and basolateral, but not central, nucleus of the amygdala) following a social competition test, in both low- and high-anxious naïve animals by comparing the expression of the immediate early transcription factor gene zif-268 mRNA following the encounter to basal conditions (Fig. 1J). We next aimed to test the causal involvement of the NAc and BLA in the establishment of a dominant rank. For this purpose, we performed independent experiments in which we pharmacologically inactivated each of these brain areas through microinfusion of muscimol, a GABAA receptor agonist, 30 min before social competition between two males matched for equivalent anxiety levels. Upon muscimol infusion in the NAc, rats exhibited reduced social dominance levels in a confrontation with another anxiety-matched male that was infused with vehicle (Fig. 1K). Importantly, this treatment did not induce changes in locomotor activity as evaluated in the open field (SI Appendix, Fig. S1I). On the contrary, inactivation of the basolateral amygdala (BLA) with muscimol had no impact on the social hierarchy outcome (Fig. 1K). We validated the effectiveness of our BLA treatment by demonstrating that it drastically inhibited fear conditioning (SI Appendix, Fig. S2), confirming the role of the BLA in this type of behavior (22). Given the prominent role of the NAc in social competition, we performed an additional experiment to gain insight into the cell types which show activation in this nucleus following a competitive encounter. Double-labeling of cFOS with markers for several neuronal types highlighted a significant activation of substance P-containing cells [known to represent medium spiny neurons (MSNs) containing the dopaminergic receptor D1 (23)] following social competition (Fig. 2 A and B) that correlated with the amount of competitive behavior (Fig. 2C). However, no social challenge-induced significant activations were observed for cholinergic cells, for cells containing enkephalin [known to represent D2-containing MSNs (23)] or for cells containing the S100 astrocytic marker (SI Appendix, Fig. S3 A–F). Therefore, these data point to the involvement of accumbal D1-containing MSNs in social competition.
Fig. 2.
D1-containing cells are activated by social competition. (A and B) cFOS activation of substance P (SubP; D1-containing cells), enkephalin (ENK; D2-containing cells), S100, and vesicular acetylcholine transporter (VAChT) in the NAc after social competition (SC) was compared with basal levels (B) and only substance P+ cells displayed cFOS activation in response to social competition. (C) cFOS activation in substance P+ cells significantly correlated with the duration of competitive offensive behavior. cFOS activation is presented as percentage of basal cFOS activation induced by social competition (n = 6 pairs per group) as mean ± SEM (*P < 0.05).
High-Anxious Rats Exhibit Reduced Mitochondrial Function in the NAc.
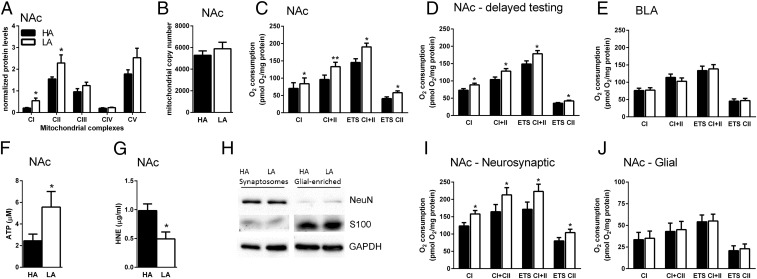
As our findings pointed to a prominent role of the NAc in social competition, we explored potential molecular pathways within the NAc differing between high- and low-anxious rats. We started by examining differential basal gene expression between high- and low-anxious rats in the NAc using microarrays and gene set enrichment analysis (GSEA). The top regulated biological processes in high- versus low-anxious rats involved metabolic functions at various cellular levels (SI Appendix, Fig. S4A) and GSEA analysis on a curated set of mitochondrial genes pointed to significant differences in genes enriched in categories related to mitochondrial function (SI Appendix, Fig. S4B and Table S1). Mitochondrial respiratory function is essential for neural processing, as it plays key roles in many essential functions required for neural homeostasis and computations (24–26). Thus, we reasoned that the disadvantage of high-anxious rats to win the competition against low-anxious rats could reflect a lower mitochondrial activity in the NAc. We first measured protein levels in the five mitochondrial complexes that comprise the electron transport chain between high- and low-anxious rats. In both complexes I and II, starting points for mitochondrial respiration, high-anxious rats exhibited lower levels of protein than low-anxious rats (Fig. 3A). We then examined whether such protein differences might be indicative of a difference in mitochondrial number. However, both PCR analysis of mitochondrial DNA levels (Fig. 3B), and mitochondrial quantification from images obtained with transmission electron microscopy (SI Appendix, Table S2) revealed no significant differences in mitochondrial number or density in the NAc between high- and low-anxious rats, indicating that the observed differences in complex I and II protein content are not due to a sheer difference in mitochondrial number but to an enrichment in these respiratory complexes per mitochondria.
Fig. 3.
High-anxious rats (HA) exhibit lower mitochondrial function that is specific to the nucleus accumbens (NAc). HA exhibit lower expression of complex I and II protein levels in the NAc than low-anxious rats (LA), n = 5–12 per group (A), but no significant difference in mitochondrial number, n = 9 per group (B). (C) HA display lower mitochondrial respiration in the NAc than LA, n = 6 per group. (D) These differences were also detected two months following anxiety characterization, n = 8 per group. (E) Mitochondrial respiration in the basolateral amygdala (BLA) does not differ between groups, n = 11 per group. ATP-levels in the NAc are lower in HA than LA, n = 9–10 per group (F), whereas ROS products are higher in HA, n = 5–6 per group (G). Synaptoneurosomes were separated from glia (H), and then mitochondrial respiration was measured in three independent experiments. The mitochondrial respiration deficit of HA is present in synaptoneurosomes (I) but not in glia-enriched fractions (J). Data are mean ± SEM. Respiration data are presented as estimated marginal means ± SEM of oxygen flux per mg tissue (*P < 0.05; **P < 0.01, Linear Mixed Model).
Accordingly, we next evaluated whether mitochondrial function in the NAc differed between high- and low-anxious rats. Using high-resolution respirometry, we found that when coupled respiration through complex I was stimulated in NAc homogenates by the addition of ADP, low-anxious rats displayed higher respiration rates than those of high-anxious rats, a difference that became more prominent upon complex II stimulation. Maximal electron transport system capacity (ETS) was also markedly higher in the NAc from low-anxious rats. Finally, the addition of rotenone revealed higher maximal respiration due to complex II activity (Fig. 3C). Linear regression analysis identified significant correlations between anxiety-like behavior on the elevated-plus maze and respiration levels following stimulation of complex II, ETS, and rotenone addition (SI Appendix, Fig. S5 A–D). The reduced mitochondrial respiratory capacity in the NAc of high-anxious animals is persistent in life, as it was also present when measured two months after anxiety characterization (Fig. 3D), fitting with the observed differences at the protein level (Fig. 3A). The addition of cytochrome c to the NAc preparations confirmed that the reported differences are not due to a differential mitochondrial vulnerability during experimental preparation (SI Appendix, Fig. S6A). Likewise, the anxiety-related effect on mitochondrial function was not a result of putative differences in body size, locomotor activity, or food intake, as these were equivalent for both high- and low-anxious animals (SI Appendix, Fig. S6 B–D). To investigate the specificity of these findings for the NAc, we examined mitochondrial respiration in the BLA. Here, unlike the NAc, high- and low-anxious rats displayed comparable respiration rates in the BLA (Fig. 3E).
We then examined whether the anxiety-related differences in NAc mitochondrial respiration had functional consequences for downstream mitochondrial outputs. First, we found that high-anxious animals exhibited significantly lower ATP concentrations in the NAc than low-anxious counterparts (Fig. 3F). Then, we measured the levels of a common ROS product in NAc homogenates, 4-Hydroxynonenal [(4-HNE; an α,β-unsaturated hydroxyalkenal produced by lipid peroxidation, with high levels suggesting increased periods of oxidative stress (27)] and found higher levels in high-anxious animals (Fig. 3G).
We then investigated the cellular specificity of the anxiety-related differences observed in NAc mitochondrial function. We performed mitochondrial respiration on synaptoneurosomal and glia-enriched fractions in three independent experiments (Fig. 3H) and found lower respiration in high-anxious compared with low-anxious animals in the synaptoneurosomal fraction only (Fig. 3 I–J). This finding is consistent with the lack of astrocytic activation induced by social competition (SI Appendix, Fig. S3C). Taken together, our results critically link anxiety with NAc mitochondrial function within the neuronal synaptic compartment (Fig. 3 I–J).
Manipulating Mitochondrial Function in the NAc Affects Social Dominance.
Next, we inquired whether mitochondrial function in the NAc could per se be causally implicated in the outcome of a social hierarchy following a dyadic encounter. Given that both complex I- and complex II-dependent respiration were found to be associated with the anxiety phenotype (Fig. 3), we microinfused specific pharmacological inhibitors for each of these complexes [for complex I: rotenone (ROT); for complex II: the competitive inhibitor malonic acid (MA) and the noncompetitive 3-nitroproprionic acid (3NP)] into the NAc of separate groups of naïve animals that were paired to another anxiety-matched male infused with the corresponding vehicle. Microinfusion of either ROT, MA, or 3NP reduced the success of treated animals to win the social contest (Fig. 4A). Importantly, these treatments did not induce side effects on social investigation or auto-grooming during social competition (SI Appendix, Table S3), or alter locomotor activity (Fig. 4B), anxiety, or sociability (SI Appendix, Fig. S7 A–D) in additional experiments. Furthermore, these inhibitor treatments did not produce neurotoxic effects, as the drugs were infused at low doses and we confirmed the absence of lesion and neuronal death (SI Appendix, Fig. S8). The effects of complex I or complex II inhibition on social competition were specific for the NAc, as infusions of the same inhibitors into the BLA had no effect on social dominance and did not affect general locomotor activity (Fig. 4 C and D). We further showed that, unlike infusion of muscimol in the BLA (SI Appendix, Fig. S2) that interferes with BLA-dependent auditory fear conditioning, microinfusion of 3NP did not affect conditioning in this task (SI Appendix, Fig. S2), discarding that neuronal inactivation could be a general mechanism whereby impairing mitochondrial function would affect putative functions from the affected brain region. Altogether, these data strongly support a key role for complex I- and II-dependent mitochondrial function within the NAc in the establishment of social dominance.
Fig. 4.
Inhibition of mitochondrial complexes I and II in nucleus accumbens (NAc) decreased social dominance. Intra-NAc infusion of rotenone (ROT, n = 12 pairs), 3-nitropropionic acid (3-NP, n = 10 pairs), or malonic acid (MA, n = 12 pairs) reduced social dominance (A), without affecting locomotion in the Open Field, n = 5–6 per group (B). Intra-BLA infusions of ROT, 3-NP, or MA had no effect on social dominance, n = 6, 9, or 10 pairs, respectively (C), or locomotion, n = 5 per group (D). Data are mean ± SEM (**P < 0.01, ***P < 0.001, Student’s t test or one-sample t test against chance level).
We then investigated whether we could reverse the disadvantage exhibited by high-anxious animals in the acquisition of social dominance by boosting NAc mitochondrial function. Nicotinamide adenine dinucleotide (NAD+) is a metabolic cofactor present in cells that has been implicated in a wide range of critical metabolic activities (28). Treatment with the NAD+ precursor nicotinamide (NAM; ref. 28), an amide form of vitamin B3 that boosts mitochondrial respiration (29), into the NAc of high-anxious rats at a time point before the social encounter and at a dose that increased accumbal mitochondrial respiration (Fig. 5A), abolished the disadvantage of high-anxious animals to become dominant against low-anxious animals (Fig. 5B). Noteworthy, anxiety, sociability, social investigation and auto-grooming remained unaffected by NAM-treatment (SI Appendix, Fig. S7 E and F and Table S3). Finally, given the higher levels of the common ROS product 4HNE observed in high-anxious rats, we investigated whether treating these animals with the antioxidant Mitoquinone mesylate (mitoQ; ref. 30) in the NAc might enhance their dominance. Infusion of MitoQ at a time (3 h before testing) and dose (10 µM) that decreases accumbal 4HNE levels (Fig. 5C) had no effect on reversing the disadvantage of high-anxious rats when competing with vehicle-infused low-anxious rats (Fig. 5D). These observations suggest that the impact of mitochondrial function in social competition described here is not mediated by oxidative stress but is related to mitochondrial respiratory capacity. Taken together, our results point to a causal link between NAc mitochondrial function and social rank.
Fig. 5.
Enhancing nucleus accumbens (NAc) energy stores via nicotinamide (NAM) infusion abolished the competitive disadvantage in high-anxious (HA) animals. (A) Intra-NAc infusion of NAM before social competition increased mitochondrial respiration in HA rats, n = 7 per group. (B) NAM-treated HA rats had similar chances to become dominant as low-anxious (LA) rats, n = 9–11 pairs. Intra-NAc infusion of mitoquinone mesylate (mitoQ) reduced local ROS products (C), n = 5, but did not enhance social competition in HA rats (D), n = 8 pairs. Data are presented as mean ± SEM (*P < 0.05, ***P < 0.001, one sample t test against baseline or chance level).
Discussion
In this study, we show that animals’ anxiety trait is predictive of the outcome of a competitive social encounter and reveal critical neurobiological mechanisms underlying individual differences in the predisposition to win or lose a social competition. Our findings establish a key role for mitochondrial function in the NAc in the attainment of social dominance. Although mitochondrial involvement has been demonstrated in different mental health conditions, including anxiety disorders, stress and depression (10–12, 31, 32), our findings go beyond and establish a role for mitochondrial function in the regulation of individual differences in social behaviors under normal, nonpathological conditions.
First, following evidence in humans linking interindividual differences in anxiety with social status (7) and competitive self-confidence under stress (8), we showed that high-anxious rats tend to become subordinate when confronted with low-anxious rats. Importantly, the anxiety phenotype was reliably established through two validated tests for anxiety in rodents, and the pervasive impact of anxiety on social competition was confirmed through repeated social encounters (Fig. 1G). As the competing rats were matched for age, size, gender, and social experience, our findings suggested a role for intrinsic differences in neural mechanisms involved in social competition between high- and low-anxious individuals.
In our search for key brain regions involved in social hierarchy establishment, we obtained evidence for the involvement of the NAc. First, we found a prominent activation of the NAc, particularly D1-containing cells, by social competition as highlighted through the assessment of the immediate early genes zif-268 and cFOS. Then, pharmacological inactivation of the NAc indicated the causal involvement of this brain region in social dominance. These findings are in line with previous evidence from lesion (33), neurochemical (34), and pharmacological (35) studies in rodents that highlight a causal involvement of the NAc in the development and/or expression of social dominance, as well as with data from human neuroimaging studies that show NAc activation under tasks involving social competition (21) or manipulation of social status (19, 20). Moreover, our findings are in agreement with current views that construe a critical role of the NAc in social operations, ranging from the processing of social information, to learning about conspecifics and making socially influenced decisions (36).
Additionally, the NAc has been classically implicated in different aspects of behavioral activation, including motivation, exertion of effort during instrumental behavior, reward-seeking and energy expenditure, as well as “vigor” of behavior (37–41). The NAc involvement seems to be particularly critical when there is ambiguity and/or uncertainty about which are the appropriate actions to be taken or under situations of instability and fluidity (42). Neural computations underlying these processes are all conceivably relevant for the evolving interactions entailed in the establishment of a social hierarchy. Importantly, a priori differences in motivation to win the social context cannot explain the outcome of the social competition between high- and low-anxious rats, as no differences in competitive behavior were observed during the early stages of the contest (Fig. 1H). Instead, the divergent ranks emerged progressively throughout the competition, indicating the potential for differential underlying metabolic capacity in the NAc.
Specifically, the key role for bioenergetics in the NAc in the differential predisposition of high- and low-anxious animals to win a social competition was identified by a number of converging data. Initial evidence in support of this concept was found in the microarray data indicating enrichment for metabolic and mitochondrial genes among those differentially expressed in the NAc between high- and low-anxious rats. Subsequently, data demonstrating lower complex I and II protein levels, mitochondrial respiration and ATP, but higher ROS products in the NAc of high anxious rats in comparison with low anxious counterparts further supported a link between variation in NAc bioenergetics and the attained social rank. The reduction in mitochondrial function was not simply a result of reduced mitochondrial number, as both high- and low-anxious rats exhibited similar mitochondrial number and densities in the NAc. Importantly, when we pharmacologically manipulated NAc mitochondrial respiration, we could directly influence social rank, which strongly supports the causal implication of differential anxiety-related NAc bioenergetics in the emergence of a dominance hierarchy. Inhibition of either complex I or complex II in the NAc markedly reduced social rank, whereas the competitive disadvantage of high-anxious animals to achieve a dominant rank against a low anxious male could be overcome by intra-NAc infusion of the mitochondrial booster vitamin B3 (NAM). Control experiments excluded that the observed differences in social dominance between animals differing in anxiety and those impinged by the pharmacological treatments were not due to broad alterations in social behavior or confounded by perturbations in locomotion or general behavior. Nutritional interventions, such as vitamin B3, have previously been proposed as therapeutics for depression and age-related neurodegenerative diseases (43, 44). Our results attribute a previously unidentified role for vitamin B3 as a potential target to deal with anxiety-related deficits in competitiveness. However, pharmacological administration of the mitochondrial antioxidant MitoQ was ineffective to reverse the competitive disadvantage of high-anxious animals. Although these data are not supportive for a role of ROS in the subordinate outcome of high-anxious rats, we cannot discard the effectiveness of other antioxidant administration regimes (i.e., other administration timing, doses, and/or chronicity). Interestingly, although there is increasing evidence linking differences in mitochondrial function and oxidative stress with clinical pathologies such as anxiety disorders (31, 45–47), here we go beyond the pathological state and show that differences in brain mitochondrial function in the NAc can be found in association with personality differences.
In our study, several control experiments targeting the BLA suggested that the observed bioenergetic effects on social competition are specific to the NAc. Although these findings might seem surprising given the classical view of the BLA as a regulatory center for anxiety, anxiety modulation by the BLA has now been demonstrated to take place at its outputs, rather than within the BLA itself (reviewed in ref. 48). Given the proposed roles of the NAc in the regulation of behavioral vigor and exertion of effort, particularly in appetitively or aversely motivated behaviors (42), it is now tempting to speculate that the detected differences in mitochondrial function in the NAc as a function of anxiety could be critically involved in the decision-making processes weighing the amount of effort and/or cost exerted against the tradeoff of the prospect to win or, more generally, in defining differences in vulnerability to persist during sustained challenge.
The fact that suboptimal mitochondrial function in high-anxious rats was found in the NAc and not the BLA, suggests that the differences in the NAc could be secondary in this brain region and not a generalized mitochondrial dysfunction. Among the possible mechanisms involved, it is tempting to consider the participation of the dopaminergic system as several studies have linked high dopamine levels with reduced mitochondrial capacity in cells (49, 50). As we found activation of D1-containing cells following social competition, it is possible that differences in dopamine, as a function of anxiety, could contribute to the observed differences in mitochondrial function.
Substantial evidence implicates the NAc in depression (51, 52) and “anergia,” or lack of energy at the core of depression and anxiety disorders (53, 54). Furthermore, mitochondrial deficiency is frequently observed in brain disorders (55), including depression (13, 14, 26, 31, 56) and anxiety (9). Moreover, individuals with mitochondrial disorders often express symptoms of depression and anxiety (10–13). High anxiety trait is a known vulnerability factor to develop depression, particularly if individuals are exposed to stress (57, 58), with stress impinging energetically costly neuronal adaptations (59, 60). Limited energy production due to reduced mitochondrial function may impair adaptive neuronal capacity to life challenges (32) and contribute to the development of psychopathologies (9, 10, 13, 14). Therefore, our results highlight differences in mitochondrial function in the NAc as a potential mechanism underlying the susceptibility or resilience to develop depression, and may open new prospects for the advancement of preventive therapeutic approaches to mood disorders.
Materials and Methods
Adult male Wistar rats (Charles River) weighing 250–275 g at the start of experiments were used. All experiments were performed with the approval of the Cantonal Veterinary Authorities (Vaud, Switzerland) and carried out in accordance with the European Communities Council Directive of 24 November 1986 (86/609EEC). Detailed descriptions of animal housing conditions, statistical analyses, and experimental methods are provided in the SI Appendix, SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank J. Grosse, I. Guillot de Suduiraut, K. Meng, E. Schranz, A. Thampi, S. Baliyan, E. T. Batzianouli, C. Maclachlan, and G. Knott for experimental assistance; and Drs. M. Kabbaj and M. Murphy for their generous gifts of the zif268 plasmid and mitoQ, respectively. This work was supported by grants from the Swiss National Science Foundation (31003A-152614; NCCR Synapsy) and intramural funding from the École Polytechnique Fédérale de Lausanne.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1512653112/-/DCSupplemental.
References
- 1.Sapolsky RM. The influence of social hierarchy on primate health. Science. 2005;308(5722):648–652. doi: 10.1126/science.1106477. [DOI] [PubMed] [Google Scholar]
- 2.Neumann ID, Veenema AH, Beiderbeck DI. Aggression and anxiety: Social context and neurobiological links. Front Behav Neurosci. 2010;4:12. doi: 10.3389/fnbeh.2010.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fuxjager MJ, et al. Winning territorial disputes selectively enhances androgen sensitivity in neural pathways related to motivation and social aggression. Proc Natl Acad Sci USA. 2010;107(27):12393–12398. doi: 10.1073/pnas.1001394107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Oliveira RF, Silva A, Canario AV. Why do winners keep winning? Androgen mediation of winner but not loser effects in cichlid fish. Proc Biol Sci. 2009;276(1665):2249–2256. doi: 10.1098/rspb.2009.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyce WT. Social stratification, health, and violence in the very young. Ann N Y Acad Sci. 2004;1036:47–68. doi: 10.1196/annals.1330.003. [DOI] [PubMed] [Google Scholar]
- 6.Allan S, Gilbert P. Submissive behaviour and psychopathology. Br J Clin Psychol. 1997;36(Pt 4):467–488. doi: 10.1111/j.2044-8260.1997.tb01255.x. [DOI] [PubMed] [Google Scholar]
- 7.Gilbert P, McEwan K, Bellew R, Mills A, Gale C. The dark side of competition: How competitive behaviour and striving to avoid inferiority are linked to depression, anxiety, stress and self-harm. Psychol Psychother. 2009;82(Pt 2):123–136. doi: 10.1348/147608308X379806. [DOI] [PubMed] [Google Scholar]
- 8.Goette L, Bendahan S, Thoresen J, Hollis F, Sandi C. Stress pulls us apart: Anxiety leads to differences in competitive confidence under stress. Psychoneuroendocrinology. 2015;54:115–123. doi: 10.1016/j.psyneuen.2015.01.019. [DOI] [PubMed] [Google Scholar]
- 9.Tyrka AR, et al. Alterations of mitochondrial DNA copy number and telomere length with early adversity and psychopathology. Biol Psychiatry. 2015 doi: 10.1016/j.biopsych.2014.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Streck EL, et al. Mitochondria and the central nervous system: Searching for a pathophysiological basis of psychiatric disorders. Rev Bras Psiquiatr. 2014;36(2):156–167. doi: 10.1590/1516-4446-2013-1224. [DOI] [PubMed] [Google Scholar]
- 11.Morava E, Kozicz T. Mitochondria and the economy of stress (mal)adaptation. Neurosci Biobehav Rev. 2013;37(4):668–680. doi: 10.1016/j.neubiorev.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 12.Anglin RE, Mazurek MF, Tarnopolsky MA, Rosebush PI. The mitochondrial genome and psychiatric illness. Am J Med Genet B Neuropsychiatr Genet. 2012;159B(7):749–759. doi: 10.1002/ajmg.b.32086. [DOI] [PubMed] [Google Scholar]
- 13.Morava E, et al. Depressive behaviour in children diagnosed with a mitochondrial disorder. Mitochondrion. 2010;10(5):528–533. doi: 10.1016/j.mito.2010.05.011. [DOI] [PubMed] [Google Scholar]
- 14.Koene S, et al. Major depression in adolescent children consecutively diagnosed with mitochondrial disorder. J Affect Disord. 2009;114(1-3):327–332. doi: 10.1016/j.jad.2008.06.023. [DOI] [PubMed] [Google Scholar]
- 15.Wang F, et al. Bidirectional control of social hierarchy by synaptic efficacy in medial prefrontal cortex. Science. 2011;334(6056):693–697. doi: 10.1126/science.1209951. [DOI] [PubMed] [Google Scholar]
- 16.Beiderbeck DI, et al. High and abnormal forms of aggression in rats with extremes in trait anxiety--involvement of the dopamine system in the nucleus accumbens. Psychoneuroendocrinology. 2012;37(12):1969–1980. doi: 10.1016/j.psyneuen.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 17.Kumaran D, Melo HL, Duzel E. The emergence and representation of knowledge about social and nonsocial hierarchies. Neuron. 2012;76(3):653–666. doi: 10.1016/j.neuron.2012.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex, or hippocampal formation lesions on established social relationships in rhesus monkeys (Macaca mulatta) Behav Neurosci. 2006;120(4):761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- 19.Zink CF, et al. Know your place: Neural processing of social hierarchy in humans. Neuron. 2008;58(2):273–283. doi: 10.1016/j.neuron.2008.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ly M, Haynes MR, Barter JW, Weinberger DR, Zink CF. Subjective socioeconomic status predicts human ventral striatal responses to social status information. Curr Biol. 2011;21(9):794–797. doi: 10.1016/j.cub.2011.03.050. [DOI] [PubMed] [Google Scholar]
- 21.Le Bouc R, Pessiglione M. Imaging social motivation: Distinct brain mechanisms drive effort production during collaboration versus competition. J Neurosci. 2013;33(40):15894–15902. doi: 10.1523/JNEUROSCI.0143-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wilensky AE, Schafe GE, LeDoux JE. Functional inactivation of the amygdala before but not after auditory fear conditioning prevents memory formation. J Neurosci. 1999;19(24):RC48. doi: 10.1523/JNEUROSCI.19-24-j0006.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Le Moine C, Bloch B. D1 and D2 dopamine receptor gene expression in the rat striatum: Sensitive cRNA probes demonstrate prominent segregation of D1 and D2 mRNAs in distinct neuronal populations of the dorsal and ventral striatum. J Comp Neurol. 1995;355(3):418–426. doi: 10.1002/cne.903550308. [DOI] [PubMed] [Google Scholar]
- 24.Nicholls DG, Budd SL. Mitochondria and neuronal survival. Physiol Rev. 2000;80(1):315–360. doi: 10.1152/physrev.2000.80.1.315. [DOI] [PubMed] [Google Scholar]
- 25.Cheng A, Hou Y, Mattson MP. Mitochondria and neuroplasticity. ASN Neuro. 2010;2(5):e00045. doi: 10.1042/AN20100019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Manji H, et al. Impaired mitochondrial function in psychiatric disorders. Nat Rev Neurosci. 2012;13(5):293–307. doi: 10.1038/nrn3229. [DOI] [PubMed] [Google Scholar]
- 27.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11(1):81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 28.Houtkooper RH, Cantó C, Wanders RJ, Auwerx J. The secret life of NAD+: An old metabolite controlling new metabolic signaling pathways. Endocr Rev. 2010;31(2):194–223. doi: 10.1210/er.2009-0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nowak R, et al. Effect of selected NAD+ analogues on mitochondria activity and proliferation of endothelial EA.hy926 cells. Eur J Pharmacol. 2010;640(1-3):102–111. doi: 10.1016/j.ejphar.2010.04.027. [DOI] [PubMed] [Google Scholar]
- 30.Murphy MP, Smith RA. Targeting antioxidants to mitochondria by conjugation to lipophilic cations. Annu Rev Pharmacol Toxicol. 2007;47:629–656. doi: 10.1146/annurev.pharmtox.47.120505.105110. [DOI] [PubMed] [Google Scholar]
- 31.Gardner A, Boles RG. Beyond the serotonin hypothesis: Mitochondria, inflammation and neurodegeneration in major depression and affective spectrum disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(3):730–743. doi: 10.1016/j.pnpbp.2010.07.030. [DOI] [PubMed] [Google Scholar]
- 32.Picard M, Juster RP, McEwen BS. Mitochondrial allostatic load puts the ‘gluc’ back in glucocorticoids. Nat Rev Endocrinol. 2014;10(5):303–310. doi: 10.1038/nrendo.2014.22. [DOI] [PubMed] [Google Scholar]
- 33.Fantin G, Bottecchia D. Effect of nucleus accumbens destruction in rat. Experientia. 1984;40(6):573–575. doi: 10.1007/BF01982337. [DOI] [PubMed] [Google Scholar]
- 34.Anstrom KK, Miczek KA, Budygin EA. Increased phasic dopamine signaling in the mesolimbic pathway during social defeat in rats. Neuroscience. 2009;161(1):3–12. doi: 10.1016/j.neuroscience.2009.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Puciłowski O, Trzaskowska E, Kostowski W, Wośko W. Inhibition of affective aggression and dominance in rats after thyrotropin-releasing hormone (TRH) microinjection into the nucleus accumbens. Peptides. 1988;9(3):539–543. doi: 10.1016/0196-9781(88)90161-1. [DOI] [PubMed] [Google Scholar]
- 36.Bhanji JP, Delgado MR. The social brain and reward: Social information processing in the human striatum. Wiley Interdiscip Rev Cogn Sci. 2014;5(1):61–73. doi: 10.1002/wcs.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Robbins TW, Everitt BJ. A role for mesencephalic dopamine in activation: Commentary on Berridge (2006) Psychopharmacology (Berl) 2007;191(3):433–437. doi: 10.1007/s00213-006-0528-7. [DOI] [PubMed] [Google Scholar]
- 38.Beeler JA, Frazier CR, Zhuang X. Putting desire on a budget: Dopamine and energy expenditure, reconciling reward and resources. Front Integr Nuerosci. 2012;6:49. doi: 10.3389/fnint.2012.00049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Niv Y, Daw ND, Joel D, Dayan P. Tonic dopamine: Opportunity costs and the control of response vigor. Psychopharmacology (Berl) 2007;191(3):507–520. doi: 10.1007/s00213-006-0502-4. [DOI] [PubMed] [Google Scholar]
- 40.Salamone JD, Correa M, Nunes EJ, Randall PA, Pardo M. The behavioral pharmacology of effort-related choice behavior: Dopamine, adenosine and beyond. J Exp Anal Behav. 2012;97(1):125–146. doi: 10.1901/jeab.2012.97-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Treadway MT, Bossaller NA, Shelton RC, Zald DH. Effort-based decision-making in major depressive disorder: A translational model of motivational anhedonia. J Abnorm Psychol. 2012;121(3):553–558. doi: 10.1037/a0028813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Floresco SB. The nucleus accumbens: An interface between cognition, emotion, and action. Annu Rev Psychol. 2015;66:25–52. doi: 10.1146/annurev-psych-010213-115159. [DOI] [PubMed] [Google Scholar]
- 43.Bodnar LM, Wisner KL. Nutrition and depression: Implications for improving mental health among childbearing-aged women. Biol Psychiatry. 2005;58(9):679–685. doi: 10.1016/j.biopsych.2005.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Liu D, et al. Nicotinamide forestalls pathology and cognitive decline in Alzheimer mice: Evidence for improved neuronal bioenergetics and autophagy procession. Neurobiol Aging. 2013;34(6):1564–1580. doi: 10.1016/j.neurobiolaging.2012.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Einat H, Yuan P, Manji HK. Increased anxiety-like behaviors and mitochondrial dysfunction in mice with targeted mutation of the Bcl-2 gene: Further support for the involvement of mitochondrial function in anxiety disorders. Behav Brain Res. 2005;165(2):172–180. doi: 10.1016/j.bbr.2005.06.012. [DOI] [PubMed] [Google Scholar]
- 46.Hovatta I, et al. Glyoxalase 1 and glutathione reductase 1 regulate anxiety in mice. Nature. 2005;438(7068):662–666. doi: 10.1038/nature04250. [DOI] [PubMed] [Google Scholar]
- 47.Rammal H, Bouayed J, Younos C, Soulimani R. Evidence that oxidative stress is linked to anxiety-related behaviour in mice. Brain Behav Immun. 2008;22(8):1156–1159. doi: 10.1016/j.bbi.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 48.Lalumiere RT. Optogenetic dissection of amygdala functioning. Front Behav Neurosci. 2014;8:107. doi: 10.3389/fnbeh.2014.00107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rosenfeld M, Brenner-Lavie H, Ari SG, Kavushansky A, Ben-Shachar D. Perturbation in mitochondrial network dynamics and in complex I dependent cellular respiration in schizophrenia. Biol Psychiatry. 2011;69(10):980–988. doi: 10.1016/j.biopsych.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 50.Brenner-Lavie H, et al. Dopamine modulates mitochondrial function in viable SH-SY5Y cells possibly via its interaction with complex I: Relevance to dopamine pathology in schizophrenia. Biochim Biophys Acta. 2008;1777(2):173–185. doi: 10.1016/j.bbabio.2007.10.006. [DOI] [PubMed] [Google Scholar]
- 51.Nestler EJ, Carlezon WA., Jr The mesolimbic dopamine reward circuit in depression. Biol Psychiatry. 2006;59(12):1151–1159. doi: 10.1016/j.biopsych.2005.09.018. [DOI] [PubMed] [Google Scholar]
- 52.Chaudhury D, et al. Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature. 2013;493(7433):532–536. doi: 10.1038/nature11713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Stahl SM. The psychopharmacology of energy and fatigue. J Clin Psychiatry. 2002;63(1):7–8. doi: 10.4088/jcp.v63n0102. [DOI] [PubMed] [Google Scholar]
- 54.Demyttenaere K, De Fruyt J, Stahl SM. The many faces of fatigue in major depressive disorder. Int J Neuropsychopharmacol. 2005;8(1):93–105. doi: 10.1017/S1461145704004729. [DOI] [PubMed] [Google Scholar]
- 55.Lin MT, Beal MF. Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature. 2006;443(7113):787–795. doi: 10.1038/nature05292. [DOI] [PubMed] [Google Scholar]
- 56.Chang CC, Jou SH, Lin TT, Liu CS. Mitochondrial DNA variation and increased oxidative damage in euthymic patients with bipolar disorder. Psychiatry Clin Neurosci. 2014;68(7):551–557. doi: 10.1111/pcn.12163. [DOI] [PubMed] [Google Scholar]
- 57.Sandi C, Richter-Levin G. From high anxiety trait to depression: A neurocognitive hypothesis. Trends Neurosci. 2009;32(6):312–320. doi: 10.1016/j.tins.2009.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Castro JE, et al. Personality traits in rats predict vulnerability and resilience to developing stress-induced depression-like behaviors, HPA axis hyper-reactivity and brain changes in pERK1/2 activity. Psychoneuroendocrinology. 2012;37(8):1209–1223. doi: 10.1016/j.psyneuen.2011.12.014. [DOI] [PubMed] [Google Scholar]
- 59.de Kloet ER, Joëls M, Holsboer F. Stress and the brain: From adaptation to disease. Nat Rev Neurosci. 2005;6(6):463–475. doi: 10.1038/nrn1683. [DOI] [PubMed] [Google Scholar]
- 60.McEwen BS. Brain on stress: How the social environment gets under the skin. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17180–17185. doi: 10.1073/pnas.1121254109. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.