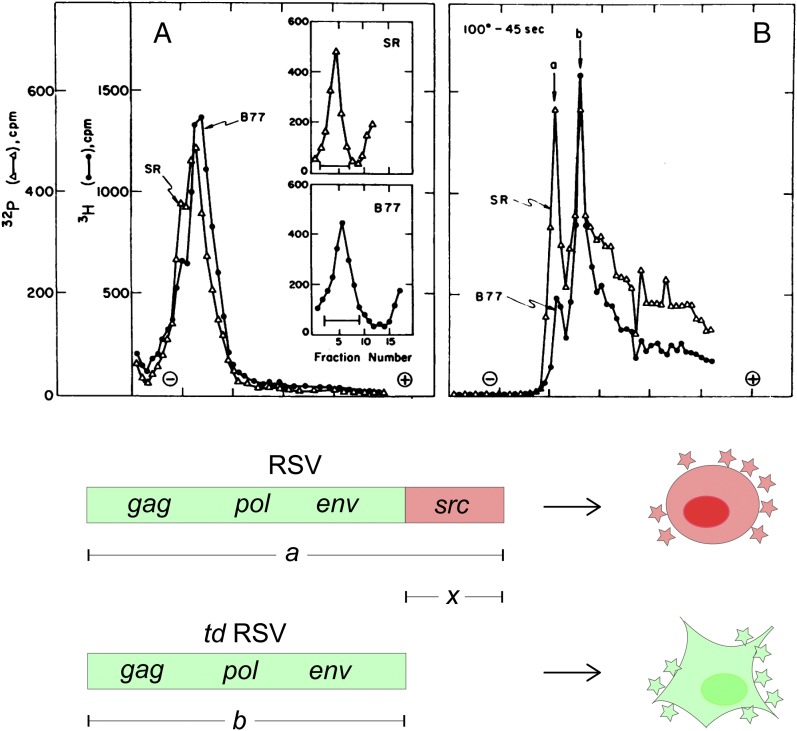
In their classic paper on the identification of the transforming principle of Rous sarcoma virus (RSV) published 1970 in PNAS (1), Peter Duesberg at the University of California, Berkeley, and Peter Vogt, then at the University of Washington, Seattle, drew a seemingly simple yet groundbreaking conclusion. When they analyzed the genomic RNAs of transforming, acutely oncogenic RSV and of transformation-defective (td) mutant derivatives, they found that all transforming virus stocks contained two classes of RNA subunits, a larger one (a) and a smaller one (b), whereas the nontransforming yet replication-competent mutants contained the smaller b subunits only. Duesberg and Vogt concluded that the larger a subunit contained the transforming principle of RSV. Based on this and on subsequent structural comparisons of the a and b subunits of biologically cloned viruses, the transforming principle was defined by the remarkably simple equation a − b = x and was later termed src (for sarcoma). The first biochemical identification of a cancer gene was achieved, initially in a chicken virus. However, the principal proof of a physical underpinning of the cancer gene hypothesis had tremendous impact on a fundamental challenge of medicine, decoding the molecular basis of human carcinogenesis.
The genetic and biochemical investigations of the chicken tumor virus RSV and the persistent search for its transforming principle are a classic paradigm in cellular and molecular cancer research (2, 3). In 1911, Peyton Rous at the Rockefeller Institute in New York discovered the first virus—later termed RSV—that could induce solid tumors in infected fowl, demonstrated by experimental transmission of sarcomas using cell-free filtrates of tumor extracts (4). This seminal discovery started the field of tumor virology (2, 3, 5). However, almost half a century had to pass before the first quantitative biological tools were developed to study the biology of RSV and its interaction with infected cells in detail. RSV is capable of transforming primary chicken embryo fibroblasts in culture, and the focus assay developed in 1958 by Howard Temin and Harry Rubin at the California Institute of Technology allowed a quantitative assessment of the virus–cell interaction leading to malignant cell transformation (6). The next crucial steps toward the identification of the underlying principle of RSV oncogenicity were based on classic genetics. The characterization of various viral strains that induced different morphologies of transformed cells suggested that the phenotype of the cancer cell is controlled by the incoming genetic information carried by the viral genome. The isolation of RSV mutants that can transform cells but do not produce infectious progeny, or vice versa, can replicate but have lost cell transforming capacity, demonstrated that viral replication and oncogenicity are genetically separable, independent functions of RSV (2, 3). A groundbreaking leap forward came from studies of conditional mutants (7, 8). In 1970, Steve Martin at the University of California, Berkeley isolated a temperature-sensitive mutant of RSV that did not transform cells at the nonpermissive temperature but replicated normally, indicating the existence of a viral gene that is necessary for cell transformation but dispensable for replication (8).
In the same year, a marvelous synergistic effort of biochemistry and virus genetics led to the first physical identification of an oncogene, reported in the classic paper by Duesberg and Vogt in PNAS (1). Their biochemical approach in the hunt for the transforming principle made use of the availability of td deletion mutants of RSV and of nontransforming viruses associated with avian sarcoma or leukemia viruses (2). In essence, the experimental design involved coelectrophoresis of viral RNAs from transforming and nontransforming avian retroviruses, including various strains of RSV and td or associated viruses. Notably, current nucleic acid technologies, like reverse transcription, blotting, cloning, or sequencing, were not yet established in those days. All of this had to be done by metabolic labeling of infected cell cultures with radiolabeled precursors ([3H]uridine or [32P]H3PO4), purification of viruses, extraction of viral RNA, polyacrylamide gel electrophoresis, gel slicing, and scintillation counting of 1-mm gel slices. The results were as clear as compelling. After heat dissociation, the 60–70S RNA complexes of all viruses able to transform chicken embryo fibroblasts resolved into two types of RNA species at variable ratios: a large a subunit and a smaller b subunit (Fig. 1). Nontransforming yet replication-competent viruses always contained b subunits only. It was concluded that the presence of genetic material in the a subunit, which is absent from the b subunit, is responsible for the oncogenic capacity of RSV. Final proof that a and b are structurally related by the equation a = b + x, and that x is indeed a contiguous segment near the 3′ end of RSV RNA (Fig. 1), came from comparative mapping of the genomes of transforming viruses, their td derivatives, and other gene-specific deletion mutants. The biochemical mapping used 2D electrophoresis-homochromatography of 32P-labeled RNase T1-resistant oligonucleotides and was done in collaborations of the Duesberg laboratory with Peter Vogt, then at the University of Southern California, Los Angeles, and with Hidesaburo Hanafusa at the Rockefeller University (9–11).
Fig. 1.
Biochemical definition of src, the first oncogene. Panels A and B, above, are from the original PNAS paper by Duesberg and Vogt (1). They show electropherograms of the 60–70S RNAs from two transforming strains of RSV, Schmidt-Ruppin (SR) and B77, before (A) and after (B) heat-dissociation. Insets in A show the final sucrose gradient purification of the RNAs before electrophoretic analysis. The heat-dissociated RNAs were resolved into two subunits with lower (a) and higher (b) electrophoretic mobility. Analyses of biologically cloned viruses revealed that the larger subunit represents the genomic RNA of transforming RSV, whereas the b subunit is the genome of transformation-defective (td) mutants spontaneously segregating from RSV (1, 10). Subsequent mapping studies (10, 11) confirmed that the genomes of RSV and of td mutants share all replicative genes (gag, pol, env) and that the size difference (a − b = x) is caused by the additional src gene at the 3′ end of the RSV genome. Cells infected by RSV become transformed (indicated by rounding) and produce virus progeny (red star symbols), whereas td RSV replicates (green star symbols) but does not transform the host cell. A and B reproduced with permission from ref. 1.
In 1976, pioneering experiments performed in the laboratory of Harold Varmus and Mike Bishop at the University of California, San Francisco, in collaboration with Peter Vogt at the University of Southern California, changed the whole field of tumor virology and cancer genetics (12). Their finding—that the src gene of RSV (v-src) is in fact a transduced allele of a cellular gene (c-src) picked up by recombination during the retroviral life cycle—is one of the most influential discoveries in cancer research. It immediately converted the purely virological matter of oncogenes to a cellular one, relevant for all animals and man, as was quickly shown by the identification of c-src in many species. Principally, any activating mutation or deregulation of cellular oncogenes, also termed proto-oncogenes in their normal nonmutated form, could now lead to cancer, with or without viral involvement. The experimental design for the discovery of c-src exploited the availability of reverse transcription and the definition of v-src by the size difference (a − b = x) of transforming and td RSV genomes (Fig. 1; and see above). Synthesis of RSV cDNA and subtractive hybridization with td RNA led to a src-specific DNA probe that was used for annealing experiments showing that normal cells contain sequences closely related to src in their genomic DNA (12). Subsequent reports on the experimental recovery of transforming viruses by recombination of td RSV carrying partial v-src deletions with cellular sequences corroborated the close v-src/c-src relationship (13). For the landmark discovery of the cellular origin of retroviral oncogenes, Bishop and Varmus were awarded the Nobel Prize in Physiology or Medicine in 1989. Following the identification of v-src and c-src, the immunological detection and characterization of the src protein product as a tyrosine-specific protein kinase with modular protein interaction domains were further groundbreaking discoveries (14–16). Moreover, the src paradigm immediately stimulated the search for the transforming principle of other highly oncogenic avian retroviruses. In two studies from the Vogt and Duesberg laboratories, also published in PNAS in 1977 and 1979, analyses of the genomes of avian acute leukemia viruses MC29 and avian erythroblastosis virus, using the biochemical approach described above, led to the discovery of specific sequences unrelated to replicative genes or to the prototypic src oncogene (17, 18). These novel oncogenes were later shown to be derived from cellular oncogenes, which today are known as major drivers of human cancer, MYC, and the ERBB/EGFR gene, respectively (2). While src, myc, and erbB were originally discovered in avian tumor viruses (2, 19), other prominent oncogenes, like ras, were identified in murine tumor viruses or in independent seminal experiments by direct transfection of human tumor cell DNA into recipient cells (2, 20).
Having spent postdoctoral time both in the Vogt and Duesberg laboratories right at the time when all of this was happening, I can vividly recall the exciting, almost adventurous spirit of the oncogene discovery days. Particularly stimulating were the joint informal meetings of the Vogt, Duesberg, and Bishop/Varmus groups held at alternating California laboratory sites, where ideas, strategies, and results were freely exchanged and crucial collaborations initiated. From the pioneering discovery of the first oncogene in a chicken virus, oncogene research has developed into a central topic in human cancer genetics. Several oncogenes originally identified in retroviruses are now recognized as major drivers in human cancers, and drugs targeted at specific oncogene functions are used in cancer therapy (2). Furthermore, many proto-oncogenes are essential genes involved in fundamental processes in normal cells, like growth, metabolism, or differentiation. Oncogenes and proto-oncogenes will remain in the focus of biology, biochemistry, and medicine.
Footnotes
The author declares no conflict of interest.
This article is part of the special series of PNAS 100th Anniversary articles to commemorate exceptional research published in PNAS over the last century. See the companion article, “Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses” on page 1673 in issue 4 of volume 67.
References
- 1.Duesberg PH, Vogt PK. Differences between the ribonucleic acids of transforming and nontransforming avian tumor viruses. Proc Natl Acad Sci USA. 1970;67(4):1673–1680. doi: 10.1073/pnas.67.4.1673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vogt PK. Retroviral oncogenes: A historical primer. Nat Rev Cancer. 2012;12(9):639–648. doi: 10.1038/nrc3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martin GS. The road to Src. Oncogene. 2004;23(48):7910–7917. doi: 10.1038/sj.onc.1208077. [DOI] [PubMed] [Google Scholar]
- 4.Rous P. A sarcoma of the fowl transmissible by an agent separable from the tumor cells. J Exp Med. 1911;13(4):397–411. doi: 10.1084/jem.13.4.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Weiss RA, Vogt PK. 100 years of Rous sarcoma virus. J Exp Med. 2011;208(12):2351–2355. doi: 10.1084/jem.20112160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Temin HM, Rubin H. Characteristics of an assay for Rous sarcoma virus and Rous sarcoma cells in tissue culture. Virology. 1958;6(3):669–688. doi: 10.1016/0042-6822(58)90114-4. [DOI] [PubMed] [Google Scholar]
- 7.Toyoshima K, Vogt PK. Temperature sensitive mutants of an avian sarcoma virus. Virology. 1969;39(4):930–931. doi: 10.1016/0042-6822(69)90030-0. [DOI] [PubMed] [Google Scholar]
- 8.Martin GS. Rous sarcoma virus: A function required for the maintenance of the transformed state. Nature. 1970;227(5262):1021–1023. doi: 10.1038/2271021a0. [DOI] [PubMed] [Google Scholar]
- 9.Lai MM, Duesberg PH, Horst J, Vogt PK. Avian tumor virus RNA: A comparison of three sarcoma viruses and their transformation-defective derivatives by oligonucleotide fingerprinting and DNA-RNA hybridization. Proc Natl Acad Sci USA. 1973;70(8):2266–2270. doi: 10.1073/pnas.70.8.2266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang LH, Duesberg P, Beemon K, Vogt PK. Mapping RNase T1-resistant oligonucleotides of avian tumor virus RNAs: Sarcoma-specific oligonucleotides are near the poly(A) end and oligonucleotides common to sarcoma and transformation-defective viruses are at the poly(A) end. J Virol. 1975;16(4):1051–1070. doi: 10.1128/jvi.16.4.1051-1070.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang LH, Duesberg PH, Kawai S, Hanafusa H. Location of envelope-specific and sarcoma-specific oligonucleotides on RNA of Schmidt-Ruppin Rous sarcoma virus. Proc Natl Acad Sci USA. 1976;73(2):447–451. doi: 10.1073/pnas.73.2.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stehelin D, Varmus HE, Bishop JM, Vogt PK. DNA related to the transforming gene(s) of avian sarcoma viruses is present in normal avian DNA. Nature. 1976;260(5547):170–173. doi: 10.1038/260170a0. [DOI] [PubMed] [Google Scholar]
- 13.Wang LH, Halpern CC, Nadel M, Hanafusa H. Recombination between viral and cellular sequences generates transforming sarcoma virus. Proc Natl Acad Sci USA. 1978;75(12):5812–5816. doi: 10.1073/pnas.75.12.5812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brugge JS, Erikson RL. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- 15.Hunter T, Sefton BM. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci USA. 1980;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sadowski I, Stone JC, Pawson T. A noncatalytic domain conserved among cytoplasmic protein-tyrosine kinases modifies the kinase function and transforming activity of Fujinami sarcoma virus P130gag-fps. Mol Cell Biol. 1986;6(12):4396–4408. doi: 10.1128/mcb.6.12.4396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Duesberg PH, Bister K, Vogt PK. The RNA of avian acute leukemia virus MC29. Proc Natl Acad Sci USA. 1977;74(10):4320–4324. doi: 10.1073/pnas.74.10.4320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bister K, Duesberg PH. Structure and specific sequences of avian erythroblastosis virus RNA: Evidence for multiple classes of transforming genes among avian tumor viruses. Proc Natl Acad Sci USA. 1979;76(10):5023–5027. doi: 10.1073/pnas.76.10.5023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bister K, Jansen HW. Oncogenes in retroviruses and cells: Biochemistry and molecular genetics. Adv Cancer Res. 1986;47:99–188. doi: 10.1016/s0065-230x(08)60199-2. [DOI] [PubMed] [Google Scholar]
- 20.Karnoub AE, Weinberg RA. Ras oncogenes: Split personalities. Nat Rev Mol Cell Biol. 2008;9(7):517–531. doi: 10.1038/nrm2438. [DOI] [PMC free article] [PubMed] [Google Scholar]