Significance
Tumor recognition by the immune system can occur spontaneously but has usually little impact on tumor growth. However, the cellular and molecular mechanisms that drive these responses could be exploited therapeutically to generate efficacious antitumor immunity. Here, we show that stimulator of IFN genes (STING), a molecule involved in cytosolic DNA sensing and required for the generation of spontaneous antitumor immune responses, can be targeted by intratumoral injection of cGAMP to boost antitumor immunity and to control tumor growth. The immune response induced by therapeutic but also spontaneous STING activation was dependent on type I IFN produced by endothelial cells in the tumor microenvironment, unraveling an unexpected role of the tumor vasculature in the initiation of spontaneous and therapeutic antitumor immunity via STING.
Keywords: STING, tumor endothelial cells, type I IFNs, CD8 T cells, antitumor immunity
Abstract
Spontaneous CD8 T-cell responses occur in growing tumors but are usually poorly effective. Understanding the molecular and cellular mechanisms that drive these responses is of major interest as they could be exploited to generate a more efficacious antitumor immunity. As such, stimulator of IFN genes (STING), an adaptor molecule involved in cytosolic DNA sensing, is required for the induction of antitumor CD8 T responses in mouse models of cancer. Here, we find that enforced activation of STING by intratumoral injection of cyclic dinucleotide GMP-AMP (cGAMP), potently enhanced antitumor CD8 T responses leading to growth control of injected and contralateral tumors in mouse models of melanoma and colon cancer. The ability of cGAMP to trigger antitumor immunity was further enhanced by the blockade of both PD1 and CTLA4. The STING-dependent antitumor immunity, either induced spontaneously in growing tumors or induced by intratumoral cGAMP injection was dependent on type I IFNs produced in the tumor microenvironment. In response to cGAMP injection, both in the mouse melanoma model and an ex vivo model of cultured human melanoma explants, the principal source of type I IFN was not dendritic cells, but instead endothelial cells. Similarly, endothelial cells but not dendritic cells were found to be the principal source of spontaneously induced type I IFNs in growing tumors. These data identify an unexpected role of the tumor vasculature in the initiation of CD8 T-cell antitumor immunity and demonstrate that tumor endothelial cells can be targeted for immunotherapy of melanoma.
Metastatic melanoma is a highly aggressive cancer with a fast increasing incidence worldwide. Unless diagnosed early and surgically resected, the disease becomes metastatic and life threatening. Both chemotherapy and irradiation are ineffective. Novel therapies that target oncogenic drivers have brought some improvements, but tumor cells escape regularly (1). Melanoma is a prototypical immunogenic tumor, as shown by the occurrence of spontaneous CD8 T-cell responses that drive tumor regressions and by the identification of CD8 T cells that recognize melanoma antigens (2, 3). Although many immunotherapeutic strategies have been developed to induce such responses, clinical efficacies have been poor. More recently, “checkpoint blockade” therapies that target T-cell–inhibitory pathways mediated by CTLA4 (4) and PD1 (5) have yielded encouraging clinical results and demonstrated that spontaneous CD8 T-cell responses in tumors can be boosted to treat melanoma.
The mechanisms that drive spontaneous antitumor immune responses are poorly understood. Type I IFNs (IFN-α and IFN-β) may play a role as the expression type I IFN-related genes in primary melanoma has been associated with spontaneous tumor regressions (6) and correlated to the tumor infiltration by specific CD8+ T cells (6, 7). Furthermore, the lack of type I IFN signaling or IFN-β expression inhibited the generation of tumor-specific CD8 T cells and accelerated tumor growth in a murine melanoma model (7–9).
Type I IFNs are typically induced upon recognition of nucleic acids in intracellular compartments. Several cytosolic DNA receptors have been identified and include DNA-dependent activator of IFN-regulatory factors (DAI) (10), gamma-interferon-inducible protein-16 (IFI16) (11), and the helicase DEAD (Asp-Glu-Ala-Asp) box protein 41 (DDX41) (12). These cytosolic receptors trigger IFN-β production via a signaling cascade that involves the master adaptor molecule stimulator of IFN genes (STING), which binds to tank-binding kinase 1 (TBK1) and induces phosphorylation of the interferon regulatory factor 3 (IRF3) (13). STING associates weakly to dsDNA (14) but strongly binds the endogenous cyclic dinucleotide GMP-AMP (cGAMP) synthesized by the cGMP-AMP synthase (cGAS) (15, 16).
Recently, spontaneous STING activation was found to be required for the spontaneous induction of antitumor immunity (17). Activation of STING occurred via tumor DNA-dependent cGAS activation and generation of endogenous cGAMP (17, 18). However, the cellular mechanism underlying this response and whether this mechanism could be exploited to generate more efficient antitumor immune responses is currently unknown.
Here, we show that intratumoral injection of exogenous cGAMP enhanced STING activation and strongly promoted the generation of antitumor CD8 T-cell responses leading to efficient growth control of injected and contralateral tumors. This response was dependent on IFN-β produced by tumor endothelial cells. In fact, endothelial cells were found to be the principal producers of type I IFN in response to both spontaneous and enforced STING activation, suggesting a role of the tumor vasculature in the initiation of antitumor immunity.
Results
Intratumoral Injection of cGAMP Promotes the Induction of CD8 T-Cell Responses and Efficiently Delays Growth of Injected Tumors.
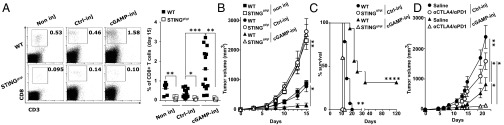
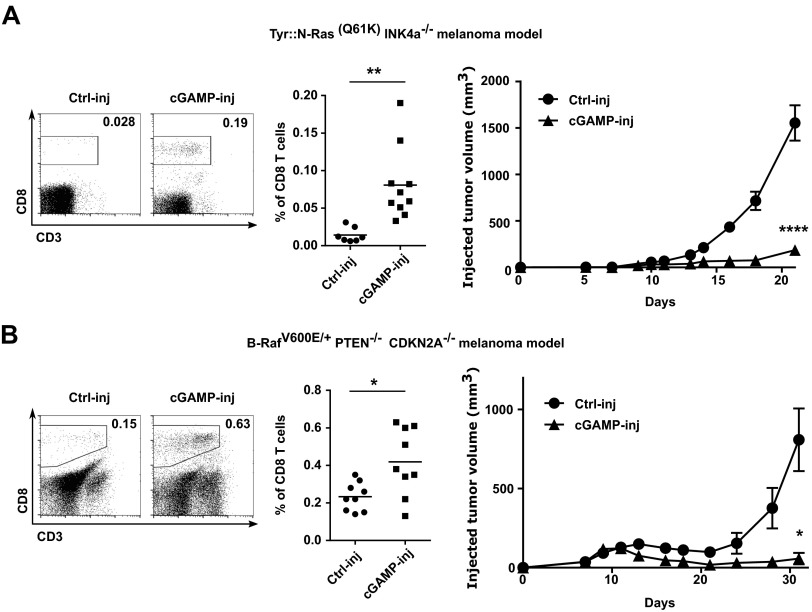
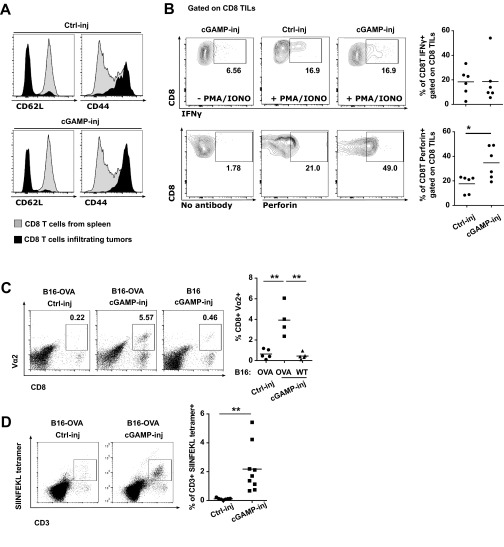
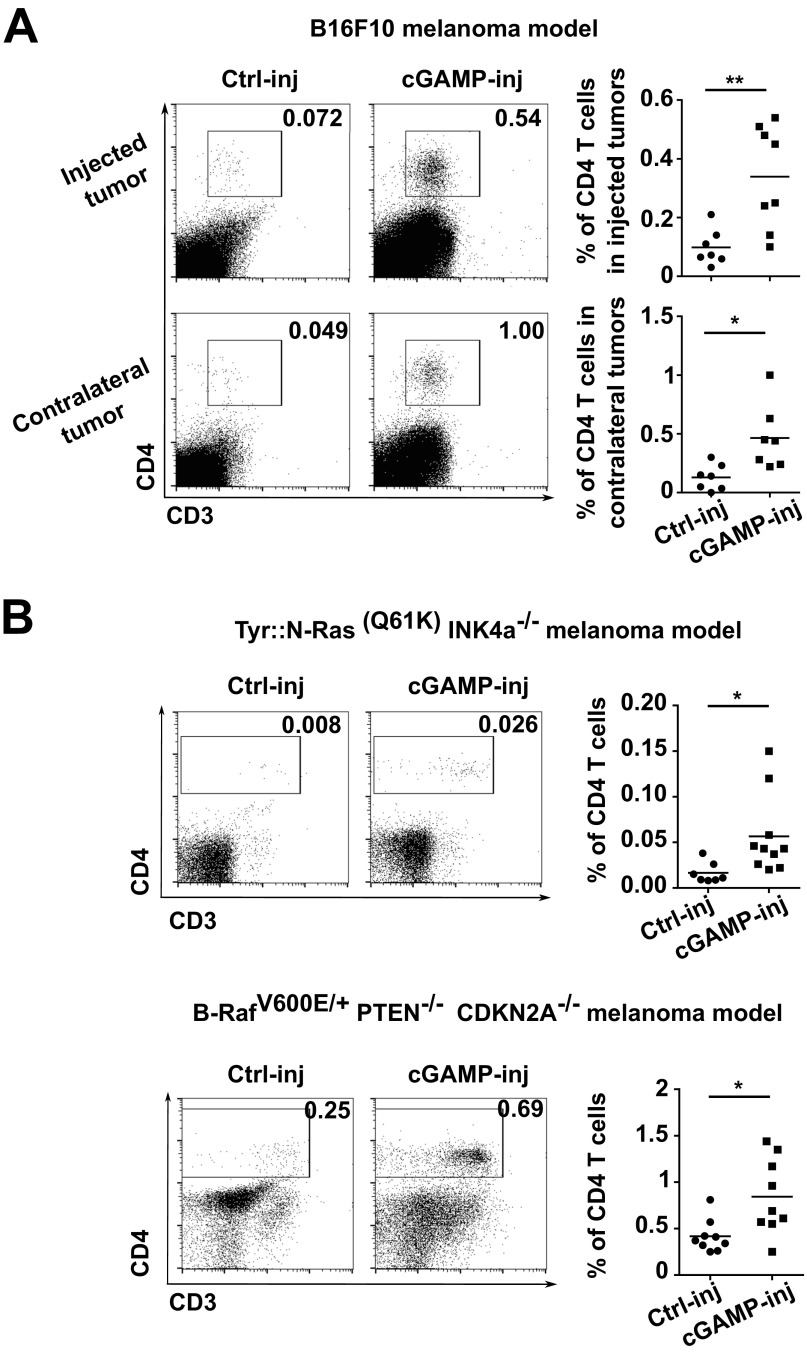
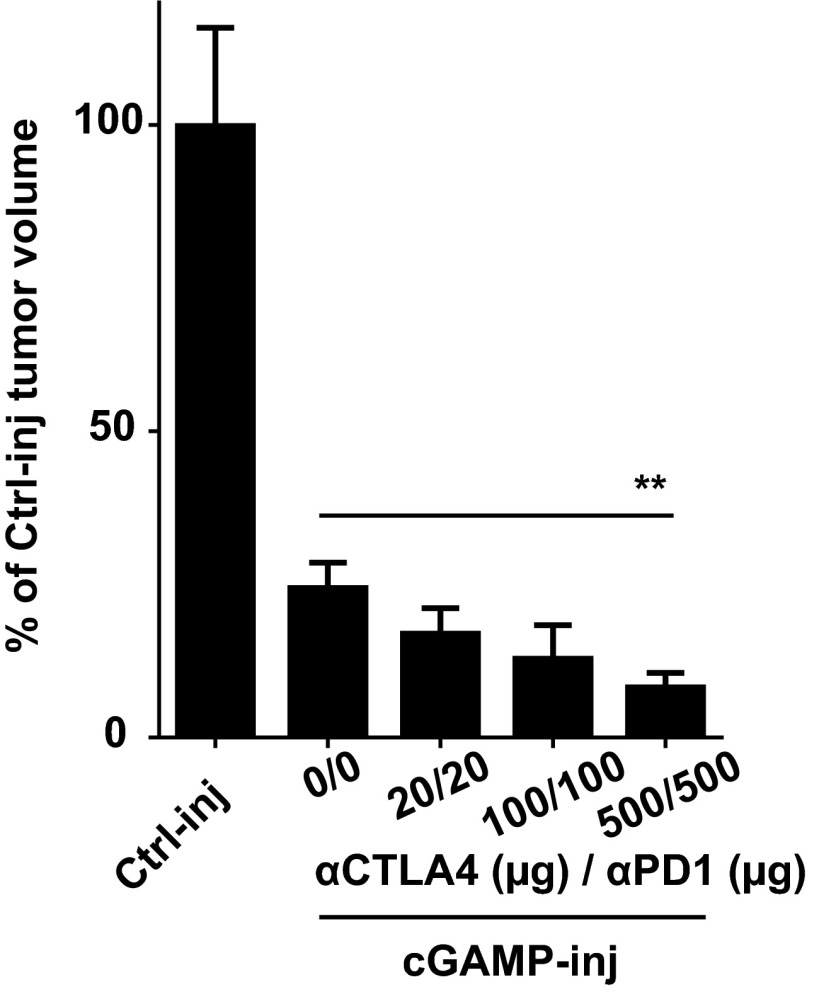
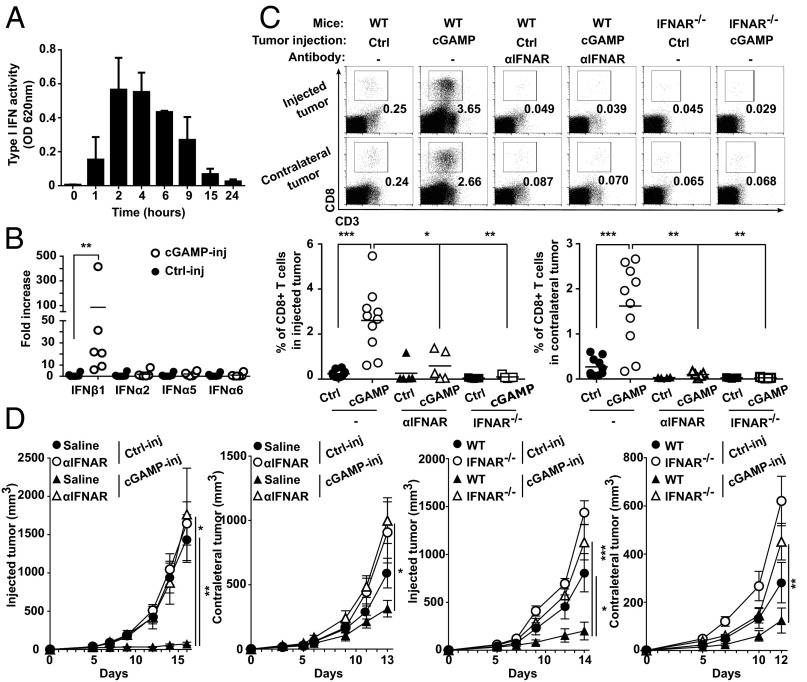
Initially, we established a mouse melanoma model to study STING-dependent induction of antitumor immunity. We engrafted B16F10 melanoma cells in wild-type mice or STING-deficient mice (STINGgt/gt) and measured tumor-infiltrating CD8 T cells as well as tumor size. CD8 T cells spontaneously infiltrating tumors were drastically reduced and tumor growth was accelerated in STINGgt/gt compared with WT mice (Fig. 1 A and B). Because this spontaneous STING-dependent CD8 T-cell response was insufficient to control the growth of tumors, we sought to investigate whether the intratumoral injection of the STING agonist cGAMP could be used therapeutically to enhance this response. We injected cGAMP into B16F10 melanoma at day 5 and day 10 after engraftment and measured CD8 T-cell in the tumors along with tumor size. Compared with control injected tumors, tumors injected with cGAMP showed a mean of fourfold increase of CD8 T cells in the tumor microenvironment (Fig. 1A) along with a significant delay in tumor growth (Fig. 1B). These effects were completely abolished in STINGgt/gt mice (Fig. 1 A and B). cGAMP also increased tumor-infiltrating CD8 T-cell numbers and delayed tumor growth when injected into engrafted melanoma derived from Tyr::N-ras(Q61K)INK4A−/− mice (19) (Fig. S1) and B-raf(V600E/+) PTEN−/− CDKN2A−/− mice (20) (Fig. S1). Tumor-infiltrating CD8 T cells displayed an effector phenotype (CD44hi CD62Llow) and were able to produce IFNγ. Compared with control-injected tumors, higher numbers of perforin-expressing CD8 T cells were found in cGAMP-injected tumors (Fig. S2) suggesting the induction of cytotoxic effector CD8 T cells. These tumor-infiltrating CD8 T cells appeared to be tumor antigen-specific as OVA specific CD8 T cells were detected in the tumors when OVA was used as a model antigen expressed by tumors (Fig. S2). Thus, intratumoral cGAMP promotes the generation of Ag-specific cytotoxic CD8 T cells that infiltrate tumors. Interestingly, intratumoral cGAMP injection also induced high numbers of infiltrating CD4 T cells in B16F10, Tyr::N-ras(Q61K)INK4A−/−, and B-raf(V600E/+) PTEN−/− CDKN2A−/− melanoma (Fig. S3). Intratumoral cGAMP improved survival of tumor-bearing mice as shown by the fact that 31% of WT mice treated with intratumoral cGAMP survived up to 3 mo, whereas control mice all died within 3 wk (Fig. 1C). The antitumor efficacy of intratumoral cGAMP was further enhanced by concomitant blockade of CTLA4 and PD1. In fact, the anti-CTLA4+anti-PD1 combo increased the antitumor activity of cGAMP in a dose-dependent manner (Fig. S4) and led to a complete inhibition of tumor growth for up to 23 d postimplantation (Fig. 1D). These data indicate that enforced STING activation by intratumoral cGAMP injection can potently increase the generation of Ag-specific effector CD8 T cells infiltrating the tumor and efficiently control its growth.
Fig. 1.
Intratumoral cGAMP promotes CD8 T-cell responses and efficiently delays growth of injected tumors. (A–C) C57BL6 or STINGgt/gt mice bearing established s.c. B16F10 tumors were either left untreated (non inj), injected intratumorally with cGAMP (cGAMP-inj), or injected with Lipofectamine alone (Ctrl-inj) at day 5 and day 10 postengraftment. Depicted are: (A) CD3+CD8+ T cells infiltrating tumors at day 15 postengraftment, measured by flow cytometry of tumor single cell suspensions. Representative plots are given (Left). Each symbol represents a separate mouse (Right). *P < 0.05, **P < 0.01, ***P < 0.001 by unpaired t test. (B) Tumor growth over time. Data are shown as mean tumor volume ± SEM of 5–6 mice per group, *P < 0.05, **P < 0.01 by two-way ANOVA. (C) Mouse survival monitored over time. Analysis include 5–16 mice per group, **P < 0.01, ****P < 0.0001 by log rank (Mantel Cox) test. (D) Mice bearing s.c. B16 tumors were treated as described above in the setting of anti-CTLA4 and anti-PD1 antibody treatment. Graph depicts tumor growth over time. Data are shown as mean tumor volume ± SEM of seven mice per group. *P < 0.05, **P < 0.01 by two-way ANOVA.
Fig. S1.
Intratumoral cGAMP injection promotes CD8 T-cell responses and efficiently delays growth of several melanoma models. (A and B) Tyr::N-Ras(Q61K)INK4a−/− melanoma cells (A) or B-RafV600E/+ PTEN−/− CDKN2A−/− melanoma cells (B) were implanted s.c. in WT mice. cGAMP (cGAMP-inj) or Lipofectamine alone (Ctrl-inj) was injected into tumors at day 5 and day 10 after engraftment. Depicted are: representative flow cytometry dot plots of CD8 T cells infiltrating tumors, quantification of tumor infiltrating CD8 T cells (*P < 0.05, **P < 0.01 by unpaired t test), and tumor growth analysis (represented as mean tumor volume ± SEM with n = 5. *P < 0.05, ****P < 0.0001 by two-way ANOVA). Data are combined from two independent experiments.
Fig. S2.
Intratumoral cGAMP injection promotes the generation of Ag-specific cytotoxic CD8 T cells that infiltrate the tumors. B16-WT or B16-OVA (if indicated) tumor cells were implanted s.c. into WT mice. (A and B) cGAMP (cGAMP-inj) or Lipofectamine alone (Ctrl-inj) was injected into tumors at day 5 and day 10. (A) Flow cytometry analysis of CD44 and CD62L expression gated on CD8 T cells infiltrating tumors at day 15. CD8 T cells from spleen of naive mice were used as control. (B) Tumor cell suspensions were cultured with Brefeldin A for 5 h in addition of PMA/Ionomycin if indicated. Data represent flow cytometry detection and quantification of IFNγ or Perforin expressing-CD8 T cells in tumor-derived cell suspension. *P < 0.05 by unpaired t test. (C and D) At day 4, 5 × 106 OT-I splenocytes were transferred intravenously. cGAMP (cGAMP-inj) or Lipofectamine alone (Ctrl-inj) was injected into the tumor at day 5 and day 10. Flow cytometry analysis and quantification of OVA-specific CD8 T cells in tumors at day 15 detected with Vα2 (C) or SIINFEKL tetramer staining (D). **P < 0.01 by unpaired t test.
Fig. S3.
Intratumoral cGAMP injection induces high numbers of tumor-infiltrating CD4 T cells. (A) B16F10 were implanted s.c. into two opposite flanks of WT mice. At day 5 and day 10, cGAMP (cGAMP-inj) or Lipofectamine alone (Ctrl-inj) was injected into only one tumor. Data represent flow cytometry analysis and quantification of CD4 T cells infiltrating injected tumor and noninjected tumor (contralateral) at day 15. *P < 0.05, **P < 0.01 by unpaired t test. (B) Tyr::N-Ras(Q61K)INK4a−/− melanoma cells or B-RafV600E/+ PTEN−/− CDKN2A−/− melanoma cells were implanted s.c. into WT mice. At day 5 and day 10, cGAMP (cGAMP-inj) or Lipofectamine alone (Ctrl-inj) were injected into the tumor. Data represent flow cytometry analysis and quantification of CD4 T cells infiltrating injected tumors. *P < 0.05, **P < 0.01 by unpaired t test.
Fig. S4.
Increasing doses of aCTLA4/aPD1 treatment improve intratumoral cGAMP efficacy. B16F10 cells were implanted s.c. into WT mice. cGAMP (cGAMP-inj) or Lipofectamine alone (Ctrl-inj) was injected into the tumors at day 5. Anti-CTLA4/anti-PD1 treatment was injected intraperitoneally twice a week at the indicated dose. Data represent the percentage of tumor volume compared with Ctrl-injected tumor at day 18. Importantly, as in Fig. 1, anti-CTLA4/anti-PD1 alone showed significantly less activity than anti-CTLA4/anti-PD1 plus cGAMP (not shown). n = 5 mice per group. **P < 0.01 by two-way ANOVA.
Intratumoral STING Activation Leads to Systemic CD8 T-Cell–Mediated Antitumor Immunity That Controls the Growth of Distant Tumors.
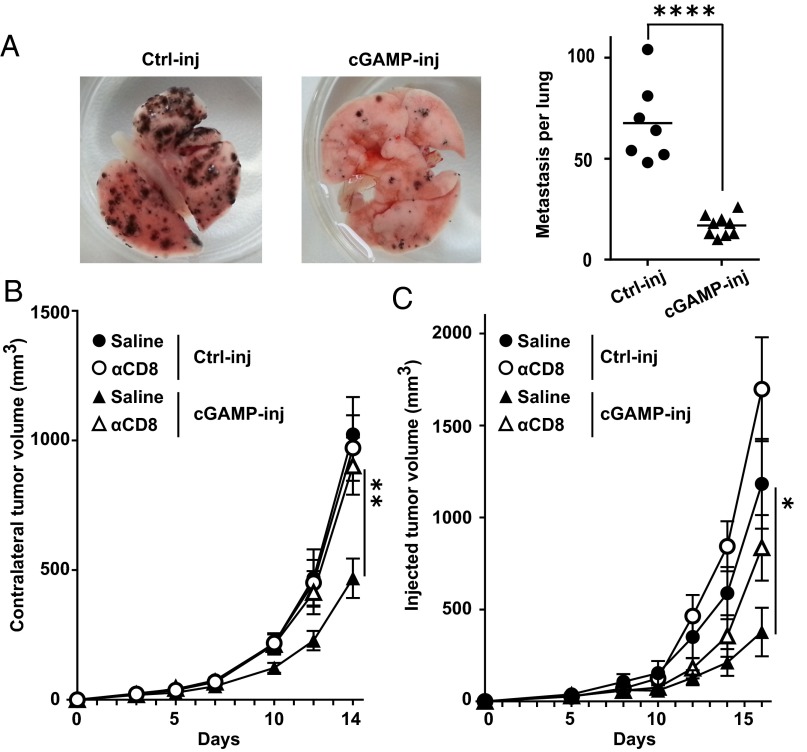
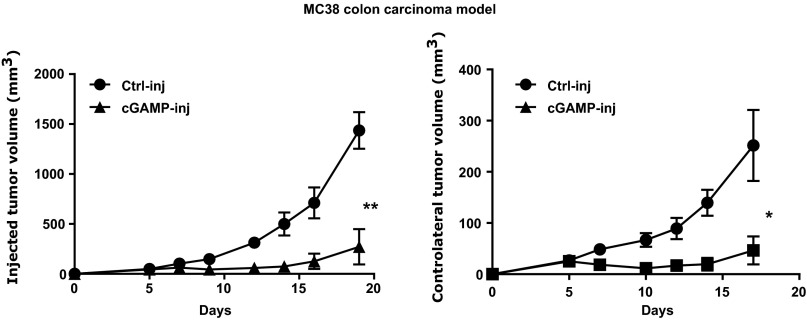
We next investigated whether, via the induction of CD8 T-cell responses, intratumoral injections of cGAMP could induce systemic antitumor immunity. First, mice bearing skin tumors that had been injected with cGAMP, received i.v. B16F10 tumor cells to induce lung metastases. Ten days later, mice were killed and the number of melanoma metastases was counted in the lungs. Intratumoral injection of cGAMP potently reduced the number of lung metastases (Fig. 2A), suggesting the induction of systemic immunity that inhibits metastasis formation. To confirm this, we engrafted B16F10 tumors into opposite flanks of the same mouse followed by treatment of only one tumor while leaving the contralateral one untreated. We found that intratumoral cGAMP treatment not only delayed the growth of injected tumors, but also delayed the growth of contralateral tumors (Fig. 2B). This effect was not limited to B16 melanoma, as intratumoral injection of cGAMP also delayed the growth of injected and contralateral MC38 colon tumor cells implanted into the skin (Fig. S5). Antibody-mediated CD8 T-cell depletion completely abolished the effect of cGAMP on contralateral tumor (Fig. 2B) but only had a partial effect on cGAMP-injected tumors (Fig. 2C). These data suggest that intratumoral cGAMP induces CD8 T-cell priming that drive systemic antitumor immunity controlling local and distant tumor growth. Interestingly, the local antitumor response to cGAMP is only partially dependent on these CD8 T cells, suggesting that additional mechanisms account for a direct antitumor activity.
Fig. 2.
Intratumoral STING activation leads to systemic CD8 T-cell–mediated antitumor immunity that controls the growth of distant tumors. (A) Mice bearing a 5-d established s.c. B16F10 tumor were treated by i.t. injection of cGAMP (cGAMP-inj) or Lipofectamine alone (Ctrl-inj) followed by i.v. injection of B16F10 melanoma cells. After 10 d, mice were killed and lung metastases counted macroscopically. Photographs of representative lungs are depicted (Left). Each symbol represents an independent mouse (Right); data are combined from two experiments. ****P < 0.0001 by unpaired t test. (B and C) Mice bearing two s.c. B16F10 tumors in opposite flanks were treated by i.t. injection of cGAMP (cGAMP-inj) or Lipofectamine alone (Ctrl-inj) in only one tumor. CD8-depleting antibody or saline were injected i.p. Graph depicts tumor growth over time of contralateral not injected tumors (B) and injected tumors (C). Data represent the mean tumor volume ± SEM of 9–10 mice per group pooled from two experiments. *P < 0.05, **P < 0.01 by two-way ANOVA.
Fig. S5.
Intratumoral cGAMP injection induces potent direct and systemic antitumor activity in the MC38 colon cancer model. MC38 colon cancer cells were implanted s.c. into two opposite flanks of WT mice. cGAMP (cGAMP-inj) or Lipofectamine alone (Ctrl-inj) was injected into one tumor at day 5. Data represent tumor growth of injected tumors and noninjected contralateral tumors, shown as the mean tumor volume ± SEM with n = 4–5. *P < 0.05, **P < 0.01 by two-way ANOVA.
The Antitumor Activity Induced by STING Is Dependent on Type I IFN Signaling.
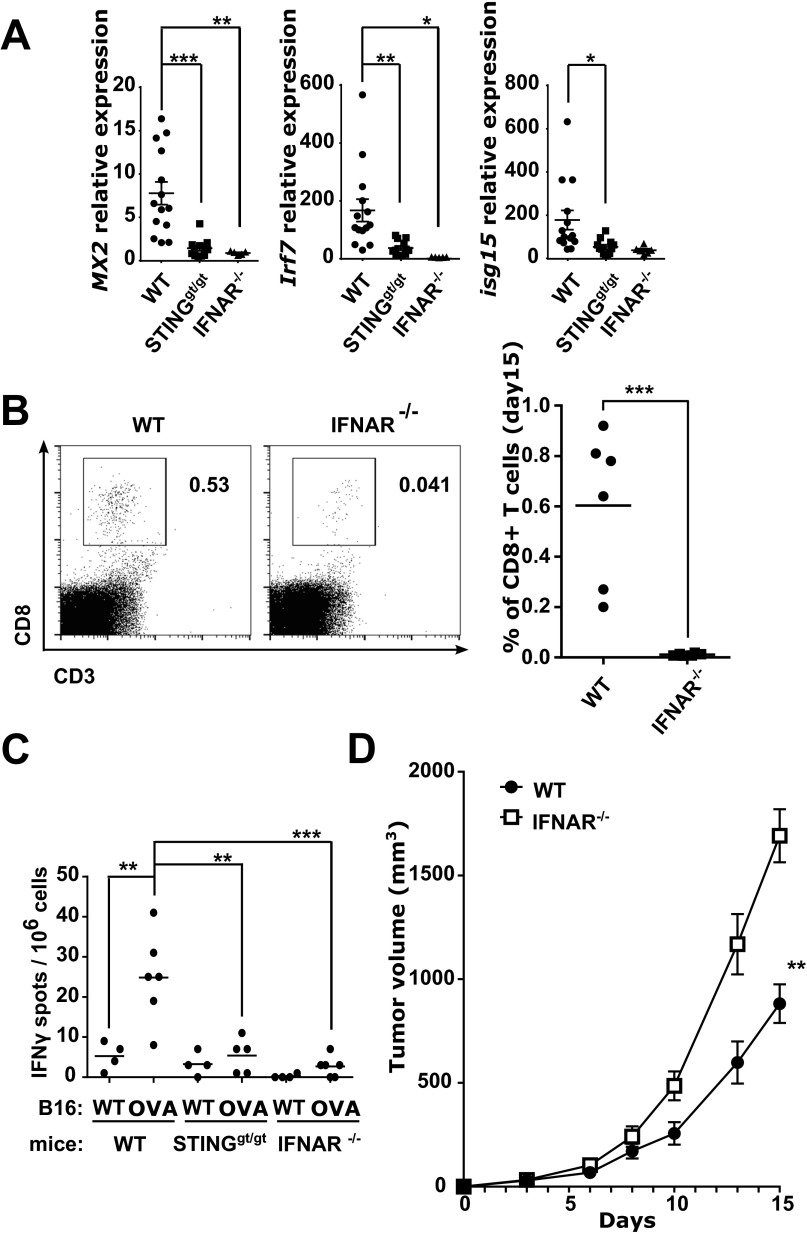
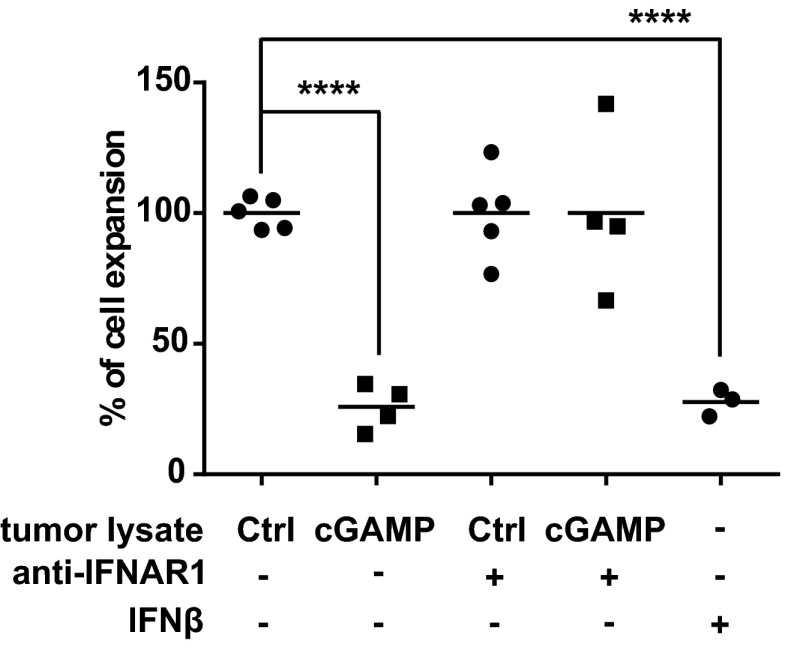
Because STING has been associated with type I IFN induction (21) we next sought to investigate the role of type I IFNs in mediating the antitumor CD8 T-cell response induced by cGAMP. As previously described (17), low levels of type I IFNs were spontaneously induced by STING signaling in growing tumors of WT mice as we detected the expression of the type I IFN-inducible genes Mx2, Irf7, and Isg15 that were abolished in STINGgt/gt mice (Fig. S6). Lack of type I IFN signaling in IFNAR−/− mice not only abolished the type I IFN signature in tumors, but also abrogated CD8 T-cell responses in the tumors (Fig. S6). We then sought to investigate whether the same type I IFN-dependent mechanism would underlie the strong antitumor CD8 T-cell responses observed following cGAMP injection. First, we measured type I IFN activity induced by intratumoral cGAMP. Strong type I IFN activity was detected starting 1 h after injection, peaking between 2 h and 4 h, and declining thereafter (Fig. 3A). mRNA expression analysis 4 h after injection revealed expression of Ifn-β1 but not of Ifn-α genes such as Ifn-α2, Ifn-α5, and Ifn-α6 (Fig. 3B), indicating that intratumoral STING activation triggers an early type I IFN activity via the induction of Ifn-β1 expression. We then assessed the role of IFN-β in the local and systemic antitumor activity induced by cGAMP. Treatment of WT mice with blocking anti-IFNAR antibodies or the use of IFNAR−/− mice to block IFNAR signaling completely abolished intratumoral CD8 T-cell numbers and antitumor activities in both injected and contralateral tumors (Fig. 3 C and D). Interestingly, despite being only partially CD8 T-cell dependent, the local antitumor activity induced by cGAMP injection was entirely mediated by type I IFNs. To test whether IFN-β expression had a direct inhibitory activity on the growth of B16 tumor cells, we generated extracts from cGAMP-injected tumors and tested their ability to block the growth of B16 tumor cells with or without neutralizing antibodies to IFNAR. These tumor extracts were indeed able to block the growth of B16 cells in a type I IFN-dependent manner (Fig. S7), a finding that is consistent with previous reports showing that type I IFNs block proliferation and induce apoptosis of B16 cells (22, 23). Together these data indicate that IFN-β is required for the generation of tumor-infiltrating CD8 T cells and for the antitumor activity induced by intratumoral cGAMP.
Fig. S6.
Spontaneous antitumor immunity in melanoma is IFNAR dependent. B16F10 or B16-OVA (if indicated) tumor cells were implanted s.c. in WT, STINGgt/gt, or IFNAR−/− mice. (A) Gene expression analysis of Mx2, Irf7, and Isg15 in tumors harvested at day 15 after engraftment. Data are combined from three independent experiments. (B) Flow cytometry analysis and quantification of tumor-infiltrating CD8+ T cells at day 15 after engraftment. Data are representative of two independent experiments. (C) At 11 d after engraftment, tumor draining lymph node cells were restimulated with 10 µg/mL SIINFEKL peptide for 24 h. The frequency of OVA-specific IFNγ-producing cells was evaluated by ELISPOT. Data are representative of three independent experiments. (D) B16F10 Tumor growth analysis in IFNAR−/− compared with WT mice. (A–C) *P < 0.05, **P < 0.01, ***P < 0.005, by unpaired t test. (D) **P < 0.01 by two-way ANOVA.
Fig. 3.
Intratumoral cGAMP induces expression of IFN-β that drives local and systemic antitumor immunity. (A and B) Mice bearing 5-d established s.c. B16F10 tumors were treated by i.t. injection of cGAMP (cGAMP-inj) or Lipofectamine alone (Ctrl-inj). Depicted are: (A) Type I IFN activity in tumor extracts at the indicated time after treatment. (B) Ifn-β1, Ifn-α2, Ifn-α5, and Ifn-α6 mRNA expression in tumors 4 h after treatment. Each symbol represents an independent mouse, **P < 0.001 by unpaired t test. (C and D) B16F10 cells were implanted s.c. into opposite flanks of WT or IFNAR−/− mice followed by i.t. injection of cGAMP (cGAMP-inj) or Lipofectamine alone (Ctrl-inj) in only one tumor. WT mice were also treated with a neutralizing αIFNAR antibody. Depicted are: (C) CD3+CD8+ T cells infiltrating tumors at day 15 postengraftment, measured by flow cytometry of tumor single cell suspensions. Representative plots are given (Upper); each symbol represents an independent mouse (Lower). *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 by unpaired t test. (D) Tumor growth over time of injected and contralateral tumors. Data are given as the mean tumor volume ± SEM with n = 5, representative of two independent experiments, *P < 0.05, **P < 0.01, ***P < 0.001 by two-way ANOVA.
Fig. S7.
Tumor extracts from cGAMP-injected tumor inhibit the growth of B16F10 cells in a type I IFN-dependent manner. Protein extracts were prepared from tumors lysed after they have been injected in vivo for 4 h with cGAMP or Lipofectamine (Ctrl). B16F10 cells were incubated with 300 µg/mL of tumor lysate proteins. Type I IFN signaling was blocked by adding 10 µg/mL of anti-IFNAR1 antibody (+) in the culture medium. As positive control, 10 units/mL of murine recombinant IFN-β was added to the culture (+). Data represent the percentage of cellular expansion compare with Ctrl conditions after 3 d of culture. Data are representative of at least three independent experiments. ****P < 0.0001 by unpaired t test.
Endothelial Cells Are the Principal IFN-β Producers in Response to Therapeutic STING Activation in Tumors.
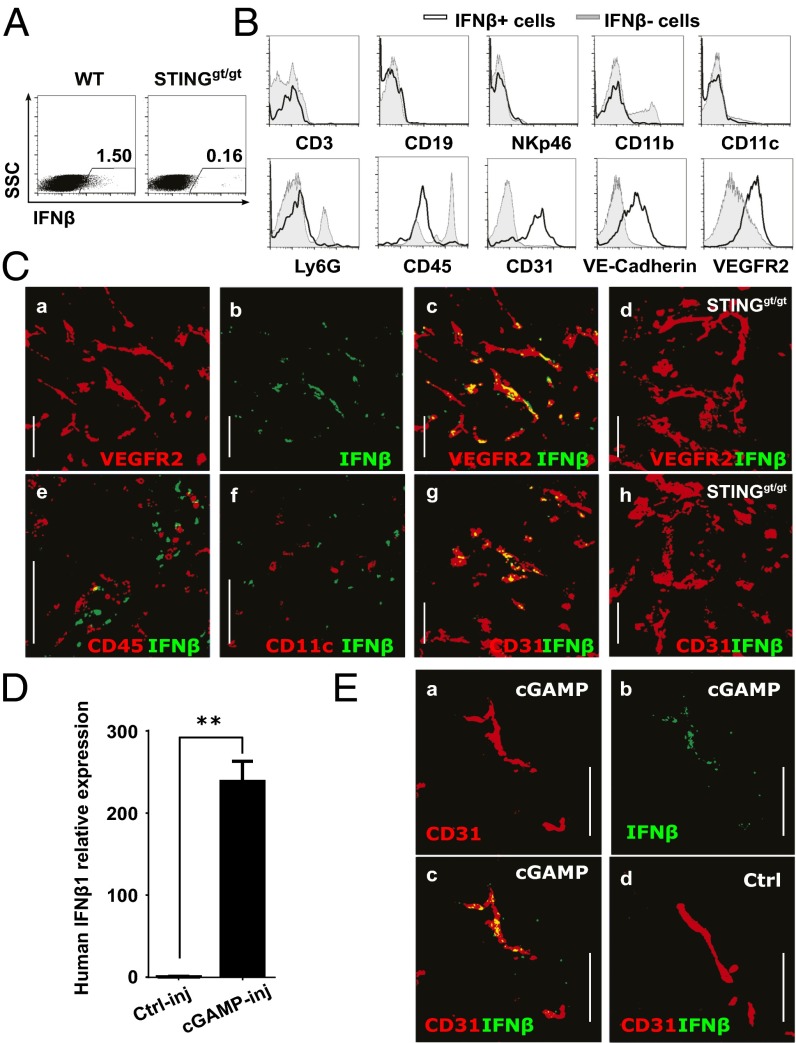
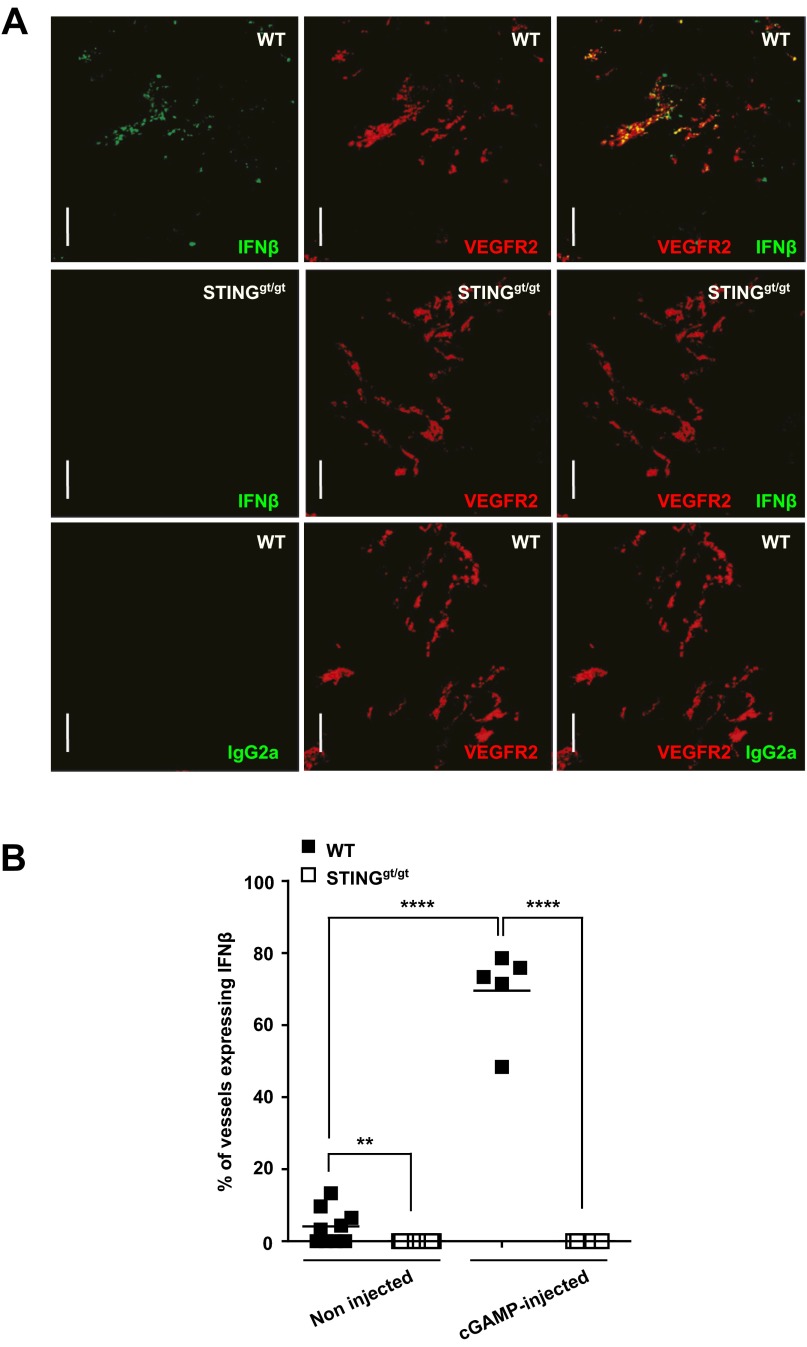
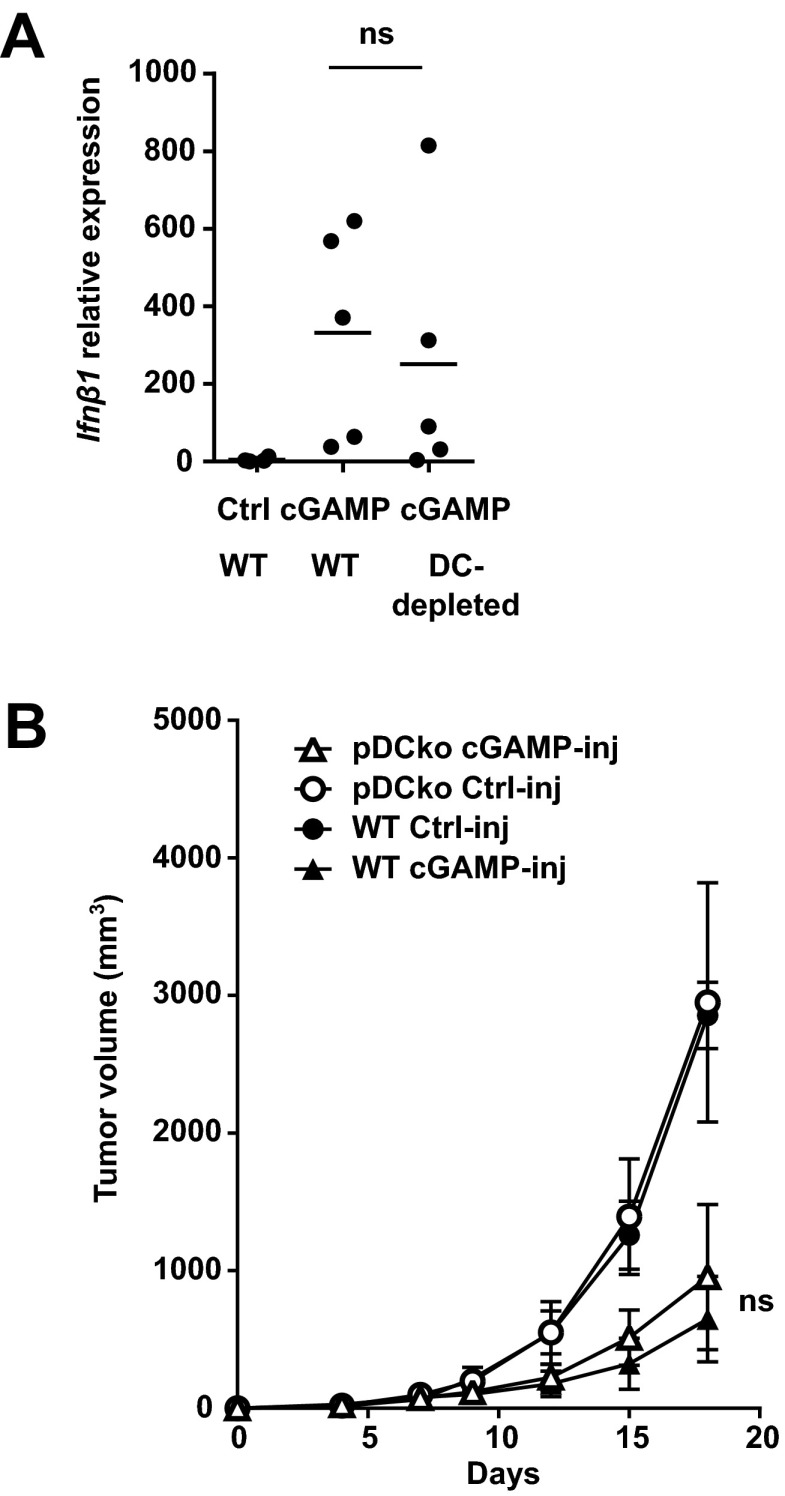
To identify the cell type responsible for the STING-mediated induction of IFN-β production in the tumor microenvironment, we performed intracellular IFN-β staining of tumor-cell–derived single cell suspensions generated after intratumoral cGAMP injection. Flow cytometry analysis revealed a small population of IFN-β–producing cells in the tumor microenvironment of engrafted wild-type mice that was not present in tumors engrafted in STING-deficient mice (Fig. 4A). Phenotypical analysis of IFN-β–producing cells revealed that IFN-β was produced by a homogeneous population that did not express lineage markers for T cells, B cells, NK cells, DC, monocyte/macrophages nor neutrophils (CD3, CD19, NKp46, CD11C, CD11b, and Ly6G, respectively) (Fig. 4B). IFN-β–producing cells expressed low levels of the hematopoietic marker CD45 and, surprisingly, expressed the specific endothelial cell markers CD31, VE-Cadherin, and VEGFR-2 (Fig. 4B). To confirm that endothelial cells are the principal target of STING activation in the tumor microenvironment, we performed immunofluorescent staining for IFN-β on cGAMP-injected tumors engrafted in wild-type mice or STING-deficient mice. IFN-β was always found to be associated with the VEGFR2+ and CD31+ tumor endothelial cells (Fig. 4C and Fig. S8) and not produced by CD45+ infiltrating immune cells including CD11c+ dendritic cells (DCs) (Fig. 4C). Importantly, IFN-β was not detected in cGAMP-injected tumors engrafted in STINGgt/gt mice (Fig. 4C and Fig. S8). Our data suggest that endothelial cells and not DCs are the principal type IFN-producing cell type in the tumor in response to STING activation. These data were confirmed by the finding that the IFN-β induction in response to intratumoral cGAMP was unaltered in CD11c-depleted mice (Fig. S9). More specifically, we confirmed that plasmacytoid dendritic cells (pDCs), which are the principal type I IFN-producing cells during antiviral immune responses, were not involved in the antitumoral activity induced by cGAMP as inhibition of tumor growth was retained upon pDC depletion in hBDCA2-DTR mice (Fig. S9). Thus, our data identify tumor endothelial cells as the principal type I IFN producers in response to therapeutic STING activation by cGAMP.
Fig. 4.
Endothelial cells represent the main IFN-β producers upon intratumoral cGAMP injection in mouse and human. (A–C) WT or STINGgt/gt mice bearing 5-d established s.c. B16F10 tumors were treated by i.t. injection of cGAMP plus Brefeldin A. Tumors were harvested after 5 h for flow cytometry and confocal microscopy analysis. (A) Intracellular IFN-β detection in tumor single cell suspensions. (B) Expression of CD3, CD19, NKp46, CD11b, CD11c, Ly6G, CD45, CD31, VE-cadherin, and VEGFR2 in IFN-β–producing cells (black line, gated as shown in A compared with nonIFN-β–producing cells (gray line). Data are representative of at least three independent experiments. (C) IFN-β expression on CD31 or VEGFR2 cells in tumor cryosections derived from WT (a–c and e–g) or STINGgt/gt mice (d and h). (Scale bar, 100 µm.) (D and E) Resected human melanoma skin metastases were injected with 2′3′-cGAMP (cGAMP-inj) or Lipofectamine alone (Ctrl-inj) and cultured in the presence of Brefeldin A (only in E) for 5 h before analysis. Depicted are: (D) Quantitative real-time PCR analysis of IFN-β1 mRNA expression in treated tumor explants. **P < 0.01 by unpaired t test. (E) Confocal microscopy of IFN-β and CD31 stained on cryosections derived from treated tumor explants. (Scale bar, 100 µm.)
Fig. S8.
Endothelial cells represent the main source of IFN-β in tumors upon therapeutic or spontaneous STING activation. (A) B16F10 cells were implanted into WT or STINGgt/gt mice. At day 5, tumors were treated with i.t. injection of cGAMP plus Brefeldin A for 5 h. Data represent confocal imaging of injected tumor cryosections stained with rat anti-mouse IFN-β, rat IgG2a isotype control, and goat anti-mouse VEGFR2 antibodies. (B) B16F10 cells were implanted into WT or STINGgt/gt mice. At day 5, tumors were treated for 5 h with Brefeldin A alone (noninjected) or Brefeldin A plus cGAMP (cGAMP-injected) before being embedded in OCT. Data represent the quantification by confocal microscopy of CD31+ vessels in tumors that express IFN-β. Each symbol represents an independent tumor. **P < 0.01, ****P < 0.0001 by unpaired t test.
Fig. S9.
IFN-β induction in response to intratumoral cGAMP is not altered in tumor of DC-depleted mice and antitumor activity induced by intratumoral cGAMP is retained in pDC-depleted mice. (A) B16F10 cells were engrafted into WT or CD11C-DTR transgenic mice. At day 4, 120 ng of diphteria toxin were i.p. injected in all mice. At day 5, 4 h after i.t. injection of cGAMP (cGAMP) or Lipofectamine alone (Ctrl), tumors were harvested for RNA extraction. Ifnβ1 gene expression was performed by quantitative PCR. ns, not significant by unpaired t test. (B) B16F10 cells were implanted s.c. into WT and hBDCA2-DTR mice. pDC depletion was achieved by systemic injection of 120 ng of diphteria toxin at day 4 and day 9. cGAMP or Lipofectamine (control) was injected into tumors at day 5 and 10. Tumor growth was monitored over time. Data show the mean tumor volume ± SEM with n = 4–5. ns, not significant by two-way ANOVA.
To determine whether cGAMP could also activate STING in human melanoma, we injected cGAMP into freshly resected melanoma skin metastases. Intratumoral cGAMP induced high levels of IFN-β expression in skin melanoma metastases compared with control injected melanoma (Fig. 4D). Immunofluorescence stainings confirmed that IFN-β was expressed predominantly by CD31+ endothelial cells in the tumor microenvironment. Thus, intratumoral cGAMP can induce type I IFNs by the human tumor vasculature (Fig. 4E).
Endothelial Cells Produce High Level of Type I IFNs upon STING Activation.
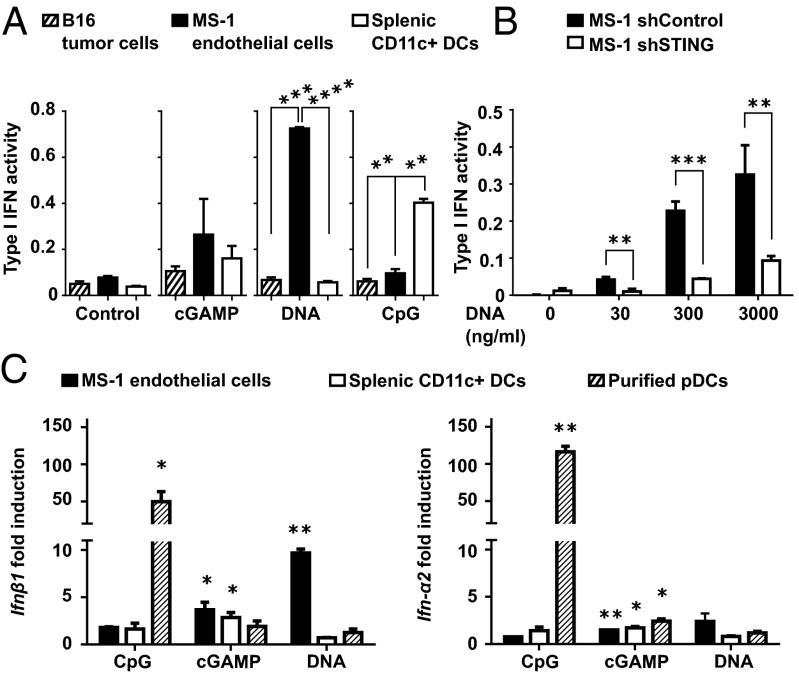
To evaluate the capacity of endothelial cells to produce type I IFNs, we performed in vitro stimulation of murine endothelial cells with cGAMP or CpGs in comparison with total CD11c+ DCs, which have previously been suggested to be the main IFN producers in the tumor microenvironment (17). We found stronger type I IFN activity in endothelial cells stimulated by cGAMP compared with DCs (Fig. 5A). By contrast, type I IFN activity was higher in DCs stimulated with CpG compared with endothelial cells (Fig. 5A). Because cytosolic transfer of extracellular tumor-derived DNA can trigger activation of STING (17), we tested whether it could also trigger type I IFN production in endothelial cells and DCs. Lipofected tumor DNA was indeed a potent inducer of type I IFN activity via STING activation of endothelial cells but not DCs (Fig. 5 A and B). IFN-β and IFN-α mRNA expression analysis confirmed that STING activation induced a predominant IFN-β expression in endothelial cells compared with DCs, whereas IFN-α mRNA was hardly detectable in both cell types (Fig. 5C). Although poorly responding to STING activation by cGAMP, purified pDCs expressed high levels of IFN-α and IFN-β mRNA upon stimulation with CpG (Fig. 5C), consistent with their specialized function as type I IFN producers in response to endosomal TLR activation. Therefore, in vitro, endothelial cells appear to produce higher amounts of IFN-β in response to STING activation compared with DCs.
Fig. 5.
Endothelial cells are specialized in producing large quantities of IFN-β in response to STING activation. (A) MS-1 endothelial cells, B16 tumor cells, and splenic CD11c+ DCs were stimulated overnight with cGAMP (5 µg/mL), tumor DNA (3 µg/mL), both complexed with Lipofectamine or with CpG-B (1 µg/mL). Type I IFN activity was measured in the culture supernatant. Data are expressed as mean ± SD and are representative of three independent experiments. **P < 0.01, ***P < 0.001, ****P < 0.0001 by unpaired t test. (B) STING-deficient (shSTING) or control (shControl) MS-1 endothelial cells were stimulated overnight with increasing concentrations of tumor DNA complexed with Lipofectamine. Type I IFN activity was measured in the culture supernatant. Data are expressed as mean ± SD of two independent experiments. **P < 0.01, ***P < 0.005 by unpaired t test. (C) MS-1 endothelial cells, spleen CD11c+ DCs, or purified spleen pDCs were activated for 5 h as described in A. Ifn-β1 and Ifn-α2 gene expression were determined by quantitative real-time PCR. Data are shown as fold induction over nonstimulated cells. *P < 0.05, **P < 0.01, by unpaired t test.
Endothelial Cells Are the Principal IFN-β Producers in Response to Spontaneous STING Activation in Tumors.
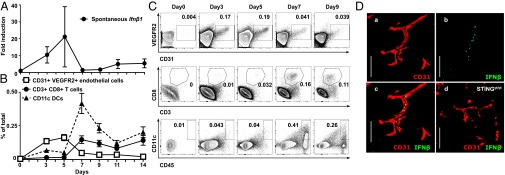
Next we sought to determine whether endothelial cells also represent the main source of spontaneous type I IFN expression in growing tumors. mRNA expression analysis revealed that Ifn-β1 was spontaneously induced in the tumors as early as day 3 after tumor engraftment, reaching maximal expression levels at day 5 (Fig. 6A). This time point was also characterized by the presence of a large number of CD45- CD31+ VEGFR2+ endothelial cells in the tumor, whereas CD45+ immune cells including CD8 T cells and CD11c+ DCs appeared later (Fig. 6 B and C). Immunofluorescence staining identified a rare population of IFN-β–expressing cells (Fig. S8), which always costained with endothelial cell markers and was completely absent in tumors of STING-deficient mice (Fig. 6D). These data suggest that tumor endothelial cells are also a main source of spontaneous type I IFN expression in the tumor microenvironment. Endothelial cell-derived type I IFNs preceded the infiltration of CD11c+ DCs and CD8 T cells in the tumor microenvironment, suggesting their role in initiating the antitumor response via type I IFN production.
Fig. 6.
Endothelial cells are the principal IFN-β producers in response to spontaneous STING activation in tumors. B16F10 melanoma cells were implanted into the flank of WT or STINGgt/gt mice. (A) Time course analysis of spontaneous Ifn-β1 mRNA expression in tumors after implantation in WT mice. Data represent the mean ± SEM of at least four independent mice. (B) Detection by flow cytometry of endothelial cells (VEGFR2+ CD31+ gated on CD45lo/neg), CD8 T cells (CD3+ CD8+), and DCs (CD45+ CD11c+) in single cell suspensions derived from tumors over time after their implantation. Data are expressed as mean ± SD of at least four independent mice. (C) Representative flow cytometry plot is shown. (D) Detection of spontaneous IFN-β production in tumors. Data show representative confocal microscopy images of 5-d-old tumors harvested from WT (a–c) or STINGgt/gt mice (d) and stained for IFN-β and CD31. (Scale bar, 100 µm.)
Discussion
Our study demonstrates that intratumoral injection of cGAMP induces potent STING activation in the tumor microenvironment and thereby inhibits tumor growth by promoting natural CD8 T-cell responses against the tumor. The cGAMP-induced antitumor activity was found to be mediated by the STING-driven induction of IFN-β in the tumor microenvironment. Interestingly, tumor endothelial cells were the main producer of IFN-β in response to cGAMP injection in both mouse and human. Endothelial cells were also found to be the main source of spontaneous IFN-β production in growing tumors. Together these data indicate that the tumor vasculature plays a key role in the initiation of spontaneous and therapeutic CD8 T-cell immunity against the tumor via STING-dependent induction of type I IFNs.
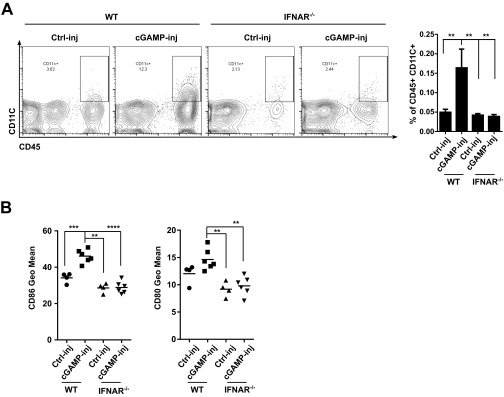
Type I IFNs play a multifaceted role in the induction of antitumor immunity. First, type I IFNs enhance tumor-specific CD8 T cells priming by promoting the recruitment and activation of DCs at the tumor site (7, 8). Accordingly, type I IFNs were found to increase the number of DCs in the microenvironment of cGAMP-injected tumors and to stimulate DC maturation as we detected a higher levels of CD80 and CD86 in DCs in lymph nodes draining cGAMP-injected tumors (Fig. S10). Second, type I IFNs may increase CD8 T-cell infiltration into cGAMP-injected tumors (6) and directly promote their survival and proliferation in the tumor microenvironment (24). However, our data suggest that the potent local antitumor efficacy is not explained by increased CD8 T-cell infiltration of cGAMP-injected tumor but rather by a direct inhibitory effect of type I IFNs on the tumor itself (22, 23).
Fig. S10.
Type I IFNs increase the number of infiltrating DCs in cGAMP-injected tumors and stimulate DC maturation in lymph node draining cGAMP-injected tumors. B16F10 cells were implanted into WT or IFNAR−/− mice. At day 5, tumors were injected with cGAMP (cGAMP-inj) or Lipofectamine alone (Ctrl-inj). (A) At day 7, CD11c+ DC number was analyzed in tumors by flow cytometry. (B) At day 7, CD11C+ DCs from tumor draining lymph nodes were analyzed by flow cytometry for CD86 and CD80 expression. **P < 0.01, ***P < 0.001, ****P < 0.0001 by unpaired t test.
DCs are typically believed to be the main source of type I IFN during immune response. Unexpectedly, our study identifies endothelial cells and not DCs as the principal IFN-producing cell in response to both spontaneous and enforced STING activation in the tumor microenvironment. One reason for this is that endothelial cells possess an enhanced capacity to produce type I IFNs in response to STING activation compared with DCs. Confirming our data, previous studies found that in vitro-stimulated endothelial cells express more type I IFNs than other immune cells such as macrophages following STING activation by viruses (25). Another reason for the preferential type I IFN production by endothelial cells may be related to their relative abundance in the tumor compared with tumor-infiltrating immune cells. Indeed, at the time of intratumoral cGAMP injection, endothelial cells in the tumor microenvironment are fourfold more abundant than DCs. Interestingly, we found exclusive expression of IFN-β but did not detect any IFN-α in the tumor microenvironment. This may be related to the weak capacity of endothelial cells to produce IFN-α upon STING activation, but may also be related to the fact that IFN-α expression requires prior IFN-β–mediated up-regulation of IRF7 (26).
In spontaneously growing tumors IFN-β is also induced early at the time of tumor vasculature formation, before the infiltration of immune cells. Here, type I IFNs produced by endothelial cells is necessary for the infiltration and activation of cross-presenting DCs and CD8 T cells in the tumor microenvironment by the mechanisms described above. A likely source of endogenous STING ligands is tumor DNA or tumor-derived cGAMP, although it is unclear how they can spontaneously reach intracellular compartments of endothelial cells. Tumors may package DNA in apoptotic bodies and transfer it to endothelial cells (27), where it activates STING upon cGAS-mediated conversion to cGAMP (15, 16). Another possibility is that tumor cGAMP is directly transferred to endothelial cells via connexin-mediated channels, as this has been previously suggested (28).
Intratumoral STING activation by cGAMP and potentially other ligands may be an attractive candidate for antitumor therapy of metastatic melanoma patients. The efficacy of intratumoral cGAMP is strongly enhanced by combination with anti-CTLA4 and anti-PD1 therapy, the currently most potent immunotherapeutic approach in melanoma (29). Intratumoral cGAMP activation is also effective in the MC38 model of colon carcinoma, suggesting that other tumors can be treated by enforced intratumoral STING activation. These data were confirmed by the finding that local tumor radiotherapy of MC38 tumors can be enhanced by intratumoral cGAMP injection (18).
Our data identify a key role of the tumor vasculature arising in growing tumors in the shaping of CD8 T-cell–mediated antitumor immunity. This finding raises the question about the effect of antiangiogenic therapies (30), which are being developed and considered to be used in combination with immune-based therapies, on antitumor immunity.
In conclusion, our study identifies STING to be the central molecular driver of spontaneous antitumor immune responses that can be exploited to generate potent antitumor immunity. We also identify endothelial cells as the principal cellular target of STING activation, uncovering an unexpected role of the tumor vasculature in the control of tumor growth via the initiation of antitumor immunity in melanoma.
Materials and Methods
Study Approval.
These experiments were approved by the Institutional Animal Care and Use Committee of the University of Lausanne.
Tumor Model and Treatment.
Mice were injected s.c. with 5 × 105 B16 melanoma cells. At 5 d after implantation, 10 μg of cGAMP complexed with Lipofectamine 2000 was injected into engrafted tumors. Tumor size was measured every 2 d using a caliper. Materials and methods are described in greater detail in Supporting Information.
SI Materials and Methods
Study Approval.
All animal experiments were approved by the Institutional Animal Care and Use Committee of the University of Lausanne. Human studies were approved by the institutional review board of the University of Lausanne and were performed in accordance with the guidelines of the Declaration of Helsinki. Melanoma metastasis explants were obtained with informed consent from patients by punch biopsies and were defined according to standard clinical and histopathological criteria.
Mice.
C57Bl6 mice were purchased from Harlan. OT-I (TCR transgenic mice specific for the Kb-SIINFEKL peptide of ovalbumin), IFNAR−/−, TLR9−/−, and CD11c-DTR mice were obtained from the SwimmR mice repository in Zurich. STING-deficient (Golden ticket or STINGgt/gt) and hBDCA2-DTR mice were purchased from The Jackson Laboratories.
Tumor Cell Lines.
B16F10 cells were obtained from the ATCC. B16F10 and B16-OVA cells were cultured in RPMI supplemented with 10% (vol/vol) FCS and 1 mM penicillin/streptomycin. MC38 cells (provided by Jeffrey Schlom, NIH) were cultured in DMEM supplemented with 10% (vol/vol) FCS and 1 mM penicillin/streptomycin. Tyr::N-ras(Q61K)INK4A−/− melanoma cell line was cultured in DMEM 10% FCS, 1 mM penicillin/streptomycin, and 5 × 10−5 M β-mercaptoethanol. B-raf(V600E/+) PTEN−/− CDKN2A−/− melanoma cell line was cultured in DMEM 10% (vol/vol) FCS, 1 mM penicillin/streptomycin, 5 × 10−5 M β-mercaptoethanol, glutamine, 1 mM sodium pyruvate, and 10 mM Hepes. Tumor cells were grown in monolayer at 37 °C in a humidified CO2 incubator. Before reaching confluence, tumor cells were harvested with 0.05% trypsin, washed, and suspended in PBS for injection.
Tumor Implantation, Treatment, and Growth Measurement.
A total number of 5 × 105 tumor cells were injected s.c. into the flank of 7- to 12-wk-old mice. At day 5 and day 10, progressing tumors were injected with 100 µL of PBS solution containing 10 µg of 3′3′-cGAMP (c[G(3′-5′)pA(3′-5′)p]) (Invivogen) complexed with 3 µL of Lipofectamine 2000 (Invitrogen). Tumor size was measured in two dimensions using a caliper. Tumor volume was calculated using the modified ellipsoid formula V = (Lxl2)/2, where L is the widest and l the smallest diamater.
In Vivo Antibody Treatments.
CD8 depleting antibody (clone 53–6.72) was purchased from BioXcell and injected intraperitoneally at the dose of 200 µg per mouse twice a week. IFNAR1 blocking antibody (clone MAR1-5A3) was purchased from BioXcell and injected at the dose of 200 µg per mouse at day −1, 0, +1 of intratumoral cGAMP injection. PD1 (clone J43) and CTLA4 (9H10) blocking antibodies were purchased from BioXcell. Anti-CTLA4 and anti-PD1 were injected intraperitoneally twice a week at the dose of 200 µg per mouse after intratumoral cGAMP injection.
Metastasis Model.
A total number of 1 × 106 tumor cells were given i.v. to generate lung metastases in mice bearing a 5-d-old s.c. tumor. At the same time, s.c. tumors were treated with intratumoral cGAMP. After 10 d, mice were killed and the number of lung metastases was counted macroscopically.
Ex Vivo Treatment of Human Melanoma Explants.
Fresh melanoma metastases were harvested by punch biopsies from a patient with in-transit skin metastases. Fresh tumor explants were injected with 100 µL PBS solution containing 10 µg of 2′3′-cGAMP (c[G(2′-5′)pA(3′-5′)p]) (Invivogen) complexed with 3 µL of Lipofectamine 2000 and incubated in a 96-well plate at 37 °C in a humidified CO2 incubator. After 5 h, tumors were harvested and snap frozen for histology and RNA extraction.
Generation and Analysis of Tumor Cell Suspensions.
Tumors were harvested, rinsed with PBS, and passed through a 70-µm cell strainer to obtain a single cell suspension. Cells were stained with antibodies against CD3 (145.2C11), CD8a (53-6-7), CD19 (1D3), NKp46 (29A1.4), CD11C (HL3), Ly6G (1A8), CD11B (M1/70), CD45 (30F11), CD31 (MEC13.3), CD144 (ebioBV13), and CD309 (Flk-1) purchased from BD Pharmingen or eBioscience. SIINFEKL tetramer was purchased from Becton Dickinson. Data were acquired on a Gallios cytometer (Beckman-Coulter), LSRII (Becton Dickinson), or FACS Calibur (Becton Dickinson), and analyzed with Flow-Jo V10 software.
Gene Expression.
Tumors were homogenized in TRIzol (Invitrogen). RNA isolation was performed with chloroform and further isopropanol precipitation. cDNA was synthesized from 1 to 2 µg of total RNA using SuperScript II reverse transcriptase (Invitrogen) according to manufacturer protocol. Gene expressions were analyzed using specific Taqman probes (Life Technologies; mouse Gapdh, Mm99999915_g1; human GAPDH, Hs02758991_g1; mouse Ifnβ1, Mm00439552_s1; human IFNβ1, Hs01077958 _s1; mouse Mx2, Mm00488995-m1; mouse Irf7, Mm00516793_g1; mouse Isg15, Mm017053338_s1), and Taqman gene expression master mix (Life Technologies). Amplifications were performed using the Step One Real-Time PCR system (Applied Biosystem). Relative gene quantifications were expressed as 2-ΔCt using Gapdh as endogenous control.
Type I IFN Activity Measurement.
Type I IFN activity was measured with B16 IFNα/β reporter cells (Invivogen). For assessment of type I IFN activity in tumors: Tumors were homogenized in 300 µL culture medium supplemented with protease inhibitor mixture (Roche). Proteins were quantified with a BCA assay (Pierce). B16 IFNα/β reporter cells were seeded in 96-well plates and stimulated with 300 µg/mL of tumor protein extracts for 16 h. Detection of type I IFNs was monitored with Quanti-blue (Invivogen) according to manufacturer instructions and absorbance at 620-nm read with a spectrophotometer. For assessment of type I IFN activity in culture supernatant: STING-knockdown B16 IFNα/β reporter cells were incubated with 40 µL of samples for 24 h prior to revelation with Quanti-blue (Invivogen).
IFN-β Detection by Immunofluorescence and Flow Cytometry.
The 5-d-old tumors were injected with 100 μL of cGAMP solution plus Brefeldin A (eBioscience). After 5 h, tumors were harvested and snap frozen for histology or processed to generate a single cell suspension for flow cytometry analysis. For immunofluorescence staining, sections (20 µm) were cut with a cryostat, thawed and mounted on Superfrost plus slides, air dried, and stored at −20 °C for further use. Sections were rehydrated in PBS, fixed with 4% (vol/vol) PFA, permeabilized with PBS 0.3% Triton-X, and incubated with blocking buffer [PBS, 5% (vol/vol) donkey serum, 0.5% BSA, 0.3% Triton-X] for 30 min at room temperature. Sections were incubated with rat anti-mouse IFN-β FITC (RMMB-1, PBL, dilution 1/25) or rabbit anti-human IFN-β (PA5-20390, Thermo Scientific, dilution 1/50) diluted in blocking buffer at 4 °C overnight. Anti-mouse CD31-biotin (MEC13.3, Pharmingen), anti-mouse VEGFR2 (Flk-1, R&D), and anti-human CD31 (JC70A, DAKO) were incubated for 2 h at room temperature in blocking buffer. Anti-mouse IFN-β FITC signal was amplified with an anti-rat Alexa 488 secondary antibody. Secondary antibodies (Molecular Probes, Invitrogen) were incubated for 1 h at room temperature in blocking buffer. Fluorescent images were acquired using ZEISS LSM 510 laser scanning confocal microscope and analyzed with ZEN 2010 software. For flow cytometry, harvested tumors were processed as described above to generate a single cell suspension. Single cell suspension was first stained with antibodies targeting cell surface markers. Intracellular detection of IFN-β was performed with intracellular fixation and permeabilization buffer set (eBioscience). After surface staining, tumor cell suspensions were fixed, washed twice with permeabilization buffer, and incubated overnight with anti-mouse IFN-β FITC (RMMB, PBL, dilution 1/25) at 4 °C. Tumor cells were analyzed on a FACS Calibur (Becton Dickinson) and analyzed with Flow-Jo V10 software.
Enrichment of CD11c DCs and pDCs.
For CD11c DC enrichment, spleens were treated with DNase1 (100 µg/mL) and Collagenase type 2 (2 mg/mL) for 30 min at 37 °C and passed through a 70-µm cell strainer to generate a single cell suspension. T and B cells were removed by magnetic separation using CD3-biotin antibody (145-2C11), CD19-biotin antibody (1D3), and antibiotin lyophilized microbeads (Miltenyi). The negative fraction was stained with anti-CD11c-biotin (N418) and antibiotin lyophilized microbeads and positively selected on magnetic column. For pDC enrichment, spleens were treated with DNase1 (100 µg/mL) and Collagenase type 2 (2 mg/mL) for 30 min at 37 °C and passed through a 70-µm cell strainer to generate a single cell suspension. pDCs were enriched from spleen by negative selection using magnetic beads (Plasmacytoid Dendritic Cell Isolation kit, Miltenyi) according to manufacturer instructions.
MS-1 Endothelial Cell Line and Generation of STING-Deficient MS-1 Endothelial Cells.
MS-1 endothelial cell line was obtained from the ATCC. MS-1 cells were cultured in DMEM supplemented with 10% FCS and 1 mM penicillin/streptomycin. STING knockdown MS-1 cells were generated using pLKO.1-puro plasmids expressing mouse STING specific shRNA or nonrelevant shRNA from Sigma (SHCLNG-NM_028261 and SHC002, respectively). MS-1 cells were cultured with lentivirus-containing supernatants for 24 h before replacing the medium. After 72 h, puromycin (10 µg/mL) was added to the culture to select infected cells. STING knockdown efficiency was verified by Western blot analysis of total cell extract using the anti-STING antibody 3337S (Cell Signaling Technology).
IFNγ ELISPOT.
IFNγ producing cells were quantified using mouse IFN gamma ELISPOT Ready-SET-Go assay from eBioscience according to manufacturer instructions.
Statistical Analysis.
All statistical analyses were performed with GraphPad Prism 6.0 software. Test, group sizes, and P values are given in the corresponding figure legends. P values <0.05 were considered statistically significant.
Acknowledgments
We are grateful to Ana Joncic, Isabelle Surbeck, and Léa Zaffalon for their excellent technical assistance; S. Tartare Deckert (C3M, INSERM U1065) and M. Bosenberg (Yale University School of Medicine) for the kind gift of the B-raf(V600E/+) PTEN−/− CDKN2A−/− primary melanoma cell line; and Prof. P. Romero (Ludwig Cancer Research) for the kind gift of the Tyr::N-ras(Q61K)INK4A−/− melanoma cell line. This work was supported by Grant KLS-3161-02-2013 from the Swiss Cancer League and Grant PO1 CA128913 from the National Cancer Institute (both to M.G.). T.V.P. was supported by Grant KLS-3406-02-2014 from the Swiss Cancer League.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1512832112/-/DCSupplemental.
References
- 1.Luke JJ, Hodi FS. Ipilimumab, vemurafenib, dabrafenib, and trametinib: Synergistic competitors in the clinical management of BRAF mutant malignant melanoma. Oncologist. 2013;18(6):717–725. doi: 10.1634/theoncologist.2012-0391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pittet MJ, et al. High frequencies of naive Melan-A/MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J Exp Med. 1999;190(5):705–715. doi: 10.1084/jem.190.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Valmori D, et al. Frequent cytolytic T-cell responses to peptide MAGE-A10(254-262) in melanoma. Cancer Res. 2001;61(2):509–512. [PubMed] [Google Scholar]
- 4.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363(8):711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hamid O, et al. Safety and tumor responses with lambrolizumab (anti-PD-1) in melanoma. N Engl J Med. 2013;369(2):134–144. doi: 10.1056/NEJMoa1305133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wenzel J, et al. Type I interferon-associated recruitment of cytotoxic lymphocytes: A common mechanism in regressive melanocytic lesions. Am J Clin Pathol. 2005;124(1):37–48. doi: 10.1309/4EJ9KL7CGDENVVLE. [DOI] [PubMed] [Google Scholar]
- 7.Fuertes MB, et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8alpha+ dendritic cells. J Exp Med. 2011;208(10):2005–2016. doi: 10.1084/jem.20101159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diamond MS, et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J Exp Med. 2011;208(10):1989–2003. doi: 10.1084/jem.20101158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jablonska J, Leschner S, Westphal K, Lienenklaus S, Weiss S. Neutrophils responsive to endogenous IFN-beta regulate tumor angiogenesis and growth in a mouse tumor model. J Clin Invest. 2010;120(4):1151–1164. doi: 10.1172/JCI37223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Takaoka A, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448(7152):501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 11.Unterholzner L, et al. IFI16 is an innate immune sensor for intracellular DNA. Nat Immunol. 2010;11(11):997–1004. doi: 10.1038/ni.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Z, et al. The helicase DDX41 senses intracellular DNA mediated by the adaptor STING in dendritic cells. Nat Immunol. 2011;12(10):959–965. doi: 10.1038/ni.2091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tanaka Y, Chen ZJ. STING specifies IRF3 phosphorylation by TBK1 in the cytosolic DNA signaling pathway. Sci Signal. 2012;5(214):ra20. doi: 10.1126/scisignal.2002521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Abe T, et al. STING recognition of cytoplasmic DNA instigates cellular defense. Mol Cell. 2013;50(1):5–15. doi: 10.1016/j.molcel.2013.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gao P, et al. Cyclic [G(2′,5′)pA(3′,5′)p] is the metazoan second messenger produced by DNA-activated cyclic GMP-AMP synthase. Cell. 2013;153(5):1094–1107. doi: 10.1016/j.cell.2013.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ablasser A, et al. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature. 2013;498(7454):380–384. doi: 10.1038/nature12306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Woo SR, et al. STING-dependent cytosolic DNA sensing mediates innate immune recognition of immunogenic tumors. Immunity. 2014;41(5):830–842. doi: 10.1016/j.immuni.2014.10.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deng L, et al. STING-dependent cytosolic DNA sensing promotes radiation-induced type I interferon-dependent antitumor immunity in immunogenic tumors. Immunity. 2014;41(5):843–852. doi: 10.1016/j.immuni.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ackermann J, et al. Metastasizing melanoma formation caused by expression of activated N-RasQ61K on an INK4a-deficient background. Cancer Res. 2005;65(10):4005–4011. doi: 10.1158/0008-5472.CAN-04-2970. [DOI] [PubMed] [Google Scholar]
- 20.Pencheva N, Buss CG, Posada J, Merghoub T, Tavazoie SF. Broad-spectrum therapeutic suppression of metastatic melanoma through nuclear hormone receptor activation. Cell. 2014;156(5):986–1001. doi: 10.1016/j.cell.2014.01.038. [DOI] [PubMed] [Google Scholar]
- 21.Ishikawa H, Ma Z, Barber GN. STING regulates intracellular DNA-mediated, type I interferon-dependent innate immunity. Nature. 2009;461(7265):788–792. doi: 10.1038/nature08476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Roh MR, et al. Difference of interferon-α and interferon-β on melanoma growth and lymph node metastasis in mice. Melanoma Res. 2013;23(2):114–124. doi: 10.1097/CMR.0b013e32835e7713. [DOI] [PubMed] [Google Scholar]
- 23.Shibata S, et al. Induction of efficient antitumor immunity using dendritic cells activated by recombinant Sendai virus and its modulation by exogenous IFN-beta gene. J Immunol. 2006;177(6):3564–3576. doi: 10.4049/jimmunol.177.6.3564. [DOI] [PubMed] [Google Scholar]
- 24.Tough DF, Borrow P, Sprent J. Induction of bystander T cell proliferation by viruses and type I interferon in vivo. Science. 1996;272(5270):1947–1950. doi: 10.1126/science.272.5270.1947. [DOI] [PubMed] [Google Scholar]
- 25.Stein SC, Lam E, Falck-Pedersen E. Cell-specific regulation of nucleic acid sensor cascades: A controlling interest in the antiviral response. J Virol. 2012;86(24):13303–13312. doi: 10.1128/JVI.02296-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ma F, et al. Positive feedback regulation of type I interferon by the interferon-stimulated gene STING. EMBO Rep. 2015;16(2):202–212. doi: 10.15252/embr.201439366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ehnfors J, et al. Horizontal transfer of tumor DNA to endothelial cells in vivo. Cell Death Differ. 2009;16(5):749–757. doi: 10.1038/cdd.2009.7. [DOI] [PubMed] [Google Scholar]
- 28.Ablasser A, et al. Cell intrinsic immunity spreads to bystander cells via the intercellular transfer of cGAMP. Nature. 2013;503(7477):530–534. doi: 10.1038/nature12640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolchok JD, et al. Nivolumab plus ipilimumab in advanced melanoma. N Engl J Med. 2013;369(2):122–133. doi: 10.1056/NEJMoa1302369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kerbel RS. Tumor angiogenesis. N Engl J Med. 2008;358(19):2039–2049. doi: 10.1056/NEJMra0706596. [DOI] [PMC free article] [PubMed] [Google Scholar]