Significance
Protein Numb controls cell fate by interacting with a number of signaling molecules, critical for maintaining neural stem cells and neuronal development in the central nervous system. However, the function of Numb has not been studied in mature neurons. Here, we found that deletion of Numb in cerebellar Purkinje cells causes deficits in motor learning, which can be ascribed to a reduced level of synaptic metabotropic glutamate 1 receptor (mGlu1). Although Numb deficiency does not alter the expression of mGlu1-associated proteins and activity-dependent plasticity of mGlu1, it inhibits intracellular trafficking of mGlu1. To our knowledge, our work is the first to demonstrate an important function of Numb in mature neurons.
Keywords: Numb, mGlu1, trafficking, Purkinje cell, cerebellum
Abstract
Protein Numb, first identified as a cell-fate determinant in Drosophila, has been shown to promote the development of neurites in mammals and to be cotransported with endocytic receptors in clathrin-coated vesicles in vitro. Nevertheless, its function in mature neurons has not yet been elucidated. Here we show that cerebellar Purkinje cells (PCs) express high levels of Numb during adulthood and that conditional deletion of Numb in PCs is sufficient to impair motor coordination despite maintenance of a normal cerebellar cyto-architecture. Numb proved to be critical for internalization and recycling of metabotropic glutamate 1 receptor (mGlu1) in PCs. A significant decrease of mGlu1 and an inhibition of long-term depression at the parallel fiber–PC synapse were observed in conditional Numb knockout mice. Indeed, the trafficking of mGlu1 induced by agonists was inhibited significantly in these mutants, but the expression of ionotropic glutamate receptor subunits and of mGlu1-associated proteins was not affected by the loss of Numb. Moreover, transient and persistent forms of mGlu1 plasticity were robustly induced in mutant PCs, suggesting that they do not require mGlu1 trafficking. Together, our data demonstrate that Numb is a regulator for constitutive expression and dynamic transport of mGlu1.
Numb was first identified in Drosophila melanogaster (1) and is evolutionarily conserved across species (2). During cell division it segregates asymmetrically in dividing cells and determines cell fate by interacting with and inhibiting Notch (2–4). Numb and Numblike, two homologs in mammals (5), are believed to play redundant roles (6). Numb contains a phosphotyrosine-binding domain (PTB), a proline-rich domain (PRR), and two Eps15 homology regions (DPF and NPF). These domains and motifs make Numb an adaptor protein capable of interacting with a number of molecules including Notch, Hedgehog, and p53 (2).
In the mammalian CNS Numb/Numblike is essential for maintaining neural stem cells during neurogenesis (7–10). Numb may play a critical role in axonal growth during the development of hippocampal pyramidal cells by mediating endocytosis of neuronal adhesion molecule L1 (11), and knocking down Numb/Numblike reduces spine density (12). Numb/Numblike is expressed not only in neuronal progenitor cells but also in postmitotic adult neurons (5); however, in mature neurons the cellular function of Numb and its role at the system level in vivo are unknown.
Because Numb is located in clathrin-coated vesicles and is cotransported with endocytic receptors (13), we hypothesized that in adult mammals it might be involved in long-term plasticity and trafficking of glutamate receptors (14). We used cerebellar Purkinje cells (PCs) as a model system to investigate these processes, because these associations have been clearly laid out in PCs, and they may reveal tractable read-outs at the behavioral level (15–18). Our data indicate that conditional deletion of Numb in PCs causes functional deficits in motor coordination, which may be ascribed to reduced trafficking of metabotropic glutamate 1 receptor (mGlu1) to perisynaptic sites at parallel fiber (PF)–PC synapses.
Results
Adult PCs Express Numb but Not Numblike.
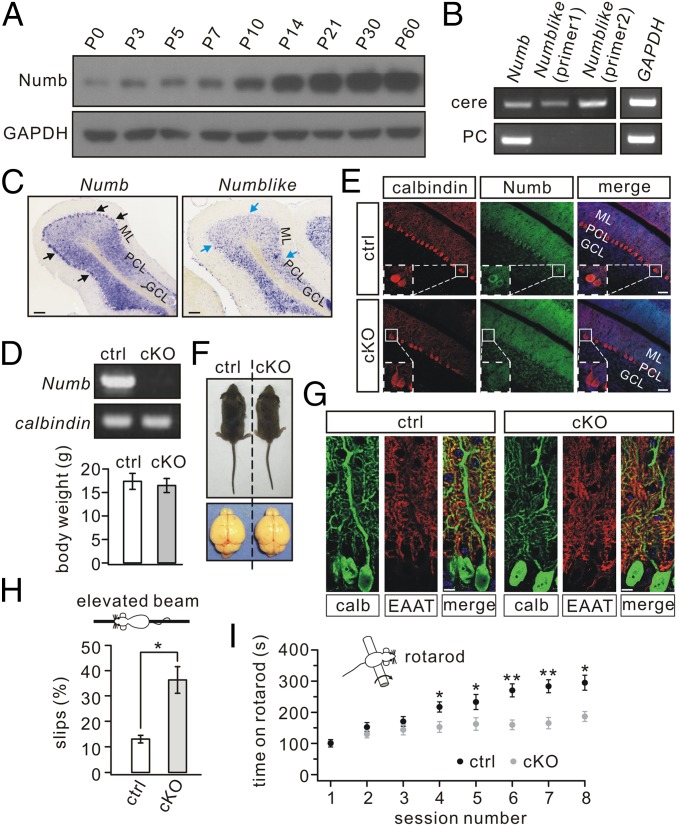
Expression of Numb was fairly weak in mice at birth but increased to a peak at approximately postnatal day (P)10 and remained constant thereafter (Fig. 1A and Fig. S1). The mRNA expressions of Numb and its close homolog Numblike (5) were examined in whole cerebella and individual PC somata using RT-PCR (19–21). Transcripts of both were detected in the whole cerebellum of P30 mice, but only Numb was found in PCs (Fig. 1B). The transcription patterns of Numb and Numblike also were examined with in situ hybridization of P30 mice. In accord with RT-PCR, Numb hybridization was localized to PC somata, but no signal for Numblike was found (Fig. 1C). The exclusive expression of Numb in P30 PCs was unexpected, because Numb and Numblike had been found to coexist in neuronal precursor cells (22).
Fig. 1.
Impaired motor coordination in Numb-cKO mice. (A) Numb expression at different postnatal stages in control cerebellum. GAPDH was the internal control. (B) Electrophoresis of Numb (214 bp), Numblike (369 bp), and GAPDH (172 bp) amplicons from cerebellar extracts (cere) (n = 5) and individual PCs (n = 7). (C) In situ hybridization with Numb and Numblike riboprobes in cerebellar sagittal sections from P30 control mice (n = 5). Note that Numb (black arrows), but not Numblike (blue arrows), was expressed abundantly in PCs. GCL, granule cell layer; ML, molecular layer; PCL, PC layer. (Scale bars: 100 μm.) (D) Electrophoresis of Numb and calbindin from individual control and Numb-cKO PCs (n = 10). (E) Immunohistochemical staining for calbindin (red), Numb (green), and DAPI (blue) in the cerebellum from control and Numb-cKO mice. Higher magnifications in dashed white boxes indicate that Numb signal is absent from Numb-cKO PCs. (Scale bars: 50 μm.) (F) Numb-cKO mice (P21) displayed normal body size and brain. Average body weights were 17.5 ± 1.7 g (control) and 16.6 ± 1.5 g (cKO) (n = 12 pairs; P > 0.05). (G) Immunostaining for calbindin (calb, red) and EAAT4 (green) shows dendrites and spine formation are normal in Numb-cKO mice compared with control. (Scale bars: 10 μm.) (H) Percentage of steps with hindpaw slips during runs on an elevated horizontal beam (n = 10 pairs). (I) Time spent on the accelerating rotarod for control and Numb-cKO mice (n = 10 pairs). *P < 0.05, **P < 0.01.
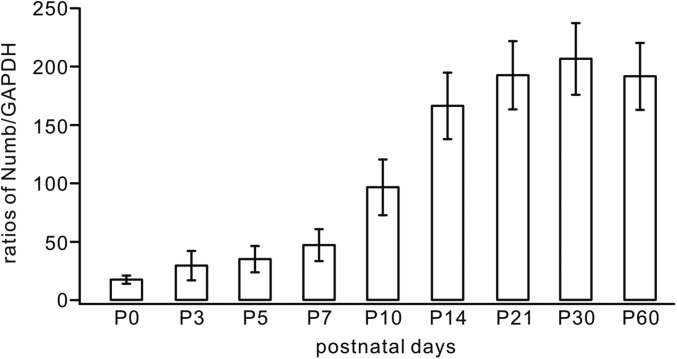
Fig. S1.
The expression of Numb in the developing cerebellum. Total protein extracted from mouse cerebella at postnatal stages probed with antibodies against Numb and GAPDH. Signal intensity ratios (Numb/GAPDH) were 18 ± 4% (P0), 31 ± 13% (P3), 37 ± 11% (P5), 48 ± 13% (P7), 98 ± 24% (P10), 168 ± 29% (P14), 194 ± 29% (P21), 208 ± 31% (P30), and 193 ± 29% (P60). n ≥ 3 for each age.
Numb Deficiency in PCs Does Not Impair Cyto-Architecture but Affects Motor Coordination.
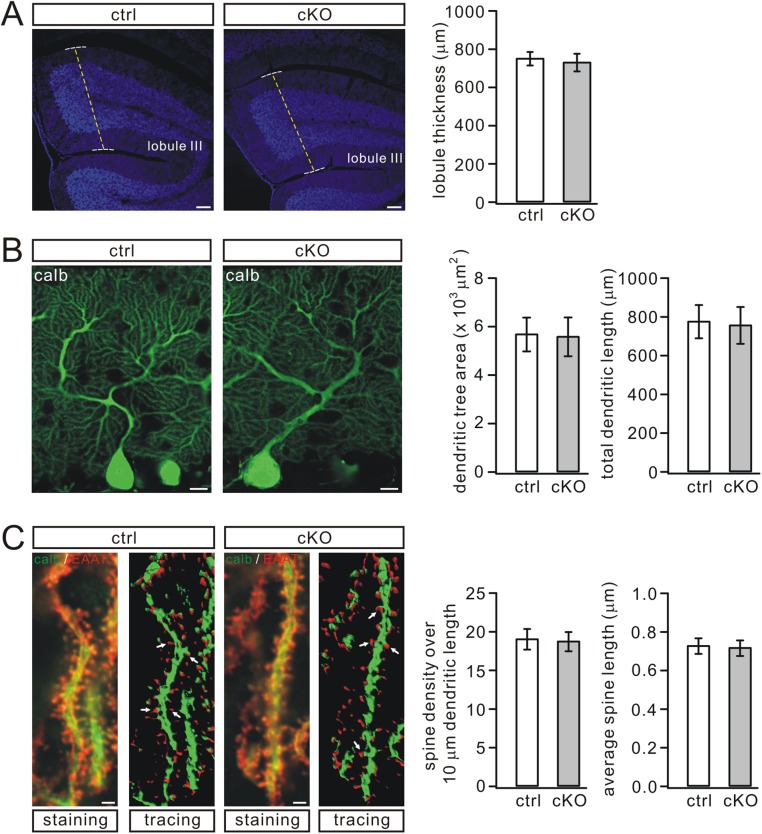
To assess potential roles of Numb at the system level, we generated conditional knockout (cKO) mice that lacked Numb specifically in PCs using the L7-promoter (Numb-cKO) (23). Deletion of Numb in PCs of Numb-cKO animals was confirmed by a lack of the Numb band following RT-PCR of mRNA extracted from their PCs (Fig. 1D) and by the lack of immunostaining in their PCs (Fig. 1E). The preserved Numb signal in the granular and molecular layers (Fig. 1E) is in line with the expression of Numb in granule cells and Bergmann glia, as shown during development (10, 24). Postnatal Numb-cKO mice (P21) appeared normal, as shown by unchanged body weight and cerebellar size (Fig. 1F). Also, their cerebellar cyto-architecture appeared normal following immunostaining for calbindin and excitatory amino acid transporter 4 (EAAT4) (25). Moreover, PC-specific Numb deficiency did not interfere with lobule thickness, dendritic branching, or number of spines (Fig. 1 E and G and Fig. S2).
Fig. S2.
PC morphogenesis is normal in Numb-cKO mice. (A) DAPI staining in the cerebellum from control and Numb-cKO mice (P21). The thickness of lobule III, which was 753 ± 35 μm (control; n = 7) or 733 ± 46 μm (Numb-cKO; n = 6), was measured as indicated by dashed lines. (Scale bars: 100 μm.) (B) Calbindin (calb) staining of PCs from control and Numb-cKO mice (P21). The average dendritic tree area was 5.7 ± 0.7 ×103 μm2 (control; n = 16) or 5.6 ± 0.8 ×103 μm2 (Numb-cKO; n = 17). Total dendritic length was 778 ± 86 μm (control; n = 16) or 759 ± 95 μm (Numb-cKO; n = 17). (Scale bars: 10 μm.) (C) Double staining with calbindin and EAAT4 on distal dendrites of PCs from control and Numb-cKO mice (P21). White arrows show spines detected using 2D reconstruction. The number of spines over a 10-μm dendritic fragment was 19.1 ± 1.34 μm (control; n = 15) or 18.8 ± 1.24 μm (Numb-cKO; n = 14). The average spine length was 0.73 ± 0.04 μm (control; n = 15) or 0.72 ± 0.04 μm (Numb-cKO; n = 14). (Scale bars: 1 μm.)
Numb-cKO mice did not show overt ataxia in standard cages (Movie S1). However, they performed poorly, with a remarkably higher number of hind-paw slips, when walking on a narrow elevated beam (Fig. 1H). Numb-cKO mice also exhibited impaired motor learning in that they showed limited improvement after four or five sessions on the accelerating rotarod compared with controls (Fig. 1I). Together, these results indicate that Numb deficiency in PCs impairs motor coordination.
Long-Term Depression Is Blocked but Long-Term Potentiation Is Normal in Numb-cKO Mice.
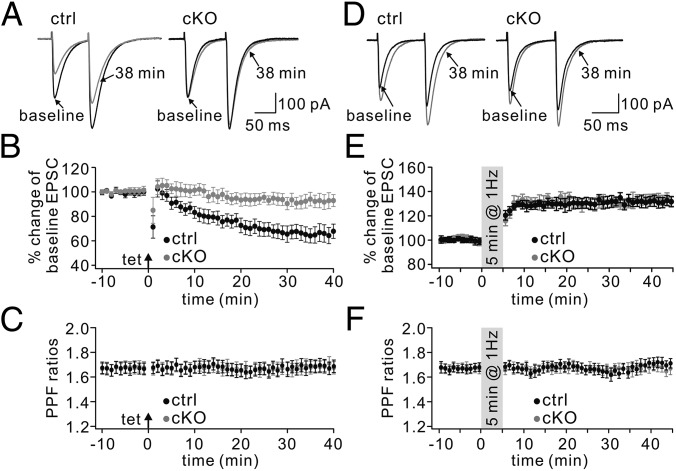
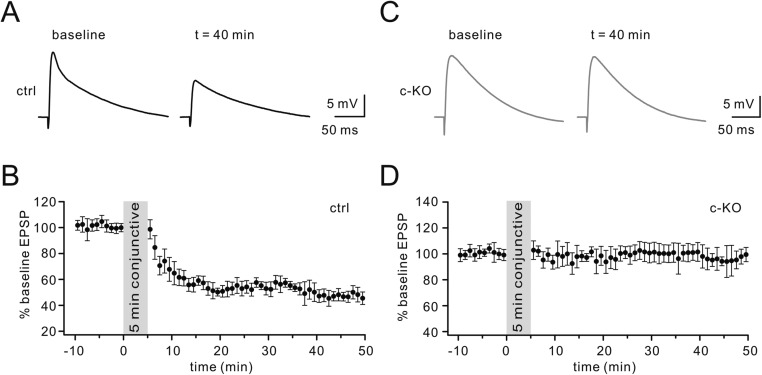
Given the location of Numb in clathrin-coated vesicles and the role of clathrin-mediated endocytosis of glutamate receptors in the expression of long-term depression (LTD) (14), we examined LTD at PF–PC synapses in Numb-cKO mice in voltage-clamp mode. As shown by paired-pulse responses to PF stimulation before and after LTD induction (Fig. 2A), control PCs showed robust PF-LTD (t = 38 min: 67 ± 7% of baseline; n = 12, P < 0.01) (Fig. 2B) in response to repetitive PF tetanus paired with PC depolarization. However, LTD induced with this protocol was blocked in Numb-cKO PCs (t = 38 min: 93 ± 6% of baseline; n = 11, P > 0.05) (Fig. 2B). Likewise, LTD of PF excitatory postsynaptic potentials (EPSPs) induced by a conjunction of double PF shocks and PC depolarization (200 ms, 1 nA) repeated at 1 Hz for 5 min in current-clamp mode was also blocked in Numb-cKO mice (Fig. S3) (26, 27). The ratio of paired-pulse facilitation (PPF) measured at an interval of 80 ms was not changed after LTD induction in control and Numb-cKO mice (Fig. 2C), indicating that presynaptic glutamate release was not affected.
Fig. 2.
Inhibited LTD but normal LTP in Numb-cKO mice. (A) Two consecutive PF EPSCs before (baseline) and after (t = 38 min) LTD induction in control (ctrl) and Numb-cKO PCs. The interval between paired EPSCs was 80 ms. (B) Time courses for percentage changes of EPSC1 amplitude in control (black) and Numb-cKO (gray) mice. Each data point was the average of three successive EPSCs evoked at 0.05 Hz. The arrow indicates LTD induction. (C) Time courses for PPF from the cells shown in B. (D) Example consecutive PF EPSCs before (baseline) and after (t = 38 min) LTP induction. (E) Time courses for percentage changes of EPSC1 amplitude in control (black) and Numb-cKO (gray) mice. (F) Time courses for PPF ratios from the subset of cells shown in E.
Fig. S3.
Induction of PF-LTD in current-clamp configuration. (A) Sample traces of PF EPSPs before and 40 min after a conjunctive stimulation in control mice (P20–P23). Each trace was an average of two consecutive EPSPs. (B) Time course of percentage changes of EPSP amplitude in control mice. (C) Sample traces averaged from two consecutive EPSPs before and 40 min after the conjunction in Numb-cKO mice (P20–P23). (D) Time course of percentage changes in EPSP amplitude in Numb-cKO mice.
Because not only PF-LTD (18, 28–31), but also PF–long-term potentiation (LTP) (17, 32–34), has been proposed as a potential factor contributing to cerebellar motor learning, we investigated the induction of PF-LTP in Numb-cKO mice using a 1-Hz tetanus protocol according to previous work (35–37). After acquiring stable excitatory postsynaptic currents (EPSCs) under voltage-clamp mode (−70 mV), a tetanus stimulation (1 Hz for 5 min) was delivered to PFs while the PC was current-clamped (Fig. 2D). The potentiation of EPSC reached 131 ± 4% of baseline in control mice (t = 38 min; n = 11, P < 0.01) (Fig. 2E). The same protocol in Numb-cKO mice evoked LTP equally successfully (132 ± 4% at t = 38 min; n = 10, P < 0.01) (Fig. 2E), suggesting that Numb is not associated with PF-LTP. The PPF ratio was unaffected after induction in both control and Numb-cKO mice (Fig. 2F), indicating that this LTP is expressed postsynaptically.
mGlu1 Is Reduced at PC Synapses in Numb-cKO Mice.
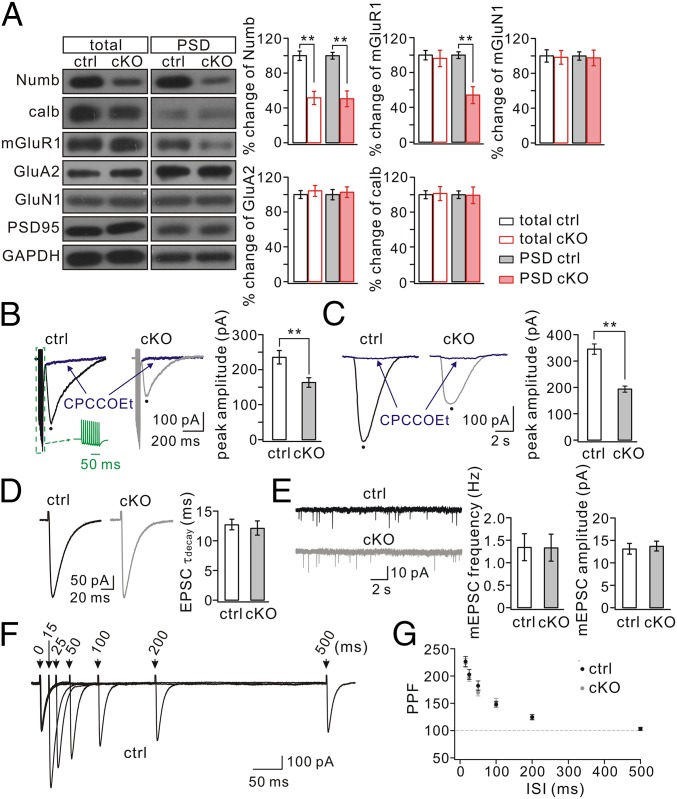
Impaired PF-LTD and motor deficits may result from altered mGlu1 expression (30, 38) and/or glutamatergic transmission (39). Therefore, expression levels of mGlu1 and ionotropic glutamate receptors were examined in relation to those of Numb and calbindin. In control cerebellum (P30) Numb was detected in the isolated synaptic fraction (Fig. 3A), in line with previous work (11). In Numb-cKO cerebellum (P30) Numb expression was significantly reduced in both total and synaptic fractions (P < 0.01) (Fig. 3A); the remaining Numb may have been from cells other than PCs, because Numb was not present in PCs of Numb-cKO mice (Fig. 1 D and E). The amount of calbindin was similar in control and Numb-cKO mice (Fig. 3A), once again indicating that PC development is normal in Numb-cKO mice. Interestingly, synaptic mGlu1 was significantly reduced in Numb-cKO mice (P < 0.01), although its total expression was not changed (Fig. 3A). Because mGlu1 is highly expressed at excitatory synapses on PCs (30, 40), this result suggests that PC ablation of Numb may directly cause the reduction of mGlu1 at PC synapses. In contrast, neither AMPA receptor (AMPAR) subunit GluA2 nor NMDA receptor (NMDAR) subunit GluN1 was changed in the whole cerebellum or at the synaptic level (P > 0.05) (Fig. 3A).
Fig. 3.
Synaptic mGlu1 is reduced in Numb-cKO mice. (A) Cerebellar (total) and PSD fractions from control (ctrl) and Numb-cKO mice were probed with antibodies to Numb, calbindin, mGlu1, GluA2, and GluN1. GAPDH and PSD95 were internal controls for total and PSD, respectively. Histograms show percentage changes of proteins in Numb-cKO mice relative to control. Control (n = 4): 100 ± 5% (total, Numb), 100 ± 4% (PSD, Numb); 100 ± 4% (total, calbindin), 100 ± 4% (PSD, calbindin); 100 ± 5% (total, mGlu1), 100 ± 4% (PSD, mGlu1); 100 ± 4% (total, GluA2), 100 ± 6% (PSD, GluA2); 100 ± 7% (total, GluN1), 100 ± 5% (PSD, GluN1). Numb-cKO (n = 4): 51 ± 7% (total, Numb), 50 ± 9% (PSD, Numb); 101 ± 8% (total, calbindin), 99 ± 9% (PSD, calbindin); 96 ± 9% (total, mGlu1), 54 ± 9% (PSD, mGlu1); 104 ± 6% (total, GluA2), 102 ± 6% (PSD, GluA2); 98 ± 8% (total, GluN1), 97 ± 9% (PSD, GluN1). (B) mGlu1 EPSCs produced by a PF burst (Green Inset: 10 pulses, 100 Hz). mGlu1 EPSCs were blocked by its antagonist CPCCOEt (100 μM). Peaks were measured as indicated by black dots. (C) Slow currents were evoked by a pulse (10 psi, 20 ms) of aCSF containing DHPG (100 μM) and were blocked by the mGlu1 antagonist CPCCOEt (100 μM). (D) Representative AMPA EPSCs in control and Numb-cKO PCs. The decay was fit with a single exponential in both cells, and mean time-constants were 12.8 ± 0.9 ms (control; n = 24) and 12.2 ± 1.2 ms (cKO; n = 30). (E) mEPSCs recorded from control (n = 10) and Numb-cKO (n = 10) PCs. mEPSC parameters were frequency, 1.4 ± 0.3 (control) and 1.3 ± 0.3 (cKO); amplitude, 13.2 ± 1.2 pA (control) and 13.8 ± 1.1 pA (cKO). (F) Superposition of PF EPSCs evoked at different intervals in a control cell. (G) PPF as a function of interstimulus interval in control (n = 11) and Numb-cKO (n = 12) cells. **P < 0.01.
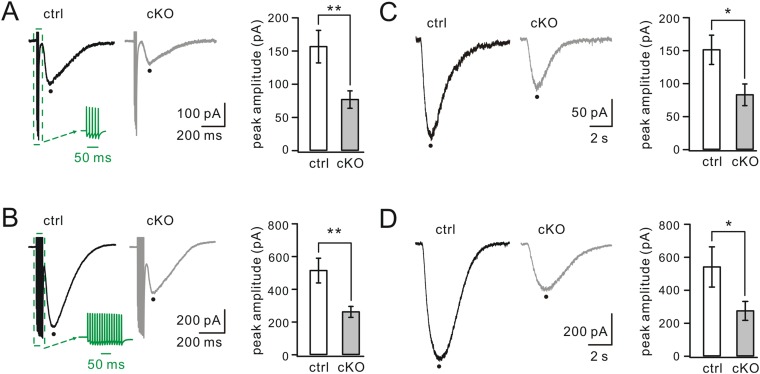
If the level of mGlu1 is reduced at PF–PC synapses, we should be able to record a specific difference in currents using whole-cell recordings. Following burst (100 Hz) stimulation of PFs under a holding voltage of −70 mV in artificial cerebrospinal fluid (aCSF) supplemented with 5 μM NBQX (an AMPAR agonist), PCs displayed fast currents mediated by AMPARs and a slow component mediated by mGlu1 (Fig. 3B) (41, 42). The peak amplitude of mGlu1 EPSC was reduced significantly in Numb-cKO mice (165 ± 12 pA; n = 14) compared with controls (237 ± 18 pA; n = 14; P < 0.01) (Fig. 3B). Moreover, we measured mGlu1 currents evoked by a brief pulse of aCSF containing the mGlu1 agonist 3,5-dihydroxyphenylglycine (DHPG; 100 μM) onto recorded PCs (41). DHPG-induced currents were markedly smaller in Numb-cKO mice (196 ± 12 pA; n = 13) than in controls (348 ± 19 pA; n = 12; P < 0.01) (Fig. 3C). Finally, the reduction of mGlu1 currents in Numb-cKO mice was confirmed further when multiple PF stimulations (5 or 15 pulses at 100 Hz) or different doses of DHPG (50 and 150 μM) were applied (Fig. S4). In contrast, AMPA currents were not affected in PCs of Numb-cKO mice, because the time constants of AMPA EPSC decay were not significantly different (P > 0.05) (Fig. 3D), both frequency and amplitude of AMPA miniature EPSCs (mEPSCs) were unaltered (P > 0.05) (Fig. 3E) (43), and the PPF of AMPA EPSCs did not change at different stimulus intervals (P > 0.05) (Fig. 3 F and G).
Fig. S4.
mGlu1 currents evoked by varying PF pulses and concentrations of DHPG. (A) mGlu1 EPSCs induced by PF burst (Green Inset: five pulses at 100 Hz). The average of peak currents was 157 ± 25 pA (control; n = 17) or 78 ± 13 pA (cKO; n = 16). (B) mGlu1 EPSCs produced by 15 PF pulses at 100 Hz (Green Inset). The average of peak currents was 517 ± 76 pA (control; n = 17) or 255 ± 33 pA (cKO; n = 17). (C) Slow currents evoked by a pulse of aCSF containing DHPG (50 μM) in PCs. The average current was 152 ± 22 pA (control; n = 8) or 84 ± 16 pA (cKO; n = 9). (D) Slow currents evoked by DHPG (150 μM) in PCs. The average currents were 544 ± 122 pA (control; n = 12) and 277 ± 57 pA (cKO; n = 10). *P < 0.05. **P < 0.01.
We next investigated whether impaired PF-LTD may be caused by altered endocannabinoid production, P/Q-type Ca2+ channels, or climbing fiber (CF) synapse elimination, all of which are involved in PF-LTD (44–46). We found that depolarization-induced suppression of excitatory synapses (DSE), which depends on Ca2+-mediated endocannabinoid production (44, 47–50), was induced normally in Numb-cKO mice (Fig. S5 A–D). In addition, PCs can produce endocannabinoid through mGlu1 signaling (51, 52). We found that the inhibition of PF EPSPs induced by high-frequency stimuli (52) did not differ in control and Numb-cKO mice (Fig. S5 E–H), suggesting that mGlu1-induced endocannabinoid production is not affected by Numb deficiency. Moreover, there was no difference in P/Q channel-mediated Ca2+ transient between control and Numb-cKO mice (Fig. S6). Finally, CF EPSCs were elicited in an all-or-none fashion in the majority of PCs in both control and Numb-cKO mice (Fig. S7), suggesting no difference in CF synapse elimination. These results support the notion that endocannabinoid signaling, P/Q-type Ca2+ channels, and CF synapse elimination in PCs do not contribute to impaired PF-LTD in Numb-cKO mice. Previous studies have reported that CF elimination is impaired in mGlu1 null-mutant mice (30, 53) but can be partially restored by transgenic expression of mGlu1 (27, 30). Our data extend these findings and suggest that partial preservation of mGlu1 can be sufficient for CF elimination.
Fig. S5.
Numb deficiency does not alter DSE at PF–PC synapses. (A) Stimulus protocol with holding potential (hp) of PCs (P17–P20) and stimulation timing (stim). The duration of depolarization to 0 mV was 50 ms. Intervals between control stimuli (1–3) were 20 s. The Δt between depolarization and test stimulus was 5 s. (B) Amplitudes of PF EPSCs derived from one control PC plotted over time for control responses (open circles) and test responses (closed circles). Numbered circles (1–4) correspond to the control and test stimuli in A. Representative EPSCs are shown on the right. (C) Amplitudes of PF EPSCs derived from one Numb-cKO PC (P17–P20) plotted over time for control and test responses. Representative EPSCs are shown on the right. (D) Percentage inhibition of test EPSCs was 33.8 ± 3.9% (control; n = 10) or 35.1 ± 4.6% (Numb-cKO; n = 10), showing no difference between them. **P < 0.01. (E) A brief inhibition of PF EPSPs was induced by a train of 10 stimuli at 50 Hz (indicated by the arrow). PF EPSPs were recorded in PCs from control and Numb-cKO mice (P10–P11) under an elevated temperature (32 °C). (F) Responses of PCs from control (Left) and Numb-cKO (Right) mice to train stimuli at 50 Hz. Vertical bars under traces indicate individual stimulations. (G) PF EPSPs recorded from control (Left) and Numb-cKO (Right) mice at 2 s before the train and 2 s and 30 s following the train. (H) Percentage changes of peak PF EPSPs are plotted in control (n = 7) and in Numb-cKO (n = 6) mice. Arrows mark the start of a brief train.
Fig. S6.
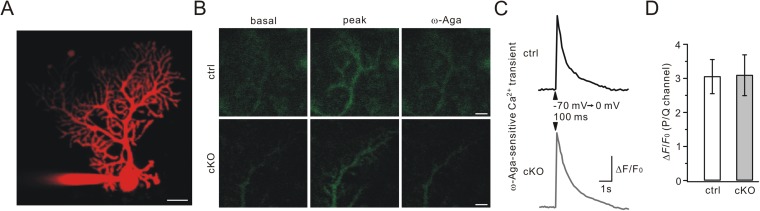
P/Q channel-mediated Ca2+ transient is not changed in Numb-cKO mice. (A) A projected z-stack image of a PC filled with Alexa Fluor 594. (Scale bar: 20 μm.) (B) Fluo-4 signals in the dendritic field of PCs from control (ctrl) and Numb-cKO mice (P21). The same microscopic fields are shown at basal (Left) and peak (Center) [Ca2+]i and after application of ω-Agatoxin IVA (Right). (Scale bars: 10 μm.) (C) ω-Agatoxin–sensitive [Ca2+]i in the cells shown in B. Arrows show the initiation of depolarization. (D) Mean amplitudes of changes in Fluo-4 fluorescence (ΔF/F0) in control and Numb-cKO mice. Ctrl: 3.0 ± 0.5 (n = 17 dendritic regions in 10 PCs). Numb-cKO: 3.1 ± 0.6 (n = 20 dendritic regions in 10 PCs).
Fig. S7.
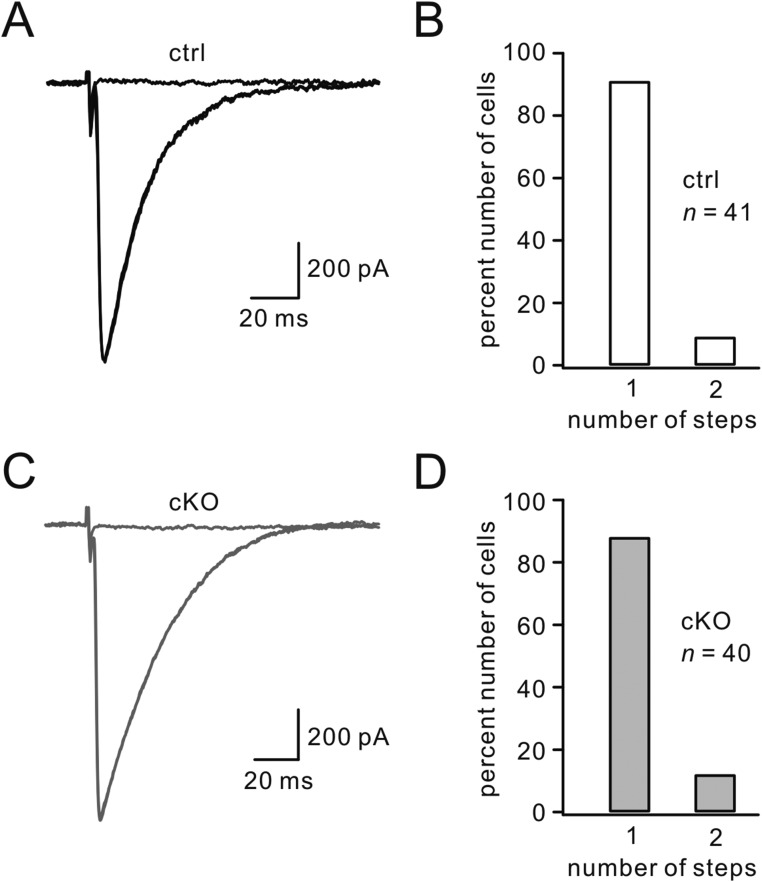
Normal CF synapse elimination in Numb-cKO mice. Tested PCs were from five control and five Numb-cKO mice between P18 and P25. (A) Sample records of CF EPSCs from control PCs. Three traces in response to different intensities are superimposed. (B) Percentages of control PCs showing number of discrete steps of CF EPSCs (1, 91%; 2, 9%). (C) Sample records of CF EPSCs from Numb-cKO PCs. Three traces in response to different intensities are superimposed. (D) Data from Numb-cKO PCs (1, 88%; 2, 12%).
Levels of mGlu1-Associated Proteins Are Normal in Numb-cKO Mice.
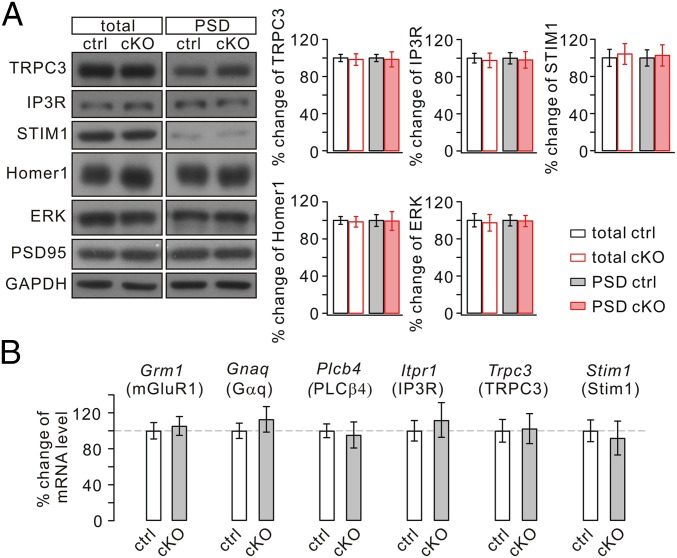
mGlu1 function may depend on associated modifying proteins (19, 54–60). Thus, the cellular changes in mGlu1 expression and currents at PC synapses might reflect a secondary process caused by an ablated interaction between Numb and one or more proteins that interact with mGlu1. We therefore investigated the levels of mGlu1-associated substrates in Numb-cKO mice. Western blots showed deletion of Numb in PCs did not affect the total or synaptic level of mGlu1-related proteins (Fig. 4A), including transient receptor potential channel 3 (TRPC3) (56, 57), inositol trisphosphate receptor (IP3R) (55), stromal interaction molecule 1 (STIM1) (58), Homer1 (59), and ERK (60). Furthermore, real-time quantitative RT-PCR was used to detect transcripts of mGlu1 (Grm1) and genes of associated proteins [Gnaq (Gαq) (19), Plcb4 (PLCβ4) (55), Itpr1 (IP3R) (55), Trpc3 (TRPC3) (56, 57), and Stim1 (Stim1) (58)] in individual PCs. Numb deficiency in PCs did not interfere significantly with mRNA levels of these genes (Fig. 4B). These results suggest that the reduced level of synaptic mGlu1 currents is not caused by deregulation of its associated proteins.
Fig. 4.
Expressions of mGlu1-associated proteins are normal in Numb-cKO mice. (A) Cerebellar (total) and PSD fraction of control (ctrl) and Numb-cKO mice were probed by immunoblotting with antibodies to TRPC3, IP3R, STIM1, Homer1, and ERK. GAPDH and PSD95 were loading controls for total and PSD, respectively. Percentage changes of signal intensities were control, 100 ± 4% (total, TRPC3), 100 ± 4% (PSD, TRPC3); 100 ± 5% (total, IP3R) 100 ± 6% (PSD, IP3R); 100 ± 8% (total, STIM1), 100 ± 8% (PSD, STIM1); 100 ± 4% (total, Homer1), 100 ± 6% (PSD, Homer1); 100 ± 7% (total, ERK), 100 ± 6% (PSD, ERK); cKO, 98 ± 6% (total, TRPC3), 98 ± 8% (PSD, TRPC3); 96 ± 8% (total, IP3R), 97 ± 8% (PSD, IP3R); 104 ± 11% (total, STIM1), 103 ± 11% (PSD, STIM1); 98 ± 5% (total, Homer1), 99 ± 9% (PSD, Homer1); 98 ± 9% (total, ERK), 99 ± 6% (PSD, ERK). n = 4. (B) Percentage changes of transcript copy numbers per cell for the indicated genes. Grm1, 100 ± 9% (n = 13, control) and 105 ± 10% (n = 14, cKO); Gnaq, 100 ± 8% (n = 13, control) and 113 ± 14% (n = 14, cKO); Plcb4, 100 ± 8% (n = 13, control) and 95 ± 14% (n = 14, cKO); Itpr1, 100 ± 11% (n = 13, control) and 112 ± 19% (n = 14, cKO); Trpc3, 100 ± 13% (n = 13, control) and 102 ± 17% (n = 14, cKO); Stim1, 100 ± 12% (n = 13, control) and 92 ± 19% (n = 14, cKO).
Numb Deficiency Affects mGlu1 Trafficking but Not Its Plasticity.
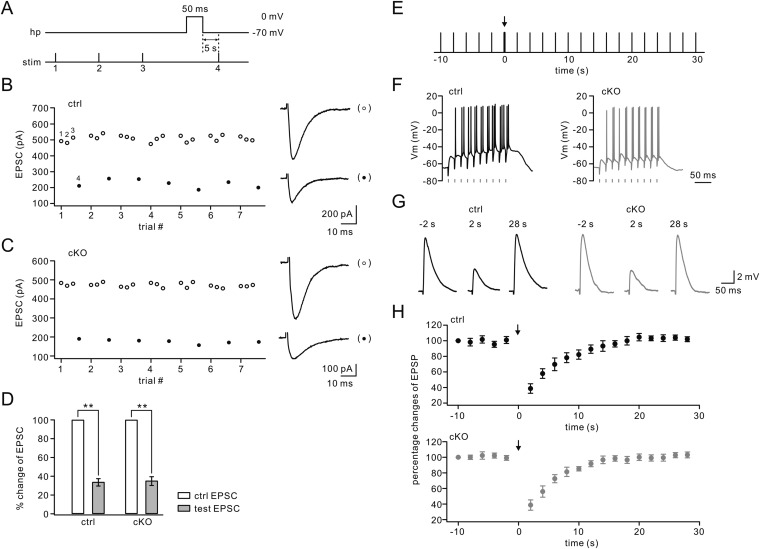
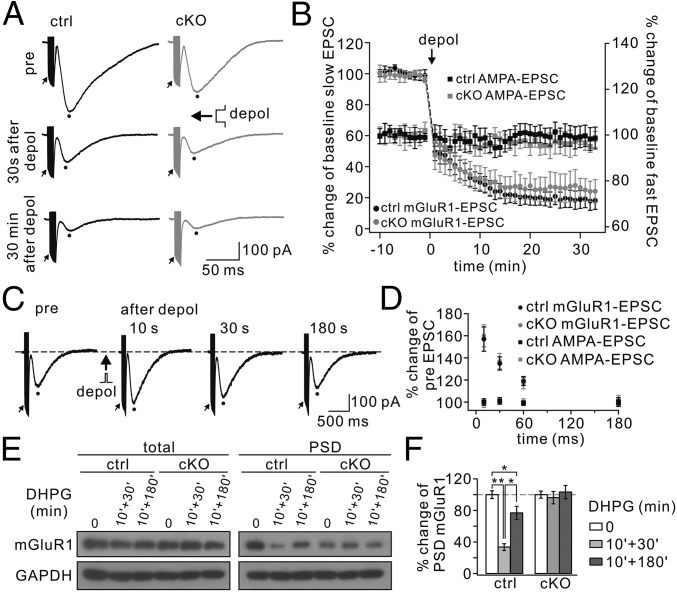
Kim et al. (61) found that mGlu1 currents themselves can exhibit persistent depression (mGluR LTD) following CF stimulation-like depolarization. Because Numb is located in clathrin-coated vesicles and is transported along with endocytic receptors (13), it might be involved in mGlu1 LTD. To address this question, we recorded mGlu1 EPSCs for a stable period and applied a 5-s step depolarization from −70 mV to 0 mV to clamped PCs. In control PCs, mGlu1 EPSCs decreased immediately (30 s after depolarization) and showed a strong depression 30 min later (Fig. 5A). The decrease in mGlu1 EPSCs was slower and the final depression was less substantial (t = 30 min: 19 ± 6% of baseline; n = 12, P < 0.01) (Fig. 5B) than reported by Kim et al. (61). Against our assumption, the amplitude of mGlu1 EPSC also was decreased after depolarization in mutant PCs (t = 30 min: 25 ± 7% of baseline; n = 12, P < 0.01) (Fig. 5 A and B), showing no difference between controls and mutants (P > 0.05). The amplitudes of AMPA EPSCs did not show any change after depolarization either in controls or in Numb-cKO mice (P > 0.05) (Fig. 5B), consistent with previous work (58). These data suggest that Numb is not directly involved in the induction of mGlu1-LTD.
Fig. 5.
mGlu1-LTD and mGlu1-STP are normal, but mGlu1 trafficking is inhibited in Numb-cKO mice. (A) mGlu1 EPSCs before and after the conditioning depolarization (depol; 5-s command to 0 mV at the soma) in control (ctrl) and Numb-cKO cells. Peaks of mGlu1 EPSCs and first AMPA EPSCs were measured as indicated by dots and arrows, respectively. (B) Time courses of percentage changes of mGlu1 EPSCs (left y axis) and AMPA EPSCs (right y axis) from control (n = 12) and Numb-cKO (n = 12) cells before and after 5-s depolarization. (C) Representative traces from one control PC showing trials before (pre), and 10, 30, and 180 s after somatic depolarization to 0 mV for 100 ms. (D) Time courses for percentage changes of mGlu1 EPSCs and AMPA EPSCs in control (n = 10) and Numb-cKO (n = 9) cells. (E) Control and Numb-cKO cerebellar slices were stimulated with 100 μM DHPG for 10 min, and mGlu1 in total and PSD fraction was immunoblotted 30 min or 180 min after DHPG challenge. GAPDH was the control. (F) Percentage changes of synaptic mGlu1 intensities were control: 100 ± 5% (0 min), 34 ± 4% (10 min + 30 min), 77 ± 8% (10 min + 180 min); cKO: 100 ± 4% (0 min), 96 ± 8% (10 min + 30 min), 103 ± 8% (10 min + 180 min). n = 4. *P < 0.05. **P < 0.01.
mGlu1 EPSCs also can undergo a transient (short-term) up-regulation (mGlu1-STP) in response to a brief depolarizing current (41, 62), so we next investigated to what extent Numb might be required for mGlu1-STP. After recording a test mGlu1 EPSC, somatic depolarization to 0 mV for 100 ms was delivered to the recorded PC. A second mGlu1 EPSC was evoked at intervals of 10 to 180 s (Fig. 5C). The conditioning depolarization produced a potentiation of mGlu1 current but had no effect on AMPA EPSCs (P > 0.05) (Fig. 5D). We found that mGlu1-STP was induced robustly in both control and Numb-cKO PCs at similar degrees across a range of intervals (Fig. 5D), indicating that Numb is not involved in mGlu1-STP.
It has been shown that mGlu1 is internalized (63–66) and recycled back into the cell membrane (65) upon ligand exposure. Because trafficking may affect receptor targeting at the cell membrane, we investigated whether Numb is involved in the internalization and recycling of mGlu1. DHPG (100 μM) was applied to acute cerebellar slices for 10 min, and the level of synaptic mGlu1 was measured 30 min (for endocytosis) or 180 min (for recycling) after DHPG application. In control slices, synaptic mGlu1 decreased to 34 ± 4% of the basal level at t = 40 min (n = 4; P < 0.01) but returned to 77 ± 8% at t = 190 min (n = 4; P > 0.05) (Fig. 5 E and F). The down- and up-regulation of mGlu1 expression in response to such agonist challenges was similar to results reported in previous work in other wild-type mice (66–68). In contrast, synaptic mGlu1 was not changed either 30 min or 180 min after DHPG application to Numb-cKO slices (t = 40 min: 96 ± 8%; t = 190 min: 103 ± 8%; P > 0.05 compared with baseline) (Fig. 5 E and F), suggesting that Numb is required for the internalization and recycling of mGlu1 in PCs.
Discussion
The present work reveals a previously unidentified role for Numb in cerebellar PCs: It regulates constitutive synaptic expression and dynamic trafficking of mGlu1 as well as LTD and motor coordination in young adult animals. Numb’s functions in neurogenesis (7–10) and development (11, 12) have been reported previously, but to our knowledge this is the first report regarding Numb function in mature neurons. We showed that (i) Numb is expressed in PCs without coexpression of Numblike, (ii) deficiency of Numb does not overtly influence PC development, (iii) PC-specific Numb deficiency impairs motor coordination, (iv) Numb deficiency specifically decreases constitutive expression of synaptic mGlu1 in PCs and blocked induction of PF-LTD, and (v) the Numb-dependent decrease of mGlu1 currents is not caused by either deregulation of associated proteins or by short-term or long-term forms of mGlu1 plasticity but probably is caused mainly by aberrant internalization and recycling.
The putative role of Numb in internalization is supported by its possible role as an endocytic adaptor (13). Indeed, Numb contains PTB, PRR, DPF, and NPF motifs (2) and is located in clathrin-coated vesicles, probably functioning in the transport of a number of receptors (13). Prevailing evidence has shown that Numb exerts comparable roles in the development of neurons in culture (11, 12) and binds with β-amyloid precursor protein in neurodegenerative diseases (69). We report here that mGlu1 expression in the isolated synaptic fraction in Numb-cKO mice is reduced, although its total expression is not altered. Furthermore, mGlu1 EPSCs evoked by strong burst stimulation of PFs (38, 41, 42) are significantly decreased in Numb-cKO mice. Because mGlu1 is expressed primarily at perisynaptic sites adjacent and linked to the postsynaptic densities (PSDs) (70, 71), the present biochemical and electrophysiological data suggest a selective reduction of mGlu1 expression at the perisynaptic sites in PCs. In contrast, synaptic GluA2 in PCs was not affected by the ablation of Numb. Because Numb is an adaptor of clathrin complex, our findings lead to a hypothesis: Cargo selection during clathrin-dependent transportation is determined not by clathrin and its general adaptors, such as AP2, but by specified adaptor proteins, such as Numb.
In addition, we present several other important findings. We found that Numb is present in PCs, but Numblike is absent. This finding challenges the idea that Numb and Numblike generally coexist in neurons, as observed in neural stem cell studies (22). Possibly, this distinction also reflects a difference in developmental stage, because previously the roles of these proteins had not been investigated extensively in mature neurons. Furthermore, we found that development of spines and dendrites of PCs is largely normal in Numb-cKO mice. These findings place previous reports on the role of Numb in cultured neurons in a different light (11, 12). Possibly, the differences can be explained by differential effects under in vivo and in vitro conditions, but further experimentation is needed.
We show that both the constitutive expression and dynamic trafficking of mGlu1 are regulated by Numb. Evidence mainly from in vitro studies has shown that intracellular trafficking of mGlu1 is subjected to regulation by multiple signaling molecules. The internalization of mGlu1 may depend on various proteins, including arrestin and dynamin (72), protein kinase C (73), caveolar lipid rafts (64, 68), and Rab8 (66). Pandey et al. (67) found that recycling of mGlu1 depends on protein phosphatase 2A and Rab11. Thus, it will be interesting to determine whether and to what extent Numb-regulated internalization and recycling of mGlu1 are mediated by these molecules.
mGlu1 currents undergo STP and LTD in response to CF stimulation or PC depolarization (41, 61, 62), but the underlying mechanisms are not fully understood. Kim et al. (41) found that mGlu1-STP is blocked by depletion of IP3-gated Ca2+ stores and postsynaptic IP3R blockade, suggesting critical roles of endoplasmic reticulum. Kim et al. (61) hypothesized that mGlu1, Gαq, or a protein that interacts with them is the molecular target of mGlu1-LTD. Here, we show that both mGlu1-STP and mGlu1-LTD remain intact, although the intracellular trafficking of mGlu1 is inhibited by Numb knockout. These findings suggest that stimulation protocols for mGlu1-STP and mGlu1-LTD do not induce dynamic transport of mGlu1 and that the plasticity of mGlu1 may be triggered by altered activity of mGlu1-associated proteins.
We found that Numb-cKO mice had impaired motor coordination, which may be directly ascribed to reduced mGlu1 currents, affecting PC excitability (30, 74). To what extent a deficit in mGlu1-dependent PF-LTD contributes to the phenotype on the accelerating rotarod remains to be determined. Many mouse mutants suffering from a lack of PF-LTD perform well on a rotarod, bringing into question a direct role of PF-LTD (17, 32). However, it is possible that a lack of PF-LTD in a motor learning process can be compensated by plastic inhibitory actions of molecular layer interneurons (33) and/or that the significance of PF-LTD and PF-LTP depends on the chemical identity of the modules involved (75, 76). Indeed, zebrin II+ and zebrin II− PCs show different intrinsic properties with probably different propensities for particular learning rules (77).
Materials and Methods
Animal experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals (78) and approved by the Animal Experimentation Ethics Committee of Zhejiang University. Mice in which exon 1 of the Numb gene was flanked by loxP sites were crossed with mice heterozygous for the L7-Cre transgene to obtain Numb-cKO mice. For all experimental details, see SI Materials and Methods. The primer sequences used for the SYBR Green probe (GenBank) are listed in Table S1. The riboprobe sequences are listed in Table S2.
Table S1.
PCR Primers
| Genes | Forward primer | Reverse primer |
| Numb | CAGTGAAGGCCGTTCTGTG | GAGTGGTGCCATCACGAC |
| Numblike (primer1) | CTATGTGCCTGAGGCATC | CCACAGGACAGACTTCAC |
| Numblike (primer2) | CTATGTGCCTGAGGCATC | CCACAGGACAGACTTCAC |
| GAPDH | TGTTACCAACTGGGACGACA | AAGGAAGGCTGGAAAAGAGC |
| Calbindin | CAGTGAAGGCCGTTCTGTG | GAGTGGTGCCATCACGAC |
| Grm1 (GenBank: NM_016976) | TTCGACATCCCACAAATCGCC | ATAGGTCCAGTTGTACCGCTT |
| Gnaq (GenBank: NM_008139) | GGTCGGGCTACTCTGACGA | ACTTGTATGGGATCTTGAGCGT |
| Plcb4 (GenBank: NM_013829) | GGACAAGTGCTAGAATGTTCCC | GAAGCCGATATTCACCAGATCC |
| Itpr1 (GenBank: NM_010585) | CGTTTTGAGTTTGAAGGCGTTT | CATCTTGCGCCAATTCCCG |
| Trpc3(GenBank: AF190645) | AGGCGCAGCAGTATGTGGA | GGCCAAAGCTCTCGTTTGC |
| Stim1 (GenBank: NM_009287) | GGCCAGAGTCTCAGCCATAG | TCCACATCCACATCACCATTAG |
Table S2.
Riboprobes
| Genes | Sequence |
| Numb-F-Notl | ATAAGAATGCGGCCGCGACCGCTGGTTAGAAGAAGTGTCA |
| Numb-R-Xbal | CCGCTCGAGCTGCTGCCAGAGATTTGTTAGGGT |
| Numblike-F-BamH1 | CGGGATCCTGCCCTCAGTCAGAAGAACTCAC |
| Numblike-R-EcoR1 | CGGAATTCGCAATTTGGGCTACAGTTCAATCTC |
SI Materials and Methods
Animals.
Animal experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals (78). Mice were kept under temperature-controlled conditions on a 12-h/12-h light/dark cycle with food and water ad libitum. Mice in which exon 1 of the Numb gene was flanked by loxP sites (https://jaxmice.jax.org/strain/005384.html) were crossed with mice heterozygous for the L7-Cre transgene (23). The resulting offspring were genotyped using PCR of genomic DNA extracted from the tail. Mice were classified as Numb-cKO when the homozygous expression of loxP sites and one Cre allele was present or as control when Cre was absent from the genome. All experiments except those shown in Fig. 1A were done in male mice at the age of P17–P30, depending on experimental requirements.
Behavioral Tests.
After habituation to the rotarod, control and Numb-cKO mice were tested twice a day at ∼8-h intervals for four consecutive days. In each session, the velocity of the rotation increased with a constant acceleration of 9 rpm starting at 5 rpm. The elevated beam balancing test was carried out and analyzed as described previously (58). The experimenters were blinded as to the genotypes of the mice until all data were integrated.
Antibodies and Reagents.
Antibodies against mGlu1 and GluN1 were purchased from BD Pharmingen; antibodies against TRPC3, IP3R, and Homer1 were from Santa Cruz; antibodies against Numb, ERK, and STIM1 were from Cell Signaling; antibodies against PSD95 and GAPDH were from Millipore; the antibody against EAAT4 was from Alpha Diagnostics; HRP-conjugated secondary antibodies were from GE Healthcare, and the anti-GluA2 antibody was a gift from Richard Huganir, Johns Hopkins University, Baltimore. DMEM, DAPI, and Alexa Fluor-conjugated secondary antibodies were from Invitrogen. Protease inhibitor mixture was from Merck Chemicals. Other chemicals were from Sigma unless stated otherwise.
Isolation of the PSD Fraction.
Cerebellar tissues were homogenized in SHEEP buffer [380 mM sucrose, 4 mM Hepes (pH 7.5), 0.1 mM EDTA, 0.1 mM EGTA supplemented with protease inhibitors] and were centrifuged at 800 × g (4 °C, 15 min). The supernatant was centrifuged at 9,200 × g (4 °C, 15 min). The final pellet was the crude PSD fraction.
Western Blotting.
Cerebellar tissues were rinsed with PBS and diluted in 1% SDS containing protease inhibitors. After the protein concentration was determined with a BCA protein assay (Bio-Rad), equal quantities of proteins were loaded and fractionated on SDS/PAGE and transferred to PVDF membranes (Immobilon-P; Millipore), immunoblotted with antibodies, and visualized by enhanced chemiluminescence (Pierce Biotechnology). The primary antibodies used (and corresponding dilutions) were mGlu1 (1:5,000), Numb (1:1,000), TRPC3 (1:500), IP3R (1:500), Homer1 (1:200), STIM1 (1:1,000), ERK (1:1,000), GluN1 (1:5,000), GluA2 (1:2,000), PSD95 (1:10,000), calbindin (1:10,000), and GAPDH (1:10,000); all secondary antibodies were diluted at 1:10,000. Film signals were digitally scanned and quantitated using ImageJ 1.42q (NIH).
Immunohistochemistry.
Sagittal frozen sections (30 μm) were cut and placed in blocking solution for 1 h at room temperature. After washing with PBS, sections were incubated with primary antibodies overnight at 4 °C and incubated with secondary antibodies for 1 h at room temperature. Primary antibody dilutions used for immunohistochemistry were Numb (1:200), EAAT4 (1:200), and calbindin (1:500). Alexa Fluor 488-conjugated goat anti-rabbit IgG and Alexa Fluor 594-conjugated goat anti-mouse IgG were diluted at 1:1,000. All antibodies were diluted in PBS containing 1% BSA and 1% normal goat serum.
Morphometric Analysis of PCs.
Sagittal cerebellar slices (10 μm) were immunostained with antibodies against calbindin and EAAT4. For dendritic branching analysis, images of PCs were captured by a confocal microscope (FV1000; Olympus), and dendritic tracing was performed using MetaMorph software (Olympus). For spine analysis, images of distal dendrites of PCs were captured by a structured illumination microscopy (SIM) super-resolution confocal microscope (N-SIM; Nikon) and were reconstructed in two dimensions using Imaris7.4 software (Bitplane). Determination of spine morphology was based on previous studies (79). Spine density was evaluated as the relative spine number over 10-μm dendritic fragments. Imaging data analysts were blinded as to experimental conditions during the measurement.
Electrophysiology.
Sagittal cerebellar slices (250 μm) prepared from P20–P23 anesthetized mice that were decapitated were placed in a submerged chamber and perfused at 2 mL/min with room temperature aCSF (in mM): 125 NaCl, 2.5 KCl, 1.25 NaH2PO4, 1 MgCl2, 2 CaCl2, 26 NaHCO3, and 25 d-glucose. GABAzine (10 μM) was added to block GABAA receptors. Whole-cell patch-clamp recordings were obtained in PCs from lobules 3–8 of the vermis cortex using an Axon MultiClamp 700B amplifier (Molecular Devices) at room temperature. Patch-clamp electrodes (3–5 MΩ) were filled with an intracellular solution composed of (in mM) 135 Cs-methanesulfonate, 10 CsCl, 10 Hepes, 0.2 EGTA, 4 Na2ATP, and 0.4 Na3GTP (pH 7.3, OSM 290) for voltage-clamp recording or 134 K-gluconate, 6 KCl, 4 NaCl, 10 Hepes, 0.2 EGTA, 4 Na2ATP, 0.3 Na3GTP, 14 Na2 phosphocreatine (pH 7.3, OSM 290) for current-clamp recording (26). Currents were digitized at 10 kHz and filtered at 3 kHz. Stimulation pipettes were filled with aCSF and placed in the middle third of the molecular layer. Recordings were excluded if series or input resistance varied by >15% during the experiment. LTD of PF EPSCs was induced by conjunction of five PF pulses at 100 Hz and a 100-ms-long depolarization of PC to 0 mV, repeated 30 times with an interval of 2 s (42, 43). LTD of PF EPSPs was induced by conjunction of double-shock PF stimulation (each 0.1 ms with an interval of 50 ms) and current pulse-induced membrane depolarization (200 ms, 1 nA), repeated at 1 Hz for 5 min (26). LTP of PF EPSCs was obtained when PFs were stimulated at 1 Hz for 5 min in current-clamp mode (37).
Ca2+ Imaging.
Confocal imaging of Ca2+ transient was performed according to previous work (20, 58, 61). Fluo-4 (200 μM; Invitrogen), a Ca2+ indicator, and Alexa Fluor 594 hydrazide (300 μM; Invitrogen), a cytosolic marker, were added to internal saline composed of (in mM) 135 Cs-methanesulfonate, 10 CsCl, 10 Hepes, 4 Mg2ATP, and 0.4 Na3GTP. After whole-cell configuration in voltage-clamp mode was established, dyes were allowed to diffuse into cells for at least 20 min. [Ca2+]i was elicited by a brief depolarization to 0 mV (100 ms), and fluorescent images from dendritic fields were recorded using a confocal microscope (A1R MP; Nikon) equipped with a 40× water-immersion objective. Fluo-4 images were recorded at 7.5 Hz with a CCD camera in frame-scan mode. Recorded frames were converted offline to stacks of images and were analyzed using ImageJ. Foreground pixels in a region of interest that applied to all frames were determined by manual thresholding and were spatially averaged to calculate F for each frame. First 10 frames were averaged and used as F0, and [Ca2+]i for each frame was thereby expressed as ΔF/F0. P/Q channel-mediated [Ca2+]i was obtained by the subtraction from total [Ca2+]i as ω-Agatoxin IVA (500 nM) (Alomone Labs), a selective P/Q channel blocker, was applied locally to recorded PCs.
RT-PCR and Real-Time Quantitative PCR.
For single-cell analysis, the contents of individual PCs were harvested as described in our previous work (20), with modifications. In brief, the tip of a conventional patch-clamp pipette was placed tightly on the soma of a selected PC. Gentle suction was applied to the pipette. After complete incorporation of the soma, the negative pressure was released, and the pipette was removed quickly from the bath. The harvested contents were subjected to RT-PCR using a OneStep Kit (Qiagen). For quantitative PCR, levels of mRNA from individual PCs and cerebellar extracts were assessed using a CFX96 system (Bio-Rad). The primer sequences used for the SYBR Green probe (GenBank) are listed in Table S1. Quantification was performed using the comparative cycle threshold (Ct) method. GAPDH was used as an internal control, to which relative gene expression was normalized.
In Situ Hybridization.
Standard in situ hybridization was performed as previously described (80). Samples were fixed in 4% (wt/vol) paraformaldehyde/PBS at 4 °C overnight, followed by 20% (wt/vol) sucrose infusion and then were embedded in Tissue-Tek O.C.T. Compound (Sakura Finetek). Samples were cryosectioned at 18 μm. Digoxigenin (DIG)-labeled RNA probes were transcribed by T7, T3, or SP6 RNA polymerase using DIG RNA Labeling Mix or Fluorescein RNA Labeling Mix (Roche Diagnostics). The riboprobe sequences are listed in Table S2.
mGlu1 Trafficking Assay.
The mGlu1 trafficking assay performed as described previously (67, 68), with modifications. In brief, acute cerebellar slices (250 μm) were incubated in aCSF containing DHPG (100 μM) for 10 min at room temperature. Subsequently, slices were chased for various times (30 min or 180 min) in plain aCSF without DHPG at room temperature. Afterward, slices for different conditions were homogenized to determine protein concentrations in total and isolated PSD fractions.
Statistics.
Data analysis was performed using Clampfit 10 (Molecular Devices) and Igor Pro-6.0 (WaveMetrics). SDs for control groups were calculated according to the average of all control data points. Error bars show mean ± SEM. P values were determined by Student’s t-test. The accepted level of significance was P < 0.05. n represents the number of cells or mice tested.
Supplementary Material
Acknowledgments
We thank Drs. Donggen Luo and Mengtong Li (Peking University) for confocal Ca2+ imaging, Dr. Xiaobing Yuan (Institute of Neuroscience, Chinese Academy of Sciences) for providing L7-Cre mice, and Dr. Richard Huganir (Johns Hopkins University) for kindly providing antibody. This work was supported by National Natural Science Foundation of China Grants 31471024, 31271148, 31200818, and 31571051; Natural Science Foundation of Zhejiang Province Grant Z15C090001; Shenzhen Committee for Technological Renovation Grant ZDSY20120617112838879; grants from the Dutch Organization for Medical Sciences (C.I.D.Z.) and Dutch Life Sciences (C.I.D.Z.); and European Research Council (ERC)-Advanced and ERC-Proof of Concept Grants (C.I.D.Z.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1512915112/-/DCSupplemental.
References
- 1.Uemura T, Shepherd S, Ackerman L, Jan LY, Jan YN. numb, a gene required in determination of cell fate during sensory organ formation in Drosophila embryos. Cell. 1989;58(2):349–360. doi: 10.1016/0092-8674(89)90849-0. [DOI] [PubMed] [Google Scholar]
- 2.Gulino A, Di Marcotullio L, Screpanti I. The multiple functions of Numb. Exp Cell Res. 2010;316(6):900–906. doi: 10.1016/j.yexcr.2009.11.017. [DOI] [PubMed] [Google Scholar]
- 3.Wakamatsu Y, Maynard TM, Jones SU, Weston JA. NUMB localizes in the basal cortex of mitotic avian neuroepithelial cells and modulates neuronal differentiation by binding to NOTCH-1. Neuron. 1999;23(1):71–81. doi: 10.1016/s0896-6273(00)80754-0. [DOI] [PubMed] [Google Scholar]
- 4.Cayouette M, Raff M. Asymmetric segregation of Numb: A mechanism for neural specification from Drosophila to mammals. Nat Neurosci. 2002;5(12):1265–1269. doi: 10.1038/nn1202-1265. [DOI] [PubMed] [Google Scholar]
- 5.Zhong W, Jiang MM, Weinmaster G, Jan LY, Jan YN. Differential expression of mammalian Numb, Numblike and Notch1 suggests distinct roles during mouse cortical neurogenesis. Development. 1997;124(10):1887–1897. doi: 10.1242/dev.124.10.1887. [DOI] [PubMed] [Google Scholar]
- 6.Johnson JE. Numb and Numblike control cell number during vertebrate neurogenesis. Trends Neurosci. 2003;26(8):395–396. doi: 10.1016/S0166-2236(03)00166-8. [DOI] [PubMed] [Google Scholar]
- 7.Zhong W, et al. Mouse numb is an essential gene involved in cortical neurogenesis. Proc Natl Acad Sci USA. 2000;97(12):6844–6849. doi: 10.1073/pnas.97.12.6844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Petersen PH, Zou K, Hwang JK, Jan YN, Zhong W. Progenitor cell maintenance requires numb and numblike during mouse neurogenesis. Nature. 2002;419(6910):929–934. doi: 10.1038/nature01124. [DOI] [PubMed] [Google Scholar]
- 9.Petersen PH, Zou K, Krauss S, Zhong W. Continuing role for mouse Numb and Numbl in maintaining progenitor cells during cortical neurogenesis. Nat Neurosci. 2004;7(8):803–811. doi: 10.1038/nn1289. [DOI] [PubMed] [Google Scholar]
- 10.Rasin MR, et al. Numb and Numbl are required for maintenance of cadherin-based adhesion and polarity of neural progenitors. Nat Neurosci. 2007;10(7):819–827. doi: 10.1038/nn1924. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura T, et al. Role of numb in dendritic spine development with a Cdc42 GEF intersectin and EphB2. Mol Biol Cell. 2006;17(3):1273–1285. doi: 10.1091/mbc.E05-07-0700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishimura T, et al. CRMP-2 regulates polarized Numb-mediated endocytosis for axon growth. Nat Cell Biol. 2003;5(9):819–826. doi: 10.1038/ncb1039. [DOI] [PubMed] [Google Scholar]
- 13.Santolini E, et al. Numb is an endocytic protein. J Cell Biol. 2000;151(6):1345–1352. doi: 10.1083/jcb.151.6.1345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wang YT, Linden DJ. Expression of cerebellar long-term depression requires postsynaptic clathrin-mediated endocytosis. Neuron. 2000;25(3):635–647. doi: 10.1016/s0896-6273(00)81066-1. [DOI] [PubMed] [Google Scholar]
- 15.Aiba A, et al. Deficient cerebellar long-term depression and impaired motor learning in mGluR1 mutant mice. Cell. 1994;79(2):377–388. [PubMed] [Google Scholar]
- 16.Schonewille M, et al. Purkinje cell-specific knockout of the protein phosphatase PP2B impairs potentiation and cerebellar motor learning. Neuron. 2010;67(4):618–628. doi: 10.1016/j.neuron.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schonewille M, et al. Reevaluating the role of LTD in cerebellar motor learning. Neuron. 2011;70(1):43–50. doi: 10.1016/j.neuron.2011.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Linden DJ. Neuroscience. From molecules to memory in the cerebellum. Science. 2003;301(5640):1682–1685. doi: 10.1126/science.1090462. [DOI] [PubMed] [Google Scholar]
- 19.Hartmann J, et al. Distinct roles of Galpha(q) and Galpha11 for Purkinje cell signaling and motor behavior. J Neurosci. 2004;24(22):5119–5130. doi: 10.1523/JNEUROSCI.4193-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu ZY, et al. AMPA receptors regulate exocytosis and insulin release in pancreatic β cells. Traffic. 2012;13(8):1124–1139. doi: 10.1111/j.1600-0854.2012.01373.x. [DOI] [PubMed] [Google Scholar]
- 21.Ji YF, et al. Upregulation of glutamate transporter GLT-1 by mTOR-Akt-NF-кB cascade in astrocytic oxygen-glucose deprivation. Glia. 2013;61(12):1959–1975. doi: 10.1002/glia.22566. [DOI] [PubMed] [Google Scholar]
- 22.Kuo CT, et al. Postnatal deletion of Numb/Numblike reveals repair and remodeling capacity in the subventricular neurogenic niche. Cell. 2006;127(6):1253–1264. doi: 10.1016/j.cell.2006.10.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barski JJ, Dethleffsen K, Meyer M. Cre recombinase expression in cerebellar Purkinje cells. Genesis. 2000;28(3-4):93–98. [PubMed] [Google Scholar]
- 24.Klein AL, Zilian O, Suter U, Taylor V. Murine numb regulates granule cell maturation in the cerebellum. Dev Biol. 2004;266(1):161–177. doi: 10.1016/j.ydbio.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 25.Dehnes Y, et al. The glutamate transporter EAAT4 in rat cerebellar Purkinje cells: A glutamate-gated chloride channel concentrated near the synapse in parts of the dendritic membrane facing astroglia. J Neurosci. 1998;18(10):3606–3619. doi: 10.1523/JNEUROSCI.18-10-03606.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Le TD, et al. Lipid signaling in cytosolic phospholipase A2α-cyclooxygenase-2 cascade mediates cerebellar long-term depression and motor learning. Proc Natl Acad Sci USA. 2010;107(7):3198–3203. doi: 10.1073/pnas.0915020107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ohtani Y, et al. The synaptic targeting of mGluR1 by its carboxyl-terminal domain is crucial for cerebellar function. J Neurosci. 2014;34(7):2702–2712. doi: 10.1523/JNEUROSCI.3542-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ito M, Kano M. Long-lasting depression of parallel fiber-Purkinje cell transmission induced by conjunctive stimulation of parallel fibers and climbing fibers in the cerebellar cortex. Neurosci Lett. 1982;33(3):253–258. doi: 10.1016/0304-3940(82)90380-9. [DOI] [PubMed] [Google Scholar]
- 29.Miyata M, Okada D, Hashimoto K, Kano M, Ito M. Corticotropin-releasing factor plays a permissive role in cerebellar long-term depression. Neuron. 1999;22(4):763–775. doi: 10.1016/s0896-6273(00)80735-7. [DOI] [PubMed] [Google Scholar]
- 30.Ichise T, et al. mGluR1 in cerebellar Purkinje cells essential for long-term depression, synapse elimination, and motor coordination. Science. 2000;288(5472):1832–1835. doi: 10.1126/science.288.5472.1832. [DOI] [PubMed] [Google Scholar]
- 31.Goossens J, et al. Expression of protein kinase C inhibitor blocks cerebellar long-term depression without affecting Purkinje cell excitability in alert mice. J Neurosci. 2001;21(15):5813–5823. doi: 10.1523/JNEUROSCI.21-15-05813.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.De Zeeuw CI, et al. Spatiotemporal firing patterns in the cerebellum. Nat Rev Neurosci. 2011;12(6):327–344. doi: 10.1038/nrn3011. [DOI] [PubMed] [Google Scholar]
- 33.Gao Z, van Beugen BJ, De Zeeuw CI. Distributed synergistic plasticity and cerebellar learning. Nat Rev Neurosci. 2012;13(9):619–635. doi: 10.1038/nrn3312. [DOI] [PubMed] [Google Scholar]
- 34.Ly R, et al. T-type channel blockade impairs long-term potentiation at the parallel fiber-Purkinje cell synapse and cerebellar learning. Proc Natl Acad Sci USA. 2013;110(50):20302–20307. doi: 10.1073/pnas.1311686110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lev-Ram V, Wong ST, Storm DR, Tsien RY. A new form of cerebellar long-term potentiation is postsynaptic and depends on nitric oxide but not cAMP. Proc Natl Acad Sci USA. 2002;99(12):8389–8393. doi: 10.1073/pnas.122206399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Belmeguenai A, Hansel C. A role for protein phosphatases 1, 2A, and 2B in cerebellar long-term potentiation. J Neurosci. 2005;25(46):10768–10772. doi: 10.1523/JNEUROSCI.2876-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wang DJ, et al. Long-term potentiation at cerebellar parallel fiber-Purkinje cell synapses requires presynaptic and postsynaptic signaling cascades. J Neurosci. 2014;34(6):2355–2364. doi: 10.1523/JNEUROSCI.4064-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brasnjo G, Otis TS. Neuronal glutamate transporters control activation of postsynaptic metabotropic glutamate receptors and influence cerebellar long-term depression. Neuron. 2001;31(4):607–616. doi: 10.1016/s0896-6273(01)00377-4. [DOI] [PubMed] [Google Scholar]
- 39.Ito M. Cerebellar circuitry as a neuronal machine. Prog Neurobiol. 2006;78(3-5):272–303. doi: 10.1016/j.pneurobio.2006.02.006. [DOI] [PubMed] [Google Scholar]
- 40.Shigemoto R, Nakanishi S, Mizuno N. Distribution of the mRNA for a metabotropic glutamate receptor (mGluR1) in the central nervous system: An in situ hybridization study in adult and developing rat. J Comp Neurol. 1992;322(1):121–135. doi: 10.1002/cne.903220110. [DOI] [PubMed] [Google Scholar]
- 41.Kim SJ, et al. Transient upregulation of postsynaptic IP3-gated Ca release underlies short-term potentiation of metabotropic glutamate receptor 1 signaling in cerebellar Purkinje cells. J Neurosci. 2008;28(17):4350–4355. doi: 10.1523/JNEUROSCI.0284-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Su LD, Sun CL, Shen Y. Ethanol acutely modulates mGluR1-dependent long-term depression in cerebellum. Alcohol Clin Exp Res. 2010;34(7):1140–1145. doi: 10.1111/j.1530-0277.2010.01190.x. [DOI] [PubMed] [Google Scholar]
- 43.Sun CL, Su LD, Li Q, Wang XX, Shen Y. Cerebellar long-term depression is deficient in Niemann-Pick type C disease mice. Cerebellum. 2011;10(1):88–95. doi: 10.1007/s12311-010-0233-2. [DOI] [PubMed] [Google Scholar]
- 44.Safo PK, Regehr WG. Endocannabinoids control the induction of cerebellar LTD. Neuron. 2005;48(4):647–659. doi: 10.1016/j.neuron.2005.09.020. [DOI] [PubMed] [Google Scholar]
- 45.Han VZ, Zhang Y, Bell CC, Hansel C. Synaptic plasticity and calcium signaling in Purkinje cells of the central cerebellar lobes of mormyrid fish. J Neurosci. 2007;27(49):13499–13512. doi: 10.1523/JNEUROSCI.2613-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Uesaka N, Kawata S, Kano M. Cellular and molecular mechanisms of synapse elimination in the Mammalian brain. Brain Nerve. 2014;66(9):1069–1077. [PubMed] [Google Scholar]
- 47.Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29(3):717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 48.Tanimura A, Kawata S, Hashimoto K, Kano M. Not glutamate but endocannabinoids mediate retrograde suppression of cerebellar parallel fiber to Purkinje cell synaptic transmission in young adult rodents. Neuropharmacology. 2009;57(2):157–163. doi: 10.1016/j.neuropharm.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 49.Wang DJ, et al. Cytosolic phospholipase A2 alpha/arachidonic acid signaling mediates depolarization-induced suppression of excitation in the cerebellum. PLoS One. 2012;7(8):e41499. doi: 10.1371/journal.pone.0041499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohno-Shosaku T, Kano M. Endocannabinoid-mediated retrograde modulation of synaptic transmission. Curr Opin Neurobiol. 2014;29:1–8. doi: 10.1016/j.conb.2014.03.017. [DOI] [PubMed] [Google Scholar]
- 51.Maejima T, Hashimoto K, Yoshida T, Aiba A, Kano M. Presynaptic inhibition caused by retrograde signal from metabotropic glutamate to cannabinoid receptors. Neuron. 2001;31(3):463–475. doi: 10.1016/s0896-6273(01)00375-0. [DOI] [PubMed] [Google Scholar]
- 52.Brown SP, Brenowitz SD, Regehr WG. Brief presynaptic bursts evoke synapse-specific retrograde inhibition mediated by endogenous cannabinoids. Nat Neurosci. 2003;6(10):1048–1057. doi: 10.1038/nn1126. [DOI] [PubMed] [Google Scholar]
- 53.Kano M, et al. Impaired synapse elimination during cerebellar development in PKC gamma mutant mice. Cell. 1995;83(7):1223–1231. doi: 10.1016/0092-8674(95)90147-7. [DOI] [PubMed] [Google Scholar]
- 54.Finch EA, Augustine GJ. Local calcium signalling by inositol-1,4,5-trisphosphate in Purkinje cell dendrites. Nature. 1998;396(6713):753–756. doi: 10.1038/25541. [DOI] [PubMed] [Google Scholar]
- 55.Canepari M, Ogden D. Kinetic, pharmacological and activity-dependent separation of two Ca2+ signalling pathways mediated by type 1 metabotropic glutamate receptors in rat Purkinje neurones. J Physiol. 2006;573(Pt 1):65–82. doi: 10.1113/jphysiol.2005.103770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hartmann J, et al. TRPC3 channels are required for synaptic transmission and motor coordination. Neuron. 2008;59(3):392–398. doi: 10.1016/j.neuron.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chae HG, et al. Transient receptor potential canonical channels regulate the induction of cerebellar long-term depression. J Neurosci. 2012;32(37):12909–12914. doi: 10.1523/JNEUROSCI.0073-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hartmann J, et al. STIM1 controls neuronal Ca²⁺ signaling, mGluR1-dependent synaptic transmission, and cerebellar motor behavior. Neuron. 2014;82(3):635–644. doi: 10.1016/j.neuron.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 59.Roche KW, et al. Homer 1b regulates the trafficking of group I metabotropic glutamate receptors. J Biol Chem. 1999;274(36):25953–25957. doi: 10.1074/jbc.274.36.25953. [DOI] [PubMed] [Google Scholar]
- 60.Karim F, Wang CC, Gereau RW., 4th Metabotropic glutamate receptor subtypes 1 and 5 are activators of extracellular signal-regulated kinase signaling required for inflammatory pain in mice. J Neurosci. 2001;21(11):3771–3779. doi: 10.1523/JNEUROSCI.21-11-03771.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin Y, Kim SJ, Kim J, Worley PF, Linden DJ. Long-term depression of mGluR1 signaling. Neuron. 2007;55(2):277–287. doi: 10.1016/j.neuron.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Batchelor AM, Garthwaite J. Frequency detection and temporally dispersed synaptic signal association through a metabotropic glutamate receptor pathway. Nature. 1997;385(6611):74–77. doi: 10.1038/385074a0. [DOI] [PubMed] [Google Scholar]
- 63.Dhami GK, Ferguson SS. Regulation of metabotropic glutamate receptor signaling, desensitization and endocytosis. Pharmacol Ther. 2006;111(1):260–271. doi: 10.1016/j.pharmthera.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 64.Francesconi A, Kumari R, Zukin RS. Regulation of group I metabotropic glutamate receptor trafficking and signaling by the caveolar/lipid raft pathway. J Neurosci. 2009;29(11):3590–3602. doi: 10.1523/JNEUROSCI.5824-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Choi KY, Chung S, Roche KW. Differential binding of calmodulin to group I metabotropic glutamate receptors regulates receptor trafficking and signaling. J Neurosci. 2011;31(16):5921–5930. doi: 10.1523/JNEUROSCI.6253-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Esseltine JL, Ribeiro FM, Ferguson SS. Rab8 modulates metabotropic glutamate receptor subtype 1 intracellular trafficking and signaling in a protein kinase C-dependent manner. J Neurosci. 2012;32(47):16933–42a. doi: 10.1523/JNEUROSCI.0625-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pandey S, Mahato PK, Bhattacharyya S. Metabotropic glutamate receptor 1 recycles to the cell surface in protein phosphatase 2A-dependent manner in non-neuronal and neuronal cell lines. J Neurochem. 2014;131(5):602–614. doi: 10.1111/jnc.12930. [DOI] [PubMed] [Google Scholar]
- 68.Hong YH, et al. Agonist-induced internalization of mGluR1alpha is mediated by caveolin. J Neurochem. 2009;111(1):61–71. doi: 10.1111/j.1471-4159.2009.06289.x. [DOI] [PubMed] [Google Scholar]
- 69.Roncarati R, et al. The gamma-secretase-generated intracellular domain of beta-amyloid precursor protein binds Numb and inhibits Notch signaling. Proc Natl Acad Sci USA. 2002;99(10):7102–7107. doi: 10.1073/pnas.102192599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scannevin RH, Huganir RL. Postsynaptic organization and regulation of excitatory synapses. Nat Rev Neurosci. 2000;1(2):133–141. doi: 10.1038/35039075. [DOI] [PubMed] [Google Scholar]
- 71.Sheng M, Kim MJ. Postsynaptic signaling and plasticity mechanisms. Science. 2002;298(5594):776–780. doi: 10.1126/science.1075333. [DOI] [PubMed] [Google Scholar]
- 72.Mundell SJ, Matharu AL, Pula G, Roberts PJ, Kelly E. Agonist-induced internalization of the metabotropic glutamate receptor 1a is arrestin- and dynamin-dependent. J Neurochem. 2001;78(3):546–551. doi: 10.1046/j.1471-4159.2001.00421.x. [DOI] [PubMed] [Google Scholar]
- 73.Mundell SJ, Pula G, Carswell K, Roberts PJ, Kelly E. Agonist-induced internalization of metabotropic glutamate receptor 1A: Structural determinants for protein kinase C- and G protein-coupled receptor kinase-mediated internalization. J Neurochem. 2003;84(2):294–304. doi: 10.1046/j.1471-4159.2003.01515.x. [DOI] [PubMed] [Google Scholar]
- 74.Coesmans M, et al. Mechanisms underlying cerebellar motor deficits due to mGluR1-autoantibodies. Ann Neurol. 2003;53(3):325–336. doi: 10.1002/ana.10451. [DOI] [PubMed] [Google Scholar]
- 75.Wadiche JI, Jahr CE. Patterned expression of Purkinje cell glutamate transporters controls synaptic plasticity. Nat Neurosci. 2005;8(10):1329–1334. doi: 10.1038/nn1539. [DOI] [PubMed] [Google Scholar]
- 76.Ebner TJ, Wang X, Gao W, Cramer SW, Chen G. Parasagittal zones in the cerebellar cortex differ in excitability, information processing, and synaptic plasticity. Cerebellum. 2012;11(2):418–419. doi: 10.1007/s12311-011-0347-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Zhou H, et al. Cerebellar modules operate at different frequencies. eLife. 2014;3:e02536. doi: 10.7554/eLife.02536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Committee on Care and Use of Laboratory Animals 1996. Guide for the Care and Use of Laboratory Animals (Natl Inst Health, Bethesda), DHHS Publ No (NIH) 85-23.
- 79.Lee KJ, Kim H, Kim TS, Park SH, Rhyu IJ. Morphological analysis of spine shapes of Purkinje cell dendrites in the rat cerebellum using high-voltage electron microscopy. Neurosci Lett. 2004;359(1-2):21–24. doi: 10.1016/j.neulet.2004.01.071. [DOI] [PubMed] [Google Scholar]
- 80.Dai ZM, et al. Stage-specific regulation of oligodendrocyte development by Wnt/β-catenin signaling. J Neurosci. 2014;34(25):8467–8473. doi: 10.1523/JNEUROSCI.0311-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.